Abstract.
The sensitivity of a point-of-care circulating cathodic antigen (POC-CCA) urine cassette test for diagnosis of Schistosoma mansoni in low-endemicity settings is poorly understood. We conducted a cross-sectional survey in 14 villages in western Côte d’Ivoire and diagnosed children aged 9–12 years for schistosomiasis. Two stool samples were subjected to triplicate Kato–Katz thick smears each for diagnosis of S. mansoni, whereas a single urine sample was examined by POC-CCA for S. mansoni, filtration for Schistosoma haematobium, and reagent strip for microhematuria. According to the Kato–Katz technique, we found 45 out of 681 children positive for S. mansoni (6.6%) with a mean intensity among infected children of 72.2 eggs per gram of stool. Point-of-care circulating cathodic antigen revealed a prevalence of S. mansoni of 33.0% when trace results were considered positive and 12.5% when trace results were considered negative. Eggs of S. haematobium were found in eight participants (1.2%), whereas the prevalence of microhematuria was 13.5%. A single POC-CCA urine cassette test revealed a several-fold higher prevalence of S. mansoni than multiple Kato–Katz thick smears in this low-endemicity area. Our findings have important ramifications for choosing an appropriate diagnostic tool in low-endemic areas that might be targeted for elimination.
INTRODUCTION
Schistosomiasis mansoni is endemic in the western part of Côte d’Ivoire.1–4 Facilitated by the Schistosomiasis Consortium for Operational Research and Evaluation (SCORE), a 5-year cluster-randomized trial was launched in 2011 in 75 villages of western Côte d’Ivoire.5 Three school-based preventive chemotherapy schedules were implemented, which considerably reduced the prevalence and intensity of Schistosoma mansoni infection already in the first year of intervention.5,6 Toward the end of the trial, school children were invited to submit three stool samples over consecutive days. Stool samples were subjected to the Kato–Katz technique that is widely used in epidemiologic investigations pertaining to intestinal schistosomiasis.5–7
Although the Kato–Katz technique is a valuable tool at the onset of large-scale control programs, it is less useful for disease surveillance in settings where the prevalence and intensity decreased after several rounds of praziquantel treatment.7,8 Indeed, the Kato–Katz method underestimates the true prevalence in low-endemicity settings and shows low sensitivity after praziquantel treatment.8–11 This urges for novel and more sensitive diagnostic approaches to monitor the prevalence and intensity of S. mansoni in areas with established control programs that implement preventive chemotherapy. The aforementioned SCORE trial provided an opportunity to assess the sensitivity of a point-of-care circulating cathodic antigen (POC-CCA) urine cassette test for the diagnosis of S. mansoni in the final year of treatment intervention when the endemicity of schistosomiasis mansoni was suspected to be low.
MATERIALS AND METHODS
Ethics.
Ethics approval was obtained from the “Comité National d’Ethique et de la Recherche” of the Ministry of Health in Côte d’Ivoire (reference no. 046/MSHP/CNER-kp; date of assignment: May 30, 2016). Local health and education authorities and village leaders were informed about the purpose and procedures of the study. Participation was voluntary, and hence, children could withdraw any time without further obligation. At the end of the study, all children and nonpregnant girls aged between 5 and 15 years were given a single 40 mg/kg oral dose of praziquantel, free of charge, following World Health Organization guidelines.12
Study design.
The main outcome of the study was the prevalence of S. mansoni, as assessed by the standard Kato–Katz technique using stool samples, to be compared with the S. mansoni prevalence based on the POC-CCA test using a single urine sample. The study was conducted in October 2016 in three regions of western Côte d’Ivoire (geographic coordinates: lat. 06°32′42.0″–07°36′54.8″ N and long. 06°44′09.8″–07°33′48.9″ W). It followed a cross-sectional design and was carried out in 14 schools purposely selected based on low prevalence (< 10%) of S. mansoni infection among 9- to 12-year-old children, as detected by the Kato–Katz method13 in the final survey of the SCORE study after two or four treatments with praziquantel over a 4-year period.4,14 Among the selected villages, Ziondrou and Gbadrou were persistent hot spots as defined according to changes in the prevalence of S. mansoni from year 1 to year 2 with slight increases of 15.6% and 10.5% after the first round of praziquantel treatment, respectively.15 These two villages were selected for assessing the main features that contribute to the increase of S. mansoni prevalence. In the remaining 12 villages, the prevalence of S. mansoni ranged from nil to 10%. All children aged 9–12 years in the 14 study villages who attended school, had no chronic disease conditions, and were nonpregnant were eligible to participate.
Stool and urine collection.
In each school, 50 children who met the eligibility criteria were randomly selected. Written informed consent was obtained from parents or guardians, whereas children provided oral assent. Children were given two pre-labeled plastic containers and were invited on the next day to provide a portion of their fresh morning stool in one container and a urine sample produced between 10:00 and 12:00 hours in the second container. For each child, two stool samples were collected on consecutive days, whereas only a single urine sample was collected on the first day.
Triplicate 41.7 mg Kato–Katz thick smears were prepared from each stool sample and examined under a microscope using a standard protocol.13 The number of S. mansoni eggs was counted and recorded for each thick smear. Mean egg counts were multiplied by a factor of 24 to obtain a measure of infection intensity, as expressed as the number of eggs per gram of stool (EPG). Children harboring S. mansoni eggs were categorized into 1) light (1–99 EPG), 2) moderate (100–399 EPG), and 3) heavy (≥ 400 EPG) intensity of infection.16 In addition, eggs of hookworm, Ascaris lumbricoides, Enterobius vermicularis, Hymenolepis spp., Taenia spp., and Trichuris trichiura were investigated, but will not be presented in this article.
Urine samples were subjected to a filtration method, counting the number of Schistosoma haematobium eggs in 10 mL of urine, visual inspection for macrohematuria, and reagent strips (Hemastix; Siemens Healthcare Diagnostics GmbH, Zurich, Switzerland; batch number: ref 2816A) for microhematuria.17 The intensity of S. haematobium was classified as light (1–49 eggs/10 mL of urine) or heavy (≥ 50 eggs/10 mL of urine).16 Schistosoma haematobium data were considered in the present research to evaluate the eventual cross-reactivity in urinary tract infection. The results of the reagent strips were stratified into negative and positive, the latter including non-hemolyzed and hemolyzed trace of blood, 1+, 2+, and 3+, according to the manufacturer’s instructions. In addition, a POC-CCA (Rapid Medical Diagnostics, Pretoria, South Africa; batch number: 50182) was performed.18 In brief, one drop of urine was added in the well of the cassette. Once completely absorbed, one drop of test buffer was added to the well and allowed to develop for 20 minutes. The tests were read by an experienced laboratory technician and scored as negative, trace, or positive.
Statistical analysis.
Data were entered into Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA). Statistical analyses were performed with STATA version IC13.1 (Stata Corporation, College Station, TX). A χ2 test was conducted and 95% confidence intervals (CIs) were calculated, considering statistical significance for P values < 0.05 and non-overlapping 95% CIs. We considered results from the Kato–Katz technique as the diagnostic “gold” standard. Prevalence of S. mansoni using three different diagnostic interpretations (i.e., multiple Kato–Katz thick smears, POC-CCA with trace considered positive and POC-CCA with trace considered negative), S. haematobium, and microhematuria were displayed by geographical location using Arc Map 10.5 (Environmental Systems Research Institute, Inc., Redlands, CA).
RESULTS
Overall, 681 children aged 9–12 years were included in the final analyses; 308 (45.2%) females and 373 (54.8%) males. Most of the children provided two stool samples (N = 641, 94.1%), whereas the remaining 40 children had a single stool sample examined. Microscopic examination of stool and urine samples revealed that 53 children (7.8%) harbored eggs of S. mansoni and S. haematobium. No S. mansoni–S. haematobium coinfection was observed.
Eggs of S. mansoni (based on six Kato–Katz thick smears for 641 children and three Kato–Katz thick smears for the remaining children) were found in 45 children, owing to an overall prevalence of 6.6% (95% CI: 4.7–8.4%). Males had a slightly higher S. mansoni prevalence than females (6.9% versus 6.1%). At the unit of the school, the prevalence of S. mansoni ranged from nil to 22.0% (Table 1). Twelve schools had a prevalence of S. mansoni below 10%, whereas the remaining two schools were above this threshold (Gbadrou and Ziondrou). The arithmetic mean egg count among S. mansoni–positive individuals was 72.2 EPG, ranging from 4.0 (Siambly) to 234.7 EPG (Gbadrou) (Table 2). Most of the infected children (86.7%) had low-intensity S. mansoni infections with less than 100 EPG.
Table 1.
Prevalence of Schistosoma mansoni and Schistosoma haematobium infections in 9- to 12-year-old children from 14 schools in western Côte d’Ivoire, in a cross-sectional survey carried out in October 2016
| Village | No. of children examined | S. mansoni | Hematuria | S. haematobium | |||||
|---|---|---|---|---|---|---|---|---|---|
| Point-of-care circulating cathodic antigen urine cassette test* | Kato–Katz method | Reagent strip | Urine filtration | ||||||
| No. (%) of children positive | 95% CI | No. (%) of children positive | (95% CI) | No. (%) of children with micro-hematuria | (95% CI) | No. (%) of children positive | (95% CI) | ||
| Baoulé Carrefour | 50 | 15 (30.0) | (16.8–43.2) | 2 (4.0) | (−1.6–9.6) | 4 (8.0) | (0.2–15.8) | 0 (0.0) | – |
| Gbadrou | 50 | 22 (44.0) | (29.7–58.8) | 6 (12.0) | (2.7–21.3) | 13 (26.0) | (13.4–38.6) | 0 (0.0) | – |
| Gregbeu | 50 | 12 (24.0) | (11.7–36.2) | 0 (0.0) | – | 0 (0.0) | – | 0 (0.0) | – |
| Klangbolably | 49 | 16 (32.7) | (19.0–46.3) | 3 (6.0) | (−0.8–13.1) | 13 (26.5) | (13.7–39.3) | 0 (0.0) | – |
| Koua | 50 | 14 (28.0) | (15.1–40.8) | 4 (8.0) | (0.2–15.8) | 6 (12.0) | (2.7–21.3) | 0 (0.0) | – |
| Mahinahi | 47 | 16 (34.0) | (19.9–48.1) | 1 (2.1) | (−2.1–6.4) | 3 (6.4) | (−0.8–13.6) | 0 (0.0) | – |
| Mona | 49 | 18 (36.7) | (22.7–50.7) | 2 (4.1) | (−1.6–9.8) | 8 (16.3) | (5.6–27.1) | 0 (0.0) | – |
| Semien | 36 | 7 (19.4) | (5.8–33.0) | 3 (6.3) | (−1.2–17.8) | 0 (0.0) | – | 0 (0.0) | – |
| Siambly | 50 | 12 (24.0) | (11.7–36.2) | 1 (2.0) | (−2.0–6.0) | 0 (0.0) | – | 0 (0.0) | – |
| Tobly Bangolo | 50 | 11 (22.0) | (10.1–33.9) | 4 (8.0) | (0.2–15.8) | 5 (10.0) | (1.4–18.6) | 0 (0.0) | – |
| Ziondrou | 50 | 25 (50.0) | (35.6–64.5) | 11 (22.0) | (10.1–33.9) | 9 (18.0) | (7.0–29.0) | 2 (4.0) | (−1.6–9.6) |
| Zouatta 2 | 50 | 22 (44.0) | (29.7–58.2) | 3 (6.0) | (−0.8–12.8) | 22 (44.0) | (29.7–58.3) | 1 (2.0) | (−2.0–6.0) |
| Zoukougbeu | 50 | 22 (44.0) | (29.7–58.2) | 3 (6.0) | (−0.8–12.8) | 8 (16.0) | (5.5–26.5) | 4 (8.0) | (0.2–15.8) |
| Zê | 50 | 13 (26.0) | (13.4–38.6) | 2 (4.0) | (−1.6–9.6) | 1 (2.0) | (−2.0–6.0) | 1 (2.0) | (−2.0–6.0) |
| Total | 681 | 225 (33.0) | (29.5–36.6) | 45 (6.6) | (4.7–8.4) | 92 (13.5) | (10.9–16.1) | 8 (1.2) | (0.3–1.9) |
Trace results considered as positive.
Table 2.
Intensity of Schistosoma mansoni and Schistosoma haematobium infections in 9- to 12-year-old children from 14 schools in western Côte d’Ivoire, in a cross-sectional survey carried out in October 2016
| Village | S. mansoni | S. haematobium | ||
|---|---|---|---|---|
| AM (EPG) | (95% CI) | Eggs/10 mL | (95% CI) | |
| Baoulé Carrefour | 12.0 | (−38.8–62.8) | 0.0 | – |
| Gbadrou | 234.7 | (−325.8–795.2) | 0.0 | – |
| Gregbeu | 0.0 | – | 0.0 | – |
| Klangbolably | 21.3 | (−1.6–44.3) | 0.0 | – |
| Koua | 13.0 | (5.0–21.0) | 0.0 | – |
| Mahinahi | 88.0 | – | 0.0 | – |
| Mona | 56.0 | (−503.1–615.1) | 0.0 | – |
| Semien | 60.0 | (−52.0–172.0) | 0.0 | – |
| Siambly | 4.0 | – | 0.0 | – |
| Tobly Bangolo | 10.0 | (−2.2–22.2) | 0.0 | – |
| Ziondrou | 76.4 | (0.3–152.4) | 6.5 | (−50.7−63.7) |
| Zouatta 2 | 72.0 | (−186.4–330.3) | 11.0 | – |
| Zoukougbeu | 49.3 | (−137.2–235.8) | 21.8 | (−5.3–48.8) |
| Zê | 36.0 | (−167.3–239.3) | 37.0 | – |
| Total | 72.2 | (11.3–133.1) | 18.5 | (5.7–31.3) |
AM (EPG) = arithmetic mean egg count (eggs per gram).
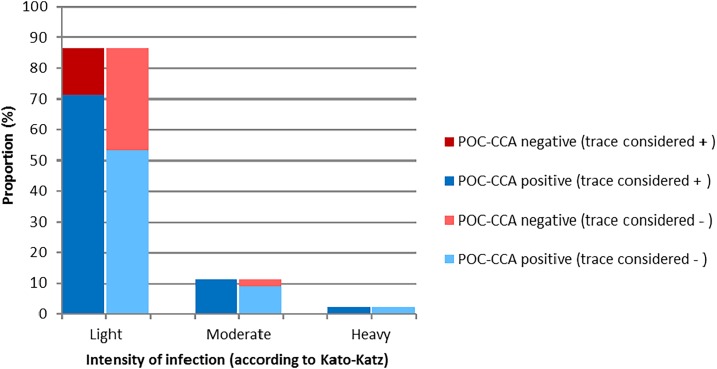
A single POC-CCA urine cassette test revealed S. mansoni prevalence of 33.0% (225 positive children; 95% CI: 29.5–36.6%) when considering trace results as positive. At the unit of the school, the S. mansoni prevalence using POC-CCA ranged from 19.4% (Semien) to 50.0% (Ziondrou). Considering trace results as negative, the respective prevalence of S. mansoni was 12.5% (85 positive children; 95% CI: 9.9–14.9%). Within the Kato–Katz positives, 29 of 45 children (64.4%) were confirmed by POC-CCA testing considering trace as negative and 38 children (84.4%) considering trace as positive. In the seven Kato–Katz–positive children who showed negative POC-CCA results (if trace included as positive), the mean egg counts were consistently below 100 EPG. Hence, if trace results were included as positive, the POC-CCA only missed light-intensity infections with an overall false-negative proportion of 15.5% (Figure 1). If considering trace results as negative, the sensitivity and specificity of the POC-CCA urine cassette test for S. mansoni diagnosis was 64.4% and 91.2%, respectively. The corresponding sensitivity and specificity if trace results were considered positive were 84.4% and 70.6%, respectively.
Figure 1.
Schistosoma mansoni infection by intensity category and point-of-care circulating cathodic antigen (POC-CCA) performance in children found positive by the Kato–Katz technique (N = 45).
No macrohematuria was observed. Reagent strip tests found 92 (13.5%) children with microhematuria. The S. haematobium prevalence, according to a single urine filtration, was very low (1.2%). Eggs of S. haematobium were found in the urine of children from only four schools, whereas microhematuria was observed in 11 schools. The arithmetic mean egg count of S. haematobium was 18.5 eggs/10 mL (95% CI: 5.7–31.3 eggs/10 mL) ranging from zero to 37.0 eggs/10 mL (Table 2). All S. haematobium–positive children had light-intensity infections.
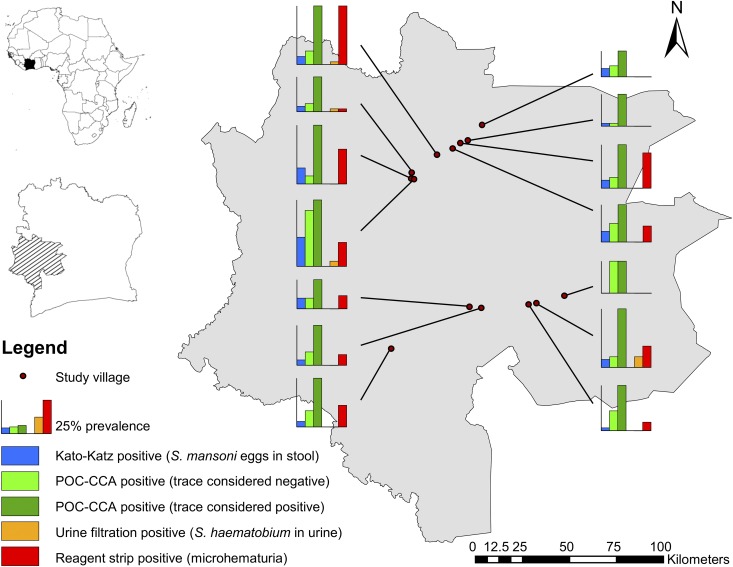
Figure 2 displays the spatial distribution of schistosomiasis in the study area, according to Kato–Katz and POC-CCA for S. mansoni, and urine filtration for S. haematobium and microhematuria using reagent strips.
Figure 2.
Map of the study area in the western part of Côte d’Ivoire showing the distribution of Schistosoma mansoni (based on Kato–Katz and point-of-care circulating cathodic antigen [POC-CCA]) and Schistosoma haematobium infections and the percentage of microhematuria positives in each of 14 schools examined in October 2016.
DISCUSSION
A preceding multi-country SCORE study revealed that a single POC-CCA urine cassette test is at least as sensitive as triplicate Kato–Katz thick smears for the diagnosis of S. mansoni.19 However, relatively little is known about the sensitivity of POC-CCA in low-endemicity settings, which are gaining in importance because of large-scale preventive chemotherapy programs.20 Indeed, in the present study, the baseline prevalence of S. mansoni at the onset of a 5-year cluster-randomized trial in 2011 in the 14 villages studied here was 19.0%,5 whereas 5 years later after two or four rounds of praziquantel administration, it had dropped to 6.6% (current data). These estimates are based on the most widely used diagnostic approach for S. mansoni—the Kato–Katz method.7 According to the POC-CCA urine cassette test with trace results considered positive, we found a 5-fold higher prevalence of S. mansoni (33.0%), whereas POC-CCA with trace results considered negative resulted in a 2-fold higher prevalence (12.5%) compared with stool microscopy. The POC-CCA has been proposed for the mapping of S. mansoni.19 Recent studies among African migrants in Europe conjectured that the POC-CCA urine cassette test might also be considered for individual diagnosis.21–23
Our findings indicate that the effect of regular deworming with praziquantel is overestimated, at least when one is using prevalence reduction based on an insensitive diagnostic method, such as the Kato–Katz technique. Our results might thus raise concern about the global strategy of schistosomiasis control, and call for more sensitive diagnostic tools, as the focus shifts from morbidity control to interrupting transmission. The POC-CCA appears to be a useful tool in low-endemicity settings.24 Our results corroborate with the findings from a recent article, analyzing data of 19 studies by comparing S. mansoni prevalence revealed by Kato–Katz and POC-CCA. Indeed, POC-CCA urine cassette test results found S. mansoni prevalence estimates that were between 1.5-fold and up to 6-fold higher than standard Kato–Katz.25 Cross-reactivity from urinary tract infection (in school-aged children in our setting most likely due to S. haematobium infection) and hematuria cannot be ruled out completely. However, we would like to point out that in two study villages (i.e., Gregbeu and Siambly), no or only one of the participating children excreted S. mansoni eggs, as detected by Kato–Katz. In contrast, a single POC-CCA revealed an S. mansoni prevalence of 24.0%. In these villages, no S. haematobium was found (neither by urine filtration nor by reagent strip), and hence, we assume the risk of potential cross-reactivity as minimal in our study setting. Higher sensitivity of the POC-CCA may also be explained by taking into account findings from animal models. These showed detectable CCA levels for S. mansoni as early as 3 weeks after experimental infection, highlighting the potential to also capture infection status with juvenile stages not yet producing eggs.26 We can further exclude discrepancies between egg excretion and remaining antigen from recently killed adult worms, because there was more than a 15-month span between the last treatment round and the current cross-sectional survey. It should also be noted that standard urine filtration and microscopy revealed a very low prevalence of S. haematobium (1.2%), whereas reagent strip testing revealed a prevalence of microhematuria of 13.5%. This observation corroborates findings from previous studies in Côte d’Ivoire and Chad, where a “background” prevalence of microhematuria of about 13% was observed in the absence of S. haematobium eggs in children’s urine.27 This result may be due to menstrual blood loss among the oldest females included in our study (e.g., aged 12 years), as this can lead to reagent strip–positive results. Yet, it should also be noted that examination of larger volumes of urine (e.g., 50 mL and more) and duplication urine might have revealed considerably higher S. haematobium prevalence rates.
Previous studies assessing the sensitivity of POC-CCA for S. mansoni diagnosis elsewhere in Côte d’Ivoire and in Cameroon showed that S. haematobium and soil-transmitted helminth infections did not influence POC-CCA results.18,28 The map in Figure 2 exemplifies how focally schistosomiasis is distributed. For example, in the three schools situated in a perimeter of less than 5 km in the northwestern part of the study area (i.e., Gbadrou, Zê, and Ziondrou), S. mansoni and microhematuria showed considerable spatial heterogeneity.
A limitation of our study is that, unlike other studies,10,11,29 we did not further stratify POC-CCA–positive results using band strengths. Our data, thus, did not allow for direct correlation of egg count and rapid test-based intensity measures. We did, however, observe that false-negative POC-CCA results were predominantly found in individuals with egg counts below 100 EPG and that they depended on the interpretation of the trace results to a similar extent as in earlier studies.29
CONCLUSION
Our results confirm that a single POC-CCA urine cassette test is considerably more sensitive than multiple Kato–Katz thick smears in a low-endemicity setting of schistosomiasis mansoni after multiple rounds of preventive chemotherapy. Point-of-care circulating cathodic antigen might, therefore, be considered as a tool for S. mansoni rapid risk profiling. In view of the findings presented here, the potential role of a POC-CCA urine cassette test for determining the impact of preventive chemotherapy with praziquantel, particularly in low-endemicity settings, should be investigated.
Acknowledgments:
We are grateful to the Schistosomiasis Consortium for Operational Research and Evaluation (SCORE) secretariat for reviewing the study protocol, and for useful comments on the manuscript draft. We thank Sadikou Touré, Raphael G. Diabré, Diabaté Salia, and Vincent A. Anzara for help with the field and laboratory work. The team of the Laboratoire de Zoologie et de Biologie Animale at the Université Félix Houphouët-Boigny is acknowledged for assistance with laboratory work. We thank the staff of the Centre Suisse de Recherches Scientifiques en Côte d’Ivoire for administrative and technical support. We are grateful to Dr. Aboulaye Meïté, Director of the Programme National de Lutte contre les Maladies Tropicales Négligées à Chimiothérapie Préventive at the Ministry of Health, and his team for the administrative support and for treating children with praziquantel. We thank teachers, parents, guardians, and children for their participation in the study. We thank the health, education, and village authorities of Guemon, Cavally, and Haut-Sassandra for their contributions.
REFERENCES
- 1.Utzinger J, N’Goran EK, Esse Aya CM, Acka Adjoua C, Lohourignon KL, Tanner M, Lengeler C, 1998. Schistosoma mansoni, intestinal parasites and perceived morbidity indicators in schoolchildren in a rural endemic area of western Côte d’Ivoire. Trop Med Int Health 3: 711–720. [DOI] [PubMed] [Google Scholar]
- 2.Raso G, Matthys B, N’Goran EK, Tanner M, Vounatsou P, Utzinger J, 2005. Spatial risk prediction and mapping of Schistosoma mansoni infections among schoolchildren living in western Côte d’Ivoire. Parasitology 131: 97–108. [DOI] [PubMed] [Google Scholar]
- 3.Assaré RK, Lai YS, Yapi A, Tian-Bi YNT, Ouattara M, Yao PK, Knopp S, Vounatsou P, Utzinger J, N’Goran EK, 2015. The spatial distribution of Schistosoma mansoni infection in four regions of western Côte d’Ivoire. Geospat Health 10: 345. [DOI] [PubMed] [Google Scholar]
- 4.Assaré RK, et al. 2014. Sustaining control of schistosomiasis mansoni in moderate endemicity areas in western Côte d’Ivoire: a SCORE study protocol. BMC Public Health 14: 1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Assaré RK, et al. 2016. Sustaining the control of Schistosoma mansoni in western Côte d’Ivoire: baseline findings before the implementation of a randomized trial. Am J Trop Med Hyg 94: 352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Assaré RK, et al. 2016. Sustaining control of schistosomiasis mansoni in western Côte d’Ivoire: results from a SCORE study, one year after initial praziquantel administration. PLoS Negl Trop Dis 10: e0004329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Utzinger J, Becker SL, van Lieshout L, van Dam GJ, Knopp S, 2015. New diagnostic tools in schistosomiasis. Clin Microbiol Infect 21: 529–542. [DOI] [PubMed] [Google Scholar]
- 8.Knopp S, Becker SL, Ingram KJ, Keiser J, Utzinger J, 2013. Diagnosis and treatment of schistosomiasis in children in the era of intensified control. Expert Rev Anti Infect Ther 11: 1237–1258. [DOI] [PubMed] [Google Scholar]
- 9.Koukounari A, Donnelly CA, Moustaki I, Tukahebwa EM, Kabatereine NB, Wilson S, Webster JP, Deelder AM, Vennervald BJ, van Dam GJ, 2013. A latent Markov modelling approach to the evaluation of circulating cathodic antigen strips for schistosomiasis diagnosis pre- and post-praziquantel treatment in Uganda. PLoS Comput Biol 9: e1003402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adriko M, Standley CJ, Tinkitina B, Tukahebwa EM, Fenwick A, Fleming FM, Sousa-Figueiredo JC, Stothard JR, Kabatereine NB, 2014. Evaluation of circulating cathodic antigen (CCA) urine-cassette assay as a survey tool for Schistosoma mansoni in different transmission settings within Bugiri District, Uganda. Acta Trop 136: 50–57. [DOI] [PubMed] [Google Scholar]
- 11.Lamberton PH, Kabatereine NB, Oguttu DW, Fenwick A, Webster JP, 2014. Sensitivity and specificity of multiple Kato-Katz thick smears and a circulating cathodic antigen test for Schistosoma mansoni diagnosis pre- and post-repeated-praziquantel treatment. PLoS Negl Trop Dis 8: e3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO , 2006. Preventive Chemotherapy in Human Helminthiasis: Coordinated Use of Anthelminthic Drugs in Control Interventions: A Manual for Health Professionals and Programme Managers. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 13.Yap P, Fürst T, Müller I, Kriemler S, Utzinger J, Steinmann P, 2012. Determining soil-transmitted helminth infection status and physical fitness of school-aged children. J Vis Exp 66: e3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ezeamama AE, et al. 2016. Gaining and sustaining schistosomiasis control: study protocol and baseline data prior to different treatment strategies in five African countries. BMC Infect Dis 16: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kittur N, Binder S, Campbell CH, King CH, Kinung’hi S, Olsen A, Magnussen P, Colley DG, 2017. Defining persistent hotspots: areas that fail to decrease meaningfully in prevalence after multiple years of mass drug administration with praziquantel for control of schistosomiasis. Am J Trop Med Hyg 97: 1810–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO , 2002. Prevention and control of schistosomiasis and soil-transmitted helminthiasis: report of a WHO expert committee. World Health Organ Tech Rep Ser 912: 1–57. [PubMed] [Google Scholar]
- 17.Plouvier S, Leroy J-C, Colette J, 1975. A propos d’une technique simple de filtration des urines dans le diagnostic de la bilharziose urinaire en enquête de masse. Med Trop 35: 229–230. [Google Scholar]
- 18.Coulibaly JT, et al. 2011. Accuracy of urine circulating cathodic antigen (CCA) test for Schistosoma mansoni diagnosis in different settings of Côte d’Ivoire. PLoS Negl Trop Dis 5: e1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colley DG, et al. 2013. A five-country evaluation of a point-of-care circulating cathodic antigen urine assay for the prevalence of Schistosoma mansoni. Am J Trop Med Hyg 88: 426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO , 2017. Schistosomiasis and soil-transmitted helminthiases: number of people treated in 2016 [Article in English, French]. Wkly Epidemiol Rec 92: 749–760. [PubMed] [Google Scholar]
- 21.Becker SL, Marti H, Zimmermann S, Vidacek D, Herrmann M, Utzinger J, Schnabel PA, Bohle RM, 2015. Application in Europe of a urine-based rapid diagnostic test for confirmation of Schistosoma mansoni infection in migrants from endemic areas. Euro Surveill 20: 21151. [DOI] [PubMed] [Google Scholar]
- 22.Chernet A, et al. 2017. Accuracy of diagnostic tests for Schistosoma mansoni infection in asymptomatic Eritrean refugees: serology and point-of-care circulating cathodic antigen against stool microscopy. Clin Infect Dis 65: 568–574. [DOI] [PubMed] [Google Scholar]
- 23.Infurnari L, Galli L, Bigoloni A, Carbone A, Chiappetta S, Sala A, Ceserani N, Lazzarin A, Castagna A, Gaiera G, 2017. The use of circulating cathodic antigen rapid test and serology for diagnosis of active Schistosoma mansoni infection in migrants in Italy, a non-endemic country: a cross sectional study. Mem Inst Oswaldo Cruz 112: 452–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Shehri H, Koukounari A, Stanton MC, Adriko M, Arinaitwe M, Atuhaire A, Kabatereine NB, Stothard JR, 2018. Surveillance of intestinal schistosomiasis during control: a comparison of four diagnostic tests across five Ugandan primary schools in the Lake Albert region. Parasitology 145: 1715–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kittur N, Castleman JD, Campbell CH, King CH, Colley DG, 2016. Comparison of Schistosoma mansoni prevalence and intensity of infection, as determined by the circulating cathodic antigen urine assay or by the Kato-Katz fecal assay: a systematic review. Am J Trop Med Hyg 94: 605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Dam GJ, Bogitsh BJ, van Zeyl RJM, Rotmans JP, Deelder AM, 1996. Schistosoma mansoni: in vitro and in vivo excretion of CAA and CCA by developing schistosomula and adult worms. J Parasitol 82: 557–564. [PubMed] [Google Scholar]
- 27.Krauth SJ, Greter H, Stete K, Coulibaly JT, Traoré SI, Ngandolo BN, Achi LY, Zinsstag J, N’Goran EK, Utzinger J, 2015. All that is blood is not schistosomiasis: experiences with reagent strip testing for urogenital schistosomiasis with special consideration to very-low prevalence settings. Parasit Vectors 8: 584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tchuem Tchuenté LA, Kuete Fouodo CJ, Kamwa Ngassam RI, Sumo L, Dongmo Noumedem C, Kenfack CM, Gipwe NF, Nana ED, Stothard JR, Rollinson D, 2012. Evaluation of circulating cathodic antigen (CCA) urine-tests for diagnosis of Schistosoma mansoni infection in Cameroon. PLoS Negl Trop Dis 6: e1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silveira AM, Costa EG, Ray D, Suzuki BM, Hsieh MH, Fraga LA, Caffrey CR, 2016. Evaluation of the CCA immuno-chromatographic test to diagnose Schistosoma mansoni in Minas Gerais state, Brazil. PLoS Negl Trop Dis 10: e0004357. [DOI] [PMC free article] [PubMed] [Google Scholar]