Abstract.
At the end phase of the Global Programme to Eliminate Lymphatic Filariasis, antibody testing may have a role in decision-making for bancroftian filariasis–endemic areas. This study evaluated the diagnostic performance of BLF Rapid™, a prototype immunochromatographic IgG4-based test using BmSXP recombinant protein, for detection of bancroftian filariasis. The test was evaluated using 258 serum samples, comprising 96 samples tested at Universiti Sains Malaysia (in-house) and 162 samples tested independently at three international laboratories in the USA and India, and two laboratories in Malaysia. The independent testing involved 99 samples from Wuchereria bancrofti microfilaria or antigen positive individuals and 63 samples from people who were healthy or had other infections. The in-house evaluation showed 100% diagnostic sensitivity and specificity. The independent evaluations showed a diagnostic sensitivity of 84–100% and 100% specificity (excluding non-lymphatic filarial infections). BLF Rapid has potential as a surveillance diagnostic tool to make “Transmission Assessment Survey”–stopping decisions and conduct post-elimination surveillance.
Lymphatic filariasis (LF), caused by the filariae worms Wuchereria bancrofti, Brugia malayi, and Brugia timori, is a neglected tropical disease endemic in 53 countries.1 Infection, typically acquired in childhood, is often asymptomatic with clinical manifestations that may present later in life.2 Although it is rarely fatal, LF is a major cause of suffering and disability, and has substantial social and economic impact. In the Global Burden of Disease study in 2016, LF accounted for 1.189 million disability-adjusted life years.3
The Global Programme to Eliminate Lymphatic Filariasis (GPELF) was established by the World Health Organization to eliminate LF as a public health problem by the year 2020. Preventive chemotherapy by mass drug administration (MDA) is the core strategy of the program, followed by morbidity management and disability prevention.4 As of 2016, an estimated 495.6 million people in 40 countries have received MDA.1
The GPELF requires diagnostic tools for mapping LF distribution, monitoring of MDA progress, making MDA stopping decisions, and conducting post-MDA surveillance, including transmission assessment surveys (TAS). The current test used for TAS in bancroftian filariasis–endemic areas is the Filariasis Test Strip (Abbott, Scarborough, ME), which is a modified version of the previous Alere BinaxNOW® Filariasis (Alere, Scarborough, ME) card test (immunochromatographic test [ICT]). It detects circulating W. bancrofti antigen in blood and has been useful in various activities of the GPELF.4 However, as LF prevalence declines, an antibody detection test may be needed to confirm interruption of transmission because filarial-specific antibodies provide an early indicator of exposure to filarial parasites.5 In brugian filariasis endemic areas, an IgG4 antibody test based on BmR1 recombinant protein (Brugia Rapid; Reszon Diagnostics International Sdn. Bhd., Selangor, Malaysia), has been useful for TAS activities.6
At Universiti Sains Malaysia (USM), we have developed a prototype antibody detection rapid test for bancroftian filariasis called BLF Rapid. It is based on immunochromatographic technology and detects the presence of specific anti-filarial IgG4 against BmSXP recombinant protein in serum samples of bancroftian filariasis patients. In this study, the main aim was to assess the diagnostic performance of BLF Rapid by evaluating its diagnostic sensitivity and specificity. It was tested at USM and five other laboratories using stored samples in the serum bank of the respective laboratories. This was an initial step in assessing the potential of BLF Rapid to assist in the LF elimination program in bancroftian filariasis endemic areas.
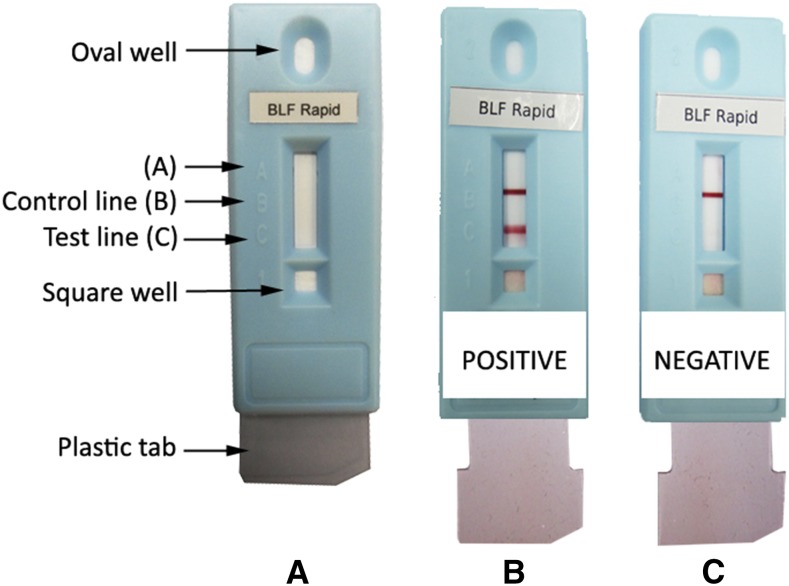
As shown in Figure 1A, BLF Rapid comprises BmSXP recombinant protein as the test line (position C) and anti-mouse IgG as the reagent control line (position B). Another commercial test which contains BmSXP is called PanLF Rapid (Reszon Diagnostics International Sdn. Bhd., Selangor, Malaysia). PanLF Rapid contains BmR1 and BmSXP test lines and is meant for use in areas with mainly brugian filariasis, but with possible co-occurrence of bancroftian filariasis, such as in Malaysia.7,8 The concentration and purity of BmSXP used in PanLF Rapid is lower than that used in BLF Rapid. The higher protein concentration in BLF Rapid increases its diagnostic sensitivity, whereas the higher protein purity ensures a high diagnostic specificity. The BLF Rapid test is performed by pipetting 30 µL of serum sample into the square-bottom well or 35 µL of whole blood is placed in the square well followed by a drop of chase buffer. The sample is allowed to flow by capillary action until the sample reaches position A. Three drops of chase buffer are added to a top oval well to release the anti-human IgG4-gold conjugate; followed by pulling a clear plastic tab at the bottom of the cassette. One drop of chase buffer is then added in the square-bottom well. This step reduces the flow rate of the gold conjugated-IgG4 down the membrane strip, thus allowing more interaction time between the gold conjugated-IgG4 and anti-filarial IgG4 that bind with BmSXP recombinant protein on the test line (position C). Results are recorded 15 minutes after the last step for serum samples or after 25 minutes for whole blood samples. The test is read as positive when two red lines (test and control lines) appear (Figure 1B). If only the control line is observed, the test is read as negative (Figure 1C).
Figure 1.
Diagrammatic representation of the BLF Rapid. (A) Unused test. (B) Positive result. (C) Negative result. This figure appears in color at www.ajtmh.org.
The diagnostic sensitivity of the prototype BLF Rapid was assessed by testing with samples that had previously been determined to be positive for bancroftian filariasis by other tests. The other tests were mainly microscopic detection of W. bancrofti microfilaria (mf) in blood and/or antigen detection tests in serum/plasma that is ICT, TropBio Og4C3 enzyme-linked immunosorbent assay (ELISA) (Cellabs Pty. Ltd., Brookvale, New South Wales, Australia), and/or AD12-ELISA.9 To assess the diagnostic specificity of BLF Rapid, it was tested with serum samples from individuals infected with other parasitic infections from non-LF areas or healthy individuals either from LF and non-LF areas. Those from LF areas were negative by both ICT and Og4C3-ELISA.
BLF Rapid was first evaluated at USM (in-house) using 96 serum samples, which included 55 samples from W. bancrofti mf-positive individuals from endemic areas in India and Egypt. Serum samples from other infections (n = 31) at USM were from patients with ascariasis, trichuriasis, hookworm infection, strongyloidiasis, toxocariasis, hydatid disease, amoebic liver abscess, and toxoplasmosis. Ten serum samples from healthy individuals were also tested. In addition, serial 2-fold dilutions of 27 of the abovementioned mf-positive samples were tested with BLF Rapid and PanLF Rapid (both contain BmSXP recombinant antigen) to determine the difference in sensitivity between the two tests.
BLF Rapid was then couriered to three international and two national (Malaysian) laboratories and were independently tested for its diagnostic sensitivity and specificity by their respective laboratory personnel using their own stored serum samples from previous studies. At the National Institutes of Health-International Center for Excellence in Research, National Institute of Research in Tuberculosis in India, 78 serum samples were tested, which included 28 samples from W. bancrofti–endemic area residents in India (different from the samples from India used at USM) who were positive by two antigen detection tests that is ICT and Og4C3 ELISA. In addition, 50 samples from healthy individuals from the same area were tested for diagnostic specificity, and these samples were negative by ICT and Og4C3-ELISA. At the Centers for Disease Control and Prevention (CDC) in Atlanta, GA, 48 samples were tested which included 10 healthy serum samples and 38 samples from W. bancrofti mf-positive individuals from endemic areas in Haiti who were previously positive by Bm14-ELISA.10 In addition, some had previous positive ICT results. At the Washington University School of Medicine in St. Louis, MO, BLF Rapid was tested with 23 positive samples from W. bancrofti mf-positive individuals from endemic areas in Sri Lanka, which were also positive, by AD12-ELISA. Ten positive samples from Malaysia were from symptomatic patients (migrant workers) suspected of LF. At the University of Malaya, Malaysia, three were mf and ICT positive and at the Insitute for Medical Research, seven were ICT positive. At the University of Malaya, two sera from other infections (toxoplasmosis and amoebiasis) and one healthy serum were also tested. The diagnostic sensitivity and specificity of BLF Rapid was calculated for each laboratory. The average percentage of the sensitivity and specificity across all laboratories, excluding USM (i.e. data from independent evaluation), was then determined. All laboratories used their stored serum samples in accordance with the guidelines of their respective human research ethics committees.
The results of the evaluations are shown in Table 1. The BLF Rapid was 100% sensitive and specific when tested at USM. Testing of 2-fold serially diluted serum samples showed that BLF Rapid detected filarial-specific antibodies in 22 of 27 (81%) samples at 1–2 higher dilution than that detected by PanLF Rapid. This showed that BLF Rapid was able to detect lower antibody titres (thus more sensitive) as compared with PanLF Rapid. The results of the independent evaluations showed that the diagnostic sensitivity of BLF Rapid ranged from 84% to 100%, with a mean of almost 94% (93/99) across the laboratories. Except for the results at CDC, all laboratories showed that BLF Rapid was 100% sensitive. Centers for Disease Control and Prevention recorded 84% (32/38) diagnostic sensitivity for BLF Rapid, three of the six false negative samples were also negative by Bm14-ELISA. The reason for the lower sensitivity of BLF Rapid when tested at CDC with samples from Haiti is unknown; however, some of the false-negative samples could be from people who were deficient in IgG4. It would be useful to test BLF Rapid with a much larger number of serum samples from Haiti, preferably using recently collected samples.
Table 1.
Diagnostic sensitivity and specificity evaluation results of BLF Rapid using stored serum samples at each laboratory
| Institution | Reactivity with different types of sera | Sensitivity (%) | Specificity (%) | |||
|---|---|---|---|---|---|---|
| Wb | Tests used to determine Wb infection | Other infections | Healthy | |||
| USM | 55/55 | mf | 0/31* | 0/10 | 100 | 100 |
| UM | 3/3 | mf and ICT | 0/2† | 0/1 | 100 | 100 |
| IMR | 7/7 | ICT | – | – | 100 | – |
| NIH-NIRT-ICER | 28/28 | ICT and Og4C3-ELISA | – | 0/50 | 100 | 100 |
| CDC | 32/38 | mf and/or ICT and/or Bm14-ELISA | – | 0/10 | 84 | 100 |
| WUSTL | 23/23 | mf and AD12-ELISA | – | – | 100 | – |
| Average‡ | 93/99 (94%) | – | – | – | – | 100 |
CDC = Centers for Disease Control and Prevention, Atlanta, GA; ICT = Filariasis card test; IMR = Institute for Medical Research, Malaysia; mf = microfilaria; NIH-NIRT-ICER = National Institutes of Health-International Center for Excellence in Research, National Institute of Research in Tuberculosis, India; UM = University of Malaya, Malaysia; USM = Universiti Sains Malaysia; Wb = Wuchereria bancrofti infection; WUSTL = Washington University School of Medicine, USA.
* Serum samples were from patients living in non-LF endemic areas with ascariasis, trichuriasis, hookworm infection, strongyloidiasis, toxocariasis, hydatid disease, amoebic liver abscess, and toxoplasmosis.
† Serum samples were from patients living in non-LF endemic areas with toxoplasmosis and amoebiasis.
‡ Average sensitivity and specificity were calculated from percentage values across all laboratories excluding USM.
A diagnostic specificity of 100% was recorded at all centers when tested with sera of subjects with non-lymphatic filarial infections. At Washington University in St. Louis, MO, BLF Rapid was also tested with patient sera from four Onchocerca volvulus and two Mansonella perstans infections, and all were positive by the rapid test. Previous studies had reported cross-reactivity of BmSXP protein with sera from people with Loa loa and O. volvulus infections.4,5 Thus, the BLF Rapid is not suitable to be used in areas co-endemic with non-lymphatic filariae.
Anti-filarial antibodies are detectable before circulating filarial antigens or mf during the course of infection.11 Because antibody response against filarial antigens is a sensitive marker of LF exposure, it can be used as a tool to assess LF transmission and to guide programmatic decisions.12,13 Thus, as prevalence declines in a bancroftian filariasis area, a rapid antibody test should be used alongside the Filariasis Test Strip antigen test to obtain data on the status of the program. An antibody test would be more sensitive in detecting exposure or infection in young children in the post-MDA period.14,15 Another prototype rapid IgG4 test for detection of bancroftian filariasis uses Wb123 recombinant protein. When compared with an IgG4-ELISA based on the same antigen, both test formats showed high diagnostic sensitivity and specificity. Furthermore, the rapid test based on Wb123 antigen has good specificity and does not detect antibodies in sera from persons infected with the non-LF filaria L. loa and O. volvulus.16
A rapid antibody test would also be very useful for post-elimination surveillance to monitor possible re-emergence of active transmission and to confirm sustained absence of LF transmission. In addition, an antibody test would aid in the detection of new infections caused by influx of migrants from an endemic region to either a non-endemic area or an area that had succeeded in eliminating LF.16 In a recent seroprevalence study among migrant workers in Malaysia, BLF Rapid was positive in 19.6% workers from India (10/51), 12.6% from Nepal (13/103), and 7.1% from Myanmar (1/14).7
In conclusion, the present study showed that BLF Rapid test is a highly sensitive and specific test for detection of bancroftian filariasis in areas not endemic for O. volvulus or L. loa. The encouraging evaluation results and the field-applicability make the test a potential diagnostic tool to complement antigen results for TAS-stopping decisions and possibly for post-elimination surveillance. Along with the Wb123-based rapid test and other prototype rapid tests that may be in the development pipeline, BLF Rapid should be further tested in the field and under operational conditions to determine the best test that can be recommended by the World Health Organization for the aforementioned purposes.
Acknowledgments:
We would like to thank Kurt C. Curtis at WUSTL for his help in performing the BLF Rapid™ tests.
REFERENCES
- 1.WHO , 2017. Global programme to eliminate lymphatic filariasis: progress report, 2016. Wkly Epidemiol Rec 92: 594–607. [PubMed] [Google Scholar]
- 2.Shenoy RK, 2008. Clinical and pathological aspects of filarial lymphedema and its management. Korean J Parasitol 46: 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2016 DALYs and HALE Collaborators , 2017. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2013; 2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390: 1260–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization , 2011. Lymphatic Filariasis: Monitoring and Epidemiological Assessment of Mass Drug Administration: A Manual for National Elimination Programmes. Geneva, Switzerland: WHO/HTM/NTD/PCT/2011.4. [Google Scholar]
- 5.Kwan-Lim GE, Forsyth KP, Maizels RM, 1990. Filarial-specific IgG4 response correlates with active Wuchereria bancrofti infection. J Immunol 145: 4298–4305. [PubMed] [Google Scholar]
- 6.Dewi RM, Tuti S, Ganefa S, Anwar C, Larasati R, Ariyanti E, Herjati H, Brady M, 2015. Brugia rapid antibody responses in communities of Indonesia in relation to the results of 'transmission assessment surveys' (TAS) for the lymphatic filariasis elimination program. Parasit Vectors 8: 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noordin R, Mohd Zain SN, Yunus MH, Sahimin N, 2017. Seroprevalence of lymphatic filariasis among migrant workers in Peninsular Malaysia. Trans R Soc Trop Med Hyg 111: 370–372. [DOI] [PubMed] [Google Scholar]
- 8.Vythilingam I, 2012. Plasmodium knowlesi and Wuchereria bancrofti: their vectors and challenges for the future. Front Physiol 3: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ismail MM, Weil GJ, Jayasinghe KS, Premaratne UN, Abeyewickreme W, Rajaratnam HN, Sheriff MH, Perera CS, Dissanaike AS, 1996. Prolonged clearance of microfilaraemia in patients with bancroftian filariasis after multiple high doses of ivermectin or diethylcarbamazine. Trans R Soc Trop Med Hyg 90: 684–688. [DOI] [PubMed] [Google Scholar]
- 10.Won KY, Sambou S, Barry A, Robinson K, Jaye M, Sanneh B, Sanyang A, Gass K, Lammie PJ, Rebollo M, 2018. Use of antibody tools to provide serologic evidence of elimination of lymphatic filariasis in the Gambia. Am J Trop Med Hyg 98: 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gass K, et al. 2012. A multicenter evaluation of diagnostic tools to define endpoints for programs to eliminate bancroftian filariasis. PLoS Negl Trop Dis 6: e1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Washington CH, et al. 2004. Spatial clustering of filarial transmission before and after a mass drug administration in a setting of low infection prevalence. Filaria J 3: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Won KY, et al. 2018. Comparison of antigen and antibody responses in repeat lymphatic filariasis transmission assessment surveys in American Samoa. PLoS Negl Trop Dis 12: e0006347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weil GJ, Ramzy RM, 2007. Diagnostic tools for filariasis elimination programs. Trends Parasitol 23: 78–82. [DOI] [PubMed] [Google Scholar]
- 15.Ramzy RM, El Setouhy M, Helmy H, Ahmed ES, Abd Elaziz KM, Farid HA, Shannon WD, Weil GJ, 2006. Effect of yearly mass drug administration with diethylcarbamazine and albendazole on bancroftian filariasis in Egypt: a comprehensive assessment. Lancet 367: 992–999. [DOI] [PubMed] [Google Scholar]
- 16.Steel C, Golden A, Kubofcik J, LaRue N, de los Santos T, Domingo GJ, Nutman TB, 2013. Rapid Wuchereria bancrofti-specific antigen Wb123-based IgG4 immunoassays as tools for surveillance following mass drug administration programs on lymphatic filariasis. Clin Vaccine Immunol 20: 1155–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]