Abstract.
Parenteral ivermectin treatment of disseminated strongyloidiasis and hyperinfection is increasing, although not licensed in humans and with limited pharmacokinetic data available. Plasma and postmortem tissue analysis in an human immunodeficiency virus (HIV)/hepatitis C virus–positive man with disseminated strongyloidiasis suggests loading subcutaneous ivermectin doses are required, from which the central nervous system is protected.
INTRODUCTION
The soil-transmitted nematode, Strongyloides stercoralis, is endemic in tropical and subtropical regions infecting more than 370 million people.1,2 Host autoinfection with filariform larvae, unique in the Strongyloides life cycle, can lead to persistent long-lasting infection. With immunosuppression, notably corticosteroids, organ transplantation and human T-cell lymphotrophic virus type 1 (HTLV-1) infection, a high intestinal burden can lead to hyperinfection, causing paralytic ileus and dissemination.2–4 Hyperinfection and dissemination carry a 50–100% mortality rate and are an emerging threat globally as immunosuppressive therapy and organ transplantation indications expand.4,5 In uncomplicated infection, ivermectin is highly active against the intestinal stages of Strongyloides. A single oral 200 μg/kg dose produced peak levels between 16 and 101 ng/mL in healthy volunteers and was 64–100% efficacious in clinical studies of gastrointestinal strongyloidiasis.6 In Strongyloides hyperinfection, ivermectin is recommended at a dose of 200 μg/kg/day orally, or rectally, until stool and/or sputum are negative for larvae for 2 weeks.4,7 However, impaired intestinal absorption may necessitate parenteral ivermectin, which is licensed only for veterinary use.7,8 Access to parenteral ivermectin and pharmacokinetic data to guide therapy is limited.9 Serial plasma and postmortem ivermectin analysis from our patient is described.
CASE REPORT
A 27-year-old man, with 6 weeks of diarrhea and 10 kg weight loss, was diagnosed with Crohn’s disease on colonoscopic biopsy but deteriorated on prednisolone 40 mg/day and azathioprine 150 mg/day and was admitted to hospital with paralytic ileus 6 weeks later. He was previously well and had visited remote indigenous communities in central Australia 1 year earlier.
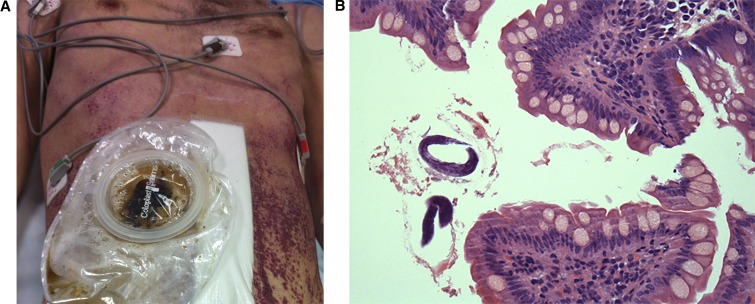
Paralytic ileus was managed with intravenous fluids, nasogastric (NG) drainage, hydrocortisone, piperacillin/tazobactam, reduced azathioprine dose, and total parenteral nutrition. Laparotomy and loop ileostomy 2 weeks after admission identified nonspecific abnormal ileal mucosa. He was transferred to our tertiary referral hospital 1 week later for mechanical ventilation, inotrope support, and antibiotic escalation for septic shock and respiratory failure. A centrally spreading abdominal purpuric macular rash was noted (Figure 1A). Further history identified a recent male sexual partner and negative human immunodeficiency virus 1 (HIV-1) serology 9 months earlier. Additional investigations identified S. stercoralis larvae in skin and ileal biopsy (Figure 1B) and bronchioalveolar lavage. No stool or ileal fluid was produced for larval analysis. Serology for HIV-1 Ag/Ab and hepatitis C virus (HCV) Ab was positive. HIV viral load was 115 million copies/mL, HIV-1 p24Ag 192 pg/mL, and CD4 340 mm−3 (17%). Hepatitis C virus viral load was 32 million copies/mL, genotype 3. Serology for hepatitis B, syphilis, and HTLV-1 was negative. Eosinopenia persisted at 0.06 × 109/L (0.4%).
Figure 1.
(A) Patient with ileostomy and purpuric macular abdominal rash characteristic of disseminated strongyloidiasis. (B) Ileal biopsy, magnification ×400, haematoxylin and eosin stain (H&E) stain demonstrating rhabtidiform Strongyloides larvae. This figure appears in color at www.ajtmh.org.
Disseminated strongyloidiasis treatment was initiated with ivermectin (200 μg/kg) NG 12 mg/day and albendazole NG 800 mg/day. However, as the patient had a nonfunctioning ileum, family and jurisdictional consent was obtained to use the veterinary parenteral preparation of ivermectin (Ivomec™; Merial, Duluth, GA). Subcutaneous ivermectin 12 mg/day was coadministered with NG ivermectin and albendazole for 14 days (Figure 2A). Therapeutic efficacy was assessed by phase-contrast microscopy of daily endotracheal aspirates for larval motility. On days 5, 6, 10, 11, and 12 of ivermectin therapy, subcutaneous ivermectin was doubled to 24 mg/day in response to persistent larval motility (Figure 2A). During this period, the patient’s course was complicated by acute kidney injury, requiring continuous venous hemodialysis; bronchopleural fistulae; sepsis; Clostridium difficile colitis treated with metronidazole, vancomycin, and tigecycline; cytomegalovirus (CMV) viremia (19,500 copies/mL) treated with ganciclovir; and pancytopenia requiring blood transfusions. Daily serum samples collected for ivermectin levels were stored at −70°C and results were obtained on days 7, 14, and 21 of ivermectin therapy.
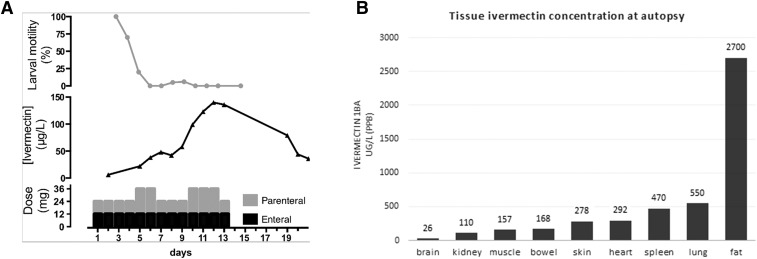
Figure 2.
(A) Ivermectin treatment timeline demonstrating relationship between days of subcutaneous and nasogastric ivermectin doses, plasma ivermectin 1Ba concentration, and bronchial larval motility. (B) Postmortem tissue analysis of ivermectin 1Ba concentration.
Subsequent clinical improvement was evidenced by withdrawal of inotropes and dialysis, a functioning ileostomy, spontaneous respiration with pressure support, and nonverbal communication, although muscle wasting was severe with flaccid atonia due to axonal degeneration identified on nerve conduction studies. Ten days after completing ivermectin treatment, cholestatic hepatitis developed with bilirubin 300 μmol/L (N < 25), ALP 500 U/L (N < 126), GGT 200 U/L (N < 50), normal AST, ALT, mildly prolonged INR 1.3 (N < 1.1), and prothrombin time 16 seconds (N 12–15 seconds), and the patient succumbed to a catastrophic gastrointestinal hemorrhage. The patient’s family consented to a postmortem.
Autopsy performed 2 days after death identified marked wasting (body mass index [BMI] 13.3 kg/m2), jaundice, an abdominal ecchymotic rash, pulmonary oedema and hemorrhage, hepatosplenomegaly, and large bowel pseudomembranes. Microscopy revealed necrotic Strongyloides organisms with associated inflammation in the left ventricular myocardium, skin, and skeletal muscle. There was diffuse alveolar damage, a fungal pulmonary abscess, cholestatic jaundice, acute tubular necrosis with myoglobinuria, and pancreatic fibrosis. Neuropathologically, there was multifocal fungal cerebritis, nonspecific microglial activation, and focal white matter necrosis. No intracerebral Strongyloides organisms were identified.
Serum collected during and after ivermectin treatment (Figure 2A) and 10 g of tissue from brain, kidney, muscle, skin, heart, spleen, lung, and liver, and omental fat collected at autopsy were stored at −70°C. Plasma and tissue ivermectin concentrations were determined by reverse phase isocratic high-performance liquid chromatography coupled with fluorescence detection (Waters Corporation, Milford, MA). Ivermectin was extracted from plasma (100 μL) or homogenized tissue (500 mg) with 100% methanol with doramectin internal standard. After centrifugation, air-dried deproteinized supernatants were derivatized with N-methylimidazole and trifluoroacetic anhydride. Ivermectin B1a was measured with a 4-point calibration curve with < 8% inter-run imprecision.
Plasma ivermectin concentration reached 50 μg/L on day 7 after doubling subcutaneous doses on days 5 and 6, and then increased to 140 μg/L on day 12 after doubling subcutaneous doses on days 10, 11, and 12 (Figure 2A). Brain ivermectin concentration was < 1% of that detected in fat (Figure 2B). Liver ivermectin concentration could not be determined because of unidentifiable assay interference, possibly bilirubin.
DISCUSSION
Islands of Strongyloides hyperendemicity exist in rural indigenous Australian communities10 probably visited by this patient. Men who have sex with men are also at increased risk of acquisition.11 A combination of unrecognized exposure to Strongyloides, HIV, and HCV in preceding months; an initial, not uncommon, histological misdiagnosis of inflammatory bowel disease,12 leading to further immunosuppression with corticosteroids; absence of eosinophilia and late recognition of a characteristic purpuric rash, all contributed to delayed diagnosis, secondary sepsis, and death. Screening for Strongyloides where exposure may have occurred recently or remotely in time or geographically, particularly if immunosuppressed, is an important consideration.
Ivermectin is highly lipophilic and protein bound, metabolized by hepatic CYP3A4, and excreted in feces with < 1% renal excretion.6,13 Parasiticidal action is mediated by high affinity binding to glutamate-gated chloride channels in invertebrate nerve and muscle cells with less affinity for mammalian gamma-aminobutyric acid –gated chloride channels. Mammalian blood–brain barrier penetration is inhibited by P-glycoprotein.14 It has a wide margin of safety with no adverse effects reported, with serum levels up to 260 ng/mL in healthy adult volunteers exposed to 1,000 μg/kg three times weekly and single doses up to 2,000 μg/kg.15
Serum ivermectin levels have been reported in eight cases of hyperinfection.7,8 In this analysis, serum ivermectin concentration between 50 and 100 μg/L was larvicidal in the lung. The slow increase in serum concentration was also observed by others. In animal studies, the ivermectin formulation’s solvent vehicle significantly affects its pharmacokinetics.16 Ivermectin may be more slowly released from the nonaqueous formulation of Ivomec™ (60% propylene glycerol/40% glycerol). In addition, there may be a larger volume of distribution in hyperinfection because of hypoalbuminemia. As such, a loading dose may be beneficial while a target concentration in this condition is not yet established. In this analysis, a serum ivermectin concentration between 30 and 50 ng/mL correlated with abolition of bronchial larval motility. Postmortem tissue analysis confirmed that ivermectin is highly concentrated in fat but does not readily cross the blood–brain barrier in disseminated strongyloidiasis. Although axonal degeneration, myopathy, and biliary stasis may be attributable to ivermectin toxicity, levels in muscle were lower than those in other unaffected tissue and are more likely to be explained by severe sepsis.
CONCLUSION
Disseminated Strongyloides infection is frequently fatal, particularly if diagnosis is delayed. Serum ivermectin analysis in this case supports the role of an initial loading dose in disseminated infection with blood–brain barrier protection even at high serum ivermectin concentration. Further human parenteral ivermectin pharmacokinetic data are needed.
Acknowledgments:
We would like to thank St. George Hospital teams from the Infectious Diseases and Immunology Department, Intensive Care Unit, Gastroenterology, Colorectal Surgery, Dermatology and Microbiology Department (SEALS) for their clinical and diagnostic support; David Looke and Tom Gottlieb for their guidance; and David Homer (Merial). We acknowledge the suffering and tragic loss of life.
REFERENCES
- 1.Bisoffi Z, et al. 2013. Strongyloides stercoralis: a plea for action. PLoS Negl Trop Dis 7: e2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Puthiyakunnon S, Boddu S, Li Y, Zhou X, Wang C, Li J, Chen X, 2014. Strongyloidiasis—an insight into its global prevalence and management. PLoS Negl Trop Dis 8: e3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mobley CM, Dhala A, Ghobrial RM, 2017. Strongyloides stercoralis in solid organ transplantation: early diagnosis gets the worm. Curr Opin Organ Transpl 22: 336–344. [DOI] [PubMed] [Google Scholar]
- 4.Mejia R, Nutman T, 2012. Screening, prevention, and treatment for hyperinfection syndrome and disseminated infections caused by Strongyloides stercoralis. Curr Opin Infect Dis 25: 458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buonfrate D, Requena-Mendez A, Angheben A, Muñoz J, Gobbi F, Van Den Ende J, Bisoffi Z, 2013. Severe strongyloidiasis: a systematic review of case reports. BMC Infect Dis 13: 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US FDA , Stromectal® (Ivermectin) Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050742s022lbl.pdf. Accessed May 5, 2018.
- 7.Barrett J, Broderick C, Soulsby H, Wade P, Newsholme W, 2016. Subcutaneous ivermectin use in the treatment of severe Strongyloides stercoralis infection: two case reports and a discussion of the literature. J Antimicrob Chemother 71: 220–225. [DOI] [PubMed] [Google Scholar]
- 8.Looke DFM, McCarthy KL, McWhinney B, Clague A, Pillans P, 2005. Strongyloides hyperinfection syndrome: low serum levels of ivermectin after oral and subcutaneous administration. Intern Med J 35: A73–A88. [Google Scholar]
- 9.Albonico M, et al. 2016. StrongNet: an international network to improve diagnostics and access to treatment for strongyloidiasis control. PLoS Negl Trop Dis 10: e0004898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Einsiedel L, Fernandes L, 2008. Strongyloides stercoralis: a cause of morbidity and mortality for indigenous people in central Australia. Intern Med J 38: 697–703. [DOI] [PubMed] [Google Scholar]
- 11.Abdolrasouli A, McMillan A, Ackers JP, 2009. Sexual transmission of intestinal parasites in men who have sex with men. Sex Health 6: 185–194. [DOI] [PubMed] [Google Scholar]
- 12.Qu Z, Kundu UR, Abadeer RA, Wanger A, 2009. Strongyloides colitis is a lethal mimic of ulcerative colitis: the key morphologic differential diagnosis. Hum Pathol 40: 572–577. [DOI] [PubMed] [Google Scholar]
- 13.Guzzo CA, Furtek CI, Porras AG, Chen C, Tipping R, Clineschmidt CM, Sciberras DG, Hsieh JY, Lasseter KC, 2002. Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. J Clin Pharmacol 42: 1122–1133. [DOI] [PubMed] [Google Scholar]
- 14.Edwards G, 2003. Ivermectin: does P-glycoprotein play a role in neurotoxicity? Filaria J 2 (Suppl 1): S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.González Canga A, Sahagún Prieto AM, Diez-Liébana MJ, Fernández Martínez N, Sierra Vega M, García Vieitez JJ, 2008. The pharmacokinetics and interactions of ivermectin in humans—a mini-review. AAPS J 10: 42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steel JW, 1993. Pharmacokinetics and metabolism of avermectins in livestock. Vet Parasitol 48: 45–57. [DOI] [PubMed] [Google Scholar]