Abstract.
Human immunodeficiency virus/tuberculosis (HIV/TB) coinfection is particularly prevalent in South Africa, where TB has been the leading cause of death for more than a decade. The 2004–2008 national rollout of antiretroviral therapy (ART) provides a unique opportunity to examine the population-level impact of ART on the TB epidemic. We performed longitudinal regression analysis to follow the evolution of TB outcomes before and after the introduction of ART using a large data set from the National Health Laboratory Service. This is the first study to produce estimates of the impact of the ART rollout by exploiting staggered timing and geographic variation in the rollout. After ART became available in a health facility, 3.7% (P < 0.0001) more patients were tested for TB and 3.2% (P < 0.0001) more received repeat testing; however, there was a steep rise in testing before the introduction of ART. Although the number of TB-positive patients increased by 4.3% (P = 0.0002) in the first year post-ART, the TB rate among tested patients fell by 2 percentage points (8%, P = 0.001) after 2 years. Sputum smear testing declined relative to more technologically advanced diagnostics post-ART. Antiretroviral therapy availability increased the attention to TB screening and drew new patients into the health-care system. Small increases in the numbers of repeat patients are indicative of retention in care. The decline in TB rates post-ART suggests that the reduction in TB risk due to improved immune functioning and health-care contact likely outweighed the increased TB risk because of the longer lifespan of ART initiators.
INTRODUCTION
The human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS) epidemic has largely fueled the resurgence of tuberculosis (TB) in sub-Saharan Africa. The relative risk of TB doubles in the first year after HIV infection and continues to rise as CD4 counts drop,1 reaching rates 20- to 37-fold higher than in those without HIV.2 Coinfection is particularly prevalent in South Africa, where coinfected patients make up 59% of TB cases compared with the 13% global average.3 Tuberculosis has been the leading cause of death in South Africa for more than a decade4 and is the leading cause of death among AIDS patients.2 In 2010, TB accounted for 11.6% of deaths in South Africa compared with 3.4% for HIV alone and HIV/TB coinfection (nineth largest cause of death).4 Today, South Africa has the highest TB incidence rate in the world, with a rate of 781 per 100,000 population compared with the global average of 140.3
Because HIV/AIDS drives the TB epidemic, investments in care for HIV/AIDS patients, such as antiretroviral therapy (ART), are likely to have significant positive spillovers to TB outcomes. The first phase of the national rollout of ART in South Africa between 2004 and 2008 provides a unique opportunity to examine the population-level impact of ART on the TB epidemic. During this period, more than US$1 billion was spent on the rollout, ART was introduced at approximately 400 health facilities, and more than 500,000 patients were enrolled on ART. This program, which dwarfed previous investments in TB care such as TB-DOTS, increased adult life expectancy by 11.3 years.5
This study estimates the impact of ART on TB and is only the second study to capture the benefits of ART investment on TB outcomes at the national level.6 We perform a longitudinal analysis that follows the evolution of TB outcomes for patients in health facilities before and after the introduction of ART in that facility using a large data set from the National Health Laboratory Service (NHLS), which includes TB test results from virtually all public health facilities in South Africa. We examine the impact of ART on TB testing patterns, the TB incidence rate among those tested, and the composition of patients tested.
This is the first study to estimate the impact of ART on the population by simultaneously applying three methods to address a comprehensive set of measured and unmeasured confounding factors. It is also the second study to estimate national-level spillover effects of ART on TB incidence for those who were not accessing ART, including both HIV-positive and HIV-negative individuals. We 1) exploit the staggered timing and geographic variation in the ART rollout around the country, 2) use facility-level longitudinal data to account for trends before the availability of ART, and 3) apply facility-level controls (multilevel analysis) to further control for time-invariant facility-level confounders. The staggered timing and geographic variation in ART comes from the National Department of Health (DOH) accreditation process, which required on-site evaluations and was, therefore, the main bottleneck in determining the sequence and pace of the ART rollout. The accreditation process focused on ensuring the equitable geographic distribution of ART facilities, which resulted in a rollout pattern that McLaren found was statistically uncorrelated with many of the potential confounding factors in this analysis, including population wealth, HIV prevalence, and local political power.7 Our methodology minimizes concerns about confounders because potential confounding factors would need to be correlated with both the sequence and the timing of the 4-year staggered rollout pattern of ART at more than 400 facilities around the country to bias our estimates. We know of no other contemporaneous national program or new technology that meets these stringent requirements to confound the estimates from our approach.
Understanding the impact of ART on TB is essential to inform the optimal design of new HIV/AIDS and TB policies and determine the necessary level of HIV/TB coordination that will maximize potential spillovers and reduce mortality from both diseases.
LITERATURE REVIEW AND HYPOTHESES
The literature identifies five potential effects of widespread ART availability on TB outcomes that can be evaluated with the NHLS data. This section presents an overview of this literature and the five hypotheses that structure our analysis. Local availability of ART will likely increase the numbers of HIV-infected individuals seeking medical care, especially those at later stages of AIDS, thereby increasing opportunities for diagnosis and treatment of TB.8–11 We predict that there will be an increase in TB testing in the period after facility accreditation (Hypothesis 1). Furthermore, though national TB treatment guidelines recommend follow-up TB tests for smear-positive patients at 2 and 5 months after the initial test, patient retention in South Africa is generally below the World Health Organization (WHO) target.12,13 Antiretroviral therapy implies lifelong clinical involvement, which provides incentives and opportunities for ongoing TB testing and improved follow-up care.14 We, therefore, expect to see an increase in the number of repeat TB testing visits after a facility receives accreditation for ART in addition to a rise in overall testing (Hypothesis 2).
Studies have demonstrated that ART access reduces infection risk among HIV-infected individuals, increases TB identification and treatment, decreases transmission and, thereby, reduces the incidence of TB in the population.14–19 One study found that nearly 20% of ART initiators in South Africa during this period had undiagnosed TB.20 The effect of ART on a TB epidemic has been shown to lag the introduction of ART by between 2 and 5 years.6,21 Reduced TB mortality in coinfected patients and the increase in TB infectiousness that accompanies the immune system recovery of coinfected ART patients may limit, but not necessarily outweigh, the forces that reduce TB in the population.22,23 We hypothesize that ART access would initially lead to an increase in the detection of TB cases but over time would reduce TB incidence in the population (Hypothesis 3).
The ART rollout was made possible by approximately $1.2 billion in additional funding.24 These increased health-care resources likely led to increased access to new laboratory resources and a greater use of more expensive, resource-intensive tests, net of other changes in TB testing technology, such as MDRTBplus (Hain Lifescience, Nehren, Germany) and GeneXpert MTB/RIF (Cepheid, Sunnyvale, CA). More resource-intensive tests are both more effective diagnostic tests for TB and more appropriate for HIV-positive patients, which would improve the accuracy of TB diagnosis and reduce loss to follow-up before treatment initiation. We therefore expect to see increased testing with TB culture, polymerase chain reaction (PCR) test, and GeneXpert MTB/RIF after a facility begins providing ART (Hypothesis 4).25
The ART-seeking population in South Africa was approximately 79% female and had a lower average age than the TB patient population before the ART rollout.26 Finally, we hypothesize that as more ART patients are tested for TB at a facility, the demographic composition of the population tested for TB will have a higher proportion of women and a lower average age than before the rollout (Hypothesis 5).
MATERIALS AND METHODS
Sources of data.
We extracted data from the NHLS database on every TB test performed on patients aged 16–64 in public health facilities for the period January 2003–December 2011 that fell within 2.5 years before or after the date of ART availability in each facility. This resulted in a sample of 10,544,350 unique patients at 4,697 facilities around the country, 429 of which were accredited to provide ART during the study period. Patient records were linked over time using patient identification numbers created by the NHLS using their proprietary linking algorithm based on patient name and other identifying information. The laboratory database records of TB diagnostic tests include information on the date, type of test performed, test result, testing facility location, and basic patient demographics. The NHLS data cover virtually all public health facilities in South Africa for this period, with the exception of KwaZulu-Natal Province, which was excluded because of data limitations.
We split the data into 3-month periods (quarters of the year) and aggregated specimen records by patient, then patient records by facility to examine facility-specific trends over time before and after the specific date that the particular facility was accredited to provide ART. This resulted in a sample size of 10,039 facility-by-quarter patient-count data points used to address all our hypotheses.
Tuberculosis-positive cases were based on the presence of at least one positive result from smear microscopy, TB culture, PCR test, or GeneXpert MTB/RIF. For smear microscopy, we considered scanty positives of three or more acid-fast bacilli per 100 immersion fields as TB-positive based on the cutoff value used in practice. For the gender analysis (Hypothesis 5), we excluded 270,573 records (1.02% of the sample) that were missing gender data before aggregating into the 10,039 facility-by-quarter data points. Repeat visits were defined as occurring at least one-quarter (91 days) after the initial date a patient was tested for TB and, therefore, include standard 5-month monitoring testing and other repeat TB testing visits. The National DOH provided dates when ART became available at each facility. Ethics approval was obtained from the University of Michigan Institutional Review Board and the University of Cape Town Faculty Ethics in Research Committee in South Africa.
Statistical analysis.
We performed the following linear regression to capture the evolution of outcomes for 10 quarters (2.5 years) before and after the date when ART became available at the facility:
| (1) |
where is the TB-related outcome for facility i in quarter t corresponding to each of our five hypotheses: number of TB tests, number of repeat patients, proportion of tests that were positive, proportion of tests by diagnostic test, and proportion of tests performed on females. is the constant that captures the sample average, is the set of indicator variables that capture the time pattern of outcomes for the 10 quarters before ART availability, and is the set of indicator variables for the 10 quarters after ART. The set of αi facility-level fixed effects (facility indicator variables) control for potentially confounding unobserved (unmeasured) time-invariant facility characteristics such as location, size, or socioeconomic status of the patient catchment area. Non-ART facilities were included to calculate nationally representative sample averages and control for time and geographic trends (year and province). Standard errors were clustered by facility.
All analysis was performed in Stata 13 (StataCorp, College Station, TX).
RESULTS
Hypothesis 1: Widespread availability of ART will create the opportunity to bring more HIV-infected individuals into medical care, thereby, increasing TB diagnosis and treatment.
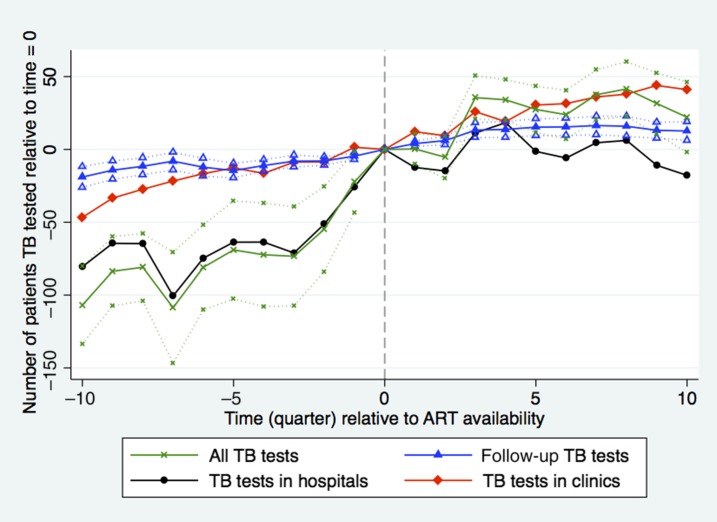
More patients were tested for TB 9 months after ART became available in a health facility relative to the date when ART became available in the facility (x axis, time = 0) (Figure 1). Nine months (three quarters) after ART became available in a facility there was a small but statistically significant 3.7% (35 patients, confidence interval [CI]: 21–50, P < 0.0001) increase in the quarterly number of patients tested for TB relative to the rate of 953 patients per quarter at the introduction of ART (-x-), and this rate remained steady through the end of the study period. Comparing the sample of hospitals-only (-•-) with the sample of clinics-only (-♦-) shows that the steep increase in patients tested in the year before ART availability was driven mainly by increased TB testing in district hospitals after the rollout was announced but before they were accredited to provide ART. The clinic sample shows little evidence of a pre-trend but a clear trend break at t = 0 when the number of patients increased at a greater rate.
Hypothesis 2: ART implies a longer lifespan and lifelong clinical involvement, which will allow for increased repeat TB testing opportunities.
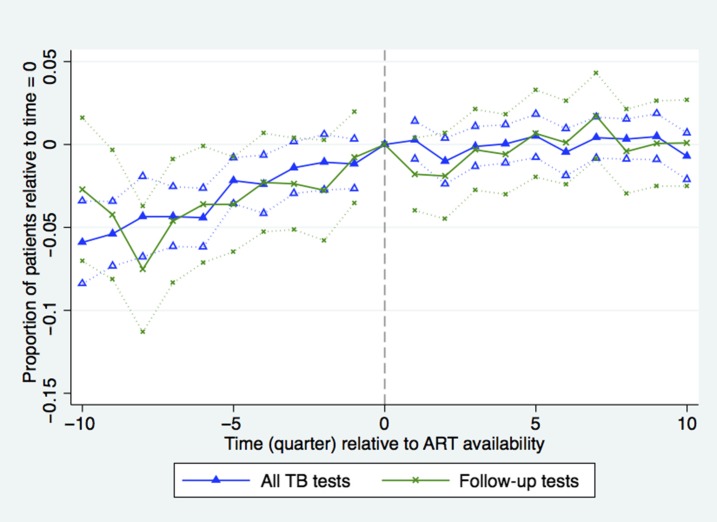
Figure 1.
Number of patients tested for tuberculosis (TB) during quarters before (δ coefficients from equation 1) and after (γ coefficients from equation 1) antiretroviral therapy (ART) introduction relative to time = 0. Time (quarter) is calculated as quarter of observation minus quarter of ART introduction. Dotted lines indicate facility-clustered, heteroskedasticity-robust 95% confidence intervals (all TB tests N = 10,039, time 0 = 953; Repeat TB tests N = 5,098, time 0 = 42; TB tests in hospitals N = 5,268, time 0 = 193; TB tests in clinics N = 5,220, time 0 = 106). This figure appears in color at www.ajtmh.org.
We found a small increase in the number of repeat TB testing visits, defined as those occurring more than 90 days from the initial testing visit, which is consistent with the ART rollout resulting in somewhat better ongoing TB monitoring and patient retention (Figure 1). Tuberculosis tests among repeat patients rose by 3.2% (14 patients, CI: 8–19, P < 0.0001) from the rate of 42 patients per quarter in the first year post-ART.
Hypothesis 3: ART availability will lower the incidence of TB in the population.
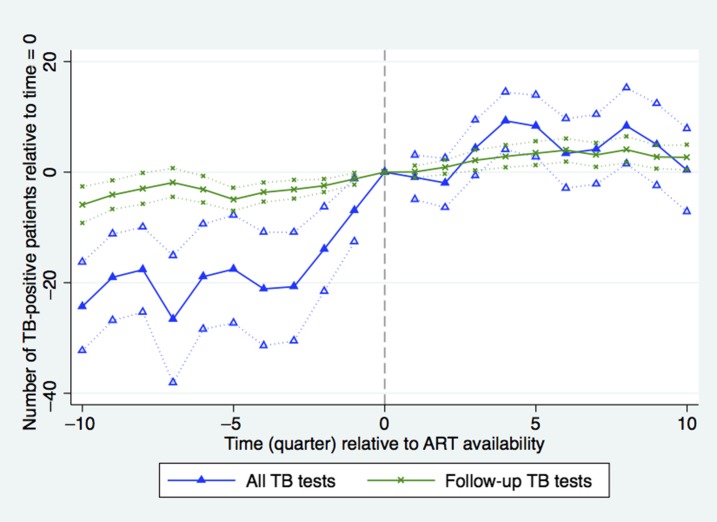
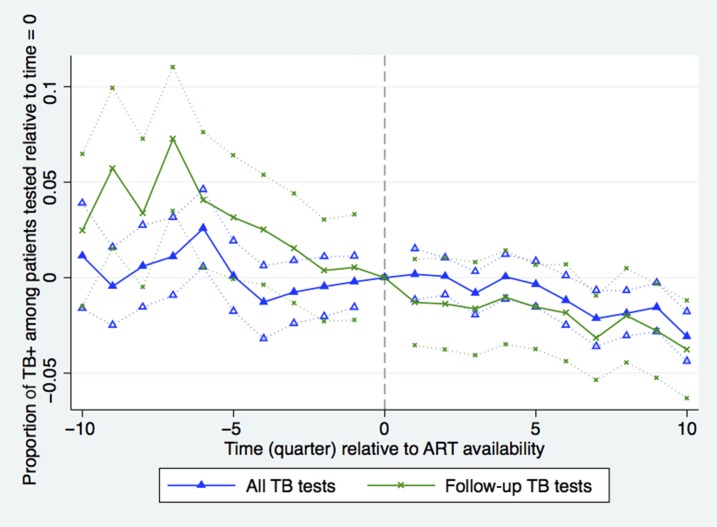
As the number of TB patients increased with the availability of ART, there was a concomitant 4.3% increase in the number of TB-positive patients tested per quarter in the first year post-ART off the rate of 215 patients per quarter (nine patients, CI: 4–14, P = 0.0002) on average, although it declined somewhat in the following year (Figure 2). Figure 3 shows that the TB rate among tested patients was fairly stable within 0.5% points of the facility average of 24% from 1 year before facility accreditation until 1.5 years after ART becomes available. Subsequently, the TB-positive rate among tested patients declined steadily in the post-ART period, falling to 22% after 2 years (CI: 21–23, P = 0.001). These patterns are most evident in the sample of new patients, so the effect is not driven by repeat patients.
Figure 2.
Number of tuberculosis (TB)-positive patients tested for TB during quarters before (δ coefficients from equation 1) and after (γ coefficients from equation 1) antiretroviral therapy (ART) introduction relative to time = 0. Time (quarter) is calculated as quarter of observation minus quarter of ART introduction. Dotted lines indicate facility-clustered, heteroskedasticity-robust 95% confidence intervals (All TB tests N = 10,039, time 0 = 215; Repeat TB tests N = 5,098, time 0 = 15). This figure appears in color at www.ajtmh.org.
Figure 3.
Proportion of tuberculosis (TB)-positive patients among patients tested for TB during quarters before (δ coefficients from equation 1) and after (γ coefficients from equation 1) antiretroviral therapy (ART) introduction relative to time = 0. Time (quarter) is calculated as quarter of observation minus quarter of ART introduction. Dotted lines indicate facility-clustered, heteroskedasticity-robust 95% confidence intervals (All TB tests N = 10,039, time 0 = 24%; Repeat TB tests N = 5,098, time 0 = 30%). This figure appears in color at www.ajtmh.org.
For TB testing of repeat patients, the steep pre-ART decline in the rate of TB-positive tests per patient leveled off slightly just after ART is introduced in a facility (Figure 3). The estimated TB rate among repeat patients fell by 1 percentage point in the first quarter after ART introduction in an apparent continuation of the pre-period trend, but then stayed approximately level until 1.5 years post-ART when it began to decline again.
Hypothesis 4: Health care resources associated with the ART rollout will lead to increased use of higher technology diagnostic tests for TB.
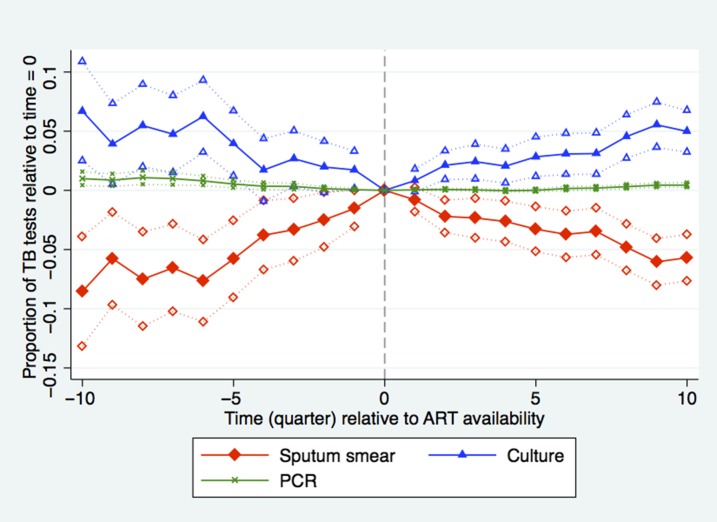
Before ART availability, the proportion of patients with at least one sputum smear test rose, whereas the proportion having had at least one culture test fell (Figure 4). However, when ART was introduced, this pattern reversed and the proportion of patients with culture tests rose over time. In the year post-ART, 76% of tests were sputum smear (CI: 74.5–77.9, P = 0.0013), 20.8% were culture (CI: 21.4–24.2, P = 0.0027) compared with 79% and 19%, respectively at t = 0. More expensive tests (GeneXpert MTB/RIF, PCR, and line probe) that may be faster and/or more accurate than smear or culture tests made up the remaining 3.2% of the sample and also increased in the post-ART period. The results are similar when we exclude 2011 from our analysis because the latest accreditation time in our sample, August 2008, predates the introduction of GeneXpert MTB/RIF.
Hypothesis 5: The demographics of the TB-tested population will more closely resemble the HIV+ population as more patients seek ART enrollment.
Figure 4.
Proportion of tuberculosis (TB) tests performed by sputum smear, culture and PCR among patients tested for TB during quarters before (δ coefficients from equation 1) and after (γ coefficients from equation 1) antiretroviral therapy (ART) introduction relative to time = 0. Time (quarter) is calculated as quarter of observation minus quarter of ART introduction. Dotted lines indicate facility-clustered, heteroskedasticity-robust 95% confidence intervals. N = 10,039 (Sputum smear time 0 = 79%, culture time 0 = 19%, PCR time 0 = 1%). This figure appears in color at www.ajtmh.org.
The proportion of patients tested for TB that are female exhibited a slight upward trend before the availability of ART and leveled off at 52%; however, there were no differences around the time of ART introduction (Figure 5). The fraction of new patients between the ages of 30–40 increased slightly post-ART, whereas the fraction between the ages of 20–30 decreased (results not shown). However, the overall average age of new patients remained constant at 38 years.
Figure 5.
Proportion of patients tested for tuberculosis (TB) who were female estimated during quarters before (δ coefficients from equation 1) and after (γ coefficients from equation 1) antiretroviral therapy (ART) introduction relative to time = 0. Time (quarter) is calculated as quarter of observation minus quarter of ART introduction. Dotted lines indicate facility-clustered, heteroskedasticity-robust 95% confidence intervals (All TB tests N = 10,039, time 0 = 53%; Repeat TB tests N = 5,098, time 0 = 50%). This figure appears in color at www.ajtmh.org.
DISCUSSION
Our results are consistent with ART availability increasing attention to TB screening and drawing new patients into the health-care system. Tuberculosis screening remained approximately level for 2 years after a facility was accredited to provide ART because HIV+ patients continue to have an elevated risk for TB even after ART initiation. The unique methodology exploiting the staggered rollout of ART, accounting for pre-period trends and including facility-level controls, increases confidence that the observed results are driven by the ART rollout rather than potential confounders. We know of no other contemporaneous program or new technology that meets the stringent requirements to confound our estimates from this approach. Hain-MDRTBPlus line probe assay was introduced following the WHO policy statement in June 2008, after the vast majority of facilities were ART accredited.27 It is, therefore, highly unlikely to confound estimates in the first year following t = 0. GeneXpert MTB/RIF rollout began in 2011 and only falls within the 2.5-year post-accreditation analysis window for very few facilities accredited late in the process.
With access to ART, individuals with HIV have a longer lifespan (but still an elevated risk of TB) and are required to visit facilities on a regular basis for follow-up HIV/AIDS care. Small observed increases in the numbers of repeat patients are indicative of this retention in care and likely reflect an increase both in screening for TB and monitoring of TB cases.
The fairly constant TB rate in the year leading up to ART availability in a facility (Figure 3) suggests that ART-eligible patients were unlikely to have sought care before ART availability. Instead, the rise in TB testing may have been because of greater concern about the disease over time. One explanation for the slow but steady decline in TB rates post-ART is that the reduction in TB risk due to improved immune functioning and more contact with health care outweighed the composite effect of the sicker ART-eligible population accessing health care and any increased TB risk because of the longer lifespan of ART initiators. The declining TB rate among repeat patients (Figure 3) slows after ART becomes available, which may reflect the reduced TB mortality among those initiating ART. Our finding of declining TB rates among tested patients is consistent with recently published data from South Africa that show declines in population-level incidence of TB associated with ART expansion but occurring with a time lag.6
Our results contribute to the debate on the impact that policies targeting HIV/AIDS have on the TB epidemic in South Africa.11,18,28,29 Other studies have shown that although the introduction of ART is associated with a subsequent reduction in the TB incidence rate among ART initiators, it also increases their lifetime likelihood of contracting TB and the length of TB infection if contracted.11 One model estimated that the risk of TB transmission in patients on ART is likely to remain high enough that ART alone is insufficient to control TB.15
The proportion of patients receiving sputum smear testing reaches its peak at the time ART is introduced to the facility and declines thereafter. More effective and more expensive TB diagnostic technology is indicated for HIV+ patients because of the low sensitivity of sputum smear testing, as well as for repeat patients because of their need for drug resistance and confirmatory testing. The unique pattern of the staggered rollout of ART exploited by the methodology lends credibility to the argument that these results are unlikely to be driven by secular technology improvements, such as the introduction of MDRTBplus or GeneXpert MTB/RIF or changes in national TB policy or TB clinic guidelines, but by the ART rollout itself.
The demographic shift in patients seeking TB testing was smaller than anticipated. The fraction of tested patients that is female showed a small but steady increase before ART introduction and then leveled off. Although South African women are more likely to seek routine health care in general, the modest shift in gender proportions may be because of a relative increase in men eligible for ART.30 The slight increase in the fraction of TB-tested patients between age 31–40 is consistent with the average age of patients who were eligible for ART during the first 4 years of the ART rollout.31 This may also reflect reduced mortality among ART initiators.
Addressing potential threats to validity.
The unique pattern of staggered timing and geographic variation in the ART rollout supports our assumption that the only thing that changed in a facility at its t = 0 is access to ART and that changes in the trends of our outcomes of interest in the months surrounding t = 0 are, therefore, because of ART access. Health facilities were accredited anytime between July 2004 and August 2008 so the data points represented in t = 5 through t = 10 do not capture a single point in time, but rather are averages calculated from facility outcomes at different points between January 2007 through February 2011. The unique pattern of the staggered rollout of ART also minimizes confounding factors due to one-time changes in other programs targeting TB such as DOTS or new technology such as MDRTBplus. To threaten the validity of our results, another program or technology would have to have been introduced in health facilities in a sequence highly correlated with the staggered pattern of the ART rollout for a substantial portion of the 429 health facilities that were accredited to provide ART during this period.
Limitations.
Though we are able to address most potential confounders using the richness of the NHLS data and the quasi-random staggered pattern in the ART rollout, our analysis has a few limitations. The NHLS does not have information on the HIV status or ART enrollment status of all patients tested for TB, so we cannot separate the direct effect of the ART rollout on ART enrollees from the indirect effect on the rest of the population. The rate of under-detection of TB in HIV-positive patients is likely to have fallen in the post-ART period, due to the shift away from sputum smear testing and toward more sensitive TB tests such as culture, especially for ART-eligible patients.32 Because our data do not have full coverage of KwaZulu-Natal, which has the highest HIV/AIDS burden, our estimates likely underestimate the magnitude of the national effect of the ART rollout. The unique methodology we use to address confounders is only valid during the early years of the rollout when the ART accreditation process was required. We cannot, therefore, address confounding for estimates of the impact of ART on TB outcomes beyond 2011.
CONCLUSION
Our results demonstrate that the national rollout of ART in South Africa was accompanied by an increase in the demand for TB testing, both from new and repeat (follow-up) patients. Our evidence suggests that within 2.5 years, ART most likely reduced TB rates among those tested, despite the fact that post-ART a greater proportion of the tested population would have been late-stage AIDS patients who were at greater risk for TB.
Neither the magnitude of positive spillovers from the 2004–2008 ART rollout to TB rates we show here nor the continued decline in TB incidence relative to increases in ART coverage between 2008 and 2012 modeled by Nanoo et al.6 appear to be large enough to control TB alone. However, the early initiation of ART for patients regardless of CD4 count is likely to improve TB control, especially if there are preventative effects of early ART on TB, which would produce a larger impact on TB rates than during the period of study.
Both HIV/AIDS and TB face similar challenges of ensuring prompt diagnosis, enrolling patients on appropriate treatment, and monitoring for side effects and drug resistance over the long-term. Closer HIV/TB integration is needed to increase demand for TB testing by HIV/AIDS patients and vice versa (such as making follow-up testing for both diseases at once more convenient) and retain patients in care for both diseases. Better integration is also required to design complementary HIV/AIDS and TB policies so as to improve the effectiveness of controlling both diseases with limited resources. HIV/TB integration saves lives. When integrated HIV/TB management and policy design capitalizes on spillovers within the system, it maximizes program impact to prevent TB and reduce mortality from both diseases.
Acknowledgments:
We thank Sue Candy, Michelle Potgieter, and Andrew Whitelaw for assistance with the data and helpful comments. The authors thank Jacob Bor, Sean Wasserman, Josh Wilde, and two anonymous reviewers for helpful comments. The authors thank Yubraj Acharya, David Ederer, Kathryn Fischer, Gaurav Khanna, Yi Mao, Alex Russov, Ryoko Sato, Reinhard Schiel, Kristefer Stojanovski, Will Story, Ben Thompson, Samuel Tzou, Jifang Zhou, and Sasha Zhou for research assistance.
REFERENCES
- 1.Sonnenberg P, Glynn JR, Fielding K, Murray J, Godfrey-Faussett P, Shearer S, 2005. How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in South African gold miners. J Infect Dis 191: 150–158. [DOI] [PubMed] [Google Scholar]
- 2.Getahun H, Gunneberg C, Granich R, Nunn P, 2010. HIV infection-associated tuberculosis: the epidemiology and the response. Clin Infect Dis 50: S201–S207. [DOI] [PubMed] [Google Scholar]
- 3.WHO , 2017. Global Tuberculosis Report. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 4.Statistics South Africa , 2013. Mortality and Causes of Death in South Africa, 2010: Findings from Death Notification Pretoria, South Africa: Statistics South Africa. Available at: http://www.statssa.gov.za/publications/p03093/p030932010.PDF. Accessed September 17, 2015.
- 5.Bor J, Herbst AJ, Newell ML, Bärnighausen T, 2013. Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science 339: 961–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nanoo A, Izu A, Ismail NA, Ihekweazu C, Abubakar I, Mametja D, Madhi SA, 2015. Nationwide and regional incidence of microbiologically confirmed pulmonary tuberculosis in South Africa, 2004–12: a time series analysis. Lancet Infect Dis 15: 1066–1076. [DOI] [PubMed] [Google Scholar]
- 7.McLaren ZM, 2015. Equity in the national rollout of public AIDS treatment in South Africa 2004–08. Health Policy Plan 30: 1162–1172. [DOI] [PubMed] [Google Scholar]
- 8.Johnson LF, 2012. Access to antiretroviral treatment in South Africa, 2004–2011. South Afr J HIV Med 13: 22–26. [Google Scholar]
- 9.Havlir DV, Getahun H, Sanne I, Nunn P, 2008. Opportunities and challenges for HIV care in overlapping HIV and TB epidemics. JAMA 300: 423–430. [DOI] [PubMed] [Google Scholar]
- 10.Abdool Karim SS, et al. 2011. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med 365: 1492–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawn SD, Harries AD, Williams BG, Chaisson RE, Losina E, De Cock KM, Wood R, 2011. Antiretroviral therapy and the control of HIV-associated tuberculosis. Will ART do it? Int J Tuberc Lung Dis 15: 571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karim SS, Churchyard GJ, Karim QA, Lawn SD, 2009. HIV infection and tuberculosis in South Africa: an urgent need to escalate the public health response. Lancet 374: 921–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLaren ZM, Sharp AR, Zhou J, Wasserman S, Nanoo A, 2017. Assessing healthcare quality using routine data: evaluating the performance of the national tuberculosis programme in South Africa. Trop Med Int Health 22: 171–179. [DOI] [PubMed] [Google Scholar]
- 14.Wood R, Lawn SD, 2011. Antiretroviral treatment as prevention: impact of the ‘test and treat’ strategy on the tuberculosis epidemic. Curr HIV Res 9: 383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girardi E, et al. Antiretroviral Therapy Cohort Collaboration , 2005. Incidence of tuberculosis among HIV-infected patients receiving highly active antiretroviral therapy in Europe and North America. Clin Infect Dis 41: 1772–1782. [DOI] [PubMed] [Google Scholar]
- 16.Middelkoop K, Bekker LG, Myer L, Whitelaw A, Grant A, Kaplan G, McIntyre J, Wood R, 2010. Antiretroviral program associated with reduction in untreated prevalent tuberculosis in a South African township. Am J Respir Crit Care Med 182: 1080–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pretorius C, et al. 2014. The potential effects of changing HIV treatment policy on tuberculosis outcomes in South Africa: results from three tuberculosis-HIV transmission models. AIDS 28: S25–S34. [DOI] [PubMed] [Google Scholar]
- 18.Suthar AB, et al. 2012. Antiretroviral therapy for prevention of tuberculosis in adults with HIV: a systematic review and meta-analysis. PLoS Med 9: e1001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams BG, Granich R, De Cock KM, Glaziou P, Sharma A, Dye C, 2010. Antiretroviral therapy for tuberculosis control in nine African countries. Proc Natl Acad Sci USA 107: 19485–19489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bassett IV, Wang B, Chetty S, Giddy J, Losina E, Mazibuko M, Bearnot B, Allen J, Walensky RP, Freedberg KA, 2010. Intensive tuberculosis screening for HIV-infected patients starting antiretroviral therapy in Durban, South Africa. Clin Infect Dis 51: 823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zachariah R, et al. 2011. Reduced tuberculosis case notification associated with scaling up antiretroviral treatment in rural Malawi. Int J Tuberc Lung Dis 15: 933–937. [DOI] [PubMed] [Google Scholar]
- 22.Manosuthi W, Chottanapand S, Thongyen S, Chaovavanich A, Sungkanuparph S, 2006. Survival rate and risk factors of mortality among HIV/tuberculosis-coinfected patients with and without antiretroviral therapy. J Acquir Immune Defic Syndr 43: 42–46. [DOI] [PubMed] [Google Scholar]
- 23.April MD, Wood R, Berkowitz BK, Paltiel AD, Anglaret X, Losina E, Freedberg KA, Walensky RP, 2014. The survival benefits of antiretroviral therapy in South Africa. J Infect Dis 209: 491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Treasury South Africa , 2007. Provincial Budgets and Expenditure Review: 2003/04–2009/10. Pretoria, South Africa Available at: http://www.treasury.gov.za/publications/igfr/2007/prov/00.%20Front%20Pages%20and%20Contents.PDF. Accessed September 17, 2015.
- 25.Meyer-Rath G, Schnippel K, Long L, MacLeod W, Sanne I, Stevens W, Pillay S, Pillay Y, Rosen S, 2012. The impact and cost of scaling up GeneXpert MTB/RIF in South Africa. PLoS One 7: e36966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosen S, Ketlhapile M, Sanne I, DeSilva MB, 2008. Characteristics of patients accessing care and treatment for HIV/AIDS at public and nongovernmental sites in South Africa. J Int Assoc Physicians AIDS Care (Chic) 7: 200–207. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization , 2008. Molecular Line Probe Assays for Rapid Screening of Patients at Risk of Multidrug-Resistant Tuberculosis. Geneva, Switzerland: World Health Organization.
- 28.Houben RM, Glynn JR, Mboma S, Mzemba T, Mwaungulu NJ, Mwaungulu L, Mwenibabu M, Mpunga J, French N, Crampin AC, 2012. The impact of HIV and ART on recurrent tuberculosis in a sub-Saharan setting. AIDS 26: 2233–2239. [DOI] [PubMed] [Google Scholar]
- 29.Dodd PJ, Knight GM, Lawn SD, Corbett EL, White RG, 2013. Predicting the long-term impact of antiretroviral therapy scale-up on population incidence of tuberculosis. PLoS One 8: e75466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muula AS, Ngulube TJ, Siziya S, Makupe CM, Umar E, Prozesky HW, Wiysonge CS, Mataya RH, 2007. Gender distribution of adult patients on highly active antiretroviral therapy (HAART) in southern Africa: a systematic review. BMC Public Health 7: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wouters E, Heunis C, Ponnet K, Van Loon F, le Roux Booysen F, van Rensburg D, Meulemans H, 2010. Who is accessing public-sector anti-retroviral treatment in the Free State, South Africa? An exploratory study of the first three years of programme implementation. BMC Public Health 10: 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reid MJ, Shah NS, 2009. Approaches to tuberculosis screening and diagnosis in people with HIV in resource-limited settings. Lancet Infect Dis 9: 173–184. [DOI] [PubMed] [Google Scholar]