Abstract.
Dengue virus (DENV) affects more than 100 countries worldwide. Dengue virus infection has been increasing in the southern Peruvian Amazon city of Puerto Maldonado since 2000. We designed this study to describe the prevalence of past DENV infection and to evaluate risk factors. In 2012, we conducted a cross-sectional serosurvey and administered a knowledge, attitudes, and practices (KAP) questionnaire to members of randomly selected households. Sera were screened for antibodies to DENV by enzyme-linked immunosorbent assay and confirmed by plaque reduction neutralization test. We created indices for KAP (KAPi). We used SaTScan (Martin Kulldorff with Information Management Services Inc., Boston, MA) to detect clustering and created a multivariate model introducing the distance of households to potential vector and infection sources. A total of 505 participants from 307 households provided a blood sample and completed a questionnaire. Fifty-four percent of participants (95% confidence interval [CI]: 49.6; 58.5) had neutralizing antibodies to DENV. Higher values of KAPi were positively associated with having DENV antibodies in the multivariate analysis (odds ratio [ORII]: 1.6, 95% CI: 0.6, 2.4; ORIII: 2.7, 95% CI: 1.3, 5.5; and ORIV: 2.4, 95% CI: 1.2, 5.0). Older groups had lower chances of having been exposed to DENV than younger people (OR20–30: 0.5, 95% CI: 0.2, 0.8; OR31–45: 0.5, 95% CI: 0.3, 0.9; and OR>45: 0.6, 95% CI: 0.3, 1.3). Multivariate data analysis from the 270 households with location information showed male gender to have lower risk of past DENV infection (OR: 0.6, 95% CI: 0.4, 0.9). We conclude that risk of DENV infection in Puerto Maldonado is related to gender, age of the population, and location.
Introduction
Dengue virus (DENV) is the most commonly distributed arbovirus, with 2–4 billion people at risk of infection across 100 countries1–4 and responsible for approximately 500,000 cases of severe disease annually.2,5 Dengue fever is primarily an urban disease. First or “primary” infection with one of the four DENV serotypes (DENV-1, -2, -3, and -4) typically results in a febrile disease with low mortality, whereas subsequent secondary infections with other serotypes are a known risk factor for the more severe forms of the disease.6 The primary vector for DENV is the anthropophilic mosquito, Aedes aegypti, which has adapted well to human living conditions, typically breeding in small containers around the home.7,8 Vector control activities focus on the removal of larval habitats and insecticide spraying to diminish the adult vector population. However, different levels of knowledge, attitudes, and practices (KAPs) of dengue prevention and vector control have been shown to influence vector infestation,9–11 making community involvement a cornerstone for vector control.12–14 As the world undergoes a global urban transition, new habitats for Ae. aegypti continue to emerge in naive populations. Communities with residents who are unaware (or poorly informed) of DENV infection prevention and control measures may be at higher risk of DENV infection.
The close relationship between the human host and mosquito vector has translated into a heterogeneous pattern of DENV distribution in urban environments,15–17 particularly where Ae. aegypti has adapted extremely well. Varying quality of human dwellings in urban settings may be associated with DENV infection risk and with socioeconomic and demographic characteristics and environmental features.18–20 However, in communities where DENV is emerging, spatial patterns of DENV may appear homogenous because of random distributions of KAPs and susceptibility to all four serotypes. Spatial analytical methods are increasingly used to investigate focal areas of present and past DENV transmission to guide vector and disease surveillance,21,22 helping to better understand DENV distribution23 and outbreak related factors,24 particularly in newly impacted communities.
Madre de Dios is the least populated region of Peru (approximately 130,000), yet has grown dramatically because of migration, primarily to the region’s capital city, Puerto Maldonado, making it a rapidly expanding urban center.25,26 Seventy percent of Madre de Dios’ inhabitants are migrants from the neighboring highland regions of Cusco and Puno, neither of which are endemic for DENV.27,28 In 2002, a study from the Peru’s National Institute for Civil Defense (INDECI, for the acronym in Spanish) reported that the city lacked approximately 1,300 housing units, which required more than 32 hectares of city expansion to meet this demand.29,30 Since that study, the population of Tambopata district, where Puerto Maldonado is located, increased by greater than 50% to approximately 78,000 in 2015.26 This pattern of human mobility also constitutes a flow of susceptible individuals into an area of DENV transmission.
The local Ministry of Health (DIRESA, for the acronym in Spanish) has established a vector control program in Puerto Maldonado.28 However, given the recent arrival of many residents and recent emergence of DENV in the region,31,32 many residents may not be familiar with the mode of transmission of the virus and the appropriate prevention control strategies, weakening the efficacy of vector control activities promoted by DIRESA. All DENV serotypes have been reported in Puerto Maldonado. Dengue infection was sporadically reported until August 2007 when more than 300 cases were detected. Since that period, annual cases have ranged from 1,000 to 3,000.31,33
Although all four DENV serotypes have been reported in Puerto Maldonado, no population studies have been conducted on the immunity profile of Puerto Maldonado’s population or the KAPs associated with DENV transmission or Ae. aegypti control. We sought to fill this knowledge through evaluation of DENV transmission spatial patterns, considering aspects such as time in city residence, availability of services, infrastructure quality, household socioeconomic status, and distance of households to potential high-risk areas (i.e., flooding areas, cemeteries, and markets) that have shown to be relevant for vector-borne diseases because of the presence of standing water conducive to Ae. aegypti breeding.20,34 The objectives of this study were to describe the past seroprevalence of DENV infection among residents of Puerto Maldonado, evaluate the influence of KAPs related to DENV control and prevention on DENV transmission, and analyze spatial patterns of DENV risk. This information should be used to help inform vector control programs in Madre de Dios and similar regions experiencing recent DENV emergence.
Materials and methods
Location.
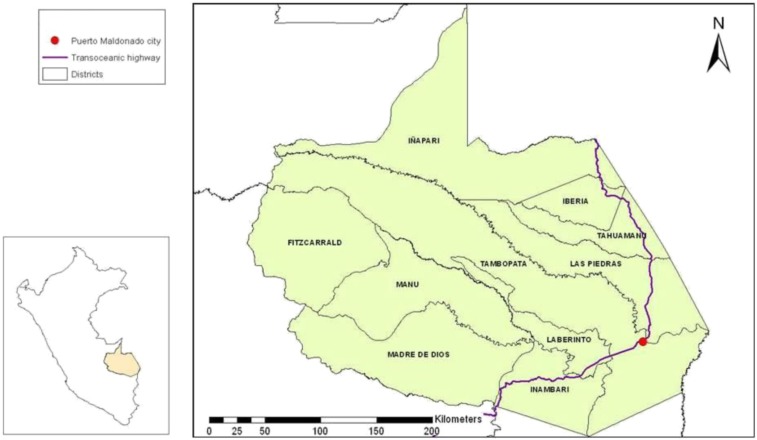
Puerto Maldonado is the capital city of Madre de Dios, located in the southern Amazon Basin of Peru (S 12°36′12.3654″, W 69°11′30.8682″) adjacent to the Madre de Dios and Tambopata rivers (Figure 1). The population of the city is approximately 78,000 people and has doubled since 1993.28
Figure 1.
Madre de Dios and Puerto Maldonado, Peru. This figure appears in color at www.ajtmh.org.
Household and Participant Selection, Serosurvey, and Questionnaire.
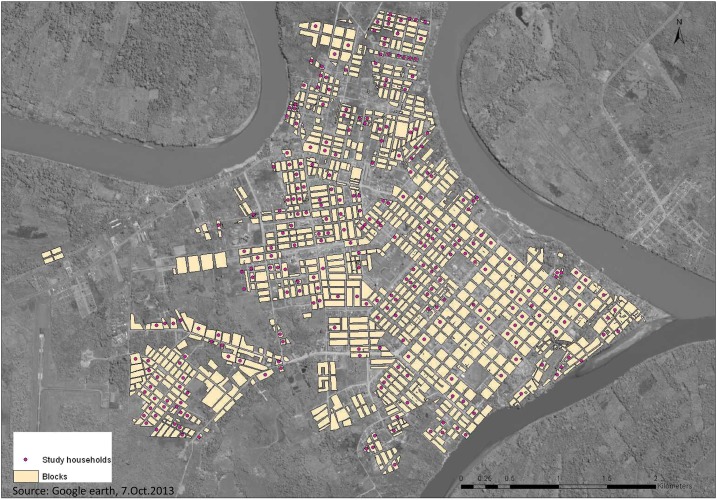
We conducted a cross-sectional survey using a household questionnaire, an individual questionnaire, and a collection of serum sample from each participant. Fieldwork was performed between July and December 2012. Households were selected from city blocks identified using an IKONOS multispectral 1/100,000 satellite image from July 2010 and a cadastral map obtained from the Municipality of Tambopata Province in January 2007. The cadastral map was updated with information from the satellite image and from current maps used by the DIRESA for the vector control program and by ground-truthing (checking accurate locations and updating information from the field). Blocks were selected randomly, and maps of the areas were created to guide fieldwork. One household per block was enrolled for the study (Figure 2). Field workers began enrollment at the residence located in the northernmost corner of the selected block. If the lot was a commercial building or the household was not willing to participate, field workers continued moving around the block clockwise to the next household that was willing to participate. If the lot selected had several residences, such as an apartment building or compound, the field team selected one of the houses or apartments using a random number table.
Figure 2.
Blocks selected from the study in Puerto Maldonado. This figure appears in color at www.ajtmh.org.
All members of the household were invited to participate, provided they were older than 6 months. The study was explained to the household heads and members. If they agreed to participate, individual consent and assent were obtained. Through the questionnaire, we collected information regarding migration history, household assets, building materials, number of dwellers, family monthly income, access to running water, garbage collection, and connection to sewage systems as well as other individual characteristics such as age, education, and occupation. The latter variable was categorized as student, household worker, blue-collar (outdoors, e.g., mototaxi driver or farmer) jobs, and white-collar (indoors, e.g., office clerks, administrators, or sales clerks) jobs.
We created three ad hoc household indices for groups of variables attributed to KAP (KAPi), infrastructure and services (ISi), and assets (Ai). All variables included in the indices were assessed based on the variability described for this population and entered with different weights, according to our criteria for KAPi and ISi and market value for Ai, for the final index. We compared different approaches to create these indices,35 including principal components analysis, but a final decision on which to use was based on equivalence of the indices, ease of use, and interpretation of the index. (see Supplemental Appendix 2 for a detailed description of the indices). A higher value indicates a better standing for all indices.
Laboratory Testing.
Blood samples were collected with standard ante brachial vein puncture and kept at 4°C until they could be transported to the U.S. Naval Medical Research Unit No. 6 (NAMRU-6) field laboratory in Puerto Maldonado, where they were centrifuged and serum collected and stored at −70°C until shipped on dry ice to the NAMRU-6 laboratory in Lima. In Lima, samples were tested first, screened for immunoglobulin G against DENV by ELISA with positives, and confirmed for neutralizing antibodies by plaque reduction neutralization (PRNT). ELISAs were performed using a modified protocol from Kuno et al.36
Plaque Reduction Neutralization Test.
Serum sample aliquots of 200 µL were inactivated in a water bath at 56°C for 30 minutes. Sera were then mixed in maintenance medium in a 2-fold serial dilution from 1/20 to 1/320 and were placed in a 24-well plate. The target virus was grown in BHK21CL15 cell lines (5 days for DENV-2, 7 days for DENV-1 and DENV-3, and 6 days for DENV-4). Viral strains used for this assay were as follows: DENV-1 (16007) C6/36 passage 9 (p-9), 02/12/2010 Asian strain, DENV-2 (16681) C6/36 p-9, 03/12/2010 Asian strain, DENV-3 (IQD1728) C6/36 p-7, 02/12/2010 Peruvian strain, DENV-4 (1036) C6/36 p-9, and 02/12/2010 Asian strain. Viruses were diluted to final working concentrations (1/2,500 for DENV-1: 1.2 × 106 plaque forming unit (PFU), 1/40,000 for DENV-2: 1.9 × 107 PFU, 1/1,000 for DENV-3: 8.0 × 105 PFU, and 1/1,500: 1.1 × 106 PFU for DENV-4) using maintenance medium (E-MEM 2% fetal bovine serum). Viral dilutions were added to the wells in the same volume as the sample dilutions (1:1) and were kept at 4°C overnight. The plaque assay was performed adding 0.5 mL of cell suspension at a concentration of 3 × 105 cells/mL. These were incubated for half an hour at 37°C and 5% CO2. Fifty microliters of viral dilution were added to each well and incubated for 3 hours at 37°C and 5% CO2. Inside a biological safety cabinet, 0.5 mL of semisolid media was added to each well and was incubated at 37°C and 5% CO2: 5 days for DENV-2, 6 days for DENV-4, and 7 days for DENV-1 and DENV-3. Media were then discarded and plates dried for color staining at room temperature for 30 minutes to 1 hour. Plates were rinsed and left to dry again; then, they were placed in a light box for plaque counting. The percentage of neutralization was obtained for each sample dilution, using the negative controls for each sample. Plaque reduction neutralization titer was calculated based on a 70% or greater reduction in plaque counts (PRNT70) using probit analysis with SPSS 20.0 (IBM Corp, Armonk, NY). End point titers are expressed as the reciprocal of the last serum dilution, showing 70% reduction in plaque counts. The following cutoff values were used: DENV-1 and 3, greater than 1/60; DENV-2, 1/80; and DENV-4, 1/40 [17].
Data Analysis.
We defined participants with primary infection as those who had PRNT results positive for only one DENV serotype, secondary infection as those who had a reaction for more than one serotype, and susceptible as those who had a negative result for all DENV serotypes or had a negative ELISA test. We categorized participants based on when they had moved to Puerto Maldonado: native, less than or equal to 15 years or more than 15 years. Different cutoffs were analyzed, but did not provide more useful information, so these were used to achieve a more balanced sample size for each group. To compare indices and monthly income, households were designated as “positive” if at least one member was PRNT positive and “seronegative” if no member was PRNT positive. For example, if a household had three members who were tested and one had a history of a secondary infection, another had had a primary infection, while the other one was seronegative, then the household was considered as a secondary infection household.
The frequencies of the variables gathered through the questionnaire were initially tested against DENV infection status using chi square analysis for categorical variables and Mann–Whitney, Kruskal–Wallis, t-test, or analysis of variance for continuous variables. Based on this preliminary analysis, variables were entered in a multilevel ordered logistic regression model to evaluate seroprevalence with three possible outcomes from a PRNT: seronegative, primary infection, and secondary infection. All analyses were performed using Stata 12.1 (StataCorp LP, College Station, TX).
Spatial Analysis.
We performed spatial analysis with different tools based on the model previously analyzed with Stata 12.1. We created a similar multilevel ordered logistic model, but included spatial coordinates. Then, we performed an exploratory analysis at the household level to assess global clustering patterns and to identify clusters with ArcGIS 10.0 (Esri, Redlands, CA). We used the average nearest neighbor analysis for positive and seronegative households to evaluate spatial clustering.37 We also explored geographical patterns of household status using an inverse weighted distance (IDW) method.38 Inverse weighted distance is a nonparametric interpolation method that estimates the influence each spatial observation may have among the others under study, considering the influence decay across distance and using the inverse squared distances between points to allocate weights.37 In a following assessment, we used incremental spatial autocorrelation (ISA) to estimate the thresholds, where clustering of positive household members may occur in our study area, and we further assessed the point patterns with Getis-Ord General G.37,38 This bivariate analysis was used to explore continuous household variables: number of members DENV antibody–positive per household, proportion of household members who are migrants, household monthly income, KAPi, ISi, and Ai. Subsequently, the Getis-Ord hot spot analysis (Gi*) was used to locate where the clustering of data occurred. This statistic for spatial association is a variation of the General G, focusing on defining the actual location of clusters.37
We conducted further analysis using SaTScan v9.3.39 As with ArcGIS, the data were solely analyzed at the household level. We introduced the significant variables identified by the ordered logistic model and the global and local tests described earlier into SaTScan to adjust for covariates. This tool is a spatial scan statistic that tests for spatial randomness using a moving circular window of variable radii across the study area. The scan statistic compares the events inside and outside the moving window and identifies where the most likely clusters are located and estimates the relative risk associated with being located inside a cluster compared with other areas. P-values were obtained using Monte Carlo hypothesis testing with 999 iterations. The test was set for an ordinal model and to include a maximum of 50% of the population in a cluster. We selected clusters that had a P-value of ≤ 0.001. To apply the SaTScan tool, we used household categories for each of the outcomes under study: seronegative household, with primary or secondary infection. Likewise, households were also categorized as migrant or native.
With this information, we reproduced a map from the threats analysis conducted by INDECI in 200230 that identified areas with temporal flooding during the rainy season (i.e., November–March) and estimated the distance from these flooding areas to each household study block (Figure 2). We also estimated the distance to features identified in the city that could be potential sources for the vector or infectious individuals, including markets, the cemetery, hospitals, and riverine borders, and entered these variables into the multilevel ordered logistic model to assess whether these features, along with individual- and household-level risk factors, had any influence on the individual risk for DENV serostatus.
Results
Overall Description of the Population.
A total of 505 participants from 307 households were enrolled (Table 1). The study population was predominantly female (61%), with an average age of 33 years. Almost half of the participants were migrants to Puerto Maldonado and had been living in the city for an average of 15 years. Non-native household heads were from Cusco (38%), other areas of Madre de Dios (36%), and Apurimac (6%), with the remaining from diverse areas including Arequipa, Lima, and Moquegua.
Table 1.
General characteristics of the study population
| N (%) | |
|---|---|
| Gender | |
| Female | 309 (61) |
| Male | 196 (39) |
| Age (year) | |
| 0–19 | 118 (23) |
| 20–30 | 130 (26) |
| 31–45 | 125 (25) |
| > 45 | 132 (26) |
| Migration | |
| Native | 269 (53) |
| Migrants | 236 (47) |
| Time living in Puerto Maldonado (year) | |
| ≤ 15 | 130 (29) |
| > 15 | 76 (17) |
| Occupation | |
| White-collar jobs | 175 (35) |
| Household activities | 126 (25) |
| Student | 119 (24) |
| Blue-collar (agriculture, farming, construction, and mechanics) jobs | 85 (17) |
| Education | |
| None | 17 (3) |
| Primary school | 126 (25) |
| Secondary school | 228 (45) |
| Technical school | 73 (15) |
| University | 50 (10) |
| Postgraduate | 1 (0) |
| Not applicable (young children/infants) | 8 (2) |
Serological Profile of the Population and Evidence of DENV Infection.
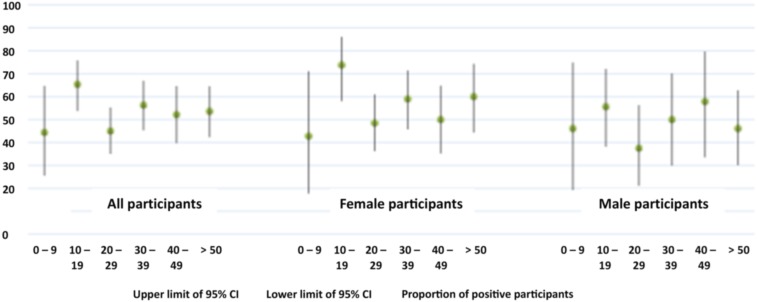
Approximately 70% (355/505) of all participants had antibodies to DENV by ELISA, of whom 54% (273/505) were confirmed by PRNT. Prevalence of past DENV infection did not significantly differ between migrants and natives of Puerto Maldonado or by age group (Figure 3, Tables 2 and 3).
Figure 3.
Prevalence of neutralizing antibodies to Dengue virus by age categories and 95% CI. This figure appears in color at www.ajtmh.org.
Table 2.
Past Dengue virus infection across age groups
| Age category (years) | Individuals (n = 505) | Seroprevalence [95% CI] | P-value* |
|---|---|---|---|
| 0–19 | 118 | 58.5 [49.0; 67.5] | 0.583 |
| 20–30 | 130 | 50.0 [41.1; 58.9] | |
| 31–45 | 125 | 52.8 [43.7; 61.8] | |
| > 45 | 132 | 55.3 [46.4; 63.9] |
Test assesses the difference in seroprevalence across age categories.
Table 3.
Past Dengue virus infection across migration groups
| Migration category (years) | Individuals (n = 505) | Seroprevalence [95% CI] | P-value* |
|---|---|---|---|
| Native | 269 | 51.9 [45.3; 58.4] | 0.118 |
| 0–15 | 151 | 52.3 [43.4; 61.1] | |
| > 15 | 85 | 60.5 [48.6; 71.5] |
Test assessing differences in seroprevalence across migration categories.
Thirty-five percent of all DENV infections were primary (Table 4). Individual characteristics such as gender, age, and education level were evaluated, but did not correlate with PRNT results. An unadjusted analysis contrasting time in Puerto Maldonado showed a trend toward an increase in the odds ratio (OR) infection of ∼3% (95% CI: 1.00; 1.05) with each increase of yearly residence (P = 0.068). In comparison, an unadjusted analysis showed that participants with an occupation within the household (i.e., housewives, retirees, unemployed, and young children) had higher odds of having antibodies to DENV than participants with white-collar (i.e., commerce and technical activities) jobs (OR: 2.12 P = 0.02; 95% CI: 1.13, 3.95). Finally, when examining the serotypes of DENV for primary infection, the most common serotype was DENV-1 (88.6%), followed by DENV-2 (6.3%), DENV-3 (4.0%), and DENV-4 (1.1%).
Table 4.
Past Dengue virus infection estimated from plaque reduction neutralization
| Status | N | % |
|---|---|---|
| Negative or susceptible | 232 | (46) |
| Primary infection* | 175 | (35) |
| Secondary infection* | 98 | (19) |
* Primary or secondary infection is considered if participants show monotypic or multitypic immunity, respectively.
Household Variables.
Households classified as negative or seronegative for DENV infection had lower KAPi scores, fewer assets (lower Ai), and lower monthly incomes (∼US$ 583 and ∼US$ 782, respectively, P < 0.001) compared with positive households (Table 5).
Table 5.
Household characteristics by Dengue virus infection status
| Variable | Household status | Number of households | Mean | Median | Interquartile range | P-value* | |
|---|---|---|---|---|---|---|---|
| 25% | 75% | ||||||
| KAPi† | Total | 307 | 0.55 | 0.46 | 0.38 | 0.79 | 0.009 |
| Negative | 112 | 0.51 | 0.43 | 0.37 | 0.68 | ||
| Positive | 195 | 0.58 | 0.50 | 0.39 | 0.82 | ||
| Primary | 116 | 0.55 | 0.46 | 0.38 | 0.77 | ||
| Secondary | 79 | 0.62 | 0.56 | 0.39 | 0.87 | ||
| ISi† | Total | 307 | 0.86 | 0.88 | 0.83 | 0.92 | 0.216 |
| Negative | 112 | 0.85 | 0.88 | 0.80 | 0.92 | ||
| Positive | 195 | 0.87 | 0.88 | 0.84 | 0.92 | ||
| Primary | 116 | 0.87 | 0.88 | 0.84 | 0.92 | ||
| Secondary | 79 | 0.87 | 0.88 | 0.84 | 0.92 | ||
| Ai‡ | Total | 307 | 7.25 | 7.62 | 6.42 | 7.66 | |
| Negative | 112 | 7.12 | 7.59 | 6.42 | 7.64 | 0.012 | |
| Positive | 195 | 7.32 | 7.62 | 6.50 | 7.70 | ||
| Primary | 116 | 7.31 | 7.62 | 6.42 | 7.70 | ||
| Secondary | 79 | 7.34 | 7.62 | 7.42 | 7.70 | ||
| Income‡ | Total | 292 | 7.30 | 7.33 | 6.91 | 7.65 | < 0.001 |
| Negative | 109 | 7.13 | 7.09 | 6.68 | 7.49 | ||
| Positive | 183 | 7.41 | 7.43 | 6.91 | 7.82 | ||
| Primary | 109 | 7.35 | 7.38 | 6.91 | 7.74 | ||
| Secondary | 74 | 7.49 | 7.53 | 6.91 | 7.97 | ||
| Proportion of migrants§ | All | 307 | 0.36 | 0.33 | 0.00 | 0.60 | 0.784 |
| Negative | 112 | 0.36 | 0.27 | 0.00 | 0.67 | ||
| Positive | 195 | 0.36 | 0.33 | 0.00 | 0.56 | ||
| Primary | 116 | 0.36 | 0.33 | 0.00 | 0.58 | ||
| Secondary | 79 | 0.36 | 0.33 | 0.00 | 0.50 | ||
Ai = indices for assets; ISi = indices for infrastructure and services; KAPi = indices for knowledge, attitudes, and practices.
Ln of Ai and of household monthly income.
Standardized to range from 0 to 1.
Ln of Ai and of household monthly income.
Migrants were living in the city for 5 years or less.
Risk Factors.
Variables evaluated in the multilevel ordered logistic model were age, gender, occupation, KAPi, ISi, Ai, monthly income (transformed with the natural logarithm), education level, and migration status. Participants between 20 and 45 years old consistently showed decreased OR of having a positive PRNT with the odds of a primary or secondary infection less than half relative to younger participants. Higher categories of KAPi score were associated with increased odds of having a positive PRNT (Table 6). Because the highest income bracket was marginally significant, we decided to keep it in the model. Time since migration to the city seemed to have some effect on risk of infection in the bivariate analysis, especially for those residents who had more than 15 years in Puerto Maldonado. The univariate model showed an association between increased OR and household occupations, but this association was not noted in the multivariate model.
Table 6.
Risk factors for Dengue virus exposure
| Model 1: risk factors | Model 2: risk factors and spatial information | Model 3: risk factors, spatial information, and potential sources | ||||
|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | OR | 95% CI | OR | 95% CI |
| Gender | ||||||
| Female | REF | REF | REF | |||
| Male | 0.66* | [0.43; 1.02] | 0.65* | [0.42; 1.00] | 0.61+ | [0.40; 0.94] |
| Age category (year) | ||||||
| 1–19 | REF | REF | REF | |||
| 20–30 | 0.46++ | [0.25; 0.85] | 0.44++ | [0.24; 0.81] | 0.42++ | [0.23; 0.77] |
| 31–45 | 0.48+ | [0.26; 0.88] | 0.42++ | [0.23; 0.78] | 0.45++ | [0.24; 0.82] |
| 46 | 0.65 | [0.34; 1.27] | 0.57 | [0.29; 1.11] | 0.59 | [0.31; 1.14] |
| Migration status | ||||||
| Native | REF | REF | REF | |||
| 15 years or less | 1.21 | [0.71; 2.07] | 1.30 | [0.76; 2.22] | 1.40 | [0.83; 2.36] |
| More than 15 years | 1.72 | [0.89; 3.32] | 1.70 | [0.88; 3.27] | 1.52 | [0.81; 2.86] |
| Income category† | ||||||
| I (lowest) | REF | REF | REF | |||
| II | 1.19 | [0.59; 2.40] | 1.19 | [0.60; 2.35] | 1.25 | [0.65; 2.42] |
| III | 1.71 | [0.86; 3.40] | 1.54 | [0.81; 2.29] | 1.49 | [0.78; 2.84] |
| IV | 1.85* | [0.91; 3.75] | 1.63 | [0.81; 3.29] | 1.58 | [0.80; 3.10] |
| KAPi category | ||||||
| I (lowest) | REF | REF | REF | |||
| II | 1.57 | [0.78; 3.14] | 1.61 | [0.81; 3.18] | 1.55 | [0.80; 3.00] |
| III | 2.71++ | [1.32; 5.54] | 2.68 | [1.33; 5.40] | 2.43++ | [1.25; 4.74] |
| IV | 2.44++ | [1.20; 4.98] | 2.37 | [1.18; 4.76] | 2.09+ | [1.07; 4.05] |
| Northing (km) | – | – | 0.88 | [0.72; 1.08] | 0.68++ | [0.52; 0.89] |
| Easting (km) | – | – | 0.72 | [0.58; 0.90] | 0.5+++ | [0.35; 0.70] |
| Distance to flooding (m) | – | – | – | – | 0.99903+++ | [0.99854; 0.99952] |
| Distance to river shore (m) | – | – | – | – | 1.00090+ | [1.00019; 1.00160] |
| Cut 1: primary infection | 3.99 | [1.31; 6.66] | −0.53 | [−1.52; 0.46] | −1.17 | [−2.20; −0.13] |
| Cut 2: secondary infection | 5.97 | [3.21; 8.72] | 1.40 | [0.39; 2.42] | 0.76 | [−0.26; 1.78] |
KAPi = indices for knowledge, attitudes, and practices; OR = odds ratio. The proportional odds/parallel lines assumption was tested with a Wald test. The models do not violate these assumptions (P = 0.750, 0.744, and 0.508, respectively).
P-values: +++ P < 0.001; ++ P < 0.01; + P < 0.05; and *P < 0.10.
The variable was transformed with the natural logarithm.
Spatial Patterns.
We obtained geographic locations for 285 households, with 270 households providing complete survey data and sera samples. We incorporated data on longitude and latitude in the multilevel ordered logistic model to assess the presence of global spatial patterns at the household level. Variables such as monthly income and migration time were no longer significant. The likelihood ratio test provided evidence that inclusion of location improved the model (P = 0.025) (data not shown) and that the inclusion of location improved the individual- and household-level model (P = 0.004), with gender becoming significant (P = 0.05). Therefore, because the introduction of location information (northing and easting) seemed relevant (data not shown), we explored large- and small-scale spatial trends.
Average nearest neighbor indicated spatial clustering among positive households (P < 0.001). This spatial pattern was explored using IDW, which identified areas with higher risk for DENV infection (map not shown). The ISA tool with the number of positive members in the household was used to determine the distance at which clustering may be occurring in this spatial pattern, which for this analysis identified a peak distance of ∼417 m (P < 0.001). This information was used for further analysis of spatial patterns using General G, which suggested clustering of households with positive members (P = 0.002), higher monthly income (P = 0.012), and lower ISi (P = 0.006). The proportion of migrants in the households (P = 0.181), KAPi (P = 0.272), and Ai (P = 0.358) were not significant. Gi* was applied on the same variables (number of positive members in the household, proportion of household members who are migrants, ISi, KAPi, Ai, and household monthly income), with all rendered areas defined as hot spots, except for ISi. Although not many hot spots were identified, an aggregation of high numbers of positive cases, higher household income, and higher KAP score occurred in the central area (∼centroid) of the city. By contrast, low values of ISi aggregated in cold spots in what could be considered as city border areas (see Supplemental Appendix 3).
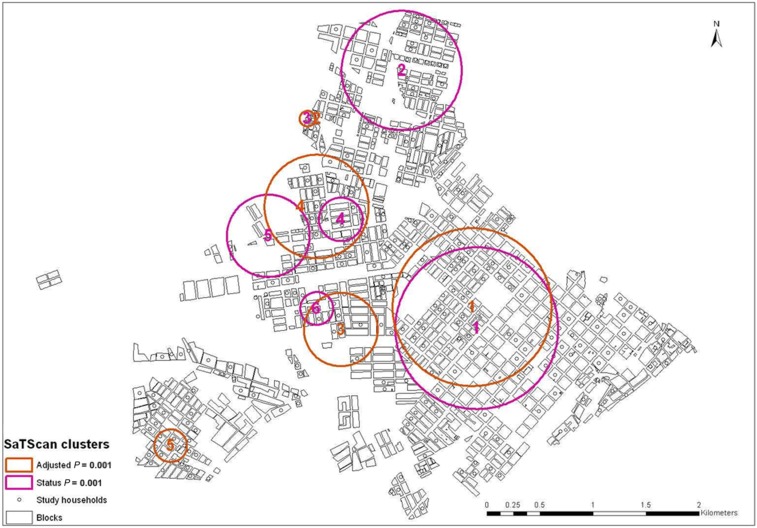
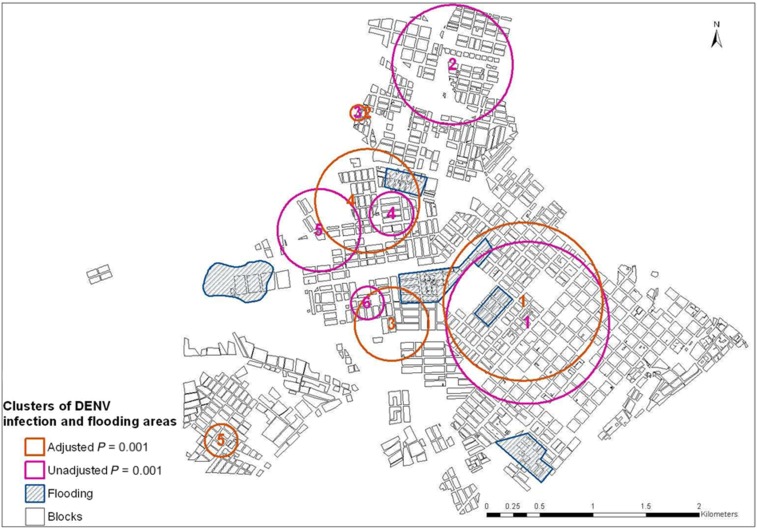
Sixty-three percent (171/270) of households were positive for DENV infection, of which 37.0% were primary infection and 26.3% were secondary infections. Sixty-two percent (168/270) were classified as migrant households. SaTScan analysis without adjustment identified six significant clusters (P = 0.001) (Figure 4). Subsequently, we assessed the location of spatial clusters for household infection status adjusting for categories of time living in Puerto Maldonado, income, and KAPi, which were shown to be significant in the ordinal model with household variables (data not shown). This analysis provided five significant clusters, which were superimposed with the clusters obtained from the unadjusted analysis, in particular the most likely cluster for both analyses. After adjusting for income, KAPi, and migration time, the most likely cluster had a radius of 0.57 km and relative risk of 1.14 and 2.31 for primary and secondary infection, respectively (Figure 5).
Figure 4.
Clusters detected with scan statistics. This figure appears in color at www.ajtmh.org.
Figure 5.
High risk clusters for Dengue virus (DENV) infection and assessed features. This figure appears in color at www.ajtmh.org.
Finally, considering the SatScan findings, we analyzed if there were specific features of the city that could be a source for exposure to the vector and clusters of infection. We evaluated several models including household and individual variables of the distance (in meters) from each study point to three markets, two hospitals, one cemetery, the distance to the river shore, and previously identified flooding areas. The distance to the river and from flooding areas was significantly associated to both primary and secondary DENV infections (Table 6). The likelihood ratio test comparing this model with the model including potential sources of infection was significant (P < 0.001). Dengue virus infection risk decreased with increasing distance from flooding areas and increased with increased distance from the river shore (Table 6). The joint information obtained from the inclusion of these spatial variables into the model point to an area of higher risk toward the center of the city, following a similar pattern from the most likely cluster obtained from the SatScan analysis (Figure 5).
Discussion
More than half of the study participants had evidence of past DENV infection. The information collected from Puerto Maldonado shows lower seroprevalence findings reported from other studies, including other Peruvian cities, such as Iquitos.16 This is probably in part because of the relatively recent introduction of DENV in the area.
The serostatus profile in Puerto Maldonado indicating similar distributions across migrant and native participants suggests the ostensible role of migrants in the serostatus to DENV among this population. We hypothesize that the constant influx of migrants is the reason for the lower seroprevalence findings, possibly “dampening” these findings among the local population, as new members without infection are continually introduced to the community. Da Silva-Nunes et al.40 found a baseline DENV antibody prevalence of 18% for rural areas of Acre, the Brazilian state bordering Madre de Dios, with a yearly increase of 3%, related to travel to endemic areas. The investigators reported an effect of migration on exposure DENV, but showed higher risk among migrants from endemic areas, in contrast to our findings of higher risk associated with residence time in the city. Human population density41 and vector indices likely have an important role in DENV transmission and may explain these discrepancies. The constant influx of migrants to Puerto Maldonado from highland regions where DENV is not known to circulate, such as Cusco and Puno, may also influence these findings, providing a continuous large pool of seronegative hosts.33 The increasing influx of migrants into Puerto Maldonado and increasing exchange with Bolivia and Brazil because of the paving of the Interoceanic Highway, completed in 2012,42 may introduce both DENV susceptible persons and potentially infected people capable of transmitting the virus.16,43 Previous studies conducted in Kamphaeng Phet, Thailand, and Iquitos, Peru, have shown the marked effect of human movement on the local dynamics and transmission of DENV.15,16,20,43
In contrast to what has been reported in other studies, we found a higher risk of DENV infection in households with higher income.44,45 We postulate that this finding relates to the challenges faced by migrants to a new location where they are initially faced with lower wage jobs than natives. Salaries may improve in time and gradually match the natives’ income46; therefore, income would act as a proxy for time spent in the city. This finding, however, was no longer significant in the final models, in which location variables were introduced, showing the stronger association of infection risk with household location. The bivariate analysis shows that housekeeping activities and female gender were risk factors for DENV infection, perhaps because these activities typically occur during the day, when Ae. aegypti bites, and are performed in proximity to larval development sites in containers around the home among residents of Puerto Maldonado.31 However, the occupation variable was not maintained in the final model because we introduced location. Hence, the specific location of the study households and the effect on DENV risk in Puerto Maldonado portray how these variables (i.e., gender, age, and location) may relate with the heterogeneous transmission of DENV that has been reported before for other areas.47 These findings have implications for emerging risks in Peru related to Zika, which shares the same vector risk profile, particularly in young adolescent women.48
We found somewhat counterintuitive evidence of a higher score knowledge of DENV, as indicated by KAPi score and risk of DENV infection. We postulate that this may result from awareness generated after having had the disease or seeing it in members of the same household. Unfortunately, knowledge about DENV and its vector does not necessarily relate to the use of preventive measures.49 Nonetheless, it should be highlighted that KAP can be targeted through outreach programs in the community, especially because household activities are related to an increased risk of DENV infection.
An important limitation to this study is its cross-sectional nature which precludes understanding the specific role of KAP in transmission dynamics. Likewise, there is potential for selection bias because households were not identified before enrollment. Households that were not available to participate or that chose not to may have had different characteristics than those that did. Sample size may also limit some of the inferences addressed in this study because several variables such as Ai or ISi may behave differently across a wider pool of participants with different migratory background. Last, the lack of entomological information leaves a gap regarding the potential effect of some of these variables on the presence of the vector in the household.
In summary, this study reveals the challenges that this emerging infection poses on the residents of Puerto Maldonado and its health authorities. Primarily, serological evidence of previous DENV infection does not seem to correspond to previous knowledge about the disease and preventive measures. Knowledge, attitudes, and practices findings can provide information to tailor specific prevention and control strategies for the area involving the community. Health authorities can focus efforts on specific strategies to improve the awareness and prevention practices of vector breeding, especially at areas of new urban development to involve them in control activities and practical demonstrations at their own homes. Larger programs of city planning can also be devised to improve infrastructure and services provided across the urban area, especially for newly developing areas. Such interventions should consider the cultural backgrounds of city dwellers to achieve desired participation and commitment.9,11 Also, there is strong evidence of spatial heterogeneities in DENV transmission in Puerto Maldonado that have been linked to specific foci and should target urban planning, especially prioritizing aspects such as improving drainage facilities across the city and providing running water plumbing and sewage in expansion areas. Although the risk increase was small, our spatial analysis showed that DENV infection risk increased with shorter household distance to areas of temporal flooding (Figure 3). We should highlight that in Puerto Maldonado, the areas of constant flooding were identified more than 10 years ago and the requirement for improved drainage facilities has not been properly addressed to date.30 These findings underscore the potential benefit of using spatial analysis tools to better target vector control and improve urban infrastructure. Furthermore, it is worth mentioning that this study also identified the cold spots for ISi (clusters of low values for ISi). Although this finding was not associated with DENV infection, this clustering occurs in areas that are considered for urban expansion. The lack of appropriate services or adequate housing could trigger higher risk areas in the future (Figure 3) as has been found in other settings.50 Finally, these findings should help target more detailed vector and incidence studies that can address spatial heterogeneities in DENV transmission in Puerto Maldonado,47 especially facing newer threats of vector-borne diseases such as Chikungunya and Zika.
Supplementary Material
Acknowledgments:
We wish to acknowledge the field personnel in Puerto Maldonado, especially Katherine Rolin, Nelly Godoy, and Abel Estela; Juan Perez and Gerardo Acosta of the U.S. NAMRU-6 Data Entry Management Unit; Lindsay Campbell for invaluable help with data processing; Hugo Razuri and Matthew Kasper for their time and support to initiate this effort; and finally, the community members of Puerto Maldonado.
Note: Supplemental Appendixes appear at www.ajtmh.org.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of Defense or the U.S. Government.
REFERENCES
- 1.Gubler DJ, 1998. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev 11: 480–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guzman A, Istúriz RE, 2010. Update on the global spread of dengue. Int J Antimicrob Agents 36 (Suppl 1): S40–S42. [DOI] [PubMed] [Google Scholar]
- 3.Guzmán MG, Kouri G, 2002. Dengue: an update Lancet Infect Dis 2: 33–42. [DOI] [PubMed] [Google Scholar]
- 4.Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, Hoen AG, Moyes CL, Farlow AW, Scott TW, Hay SI, 2012. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis 6: e1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO Dengue and Severe Dengue. Geneva, Switzerland: WHO; Available at: http://www.who.int/mediacentre/factsheets/fs117/en/. Accessed December 27, 2017. [Google Scholar]
- 6.Monath TP, 1994. Dengue: the risk to developed and developing countries. Proc Natl Acad Sci USA 91: 2395–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrera R, Amador M, Diaz A, Smith J, Munoz-Jordan JL, Rosario Y, 2008. Unusual productivity of Aedes aegypti in septic tanks and its implications for dengue control. Med Vet Entomol 22: 62–69. [DOI] [PubMed] [Google Scholar]
- 8.Harrington LC, et al. 2005. Dispersal of the dengue vector Aedes aegypti within and between rural communities. Am J Trop Med Hyg 72: 209–220. [PubMed] [Google Scholar]
- 9.Lloyd LS, Winch P, Ortega-Canto J, Kendall C, 1992. Results of a community-based Aedes aegypti control program in Merida, Yucatan, Mexico. Am J Trop Med Hyg 46: 635–642. [DOI] [PubMed] [Google Scholar]
- 10.Leontsini E, Gil E, Kendall C, Clark GG, 1993. Effect of a community-based Aedes aegypti control programme on mosquito larval production sites in El Progreso, Honduras. Trans R Soc Trop Med Hyg 87: 267–271. [DOI] [PubMed] [Google Scholar]
- 11.Winch PJ, Leontsini E, Rigau-Pérez JG, Ruiz-Pérez M, Clark GG, Gubler DJ, 2002. Community-based dengue prevention programs in Puerto Rico: impact on knowledge, behavior, and residential mosquito infestation. Am J Trop Med Hyg 67: 363–370. [DOI] [PubMed] [Google Scholar]
- 12.Vanlerberghe V, Toledo ME, Rodríguez M, Gomez D, Baly A, Benitez JR, Van der Stuyft P, 2009. Community involvement in dengue vector control: cluster randomised trial. BMJ 338: b1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ault SK, 1994. Environmental management: a re-emerging vector control strategy. Am J Trop Med Hyg 50 (Suppl 6): 35–49. [DOI] [PubMed] [Google Scholar]
- 14.Escobar-Mesa J, Gómez-Dantés H, 2003. Determinants of dengue transmission in Veracruz: an ecological approach to its control [article in Spanish]. Salud Publica Mex 45: 43–53. [PubMed] [Google Scholar]
- 15.Morrison AC, et al. 2004. Temporal and geographic patterns of Aedes aegypti (Diptera: Culicidae) production in Iquitos, Peru. J Med Entomol 41: 1123–1142. [DOI] [PubMed] [Google Scholar]
- 16.Morrison AC, et al. 2010. Epidemiology of dengue virus in Iquitos, Peru 1999 to 2005: interepidemic and epidemic patterns of transmission. PLoS Negl Trop Dis 4: e670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Mattos Almeida MC, Caiaffa WT, Assunção RM, Proietti FA, 2007. Spatial vulnerability to dengue in a Brazilian urban area during a 7-year surveillance. J Urban Health 84: 334–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Almeida AS, de Medronho RA, Valencia LIO, 2009. Análise espacial da dengue e o contexto socioeconômico no município do Rio de Janeiro, RJ. Rev Saúde Pública 43: 666–673. [DOI] [PubMed] [Google Scholar]
- 19.Mondini A, Chiaravalloti-Neto F, 2008. Spatial correlation of incidence of dengue with socioeconomic, demographic and environmental variables in a Brazilian city. Sci Total Environ 393: 241–248. [DOI] [PubMed] [Google Scholar]
- 20.Tsuda Y, Suwonkerd W, Chawprom S, Prajakwong S, Takagi M, 2006. Different spatial distribution of Aedes aegypti and Aedes albopictus along an urban–rural gradient and the relating environmental factors examined in three villages in northern Thailand. J Am Mosq Control Assoc 22: 222–228. [DOI] [PubMed] [Google Scholar]
- 21.Lagrotta MT, Silva Wda C, Souza-Santos R, 2008. Identification of key areas for Aedes aegypti control through geoprocessing in Nova Iguaçu, Rio de Janeiro state, Brazil. Cad SaÃode PÃoblica 24: 70–80. [DOI] [PubMed] [Google Scholar]
- 22.Morrison AC, Getis A, Santiago M, Rigau-Perez JG, Reiter P, 1998. Exploratory space-time analysis of reported dengue cases during an outbreak in Florida, Puerto Rico, 1991–1992. Am J Trop Med Hyg 58: 287–298. [DOI] [PubMed] [Google Scholar]
- 23.Kuan G, Gordon A, Avilés W, Ortega O, Hammond SN, Elizondo D, Nuñez A, Coloma J, Balmaseda A, Harris E, 2009. The Nicaraguan pediatric dengue cohort study: study design, methods, use of information technology, and extension to other infectious diseases. Am J Epidemiol 170: 120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali M, Wagatsuma Y, Emch M, Breiman RF, 2003. Use of a geographic Information system for defining spatial risk for dengue transmission in Bangladesh: role for Aedes albopictus in an urban outbreak. Am J Trop Med Hyg 69: 634–640. [PubMed] [Google Scholar]
- 25.INEI , 2009. Perú: Migraciones Internas 1993–2003. Lima, Peru: Taller de la Oficina Técnica de la Difusión del INEI. [Google Scholar]
- 26.Sociales INEI (Perú) DT de D e I 2008. Perfil Sociodemográfico Del Perú: Censos Nacionales 2007: XI de Población y VI de Vivienda. Lima, Peru: INEI. [Google Scholar]
- 27.DIRESA Madre de Dios , 2006. Análisis de la Situación de Salud de Madre de Dios. Puerto Maldonado, Madre de Dios, Peru: Dirección de Epidemiología. [Google Scholar]
- 28.DIRESA Madre de Dios , 2007. Análisis de la Situación de Salud de Madre de Dios. Puerto Maldonado, Madre de Dios, Peru: Dirección de Epidemiología. [Google Scholar]
- 29.Palomino L, Mosqueira C, Kuroiwa J, Pérez Galleno A, Zerga A, 2008. Programa ciudades sostenibles, plan de acción 2007–2011: integración de los sectores público y privado. Rev Com Andino Para Prev Aten Desastres CAPRADE 3: 28–32. [Google Scholar]
- 30.Kuroiwa Huroich J, 2002. Mapa de Peligros de la Ciudad de Puerto Maldonado. Zerga Ocana A, ed. Tambopata, Madre de Dios, Peru: INDECI; Available at: http://bvpad.indeci.gob.pe/doc/estudios_CS/Region_Madre_de_Dios/tambopata/puertomaldonado.pdf. Accessed October 1, 2012. [Google Scholar]
- 31.Forshey BM, et al. 2010. Arboviral etiologies of acute febrile illnesses in western South America, 2000–2007. PLoS Negl Trop Dis 4: e787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DIRESA , 2014. Boletín de Información Técnica. 2009. Puerto Maldonado, Madre de Dios, Peru: DIRESA. [Google Scholar]
- 33.DIRESA Madre de Dios DIRESA Puno , 2013. Plan Macroregional Sur-Oriente de Lucha contra el Dengue. [Google Scholar]
- 34.Morrison AC, Astete H, Chapilliquen F, Ramirez-Prada G, Diaz G, Getis A, Gray K, Scott TW. 2004. Evaluation of a sampling methodology for rapid assessment of Aedes aegypti infestation levels in Iquitos, Peru. J Med Entomol 41: 502–510. [DOI] [PubMed] [Google Scholar]
- 35.Morris SS, Carletto C, Hoddinott J, Christiaensen LJM, 2000. Validity of rapid estimates of household wealth and income for health surveys in rural Africa. J Epidemiol Community Health 54: 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuno G, Gómez I, Gubler DJ, 1991. An ELISA procedure for the diagnosis of dengue infections. J Virol Methods 33: 101–113. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell A, 2009. Spatial Measurements and Statistics. Redlands, CA: ESRI Press. [Google Scholar]
- 38.Waller LA, Gotway CA, 2004. Applied Spatial Statistics for Public Health Data. Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- 39.Kulldorff M, 2010. SaTScan-Software for the Spatial, Temporal, and Space-Time Scan Statistics. Boston, MA: Harv Med Sch Harv Pilgr Health Care. [Google Scholar]
- 40.da Silva-Nunes M, et al. 2008. Risk factors for dengue virus infection in rural Amazonia: population-based cross-sectional surveys. Am J Trop Med Hyg 79: 485–494. [PubMed] [Google Scholar]
- 41.Chowell G, Torre CA, Munayco-Escate C, Suárez-Ognio L, López-Cruz R, Hyman JM, Castillo-Chavez C, 2008. Spatial and temporal dynamics of dengue fever in Peru: 1994–2006. Epidemiol Infect 136: 1667–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.COSIPLAN , 2012. Iniciativa para la Integración de la Infraestructura Regional Suramericana (IIRSA). Ficha del Proyecto: Pavimentación Iñapari–Puerto Maldonado–Inambari, Inambari–Juliaca/Inambari–Cusco. Eje Perú–Brasil–Bolivia. Available at: http://www.iirsa.org/proyectos/detalle_proyecto.aspx?h=319&x=9&idioma=ES. Accessed October 1, 2012.
- 43.Adams B, Kapan DD, 2009. Man bites mosquito: understanding the contribution of human movement to vector-borne disease dynamics. PLoS One 4: e6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brunkard JM, et al. 2007. Dengue fever seroprevalence and risk factors, Texas–Mexico border, 2004. Emerg Infect Dis 13: 1477–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bartley LM, Carabin H, Vinh Chau N, Ho V, Luxemburger C, Hien TT, Garnett GP, Farrar J, 2002. Assessment of the factors associated with Flavivirus seroprevalence in a population in southern Vietnam. Epidemiol Infect 128: 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borjas GJ, Bronars SG, Trejo SJ, 1992. Assimilation and the earnings of young internal migrants. Rev Econ Stat 74: 170–175. [Google Scholar]
- 47.Favier C, Schmit D, Müller-Graf CD, Cazelles B, Degallier N, Mondet B, Dubois MA, 2005. Influence of spatial heterogeneity on an emerging infectious disease: the case of dengue epidemics. Proc Biol Sci 272: 1171–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faria NR, et al. 2016. Zika virus in the Americas: early epidemiological and genetic findings. Science 352: 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Itrat A, Khan A, Javaid S, Kamal M, Khan H, Javed S, Kalia S, Khan AH, Sethi MI, Jehan I, 2008. Knowledge, awareness and practices regarding dengue fever among the adult population of dengue hit cosmopolitan. PLOS One 3: e2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Benthem BH, Vanwambeke SO, Khantikul N, Burghoorn-Maas C, Panart K, Oskam L, Lambin EF, Somboon P, 2005. Spatial patterns of and risk factors for seropositivity for dengue infection. Am J Trop Med Hyg 72: 201–208. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.