Abstract
Meiotic drivers are selfish alleles that subvert gametogenesis to increase their transmission into progeny. Drivers impose a fitness cost, putting pressure on the genome to evolve suppressors. Here we investigate the wtf gene family from Schizosaccharomyces pombe, previously shown to contain meiotic drivers in wild isolates. We discovered that wtf13 found in lab stocks is a meiotic driver. wtf13 kills spores that do not inherit it by generating both a diffusible poison and a spore-specific antidote. Additionally, we demonstrate that wtf13 is suppressed by another wtf gene, wtf18-2, that arose spontaneously in the lab and makes only an antidote. Wtf18-2 does not act indiscriminately to prevent spore destruction. Instead, it rescues only the spores that inherit wtf18-2. In this way, wtf18-2 selfishly gains a transmission advantage of its own while dampening the drive of wtf13. This establishes a novel paradigm for meiotic drive suppressors and provides insight into the mechanisms and evolution of drive systems.
Author summary
Killer meiotic drivers are selfish genes that cause infertility by destroying the gametes (e.g. sperm) that do not inherit them. This allows them to be transmitted into up to 100% of the surviving gametes. Because of the significant fitness cost that these killer meiotic drivers impose to the organism, suppressors of drive are expected to evolve. We and others recently discovered that members of the wtf gene family in natural isolates of fission yeast are killer meiotic drivers. These genes generate a poison to kill gametes, but rescue those that inherit the driver with an antidote. In this work, we discovered that an additional wtf gene, wtf13 found in intensely studied lab strains is also a meiotic driver. This driver is only capable of driving in the absence of another wtf gene, wtf18-2, which works by mimicking the antidote of wtf13. By protecting only the spores that inherit the gene, wtf18-2 selfishly gains a transmission advantage of its own, establishing a novel paradigm for drive suppressors.
Introduction
A founding principle of genetics is that the two alleles carried by a diploid are transmitted at Mendelian (equal) frequencies into the gametes [1]. The mechanisms of meiotic chromosome segregation and gametogenesis are often assumed to be unbiased and to therefore guarantee Mendelian transmission. These processes, however, are not perfect and are vulnerable to exploitation by ‘selfish’ genetic parasites that can bias their own transmission to the next generation, often at the expense of the rest of the genome [2]. These selfish alleles are known as meiotic drivers. Although the term was initially used to describe alleles that could bias the meiotic divisions in their favor, it soon expanded to include alleles that act after the meiotic divisions to destroy the gametes that do not inherit them [2, 3].
Meiotic drivers have been observed across eukaryotes from plants to humans [4–6]. While the true prevalence of meiotic drivers is unknown (and perhaps underappreciated), the detection of meiotic drive has accelerated in recent years, as next generation sequencing enabled the detection of drivers not linked to obvious phenotypes [7–11].
Meiotic drive typically compromises organismal fitness [12–15]. This is because Mendelian transmission facilitates natural selection of the best adapted alleles by ensuring each allele has a fair chance to be tested. Additionally, meiotic drivers can inadvertently cause deleterious alleles that are linked to drivers to be maintained or spread in a population. For example, several drive loci are genetically linked to recessive mutations that cause sterility or even lethality [13, 16–18]. One class of meiotic drivers, the killer meiotic drivers, can even cause infertility directly. These drivers act by actively destroying meiotic products that do not inherit the drive allele from a heterozygous parent [6, 19].
Genomic loci that are unlinked from drive alleles can suffer direct and indirect fitness costs imposed by drivers. This puts meiotic drivers in genetic conflict with the rest of the genome, especially alleles that are driven against and transmitted to less than half of the functional gametes. Alleles that are unlinked from the driver that suppress drive can therefore be favored by natural selection, which in turn, selects for drive alleles that evade suppression [4, 12, 20]. The evolutionary dynamic between meiotic drivers and their suppressors is akin to that observed between viruses and immune factors: both sides are predicted to rapidly evolve in an ongoing molecular arms race [21, 22]. This race between drivers and suppressors is predicted to shape the evolution of fundamental aspects of eukaryotic cells including chromosome structure and segregation [23–25].
A handful of meiotic drive alleles have been identified, and the phenotypic effects of many more have been observed. Very little, however, is known about the molecular mechanisms by which they function [4–6, 9, 13, 26–33]. Additionally, while the actions of drive suppressors have been detected in many cases, only three suppressors of drive have been identified [4, 6, 9, 11, 13, 26–37].
The wtf gene family of fission yeast offers an excellent opportunity to study the mechanisms and evolution of meiotic drive genes [38, 39]. In the reference strain of S. pombe (strain L972, denoted here as Sp), there are 25 wtf genes, including several pseudogenes [40]. Four different wtf genes were recently identified as meiotic drivers in two different S. pombe group isolates, S. kambucha (denoted here as Sk) and CBS5557 [7, 8]. These genes all act to kill spores (yeast meiotic products) that do not inherit them such that >70% of the viable spores generated by wtf+/- heterozygotes are wtf+ [7, 8].
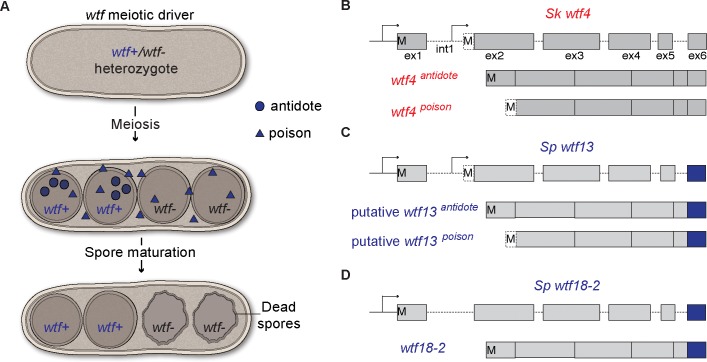
At least one such gene, wtf4 from S. kambucha, causes meiotic drive by a poison and antidote mechanism (Fig 1A). The wtf4 gene has two alternative transcriptional start sites (Fig 1B). The two transcripts are in the same reading frame and are largely overlapping, but they encode distinct poison and antidote proteins. The Wtf4poison protein is trans-acting and can kill all spores generated by diploids encoding the locus. However, from the four spores produced after meiosis, the two spores that inherit the wtf4 gene generally survive because they are protected by the cis-acting Wtf4antidote protein (Fig 1A) [7]. Other driving wtf genes are predicted to act via an analogous poison-antidote mechanism [8, 38].
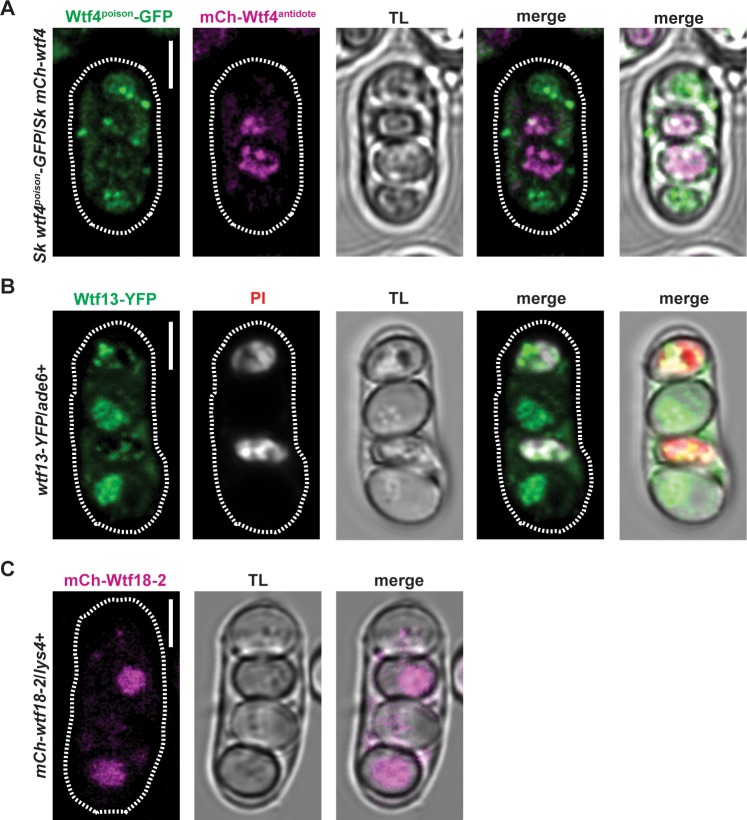
Fig 1. Wtf poison and antidote meiotic drive.
(A) Model for how wtf meiotic drivers enhance their transmission into functional spores when heterozygous (wtf+/wtf-). wtf meiotic drivers generate both a poison and an antidote. The poison spreads throughout the ascus (sac that holds the spores). The antidote is specifically enriched within the spores that inherit the driver allele. Spores that inherit the wtf+ allele are thus overrepresented in the viable spore population. (B) Sk wtf4 uses alternate transcriptional and translational start sites to generate two transcripts. The short transcript encodes a poison from five exons and the longer transcript encodes an antidote to the poison in six exons [7]. (C) Analogous to Sk wtf4, Sp wtf13 makes two transcripts [41]. (D) Sp wtf18-2 generates one transcript with six exons. To emphasize the high amino acid identity shared by exon six of Sp wtf13 and Sp wtf18-2, those exons are depicted in blue. M’s represent the translational start sites.
Not all intact wtf genes, however, appear to encode two transcripts. Many wtf genes, including twelve in the reference genome, encode only one transcript [8, 39, 41]. In addition, all three tested intact wtf genes predicted to have one transcript failed to cause drive [7]. These results led us to predict that at least some of these wtf genes encode only antidotes and that they could act as suppressors of wtf drive genes [7, 38].
Here, we show that Sp wtf13 is a poison-antidote meiotic driver. In addition, we show that Sp wtf18-2 encodes a protein that suppresses Sp wtf13 via molecular mimicry of the Sp Wtf13antidote protein. Importantly, Sp wtf18-2 only rescues gametes that inherit it from destruction by the Sp Wtf13poison protein. In other words, wtf18-2 benefits from the infertility caused by wtf13. This represents a novel type of drive suppressor in that it parasitizes the driver to gain a non-Mendelian transmission advantage of its own.
Results
S. pombe wtf13 is an autonomous poison-antidote meiotic drive gene
Our previous work suggested that a complex network of meiotic drivers and suppressors of drive are distributed across both Sp and Sk chromosome 3’s. This inference stems from the phenotypes of a series of distinct Sk/Sp hybrid diploids. Each of these diploids is heterozygous for one pure Sk copy and one independently-derived recombinant (Sk/Sp mosaic) copy of chromosome 3. In some of these diploids, the mosaic chromosome was transmitted to more than 50% (73–97%) of the viable haploid spores. This phenotype suggested the existence of an Sp drive allele within the Sp-derived portion of those mosaic chromosomes (S1 Fig) [7, 18].
To identify the hypothesized Sp driver, we focused on a candidate region (between positions 1,397,380 and 1,810,659) shared by all the driving mosaic chromosomes (S1 Fig). We specifically searched this region for a wtf gene with a sequence similar to that of a known wtf drive gene. We found that this region contains the Sp wtf13 gene that shares 78% amino acid identity with the Sk wtf4 drive gene (S2A Fig). In addition, Sp wtf13 has two types of transcripts: a long transcript that includes six exons and a shorter five exon transcript that begins within what is intron 1 of the long transcript (Fig 1C, S3A Fig) [41]. The long and short transcripts of Sp wtf13 are reminiscent of those that encode the antidote and poison proteins, respectively, of Sk wtf4 (Fig 1B and 1C). We therefore predicted that Sp wtf13 is a meiotic driver and that the long transcript encodes an antidote, while the short transcript encodes a poison.
We decided to test the phenotype of Sp wtf13 in an Sk genetic background, as the effects of drive are often uncovered in divergent backgrounds. Sk encodes a wtf13 allele that is only 83% identical to the Sp allele (S2B Fig). This divergence is much higher than expected given the near sequence identity of the Sk and Sp genomes (>99% identity) [18, 42]. The Sk wtf13 allele, however, does not appear to be a meiotic driver as it lacks an in-frame start codon near the start of exon 2 that is shared by all known drivers (S2B Fig, right).
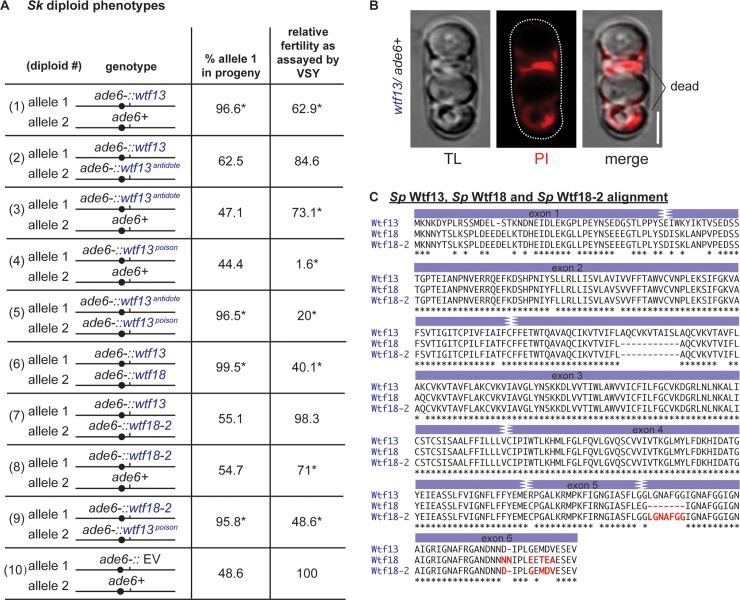
To test Sp wtf13, we integrated the gene linked to a drug resistance marker into the ade6 marker locus on Sk chromosome 3. This generated a strain that exhibits adenine auxotrophy (ade6-) and resistance to a drug (geneticin or hygromycin B). We then mated this haploid strain to a wild-type Sk haploid to generate hemizygous Sp wtf13/ade6+ diploids. We then induced this diploid to sporulate (make meiotic products) and assayed its phenotype by following the ade6 and drug resistance markers (Fig 2A, diploid 1). We found that the fertility (as measured by the viable spore yield assay [43]) of the Sp wtf13/ade6+ diploids was significantly less than that of the empty vector/ade6+ control (Fig 2A, diploid 1 and 10, respectively, S2 Table).
Fig 2. Sp wtf13 is a meiotic driver that is suppressed by Sp wtf18-2.
(A) Allele transmission and fertility of Sk diploids with the indicated constructs integrated at the ade6 marker locus. Alleles were followed using drug resistance markers (kanMX4 or hphMX6). Diploids 1–9 were compared to a wild-type empty-vector (EV) control (diploid 10) to detect biased allele transmission and differences in fertility as measured by viable spore yield. * indicates p-value of < 0.05 (G-test [allele transmission] and Wilcoxon test [fertility]). Fertility was normalized to the empty-vector control and reported as percent. More than 200 viable haploid spores were genotyped for each cross. Spores that inherited both markers and were thus geneticinR Ade+, hygromycinR Ade+ or hygromycinR geneticinR were excluded from the analyses. Raw data can be found in S1 and S2 Tables. (B) Transmitted light (TL) and fluorescent images from a tetrad produced by a wtf13/ade6+ heterozygous diploid (diploid 1) stained with propidium iodide (PI). Dead cells that lose membrane integrity stain with PI, but viable cells exclude the dye. 86% of stained tetrads exhibited this pattern (n = 58, S4 Fig). The scale bar represents 3μm. (C) Amino acid sequence alignment of Sp Wtf13 (long isoform), Sp Wtf18 and Sp Wtf18-2. In red are the amino acid differences between Wtf18 and Wtf18-2. Those red residues are all identical between Sp Wtf13 and Sp Wtf18-2.
Killer meiotic drivers are expected to kill the two spores that lack the driver in each tetrad (four products of a single meiosis). To determine the pattern of spore death in tetrads generated by Sp wtf13/ade6+ diploids, we used propidium iodide (PI) dye. PI is excluded from viable cells but can penetrate dead spores that have lost membrane integrity [7]. 86% of the tetrads contained two (live) spores that excluded PI and two (dead) spores that did not (n = 58, Fig 2B, S4A Fig). In addition, we found that the Sp wtf13/ade6+ diploids transmitted the Sp wtf13 allele to 97% of the viable haploid spores (Fig 2A, diploid 1). Together, these phenotypes are consistent with Sp wtf13 acting as a meiotic driver that kills the two spores that do not inherit the gene from the Sp wtf13/ade6+ hemizygous diploid. In addition, they demonstrate that Sp wtf13 is not suppressed in Sk.
In additional experiments, we found that Sp wtf13 uses a poison-antidote mechanism analogous to the previously described Sk wtf4. The short transcript of Sp wtf13 encodes a poison and the long transcript encodes an antidote to the poison. By mutating the start codons in each transcript, we were also able to isolate the two functions of Sp wtf13 in wtf13antidote and wtf13poison alleles (Fig 2A, diploids 2–5).
The Sp wtf13antidote/ade6+ Sk diploids did, however, have reduced fertility relative to the empty vector control (Fig 2A, compare diploids 3 and 10). In addition, the Sp wtf13antidote allele only partially rescued the toxicity associated with the Sp wtf13poison allele (Fig 2A, compare the fertility of diploids 1, 4 and 5). A similar antidote-only allele made by deleting intron 1 (wtf13intron1Δ) showed the same phenotypes as Sp wtf13antidote (S1 and S2 Tables, diploids 21–23). We tested if the pattern of spore killing caused by the wtf13antidote allele was similar to that of Sp wtf13 via PI-staining of Sp wtf13antidote/ade6+ heterozygous diploids. Most asci (72%) had 4 unstained spores. Unlike Sp wtf13/ade6+, the stained spores were usually found alone in a tetrad (S4C Fig). The cause of this spore viability defect is unknown. This phenotype was not detected with the analogous Sk wtf4antidote allele [7].
S. pombe wtf18-2 suppresses meiotic drive of S. pombe wtf13
We previously predicted that wtf genes that contained only the first transcriptional start site could be suppressors of drive. Given the high amino acid identity between the known Wtfantidote proteins and the Wtfpoison proteins they suppress, we hypothesized that the specificity between poison and antidote proteins is conferred by shared amino acid sequences [7, 38]. To identify potential suppressors of Sp wtf13, we looked for antidote-like wtf genes similar in sequence to Sp wtf13.
This search identified Sp wtf18, which shares 88% amino acid identity with the short (poison) isoform of Sp wtf13 (Fig 2C). We next analyzed published long-read RNA sequencing data and confirmed that Sp wtf18 generates only long (antidote-like) transcripts (Fig 1D and S3B Fig) [41].
To test if Sp wtf18 can suppress Sp wtf13, we first cloned the gene using genomic DNA from our lab stocks. Interestingly, despite the generally assumed isogeny of Sp lab stocks (which are derived from strain L972), we found that the Sp wtf18 allele found in some of our lab stocks was different from the allele found in the reference genome [44]. We call the alternate allele Sp wtf18-2. There are seven amino acid differences and a seven-amino acid insertion encoded in exon 6 of wtf18-2 compared to the wtf18 allele (Fig 2C). Sp wtf18-2 is more similar (92% amino acid identity) to the short isoform of Sp wtf13 than is Sp wtf18 (88% identity). In addition, exon 6 of Sp wtf18-2 is identical to the last exon of Sp wtf13 (Fig 2C).
To test if Sp wtf18 is a suppressor of the Sp wtf13 driver, we first integrated the Sp wtf18 allele into Sk at ade6. We then crossed the strain to the Sk strain described above with Sp wtf13 at the same locus. This generated Sp wtf13/Sp wtf18 heterozygotes (Fig 2A, diploid 6). We found that these diploids had the same phenotype as the Sp wtf13/ade6+ hemizygotes: reduced fertility and drive of Sp wtf13 (Fig 2A, compare diploids 1 and 6). These results indicate that the Sp wtf18 allele is not a suppressor of Sp wtf13.
We next tested if the Sp wtf18-2 allele could suppress Sp wtf13 using the same approach described above (Fig 2A, diploid 7). We found that Sp wtf13/Sp wtf18-2 heterozygotes had the same fertility as the empty vector control and showed Mendelian allele transmission (Fig 2A, compare diploids 1, 7 and 10). These results demonstrate that Sp wtf18-2 acts as a suppressor of the Sp wtf13 meiotic driver.
We next tested if, like Spok1 in the filamentous fungus Podospora anserina, Sp wtf18-2 could act not only as a suppressor, but also as a driver in heterozygotes [11]. Sp wtf18-2 did not bias allele transmission, showing that wtf18-2 is not an autonomous driver in the Sk strain background (Fig 2A, diploid 8). Interestingly, the Sp wtf18-2/ade6+ diploids, similar to the wtf13antidote allele, had significantly lower fertility than the empty vector control (Fig 2, diploid 8 and S4D Fig). This suggests that this allele imposes a fitness cost in the Sk background that we did not observe in the presence of the Sp wtf13 driver.
We reasoned that if wtf18-2 was working like the wtf13antidote, it too could rescue the toxicity caused by the wtf13poison. To test this, we generated diploids heterozygous for wtf18-2 and wtf13poison at the ade6 marker locus (Sp wtf18-2/Sp wtf13poison). Consistent with our hypothesis, we observed transmission bias (96%) favoring the wtf18-2 allele and an increase in fertility (Fig 2A, compare diploids 4 and 9).
Shared sequence identity of residues encoded in exon 6 promotes the ability of suppressors to neutralize drivers
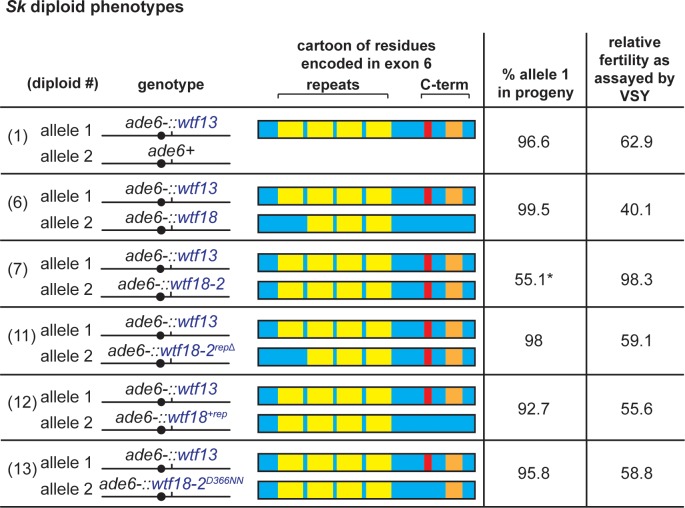
We next wanted to leverage the differential abilities of wtf18 alleles to suppress Sp wtf13 to provide insight into what is required for suppression. The two alleles of wtf18 found in Sp are nearly identical outside of exon 6 (Fig 2C and S5 Fig). Exon 6 is identical between Sp wtf18-2 and Sp wtf13. Sp wtf18 exon 6 differs in that it has 3 copies of a seven amino acid repeat sequence, whereas the other genes have 4 copies [39]. The C-terminal region is also different in Sp wtf18, relative to Sp wtf13 and Sp wtf18-2.
To probe the relevance of these changes on the ability to suppress Sp wtf13, we mutated Sp wtf18-2 to make it look more like Sp wtf18. We found that either deleting one copy of the repeat unit or changing the entire C-terminal region both disrupted the ability of Sp wtf18-2 to suppress Sp wtf13 (Fig 3, diploids 11 and 12). Some more subtle changes to the C-terminal region were tolerated (S6 Fig, diploids 24 and 25), whereas the D366NN change in Sp wtf18-2 alone was sufficient to prevent suppression of Sp wtf13 (Fig 3, diploid 13).
Fig 3. Mutations in exon 6 alter specificity between the Sp wtf13 driver and the Sp wtf18-2 suppressor.
A cartoon of the C-terminal region, allele transmission and fertility of Sk diploids is shown. In the cartoon, the yellow boxes depict the number of repeat units for each allele. The red and orange boxes depict the differences between the residues at the most C-terminal region of the proteins. The diploid numbers carry over between Figures, meaning that the data for diploids 1, 6 and 7 are repeated from Fig 2. To detect if the different alleles generated still had the ability to suppress the drive phenotype, diploids 6, 7, 11–13 were compared to diploid 1. Fertility was normalized to the wild-type control (Fig 2, diploid 10). More than 200 haploid spores were genotyped for each diploid. * indicated p-value < 0.05 (G-test [allele transmission]). None of the fertility values were significantly different compared to the control (diploid 1). All raw data can be found in S1 and S2 Tables.
Additionally, we found that we could make a novel driver by deleting one unit of the exon 6 repeat in Sp wtf13 (S6 Fig, diploid 26). We found that this driver could be suppressed by alleles of wtf18 that had matching repeats (units and sequence, S6 Fig, diploid 29 and 30). Interestingly, this driver could also be suppressed by Sp wtf18, even though the residues in the C-terminal region did not match (S6 Fig, diploid 30).
Curiously, the Sp wtf13repΔ allele was not suppressed by the endogenous Sk wtf18 allele present in the strain background used for this analyses (with three repeat units and matching C-terminal region; S6 Fig, diploid 26). We hypothesized the Sk wtf18 could be silenced at its endogenous locus. To test our hypothesis, we integrated a copy of the Sk wtf18 allele at ade6. Consistent with our hypothesis, Sk wtf18 suppressed the Sp wtf13repΔ driver when both were at ade6 (S6 Fig, diploid 31). Overall, our experiments demonstrate that sequence identity of residues encoded in exon 6 between drivers and their suppressors is functionally important. Depending on the driver, however, some mismatches between the drivers and suppressor sequences can be tolerated in the C-terminal region.
At their endogenous loci, Sp wtf13 acts as a driver and Sp wtf18-2 acts as a suppressor
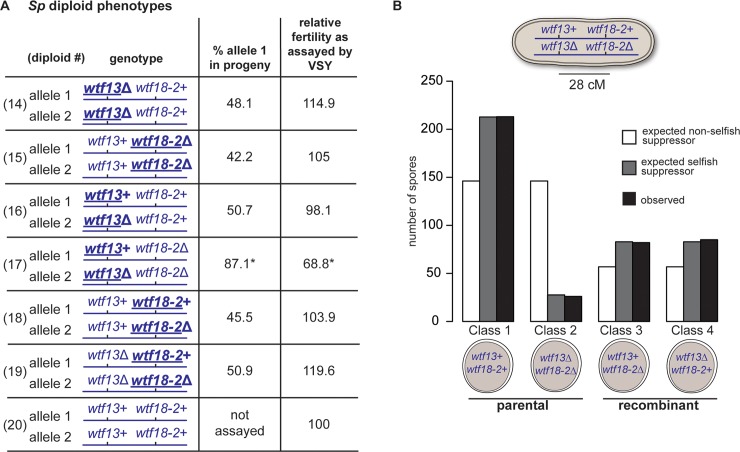
We next tested the phenotypes of Sp wtf13 and Sp wtf18-2 in their native Sp genomes at their endogenous loci by characterizing deletion mutants. We found that neither gene was required for sexual reproduction (Fig 4A, diploids 14 and 15). We also confirmed our observations from the Sk background in that Sp wtf13 acted as a driver and it was suppressed by Sp wtf18-2, but not Sp wtf18 (Fig 4A, compare diploids 16 and 17 and S7 Fig).
Fig 4. Sp wtf13 drives only in the absence of the Sp wtf18-2 suppressor in Sp.
(A) Allele transmission and fertility of Sp diploids is shown. The allele transmission reported here is from the bold underlined locus which was assayed using drug resistance markers (kanMX4 and/or hphMX6). To detect biased allele transmission, diploids 16 and 17 were compared to diploid 14 as a control. Diploids 18 and 19 were compared to diploid 15 as a control. All diploids (14–19) were compared to the wild-type control diploid (20) to detect any reduced fertility. Fertility was normalized to the wild-type control diploid (diploid 20). Allele transmission in diploid 20 was not assayed due to the lack of markers. More than 200 viable spores were genotyped for each diploid. * indicates p-value of < 0.05 (G-test [allele transmission] and Wilcoxon test [VSY]). Raw data can be found in S2 and S3 Tables. (B) The number of spores with the indicated genotypes recovered from Sp wtf13+/Sp wtf13Δ, Sp wtf18-2+/Sp wtf18-2Δ double heterozygotes is shown. wtf13 and wtf18-2 loci are 28cM (centiMorgans) apart, thus the parental classes are expected to be found at a higher frequency than the recombinant classes. The number of spores expected on the basis of the two models presented in the text is shown. In the first model (white boxes), the suppressor is non-selfish in that it rescues spores that do not inherit the suppressor. In the second model (grey boxes), the suppressor is selfish in that it only rescues spores that inherit the suppressor. Observed values are shown in black (n = 406). Raw data can be found in S4 Table.
In the Sk background, we observed a fitness cost of the Sp wtf18-2 allele in the absence of Sp wtf13 (Fig 2A, diploid 8). We did not observe any such cost in Sp, suggesting that phenotype is specific to the Sk strain background or the constructs used in those experiments (Fig 4A, diploids 18 and 19).
Sp wtf18-2 rescues only Sp wtf13-poisoned spores that inherit Sp wtf18-2
The characterized Sk Wtf4antidote protein is enriched in the spores that inherit the locus and only rescues those spores from the Sk Wtf4poison [7]. Similarly, the Sp Wtf13antidote only rescues the spores that inherit the Sp wtf13 locus (Fig 2). We hypothesized that the Sp Wtf18-2 protein would act in a similar way to these antidotes. In other words, we predicted only those spores that inherit Sp wtf18-2 would be protected from killing by the Sp wtf13 driver. This is a stark departure from the prevailing hypothesis about how suppressors of killer meiotic drivers work: indiscriminately rescuing all gametes.
To distinguish these possibilities, we assayed Sp wtf13 and Sp wtf18-2 double heterozygote mutants (wtf13+ wtf18-2+ /wtf13Δ wtf18-2Δ). These diploids will generate four different classes of spores: 1) wtf13+ wtf18-2+, 2) wtf13Δ wtf18-2Δ, 3) wtf13+ wtf18-2Δ and 4) wtf13Δ wtf18-2+. The two genes are linked (28 cM, see Methods), so not all spore types should be generated in equal frequencies. If the Sp wtf18-2 suppressor protects only the spores that inherit it, the spores that do not inherit the driver (Sp wtf13) or the suppressor will die (Fig 4B, Class 2). In contrast, if wtf18-2 protects every spore, there should be no spore killing in these diploids.
We observed that the spores that lacked both the driver and the suppressor (Fig 4B, Class 2) were underrepresented (8-fold) compared to the spores that had both the meiotic driver and its suppressor (Fig 4B, Class 1). Importantly, the drive of Sp wtf13 amongst the parental classes (Fig 4B, Classes 1 and 2) was 89%, which is indistinguishable from the 87% drive of Sp wtf13 observed in diploids lacking Sp wtf18-2 (Fig 4A, diploid 17). We observed analogous results using totally unlinked tagged alleles of Sp wtf13 and Sp wtf18-2 (S4 Table, diploid 32).
These results demonstrate that the wtf18-2 suppressor is spore-specific, like the antidote of Sk wtf4. In other words, Sp wtf18-2 acts like a selfish element by protecting only the spores that inherit Sp wtf18-2. Interestingly, this tactic allows Sp wtf18-2 allele to also gain a transmission advantage (73.4% transmission) in this diploid equivalent to that enjoyed by the Sp wtf13 drive allele (72.7%, Fig 4B). Effectively, Sp wtf18-2 parasitizes the ability of Sp wtf13 to drive. In the process, Sp wtf18-2 dampens the transmission advantage enjoyed by Sp wtf13.
Wtf18-2 mimics the Wtf13antidote
To assay the relationship between Sp Wtf13poison, Sp Wtf13antidote and Sp Wtf18-2 proteins, we generated fluorescently tagged alleles. The tagged Sp wtf13 separation-of-function (i.e. antidote-only and poison-only) alleles we made were not functional. The Sp wtf13antidote-YFP allele we generated was unable to suppress drive of Sp wtf13 and the fertility of diploids hemizygous for the Sp wtf13poison-mTurquoise2 allele we generated was ten-fold higher than we observed for diploids hemizygous for the untagged Sp wtf13poison allele.
Instead, we generated an allele that tags both proteins at the shared C-terminus with YFP. We introduced this allele at ade6 in Sk, as described above. The Sp wtf13-YFP allele does cause meiotic drive (71% transmission in Sp wtf13-YFP/ade6+ heterozygotes, S1 Table, diploid 33), but the phenotype is weaker than the untagged allele (97% transmission, Fig 2A, diploid 1). We also generated a second Sp wtf13-YFP allele integrated at the lys4 locus in Sk. For unknown reasons, this allele had the same phenotype as the untagged allele (96% transmission in Sp wtf13-YFP/lys4+ diploids; S1 Table, diploid 34). It is not clear why the antidote in Sp wtf13-YFP is functional, whereas the Sp wtf13antidote-YFP was not.
In tetrads generated by diploids heterozygous for either Sp wtf13-YFP allele, we observed a cytoplasmic haze of YFP and YFP puncta throughout the spore-sac (Fig 5B, S8 Fig). We infer this localization represents the Wtf13poison, based on comparison to the Sk Wtf4poison (Fig 5A, green). In addition to the presumed poison localization, we observed Sp Wtf13-YFP enriched in two of the four spores (Fig 5B, S8 Fig). This localization is reminiscent of the previously characterized Sk Wtf4antidote protein, which is enriched in the spores that inherit the allele (Fig 5A, magenta). Like the Sk Wtf4antidote, the Sp Wtf13-YFP signal is enriched in the two spores that inherit the Sp wtf13-YFP allele and survive as the other two spores generally stain with PI (Fig 5B, S8 Fig). We therefore infer the spore-enriched Sp Wtf13-YFP population represents the Wtf13antidote.
Fig 5. Sp Wtf18-2 shows an antidote-only localization pattern.
Localization of Sk Wtf4, Sp Wtf13 and Sp Wtf18-2 fluorescently tagged proteins in tetrads. (A) Tetrad showing the localization of Sk Wtf4antidote (magenta) and Wtf4poison (green) and transmitted light (TL) using transgenes in Sp [7]. (B) Analogous to the Wtf4antidote, Sp Wtf13-YFP (green) is enriched in two out of the four spores. Similar to the Wtf4poison, Wtf13-YFP also contains diffuse cytoplasmic signal and puncta throughout the tetrad. This image is of the weaker Sp wtf13-YFP transgene introduced at ade6 in Sk. We observe the same localization in the stronger Sp wtf13-YFP allele at lys4 (S8 Fig). Wtf13-YFP is enriched in the two (living) spores that do not stain with PI. This indicates that the YFP is enriched in the spores that inherit wtf13-YFP, as that allele is overrepresented amongst the viable spores. This image was linearly unmixed. (C) mCh-Wtf18-2 (magenta) is enriched in half of the spores generated by mCh-wtf18-2/lys4+ heterozygotes. This image was linearly unmixed (see Materials and Methods, S9A Fig). Brightness and contrast were adjusted differently for each image and the images were smoothed using Gaussian blur. The scale bar represents 3μm.
To detect the localization of Sp Wtf18-2, we generated an Sp mCherry-wtf18-2 allele and introduced the construct at the lys4 locus in Sk. The Sp mCherry-wtf18-2 allele is functional as it rescues the drive phenotype of the weaker wtf13-YFP allele integrated at ade6 (S4 Table, diploid 32). mCherry-wtf18-2 also partially rescues the drive phenotype of the stronger Sp wtf13-YFP allele at lys4 (S1 Table, diploid 35). In tetrads generated by diploids heterozygous for Sp mCherry-wtf18-2, we observed Sp mCherry-Wtf18-2 enriched in two out of the four spores (Fig 5C, S9A Fig).
The localization pattern of Sp mCherry-Wtf18-2 suggested that, like the Wtfantidote proteins, Wtf18-2 is enriched in the spores that inherit the locus. We tested this in diploids heterozygous for Sp mCherry-wtf18-2 and Sp wtf13-YFP at lys4 (Fig 6A). Two spores from these diploids will inherit Sp mCherry-wtf18-2 and the other two will inherit Sp wtf13-YFP. We can tell which spores inherited Sp wtf13-YFP because the protein is enriched in those spores (Fig 5B and S8 Fig). The spores without YFP enrichment, therefore, represent those that inherited Sp mCherry-wtf18-2. We observed that Sp mCherry-wtf18-2/Sp wtf13-YFP heterozygous diploids generated tetrads in which two spores were enriched with YFP and the others displayed enriched mCherry signal (Fig 6B and S9B Fig). These results support the hypothesis that Sp Wtf18-2 is enriched in the spores that inherit the allele.
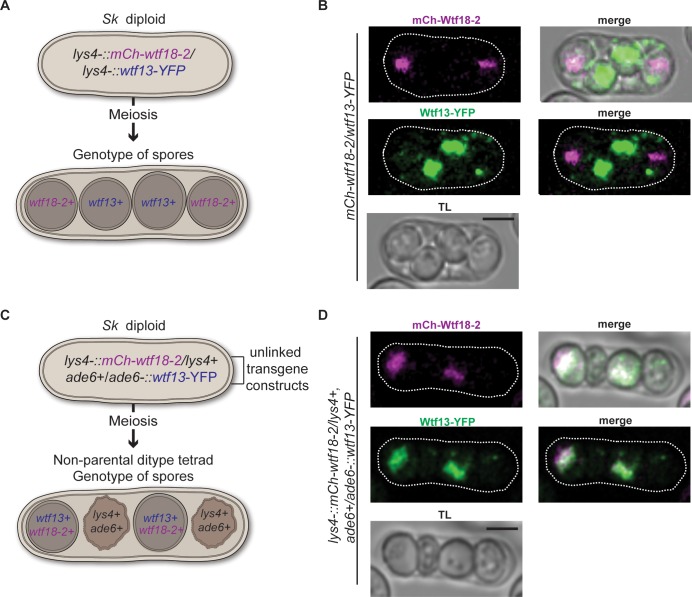
Fig 6. Sp mCh-Wtf18-2 and Sp Wtf13-YFP colocalize.
(A) A cartoon depicting the assayed Sp wtf13-YFP/ Sp mCh-wtf18-2 heterozygous diploids. Both transgene constructs are integrated at lys4 in Sk. (B) The localization of Sp Wtf13-YFP (green) and Sp mCh-Wtf18-2 (magenta) is shown in a tetrad. (C) A cartoon of the assayed diploid heterozygous for Sp mCh-wtf18-2 and Sp wtf13-YFP is shown. mCh-wtf18-2 was integrated at lys4 and Sp wtf13-YFP was integrated at ade6 in Sk. lys4 and ade6 are unlinked, so the alleles will segregate randomly into spores yielding three tetrad types (S10 Fig). The genotype of a non-parental ditype tetrad is shown in the cartoon. (D) The localization of Sp mCh-Wtf18-2 (magenta) and Sp Wtf13-YFP (green) is shown in a non-parental ditype tetrad. The images were linearly unmixed (S9B and S9C Fig) and smoothed using Gaussian blur. The scale bar represents 3μm.
The strong similarity between the pattern of localization of Sp mCherry-Wtf18-2 and what we infer to be the Sp Wtf13antidote-YFP suggested those proteins may localize to the same region. To test this idea, we generated Sk diploids hemizygous for tagged alleles of both Sp wtf13 and Sp wtf18-2. The two tagged alleles (Sp wtf13-YFP and Sp mCh-wtf18-2) were integrated at unlinked loci (ade6 and lys4, respectively), so they should independently segregate into spores (Fig 6C). In a subset of tetrads, two spores will inherit both tagged alleles (Fig 6C and S10A Fig). In these tetrads, we observed co-localization between the YFP and mCherry signals (Fig 6D, S9C, S10B and S11 Figs). These results indicate that the Sp Wtf13antidote and Sp Wtf18-2 proteins co-localize.
Discussion
wtf13 is an autonomous poison-antidote meiotic driver
This study identifies the S. pombe wtf13 gene as an autonomous killer meiotic driver (Fig 2). In general, Sp wtf13 acts very much like the previously characterized Sk wtf4 driver. Sp wtf13 joins four other proven wtf meiotic drive genes found in wild isolates of the S. pombe group: wtf4 and wtf28 in Sk, and cw9 and cw27 in CBS5557 [7, 8]. The dual-transcript poison-antidote mechanism used by Sp wtf13 appears to be homologous to the mechanisms employed by the other wtf drivers.
Our results are consistent with the idea that an ancestral wtf gene was a dual-transcript poison-antidote meiotic driver. Novel duplications of this ancestral meiotic driver to unlinked loci (e.g. the ‘R’ locus in Fig 7) could have been selected based on their abilities to cause drive and protect spores from the poison of the ancestral driver (e.g. at the ‘A’ locus in Fig 7). This advantage would only manifest, however, upon outcrossing to a strain that is sensitive to the driver. It is important to note that population genetic analyses suggest S. pombe rarely outcrosses [45, 46]. However, the decreased recombination rates and pervasive meiotic drive observed in outcrossed S. pombe could both minimize signatures of outcrossing [18]. Our results also support the idea that other intact wtf genes which make two transcript classes in Sp (wtf4, wtf19 and wtf23) are also likely able to cause meiotic drive, in the absence of suppressors.
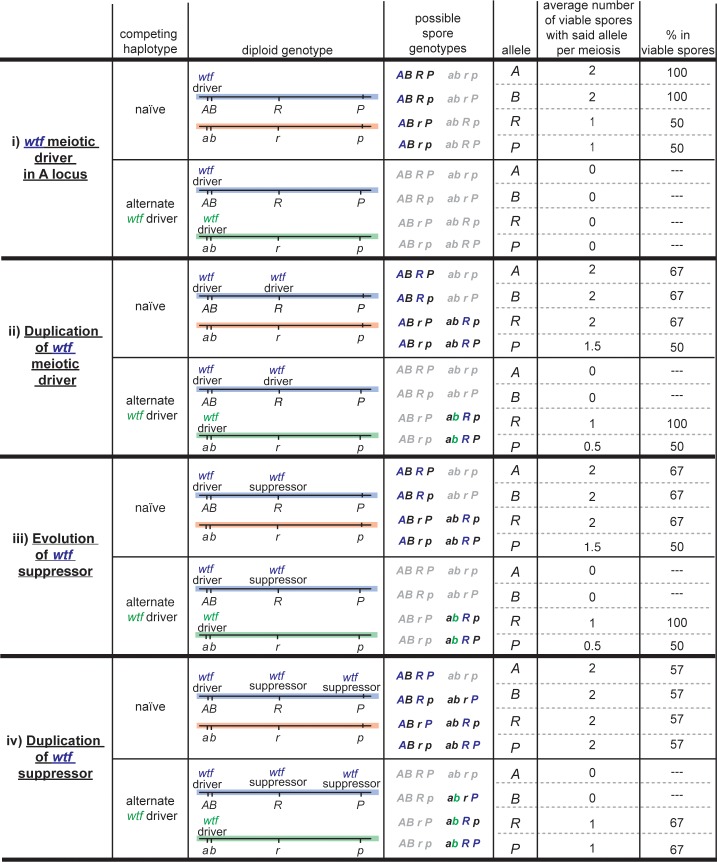
Fig 7. Hypothetical expansion of the wtf gene family.
The colored lines represent chromosomes with 4 key loci (A, B, R and P). The spore genotypes are depicted in gray if the spores are inviable. At each level of the table (i-iv), the blue chromosome is the ‘experimental’ chromosome. (i) The blue chromosome has one meiotic driver at the A locus. (ii) The meiotic driver at the A locus generated a duplicate copy at the R locus on the blue chromosome. (iii) The poison function of the meiotic driver at R degenerated leaving an antidote-only suppressor in its place on the blue chromosome. (iv) The wtf suppressor at the R locus generated a duplicate gene at the P locus on the blue chromosome. In each scenario (i-iv), we consider the evolutionary success of the alleles on the blue chromosome when competed against two alternate chromosomes for transmission into the viable spores: 1) a naïve chromosome with no meiotic drivers (red) and 2) a chromosome (green) with a wtf driver that is distinct from the one on the blue chromosome (i.e. the antidote from the driver on the green chromosome does not neutralize the poison generated by the driver on the blue chromosome and vice versa). The last three columns of the table list the average number of spores bearing the indicated allele from the blue chromosome per meiosis and the fraction of the viable spores expected to inherit the indicated allele. The A and B loci are absolutely linked, but all other loci are unlinked. We assumed the wtf meiotic drivers kill 100% of the spores that do not inherit a copy of the gene and the suppressors are 100% efficient at rescuing spores that inherit a suppressor.
wtf18-2 is an antidote-only suppressor of the wtf13 driver
This study also demonstrates that Sp wtf13 can be fully suppressed by another gene, Sp wtf18-2 (Figs 2, 3 and 4). The Sp wtf18-2 allele arose spontaneously in the lab of Harmit Malik in a routine strain-building cross (without meiotic drive) between two Sp isolates. Given the 100% DNA sequence identity between Sp wtf18-2 and Sp wtf13 from exon 6 up to 75 bp downstream of the coding sequence, we propose the Sp wtf18-2 allele likely arose from non-allelic gene conversion with Sp wtf13 (Figs 2C and 8A). Indeed, non-allelic gene conversion is common within the wtf gene family and recombination events between these two loci have been reported [8, 39, 47].
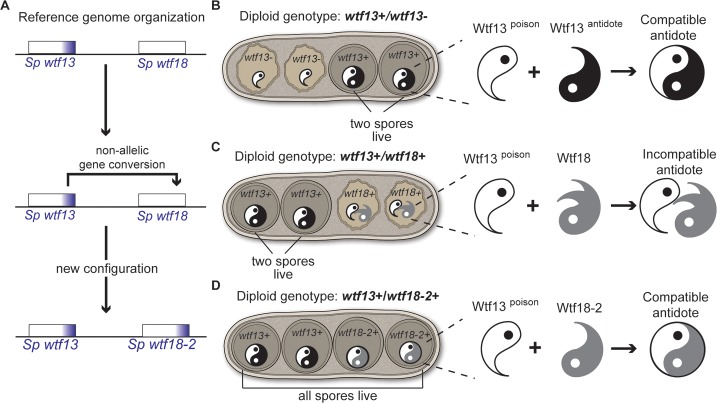
Fig 8. Model for wtf drive suppression.
(A) Sp wtf18-2 likely arose from a non-allelic gene conversion event between the Sp wtf18 allele present in the reference genome and Sp wtf13. This gene conversion event provided Sp wtf18 with the exon 6 present in Sp wtf13. The non-allelic conversion event happened from positions 1,580,047–1,580,343 to positions 1,806,929–1,807,206. This track includes part of wtf13 exon 5, intron 5, exon 6 and 3’ UTR region. (B) In wtf13 heterozygous diploids (wtf13+/ wtf13-), Sp Wtf13poison has the ability to kill every spore, but the Sp Wtf13antidote recognizes the Sp Wtf13poison protecting the spores that inherit the Sp wtf13+ allele. (C) In Sp wtf13+/ Sp wtf18+ diploids, the spores that inherit the wtf13+ allele are protected from the Wtf13poison by the Sp Wtf13antidote. The two spores that inherit the Sp wtf18+ allele die because Wtf18 does not neutralize the Sp Wtf13poison. (D) In Sp wtf13+/ Sp wtf18-2+ diploids, the spores that inherit the Sp wtf18-2+ allele are protected from the Wtf13poison because Sp Wtf18-2 makes an antidote capable of neutralizing the Sp Wtf13poison. Both Sp wtf13 and Sp wtf18 alleles are depicted at the same locus on opposite haplotypes, like the experiments in Fig 2A.
The phenotypes of many drive suppressors have been observed, but these drive systems are generally in non-model organisms with historically limited genetic tools [13, 27, 29, 35, 48, 49]. Many of the suppressors are also found on Y chromosomes, which precludes using traditional recombination mapping to identify the causative alleles. Because of these challenges, Sp wtf18-2 is only the fourth cloned suppressor of meiotic drive. Two of the known suppressors, the Tmy and Nmy genes are from D. simulans and both suppress the Dox driver. These suppressors were derived from Dox via retrotransposition and suppress it via a small interfering RNA (siRNA) mechanism [34, 36, 37].
The other known drive suppressor, Spok1 of the filamentous fungus Podospora anserina, is also related to the gene it suppresses, Spok2 [11]. Unlike Nmy, however, Spok1 is a protein coding gene that can itself cause meiotic drive by a poison and antidote mechanism [11]. It is possible that the antidote encoded by Spok1 can suppress both the Spok1 and Spok2 poisons, but the mechanism of suppression is also unknown.
Like Nmy, Tmy and Spok1, wtf18-2 shares homology with the driver it suppresses. This result supports our previously proposed model in which single transcript wtf genes could act as suppressors of wtf drivers [7, 38]. Specifically, we posited that the single transcript wtf genes were born initially via the degradation of an intact driver. For example, Fig 7 illustrates a hypothetical scenario in which one copy of a duplicated wtf driver (at the ‘R’ locus) loses the ability to make a poison and can only act as an antidote against the paralog (at the ‘A’ locus), becoming a wtf suppressor. In our example, this would be a neutral fitness transition for the ‘R’ locus: it gains the same transition advantage and produces the same number of viable spores as when the locus encoded an intact duplicate of the driver at the ‘A’ locus in the two genetic contexts presented (Fig 7, compare ii to iii). As discussed above, this analysis pertains to outcrossing, which may be rare in S. pombe [45, 46].
It is interesting to consider if repeated driver decay is responsible for the high number (12) of intact single transcript wtf genes relative to intact potential drivers (4) in the reference Sp genome. With this question in mind, we carried our hypothetical example further. We reasoned that the suppressor at the ‘R’ locus could also generate an unlinked duplicate of itself to an unlinked locus (‘P’ in Fig 7). Interestingly, this would increase the fitness of the ‘P’ locus in the outcrossing scenarios considered (Fig 7, compare iii to iv). It would on average produce more viable spores per meiosis and be transmitted to a greater fraction of the viable spores (i.e. have fewer competitors). This suggests that duplication of suppressors, in addition to driver decay, could also have contributed to the expansion of the wtf gene family and the high number of single transcript wtf genes [7, 8, 40].
Wtf18-2 suppresses drive via molecular mimicry of the Wtf13antidote
Our hypothesized origins of the single transcript wtf genes as degraded intact drivers point to a possible molecular mechanism. Specifically, if a single transcript wtf gene encodes an antidote protein, it could suppress a driver by mimicking the antidote of that driver (Fig 8B and 8D). Consistent with this idea, it was the high degree of shared amino acid identity with the Wtf13poison that led to our discovery of the Wtf18-2 suppressor (Fig 2C).
In addition to sequence similarity, we demonstrated that the Wtf18-2 protein expression pattern is indistinguishable from that of the Wtf13antidote. Both proteins are highly enriched within the spores that encode them (Figs 5 and 6). Finally, we demonstrate that the Wtf18-2 and Wtf13antidote proteins co-localize to the same subcellular region within spores that inherit both genes (Fig 6D and S9–S11 Figs). Overall, our results support a model in which Wtf18-2 suppresses drive by mimicking the Wtf13antidote protein (Fig 8B and 8D).
How Wtfpoison proteins kill and how Wtfantidotes prevent killing is still unknown. We and others have proposed that the poison proteins kill by oligomerizing to form a pore that could disrupt vital cellular membranes because the proteins have multiple predicted transmembrane domains (S12 Fig) [7, 8, 38]. The poison proteins can assemble into small aggregates (visible as foci in Figs 5 and 6). We speculate that these aggregates could be oligomers capable of disrupting membranes within spores. We have also proposed that the antidotes use their shared amino acid sequences to interact with the poison proteins and somehow disrupt pore formation. Our results are consistent with the idea that homotypic interactions are important, although some sequence differences between the driver and suppressor are tolerated (Fig 3 and S6 Fig).
This emerging theme of shared identity between drivers and their suppressors could guide the discovery of novel suppressors as new drivers are cloned. Additionally, this work and the work of others have shown that one potential way to engineer a suppressor of drive is to use the sequence of the driver itself. This idea will be particularly important to consider when trying to understand, predict, or alter the evolution of synthetic gene drive systems.
wtf18-2 selfishly suppresses the selfishness of wtf13
Unlinked suppressors of selfish meiotic drivers are often assumed to be beneficial alleles that are selected because they increase the fitness of their hosts [12]. The Nmy suppressor of Dox fits this paradigm. Nmy ensures Mendelian sex chromosome representation in the gametes, even in the 50% of gametes that do not inherit Nmy from a Nmy/nmy heterozygote [34, 36, 37]. In other words, the benefits of Nmy are shared by the gametes that do not inherit the suppressor. The wtf18-2 suppressor, on the other hand, represents a more ‘selfish’ strategy that is likely a relic of the gene’s evolutionary history. Wtf18-2 only suppresses drive in the spores that inherit the wtf18-2 allele. This tactic allows wtf18-2 to exploit the Wtf13poison protein to gain a transmission advantage of its own (Fig 4B). While this selfish suppression strategy would increase host fitness (when the driver and suppressor are not absolutely linked in cis), the benefits of suppression are not shared by the gametes that do not inherit the suppressor.
Meiotic drivers drive infertility
wtf genes are known to be rapidly evolving in natural isolates [7, 8, 39]. Such rapid evolution is thought to be a hallmark of genes in conflict, such as meiotic drivers and their suppressors [4, 21, 22]. This work supports the idea that the rapid evolution of non-driving wtf genes is due to their role as suppressors of meiotic drive. The rapid evolution of wtf genes leads to heterozygosity of these genes in crosses between natural isolates. This wtf heterozygosity is known to be a major driver of infertility between different isolates of S. pombe [7, 8, 18]. Our work suggests the rapid evolution of this gene family is also generating variation within lab stocks that are generally considered isogenic. This heterozygosity could uncover cryptic drivers and introduce major artifacts into studies of S. pombe reproduction and fertility. More broadly, infertility caused by meiotic drivers may be common in eukaryotes [50–52].
Finally, this work demonstrates how even strong meiotic drivers can remain hidden despite intensive genetic investigation. This example underscores the challenges inherent to detecting similarly cryptic meiotic drivers in more complex systems, including humans. Furthermore, it highlights the power of investigating the evolutionary dynamics of drivers in tractable model systems.
Materials and methods
Plasmid construction: Integrating vectors
The genotype of the plasmids used can be found in S5 Table. To integrate transgenes linked to kanMX4 into the Sk genome at ade6+, we used the ade6-targeting plasmid (pSZB188) described in [7]. For this study, we also made a cloning vector (pSZB386) that would allow us to similarly introduce transgenes linked to hphMX6 at ade6. To make pSZB386, we first made a mutant ade6 allele (with internal, 5’ and 3’ deletions in addition to introducing a central KpnI restriction site). To make the mutant ade6 allele, we amplified the 5’ end of the ade6 gene using oligos 588 and 589 (S6 Table). We then stitched this fragment to a second fragment of the gene that was amplified using oligos 590 and 591 using overlap PCR. We then digested the amplified fragment with BamHI and XhoI and cloned it into the BamHI and SalI sites of pAG32 [53].
To similarly integrate constructs linked to drug resistant markers at lys4, we generated the lys4-targeting vectors pSZB329 (kanMX4) and pSZB322 (hphMX6). To generate these vectors, we first purchased a mutant allele of lys4 as a gBlock from IDT (Coralville, IA). This lys4 allele lacks 240 bp from the beginning and end of the gene. The allele also has 50 bp deleted from the center of the gene that is replaced with a KpnI site. We blunt-cloned the gBlock into the PvuII sites of pFA6 and pAG32 to generate pSZB329 and pSZB322, respectively [53].
Plasmid construction: Integrating vectors with Sp wtf13 alleles
To make a construct that would allow us to introduce Sp wtf13 into Sk linked to hphMX6 at ade6, we first amplified the gene from Sp genomic DNA using oligos 879 and 880. We then digested the PCR product with SacI, and ligated it into the SacI site of pSZB188 and pSZB386 to generate pSZB368 and pSZB495, respectively.
We made the Sp wtf13-YFP allele using overlapping PCR. We first amplified the Sp wtf13 gene from Sp genomic DNA using the forward oligo 1115 and a reverse oligo 1125 which anneals right before the stop codon. We then amplified YFP from pFA6-EYFP-HIS3MX6 [54] using oligos 1123 and 634. We then stitched both fragments using overlapping PCR. We next digested the resulting PCR product with SacI and ligated it into the SacI sites of pSZB188 and pSZB329 to generate pSZB522 and pSZB706, respectively.
To generate an antidote-only allele of Sp wtf13, we mutated the start site (ATG→TAC) utilized by the shorter transcript using PCR. First, using pSZB496 (same as pSZB495) as a template, we amplified both a 5’ fragment (using oligos 1115 and 1395) and a 3’ fragment (using oligos 1394 and 879) of the Sp wtf13 gene. We then stitched the two fragments together using overlap PCR. We then digested the PCR product with SacI and ligated it into the SacI site of pSZB188 to generate pSZB702 and pSZB703.
We took a rather indirect path to generate the poison-only allele of Sp wtf13. We first made a tagged wtf13-mTurq2 allele. We made this allele by amplifying Sp wtf13 from Sp genomic DNA using the forward primer 1115 and the reverse primer 1126. We also amplified mTurquoise2 from pFA6-mTurq2-URA3MX [55] using primers 1124 and 634. Using overlapping PCR, we next stitched the wtf13 and mTurq2 PCR fragments together. We digested the resulting PCR product with SacI and ligated it into the SacI site of pSZB386 to generate pSZB526. We then used a gBlock from IDT containing 250 bp of Sp wtf13 in which both methionine codons in exon 1 were mutated (ATG→TAG). Using the QuickChange II XL Site-Directed Mutagenesis Kit (Agilent Technologies) and the gBlock, we introduced the two mutations in exon 1 of pSZB526 to generate pSZB565. Although this allele was non-functional, we used the mutations in pSZB565 to make the Sp wtf13 poison-only separation-of-function allele. We amplified the 5’ end of the gene (including the start site mutations) using oligos 1115 and 985 and pSZB565 as a template. We also amplified the 3’ of the gene, using pSZB495 as a template, using oligos 601 and 879. Using overlapping PCR, we stitched these two fragments to generate an Sp wtf13 allele with both methionine codons mutated in exon 1. We then digested the PCR product with SacI and ligated it into the SacI site of pSZB386, to generate pSZB686.
To make the wtf13repΔ allele, we ordered a gBlock from IDT that contained from exon 5 to 1,078 bp downstream of the stop codon of Sp wtf13. In this gBlock, we deleted the 21 bp that encode the first repeat unit (LGNAFGGΔ). We amplified the 5’ region of the Sp wtf13 gene from template pSZB368 using oligos 880 and 1530, which amplified the gene up to exon 5. We then used overlapping PCR to stitch this fragment with the gBlock, which generated an Sp wtf13 allele with the first repeat unit deleted. We digested this allele with SacI and ligated it into the SacI site of pSZB386 to make pSZB806.
Plasmid construction: Integrating vectors with Sp wtf18 alleles
To make the Sp wtf18 kanMX4 ade6-targeting vector, we amplified the wtf18 gene from genomic DNA (strain SZY44) using oligos 1037 and 1039. We then digested this fragment with SacI and cloned it into the SacI site of pSZB188 to generate pSZB483. We also cloned this fragment into the SacI site of pSZB386 to generate pSZB485. We amplified Sp wtf18-2 from genomic DNA of strain SZY643 using oligos 1039 and 1202. We then digested the PCR product with SacI and ligated it into the SacI site of pSZB188 to make SZB566. We amplified the Sk wtf18 allele from Sk genomic DNA (strain SZY13) using oligos 1037 and 1039. We digested this PCR product using SacI and then ligated it at the SacI site of pSZB188 to generate pSZB470.
We used overlap PCR to generate the Sp wtf18-2 allele with an mCherry tag on the N-terminus. First, we ordered a gBlock from IDT containing the Sp wtf18-2 promoter linked to mCherry, five glycine codons, and the first 149 bp of Sp wtf18-2 exon 1 [56]. We amplified this fragment using oligos 1039 and 1254. We then used pSZB566 as a template to amplify Sp wtf18-2 using oligos 1255 and 1202. Using overlap PCR, we stitched these fragments together. We then cut the resulting PCR product with SacI and ligated it into the SacI site of pSZB322 to generate pSZB625.
We used the QuickChange II XL Site-Directed Mutagenesis Kit from Agilent Technologies with pSZB566 as a template and different set of oligos to generate the different Sp wtf18-2 mutants. We made an Sp wtf18-2 allele that had the first repeat unit deleted (Sp wtf18-2 repΔ) using oligos 1659 + 1660 to make pSZB673. To make the wtf18-2D366NN allele, we used oligo 1661 with oligo 1662 to generate pSZB674. To make the wtf18-2D366N allele (referred to as Sp wtf18-2mut1 in S6 Fig), we used oligos 1663 and 1664 and made pSZB675. Finally, to make the wtf18-2G370E, M372T, D373E, V374A allele (referred to as Sp wtf18-2mut2 in S6 Fig), we used oligo 1665 + 1666 to make pSZB688.
We generated the wtf18+repeat1 allele using overlapping PCR. We ordered from IDT a 599 bp gBlock containing from exon 5 to the end of the 3’ UTR of wtf18 where we introduced the 21 bp that encode for the first repeat unit (LGNAFGG) after residue 332. We amplified wtf18 from pSZB485 using oligos 1039 and 598 and stitched the product to the gBlock we ordered using overlapping PCR. We then digested this PCR product with SacI and cloned it into the SacI site of pSZB188 to generate pSZB807 and pSZB808.
Strain construction: Introducing alleles into yeast
All strain names and genotypes are described in S7 Table. We digested the integrating plasmids described above with KpnI (which cuts within the mutant ade6 or lys4 cassettes) and transformed the linear plasmids into yeast using a standard lithium acetate protocol. We selected drug resistant (geneticin or hygromycin) transformants. For alleles generated with the ade6 targeting vectors, we picked red (ade6-) colonies to ensure proper integration of the transgenes at ade6. For alleles generated with lys4 targeting vectors, we screened for lys- colonies to ensure proper integration at lys4.
Deleting wtf13 and wtf18-2 loci in S. pombe
To delete wtf13 at its endogenous locus, we generated DNA deletion cassettes via PCR. First, we amplified both the sequence (~1,200 bp) upstream of Sp wtf13 using oligos 1048 and 1049, and the sequence downstream (~1,100 bp) of Sp wtf13 using oligos 1052 and 1053. We also used oligos 1050 and 1051 to separately amplify the drug resistance genes kanMX4 and hphMX6 (from pFA6a and pAG32, respectively [53]). We then used overlap PCR to stitch the three PCR fragments together to make cassettes in which a drug resistance gene was flanked by the sequences up and downstream of Sp wtf13. We then used standard lithium acetate transformation to introduce the deletion cassettes into the genome in strain SZY643, selecting for drug resistant colonies (wtf13Δ::kanMX6 or wtf13Δ::hphMX6). This generated strains SZY1444, SZY1445, SZY1440 and SZY1441. We verified the proper deletion of the gene by amplifying the locus using PCR with oligos external to the targeting cassette (oligos 1058 and 1061).
We used a similar approach to delete Sp wtf18-2. We first generated wtf18-2Δ::kanMX6 and wtf18-2Δ::hphMX6 deletion cassette using overlap PCR. We used oligos 1078 and 1079 to amplify the upstream region (~1,000 bp) of wtf18-2 and oligos 1082 and 1083 to amplify the downstream region (~1,000 bp) of the wtf18-2 locus. We also amplified the drug resistance genes kanMX4 and hphMX6 (from pFA6a and pAG32, respectively [53]) with oligos 1080 and 1081. We then stitched all the fragments together using overlap PCR to make the DNA deletion cassettes with the regions upstream and downstream of Sp wtf18-2 flanking a drug resistance gene. We then used standard lithium acetate transformation to introduce the deletion fragments into yeast (strains SZY1442 and SZY1446). We selected for drug resistant colonies and verified strains SZY1481 and SZY1483 had the proper deletion by amplifying the locus with oligos (1113 and 1114) external to the targeting cassettes.
Crosses for assaying fertility and meiotic drive
We generated stable diploids similar to the description in [18]. We first mixed 300 μl of a saturated culture of each parental haploid strain in a microcentrifuge tube. Then, we spun down the cells and spread them onto SPA (1% glucose, 7.3mM KH2PO4, vitamins and agar) and SPAS (SPA + 45 mg/l adenine, histidine, leucine, uracil and lysine) for ~12 hours at 25°C. We then scraped the cells off the SPA (or SPAS) plates and struck them out onto selective media (for most of the crosses: minimal yeast nitrogen-based plates). The parental strains contained complementary auxotrophies that allowed us to select for heterozygous diploids. We grew diploid cells in YEL (0.5% yeast extract, 3% glucose, 250 mg/l adenine, histidine, leucine, uracil and lysine) at 32°C with shaking overnight. We spread 50 μl of the saturated culture onto SPA to induce sporulation. We also diluted the culture and spread it onto YEA (same as YEL + agar) plates to determine the colony forming units (CFU) and to assess if the culture contained heterozygous diploids which we determined via replica plating colonies after 2–3 days of growth at 32°C. Cultures determined to not be comprised of heterozygous diploids were not assayed further. After three days on SPA, we scraped off all the cells. We then incubated the cells with glusulase (snail gut enzyme) to digest the ascus wall and release the spores. We also treated the cells with 60% ethanol to kill the remaining vegetative cells. We plated diluted spores on YEA and incubated the plates at 32°C for five days. We scored the CFU of the cells plated on YEA and assayed fertility using the viable spore yield assay (VSY = [number of spores/ number of diploids] [43]). To assay allele transmission, we determined the genotype of the progeny at control and test loci via replica plating. We generated at least two independent diploids (but usually more) and genotyped more than 200 spores per cross. We used a G-test to detect biased allele transmission compared to our control sample (S1, S3 and S4 Tables). To determine if there were any changes in the fertility of the different crosses, we assayed at least three diploids using VSY. We then used the Wilcoxon test and compared them to control diploids. All the raw values we utilized for the Wilcoxon test can be found in S2 Table.
Linkage between wtf13 and wtf18 loci
To determine the recombination frequency between wtf13 and wtf18-2, we used the progeny made by the Sp wtf13 wtf18-2 double heterozygote diploid (wtf13+ wtf18-2+ /wtf13Δ wtf18-2Δ; S4 Table) made by mating strains SZY643 and SZY1481 [or SZY1482]. Drive of the Sp wtf13 allele occurs in this diploid, which prevented us from using all the progeny to calculate recombination frequencies. Instead, we only used wtf13+ progeny which are not killed by the driver. We then divided the number of recombinants (wtf13+ wtf18-2Δ) by the total number of wtf13+ progeny. We had 82 recombinants and 213 parental wtf13+ progeny (S4 Table). This gave us the 28% recombination frequency.
Sp wtf18 alleles
The Sp wtf18 allele from the reference genome is present in the lab stock strain SZY44. The Sp wtf18-2 allele was discovered in our lab stock strain SZY643. This strain is a segregant from a cross between SZY631 and SZY629, which are themselves segregants from a cross between GP52 and GP7596. The GP strains were obtained from the collection of G.R. Smith. We sequenced the wtf18 locus from SZY631, SZY629, GP52, and GP7596. We determined that the wtf18-2 mutant appeared in SZY629.
Alignments
To determine the sequence identity between our proteins, we aligned the amino acid sequences using Geneious 10.1.3 (https://www.geneious.com). Using the Geneious alignment tool, we performed global alignments with free end gap with the default parameters. We deposited the sequence of the Sp wtf18-2, Sk wtf18 and Sk wtf13 alleles to Genbank under accession numbers MH029505, MH029506 and MH029507, respectively.
Transmembrane domain prediction
We used the topology prediction program TMHMM2.0 [57] to predict the topology of Sp Wtf13, Wtf18 and Wtf18-2 proteins and the number of transmembrane helices in each protein.
Cytology
To detect if spores were being killed, we stained the asci sporulated on SPA plates with propidium iodide (PI, 1mg/ml) [7]. We scraped the cells off the plate and mixed them with 50 μl of diluted PI (1:50 in ddH20). We then incubated the cells at room temperature for 20 minutes. We then plated the cells onto a 35mm glass culture dish (MatTek) pre-coated with lectin to immobilize the cells [58]. We then imaged the cells on an LSM-780 (Zeiss) AxioObserver microscope with a 40X C-Apochromat water-immersion (Zeiss, NA = 1.2). We imaged the PI-stained cells in photon-counting channel mode with 561 nm excitation and collected the PI fluorescence through a 562–642 nm filter.
We also acquired the Sk wtf4 images on the same LSM-780 microscope, with a 100x alpha Plan-Apochromat (Zeiss, NA = 1.46) objective. We acquired the images in photon-counting channel mode. We excited GFP at 488 nm and collected the fluorescence through a 481–552 nm bandpass filter. We excited mCherry at 561 nm and collected its fluorescence through a 572–735 nm bandpass filter.
For imaging asci, we induced meiosis of the diploid cells in liquid culture using the protocol described in Zanders et al [18]. Briefly, we made diploid cultures in YEL and incubated them at 32°C overnight. We then diluted the saturated culture 1:50 into 5 ml of PM media (8mM NA2HPO4, 2% glucose, 0.5% NH4Cl, EMM2 salts, vitamins and minerals) shaking at 32°C for 16 hours. To induce meiosis, we spun down 500 μl of the PM culture in a microcentrifuge tube and resuspended the cells in 5 ml of PM-N media (8mM NA2HPO4, 1% glucose, EMM2 salts, vitamins and minerals). We then incubated the samples shaking at 28°C and imaged the cells ~24 hours after the induction of sporulation. For analyses using YFP and PI, we acquired images on the same LSM-780 microscope, also with the same 40x C-Apochromat water-immersion objective. We acquired images sequentially, first in photon counting lambda mode with YFP excitation at 488 nm and with the fluorescence collected over the entire visible range. We then acquired PI images in photon counting channel mode with excitation at 561 nm and with the fluorescence collected over a bandpass of 597–735 nm. To obtain the true YFP signal from confounding autofluorescence, we linearly unmixed the YFP lambda mode data using YFP and yellow autofluorescence spectra. We recombined the unmixed YFP in ImageJ with the channel mode PI image.
To determine if Sp Wtf13-YFP co-localized with Sp mCh-Wtf18-2 (Fig 6C), we induced meiosis in diploids carrying those tagged constructs using the liquid sporulation protocol described above. Here, we also acquired the images on the LSM-780 microscope, with the objective described above, using photon-counting lambda mode with YFP and mCherry excited off-peak at 488 and 633 nm, respectively. This was done to eliminate, as much as possible, overlapping autofluorescence spectra. We collected the emission from the entire visible spectral region (~400–700 nm). After acquisition, we linearly unmixed the samples using an in-house written plug-in in ImageJ (https://imagej.nih.gov/ij/). To obtain the true fluorescence signal from the samples, we imaged control strains to obtain the YFP and mCherry spectra.
To detect if the mCherry from the Sp mCh-Wtf18-2 was enriched in two out of the four spores of the spore sac in a heterozygote (Fig 5C), we manually drew region of interest (ROIs) around the spores based only on transmitted light. Then, we plotted a histogram of the raw average intensities, which was weakly bimodal. We found a threshold between the peaks at an intensity of 3. We counted as positive every spore with an average intensity above 3, and negative every spore with an intensity less than 3. Every positive spore was given a score of 1 and an average value for that ascus was calculated. Enrichment in two out of the four spores will yield an average of 0.5 (2 positive out of 4 spores in the ascus). The analysis of the data can be found at the Stowers Original Data Repository (http://www.stowers.org/research/publications/libpb-1270).
To determine if mCherry and YFP co-localized inside the spores (Fig 6D, S9–S11 Figs) in Sk diploids hemizygous for both Sp wtf13-YFP and Sp mCh-wtf18-2, we manually drew ROIs around each of the spores in the ascus, again based only on transmitted light. We determined positive and negative mCherry and YFP spores as described above. In this case, positive YFP spores had an average intensity greater than 10 and mCherry spores had an average intensity greater than 5. We then determined how many spores were mCherry and YFP positive. The number of YFP and mCherry positive spores was then corrected to account for the double counting of the double positive spores by subtracting the number of double positive spores from both the YFP and mCherry positive spores. For example, in an ascus that has 1 mCherry positive, 1 YFP positive and 1 double positive, there are in total 2 mCherry positive, 2 YFP positive and 1 double positive spores. Subtracting 1 (double positive) from each of the mCherry and YFP positive spores yields the correct number of spores of each type. We then counted all the spores that fell into the parental ditype (PD) (2 mCherry positive, 2 YFP positive), non-parental ditype (NPD) (2 double positive) and tetratype (TT) (1 mCherry positive, 1 YFP positive, 1 double positive) tetrads. For each, we then calculated the percent of asci that were classified as each. Our unbiased approach identified PD, NPD and TT at the expected (1:1:4) frequencies. The analysis of the data can be found at the Stowers Original Data Repository (http://www.stowers.org/research/publications/libpb-1270).
Supporting information
Cartoons of the driving-mosaic chromosomes. % transmission refers to transmission of the mosaic chromosome into spores of mosaic/Sk heterozygotes homozygous for rec12Δ. rec12Δ mutants fail to initiate meiotic recombination, so chromosomes get transmitted whole through meiosis. Sp DNA is depicted in blue, Sk DNA in red. The numbers under the chromosomes are the breakpoints between the Sp and Sk regions. These data were published in [7]. All driving chromosomes show the Sp DNA region between 1,397,380 and 1,810,659 which includes Sp wtf13. All driving chromosomes also share Sk DNA between 252,650–763,460 and after 1,810,859. The second region includes the wtf18 locus, so all the driving chromosomes have Sk wtf18.
(TIF)
Sk allele names are depicted in red. Sp allele names are depicted in blue. (A) Alignment of the long isoforms of Sk Wtf4 and Sp Wtf13. Sk Wtf4 and Sp Wtf13 share 78% amino acid identity (left). Both genes have two transcriptional and translational start sites (right). (B) Alignment of the long isoform of Sp Wtf13 and Sk Wtf13 (83% amino acid identity) (left). Sk wtf13 has only one transcriptional and translational start site (right). (C) Alignment of the proteins encoded by the Sp wtf18 allele present in the reference genome and the Sk wtf18 allele (97% identity) (left). Both wtf18 genes have one transcriptional and translational start site (right). (D) Alignment of the Wtf18 protein from Sk and the Wtf18-2 protein from Sp. Sk Wtf18 and Sp Wtf18-2 share 98% amino acid identity. M’s represent the translational start sites.
(TIF)
Alignment of long-read RNA sequencing data from Kuang et al to Sp wtf13 (A) and Sp wtf18 (B) [41]. Our annotation of the genes is shown on top (grey) and the PomBase annotation is shown below (blue). M’s represent the translational start sites. In red and orange, are the meiotic transcripts sequenced by [41]. Only transcripts with 11 reads or more are depicted. If the transcript had more than 50 reads, the transcript is shown in red. The data were visualized using IGV (http://www.broadinstitute.org/igv). Exons are depicted as thick boxes with white arrows, introns as thin lines with blue arrows, and the UTRs as thin lines. No transcripts with more than 11 reads were reported at six hours for Sp wtf18. The PomBase gene model for Sp wtf13 and wtf18 is different than the one suggested by the RNA sequencing data [59]. Our annotation predictions are consistent with those of [8] which were made computationally. We use the gene models suggested by the data (grey).
(TIF)
Representative image (top) and quantification (bottom) of PI stained tetrads from the indicated diploids. (A) Sp wtf13/ade6+ (n = 58 4-spore asci). (B) empty vector/ade6+ (n = 79 4-spore asci). (C) Sp wtf13antidote/ade6+ (n = 123 4-spore asci). (D) Sp wtf18-2/ade6+ (n = 116 4-spore asci). The images were smoothed using Gaussian blur. The scale bar represents 3μm. The pattern of spore death produced by Sp wtf13/ade6+, Sp wtf13antidote/ade6+ and Sp wtf18-2/ade6+ diploids were all significantly different from that of the empty vector/ade6+ control diploids (G-test, p-value< 0.0001).
(TIF)
Sk allele names are depicted in red. Sp allele names are depicted in blue. Alignment of Sp Wtf13 (long isoform), Sp Wtf18, Sp Wtf18-2 and Sk Wtf18.
(TIF)
Allele transmission and fertility of Sk diploids with the indicated Sp wtf13 and Sp wtf18 mutant alleles integrated at the ade6 marker locus. Cartoons depicting the mutations made at the C-termini of the proteins are shown. Alleles were followed using drug resistance markers (kanMX4 or hphMX6). Diploids 1, 6 and 7 are repeated from Fig 2. Diploids 6, 7, 24 and 25 were compared to diploid 1 to detect suppression of the drive phenotype of Sp wtf13 and differences in fertility as measured by viable spore yield. Diploids 27–31 were compared to diploid 26 as control to detect if these alleles could suppress the drive phenotype of Sp wtf13repΔ. * indicates p-value of < 0.05 (G-test [allele transmission] and Wilcoxon test [fertility]). Fertility was normalized to the empty-vector control (Fig 2, diploid 10) and reported as percent. More than 200 viable haploid spores were scored for each cross. Spores that inherited both markers and were thus hygromycinR Ade+ or hygromycinR geneticinR were excluded from the analyses. Raw data can be found in S1 and S2 Tables. The yellow boxes are the repeat units found at the C-terminus. The wtf18-2mut1 allele is wtf18-2 (D366N). The Sp wtf18-2mut2 allele is Sp wtf18-2 (G370E, M372T, D373E, V374A). For the Sp wtf18+rep allele, the first repeat unit (LGNAFGG) was inserted between residues 332 and 333. In both Sp wtf13repΔ and wtf18-2repΔ, the first repeat unit was deleted. The wtf18 allele from Sk is shown in red (contains three repeats units; the first yellow box is depicted with a brown line to indicate a different residue in the first unit compared to wtf13repΔ, see S5 Fig).
(TIF)
Using two different double heterozygotes, we showed that Sp wtf18 from the reference genome is unable to suppress drive from Sp wtf13. The first double heterozygotes (top) included wtf13+ wtf18+ on one haplotype and wtf13Δ wtf18-2+ on the other. In these diploids, the wtf13Δ spores are expected to also inherit the wtf18-2 suppressor 72% of the time due to linkage (28 cM) between the loci. The second diploids (bottom) we tested had wtf13+ wtf18-2+ on one haplotype and wtf13Δ wtf18+ on the other. In these diploids, the wtf13Δ spores are expected to inherit the wtf18 allele 72% of the time. The table shows the expected results if both alleles of wtf18 are suppressors and if only wtf18-2 is a suppressor. The expected results are based on the assumption that the suppressor only rescues the spores which inherit the suppressor (demonstrated in Fig 4B). The observed results fit the model in which only wtf18-2 is a suppressor of wtf13. The wtf13Δ allele was followed using the hphMX6 drug resistance cassette.
(TIF)
Example of the localization of Wtf13-YFP (green). Propidium iodide (PI) was used to detect spores that have lost membrane integrity. Sp wtf13-YFP transgene was integrated at lys4 in Sk. The drive phenotype of this allele is indistinguishable from the untagged Sp wtf13 allele (Data in S1 Table, compare diploid 34 to diploid 1). This allele exhibit the same localization as the weaker Sp wtf13-YFP allele shown in Fig 5B. The images were smoothed using Gaussian blur. The scale bar represents 3μm.
(TIF)
(A) Linear unmixing (see Materials and Methods) of the representative image presented in Fig 5C is shown. mCh-wtf18-2 was integrated at lys4 in Sk. (B) Linear unmixing of the representative image in Fig 6B is shown. The transgene constructs were both integrated at lys4 in opposite haplotypes. (C) Linear unmixing of the representative image in Fig 6D is shown. wtf13-YFP was integrated at ade6 in Sk. mCh-wtf18-2 was integrated at lys4 in Sk. The autofluorescence images are shown with the same intensity as their respective channels. The brightness and contrast were adjusted differently for images A, B and C and the images were smoothed using Gaussian blur. Scale bar represents 3μm.
(TIF)
(A) The cartooned diploid was generated by integrating Sp wtf13-YFP at ade6 and Sp mCh-wtf18-2 at lys4 in Sk. The ade6 and lys4 loci are unlinked, thus the alleles will segregate randomly. This diploid will generate: parental ditype (PD), non-parental ditype (NPD) and tetratype (TT) tetrads. Due to random assortment, we expect PD, NPD and TT at a 1:1:4 ratio. Consistent with this, we determined that there were 15% PD, 17% NPD and 68% TT tetrads (See Material and Methods). (B) Examples of the localization of mCh-Wtf18-2 (magenta) and Wtf13-YFP (green) in representatives of the three classes of tetrads are shown. The brightness and contrast were adjusted differently for each image and images were smoothed using Gaussian blur. The scale bar represents 3μm.
(TIF)
Examples of the localization of mCh-Wtf18-2 (magenta) and Wtf13-YFP (green) in non-parental ditype tetrads from diploids heterozygous for Sp mCh-wtf18-2 and Sp wtf13-YFP. The images were processed to remove autofluorescence using linear unmixing, scaled to the same size, and smoothed using Gaussian blur. The brightness and contrast were adjusted differently for each image. The NPD tetrads from Fig 6D and S9C Fig as well as S10B Fig are also represented in this Supplemental Figure for easy comparison. The scale bar represents 2μm.
(TIF)
Plots show the probabilities of inside, outside and transmembrane helices in (A) Wtf13antidote, (B) Wtf18-2, (C) Wtf18, (D) Wtf13repΔ, (E) Wtf18-2repΔ and (F) Wtf18+rep. Outside is depicted in cyan, inside in magenta and the transmembrane helices in black. At the top of every plot there is the prediction of the transmembrane protein topology.
(TIF)
Each of the horizontal lines represents the relevant genotype and allele transmission of the indicated diploid. The first column represents the diploid number, which matches the numbers in Fig 2 and Fig 3. In columns 2–5, the strain number (SZY) and relevant genotype of the haploid parent strains used to determine the allele transmission at the drive locus (ade6 or lys4). Sp alleles are labeled in blue. Sk alleles are labeled in red. Columns 6 and 7 indicate which phenotypes were followed at the control locus (ura4) and the number of progeny that showed the indicated phenotype. Columns 9 and 10 indicate the phenotypes that were followed at the drive loci (ade6 or lys4) and the number of haploid progeny that exhibited the indicated phenotype. Some of the progeny inherited both markers from the parent strains. The number of those progeny (geneticinR Ade+, hygromycinR Ade+ or hygromycinR geneticinR) is presented in column 11 and the percent of the progeny with this phenotype is shown in column 12. Column 13 shows the fraction of the haploid progeny that inherited the genotype of allele 1. Column 14 shows the fraction of the haploid progeny that inherited the genotype of allele 2. Column 15 shows the total progeny assayed excluding progeny that inherited both markers. Column 16 shows the total progeny including progeny that inherited both markers. Column 17 shows the total number of diploids assayed. Last column shows the p-value calculated by comparing diploids 1–9, 21–23 to control diploid 10 using a G-test. Diploids 11–13, 24 and 25 were compared to diploid 1 using a G-test. Diploids 27–31 were compared to control diploid 26.
(PDF)
Each of the rows represents the diploid assayed, which matches the diploid number in Figs 2, 3 and 4. The numbers underneath the diploid number are SZY numbers of the haploid parent strains. All the viable spore yield values are shown for each diploid. Diploids 1–9, 11, 12, 13, 21, 22–31 were normalized to control diploid 10. Diploids 14–19 were normalized to diploid 20. To determine any fertility defect in Fig 2, we compared diploids 1–9, 21, 22 and 23 to control diploid 10. To test if the wtf18 alleles rescued the fertility phenotype caused by the wtf13 driver, we compared diploid 6, 7, 11, 12 and 13 from Fig 3, and diploids 24 and 25 from S6 Fig to diploid 1. In S6 Fig, diploids 27–31 were compared to diploid 26 as control. Diploids in Fig 4 were all compared to diploid 20. We calculated the p-value for each diploid using the Wilcoxon test.
(PDF)
Each of the horizontal lines represents the relevant genotype and allele transmission of the indicated diploid. The first column represents the diploid number, which matches the numbers in Fig 4. In columns 2–5, the strain number (SZY) and relevant genotype of the haploid parent strains used to determine the allele transmission at the Sp wtf13 or Sp wtf18-2 loci. Columns 6 and 7 indicate which phenotypes were followed at the control locus (ura4) and the number of progeny that showed the indicated phenotype. Columns 9 and 10 indicate the phenotypes that were followed at the Sp wtf13 or Sp wtf18-2 loci (bolded) and the number of haploid progeny that exhibited the indicated phenotype. Column 11 shows the fraction of the haploid progeny that inherited the genotype of allele 1. Column 12 shows the fraction of the haploid progeny that inherited the genotype of allele 2. Column 13 shows the total progeny assayed. Column 14 shows the total number of diploids assayed. Last column shows the p-value calculated by comparing diploids 16 and 17 to control diploid 14, and diploids 18 and 19 to control diploid 15. P-values were calculated using a G-test.
(PDF)
Each of the horizontal lines represents the relevant genotype and allele transmission of the indicated diploid. The first column shows the Fig where these data were represented, or the diploid number referred to in the text. In columns 2–5, the strain number (SZY) and relevant genotype of the haploid parent strains used to determine the allele transmission are shown. Diploids with the indicated genotype will generate four different types of spores (column 6–9). Columns 6 and 7 are the parental classes and columns 8 and 9 are the recombinant classes. Column 10 shows the total progeny assayed. Column 11 shows the total number of diploids assayed.
(PDF)
(PDF)
(PDF)
Notes: The ura4-X allele in SZY562 and SZY581 listed in the table is either ura4-D18 or ura4-294. Sp wtf13 (M1X, M12X) is the Sp wtf13 poison allele. Sp wtf18-2 (D366N) is the Sp wtf18-2mut1 allele used in S6 Fig. The Sp wtf18-2 (G370E, M372T, D373E, V374A) allele is Sp wtf18-2mut2 in S6 Fig. The Sp wtf18-2 (p.333-339Δ) allele contains the first repeat unit deleted. Sp wtf18 (G332_I333insLGNAFGG) is the wtf18+rep allele in which we inserted the first repeat unit between residues 332 and 333. Sp wtf18-2 (D366N, D366_I367insN) is the Sp wtf18-2D366NN allele used in Fig 3. Sp wtf13 (p.343-349Δ) is an allele containing the deletion of the first repeat unit. We are listing the mutations in the protein. Sp wtf13 (358A>T, 359T>A, 360G>C) is the Sp wtf13 antidote allele. We are listing the mutations in the nucleotide sequence.
(PDF)
Acknowledgments
We are grateful to Mia Levine, Robert Unckless, Sue Jaspersen, Philippe Silar, an anonymous reviewer and members of the Zanders lab for their helpful comments on the manuscript. This work was performed to fulfill, in part, requirements for MABN‘s thesis research in The Graduate School of the Stowers Institute for Medical Research.
Data Availability
Original data underlying this manuscript can be accessed from the Stowers Original Data Repository at http://www.stowers.org/research/publications/libpb-1270.
Funding Statement
This work was supported by the following awards to SEZ: The Stowers Institute for Medical Research (https://www.stowers.org), March of Dimes Foundation Basil O'Connor Starter Scholar Research Award No. 5-FY18-58 (https://www.marchofdimes.org), and the National Institutes of Health (NIH) under the award numbers R00GM114436 and DP2GM132936 (https://www.nih.gov). MABN was also supported by NIH under award number F99CA234523. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Abbott S, Fairbanks DJ. Experiments on plant hybrids by Gregor Mendel. Genetics. 2016;204(2):407–22. 10.1534/genetics.116.195198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sandler L, Novitski E. Meiotic drive as an evolutionary force. The American Naturalist. 1957;91(857):105–10. [Google Scholar]
- 3.Zimmering S, Sandler L, Nicoletti B. Mechanisms of meiotic drive. Annu Rev Genet. 1970;4:409–36. 10.1146/annurev.ge.04.120170.002205 [DOI] [PubMed] [Google Scholar]
- 4.Burt A, Trivers R. Genes in conflict: the biology of selfish genetic elements Cambridge, Mass.: Belknap Press of Harvard University Press; 2006. viii, 602 p., 8 p. of plates p. [Google Scholar]
- 5.Lindholm AK, Dyer KA, Firman RC, Fishman L, Forstmeier W, Holman L, et al. The ecology and evolutionary dynamics of meiotic drive. Trends Ecol Evol. 2016;31(4):315–26. 10.1016/j.tree.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 6.Bravo Núñez MA, Nuckolls NL, Zanders SE. Genetic villains: killer meiotic drivers. Trends in Genetics. 2018;34(6):424–33. 10.1016/j.tig.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nuckolls NL, Bravo Núñez MA, Eickbush MT, Young JM, Lange JJ, Yu JS, et al. wtf genes are prolific dual poison-antidote meiotic drivers. eLIFE. 2017;6:e26033 10.7554/eLife.26033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu W, Jiang Z, Suo F, Zheng J, He W, Du L. A large gene family in fission yeast encodes spore killers that subvert Mendel’s law eLIFE. 2017;6:e26057 10.7554/eLife.26057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Didion JP, Morgan AP, Clayshulte AM, McMullan RC, Yadgary L, Petkov PM, et al. A multi-megabase copy number gain causes maternal transmission ratio distortion on mouse chromosome 2. PLoS Genet. 2015;11(2):e1004850 10.1371/journal.pgen.1004850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei KH, Reddy HM, Rathnam C, Lee J, Lin D, Ji S, et al. A pooled sequencing approach identifies a candidate meiotic driver in Drosophila. Genetics. 2017;206(1):451–65. 10.1534/genetics.116.197335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grognet P, Lalucque H, Malagnac F, Silar P. Genes that bias Mendelian segregation. PLoS Genet. 2014;10(5):e1004387 10.1371/journal.pgen.1004387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crow JF. Why is Mendelian segregation so exact? Bioessays. 1991;13(6):305–12. 10.1002/bies.950130609 [DOI] [PubMed] [Google Scholar]
- 13.Larracuente AM, Presgraves DC. The selfish Segregation Distorter gene complex of Drosophila melanogaster. Genetics. 2012;192(1):33–53. 10.1534/genetics.112.141390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price TAR, Wedell N. Selfish genetic elements and sexual selection: their impact on male fertility. Genetica. 2007;132(3):295 10.1007/s10709-007-9173-2 [DOI] [PubMed] [Google Scholar]
- 15.Sutter A, Lindholm AK. Detrimental effects of an autosomal selfish genetic element on sperm competitiveness in house mice. Proceedings of the Royal Society B: Biological Sciences. 2015;282(1811). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dyer KA, Charlesworth B, Jaenike J. Chromosome-wide linkage disequilibrium as a consequence of meiotic drive. Proc Natl Acad Sci U S A. 2007;104(5):1587–92. 10.1073/pnas.0605578104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schimenti J. Segregation distortion of mouse t haplotypes the molecular basis emerges. Trends Genet. 2000;16(6):240–3. [DOI] [PubMed] [Google Scholar]
- 18.Zanders SE, Eickbush MT, Yu JS, Kang JW, Fowler KR, Smith GR, et al. Genome rearrangements and pervasive meiotic drive cause hybrid infertility in fission yeast. eLIFE. 2014;3:e02630 10.7554/eLife.02630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crow JF. The ultraselfish gene. Genetics. 1988;118(3):389–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartl DL. Modifier theory and meiotic drive. Theoretical Population Biology. 1975;7(2):168–74. [DOI] [PubMed] [Google Scholar]
- 21.Daugherty MD, Malik HS. Rules of engagement: molecular insights from host-virus arms races. Annu Rev Genet. 2012;46:677–700. 10.1146/annurev-genet-110711-155522 [DOI] [PubMed] [Google Scholar]
- 22.McLaughlin RN Jr., Malik HS. Genetic conflicts: the usual suspects and beyond. J Exp Biol. 2017;220(Pt 1):6–17. 10.1242/jeb.148148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pardo-Manuel de Villena F, Sapienza C. Female meiosis drives karyotypic evolution in mammals. Genetics. 2001;159(3):1179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henikoff S, Ahmad K, Malik HS. The centromere paradox: stable inheritance with rapidly evolving DNA. Science. 2001;293(5532):1098–102. 10.1126/science.1062939 [DOI] [PubMed] [Google Scholar]
- 25.Dawe RK. Maize Centromeres and Knobs (neocentromeres) In: Bennetzen JL, Hake S, editors. Handbook of Maize: Genetics and Genomics. New York, NY: Springer; New York; 2009. p. 239–50. [Google Scholar]
- 26.Ottolini CS, Newnham LJ, Capalbo A, Natesan SA, Joshi HA, Cimadomo D, et al. Genome-wide maps of recombination and chromosome segregation in human oocytes and embryos show selection for maternal recombination rates. Nat Genet. 2015;47(7):727–35. 10.1038/ng.3306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dawe RK, Lowry EG, Gent JI, Stitzer MC, Swentowsky KW, Higgins DM, et al. A Kinesin-14 motor activates neocentromeres to promote meiotic drive in maize. Cell. 2018;173(4):839–50.e18. 10.1016/j.cell.2018.03.009 [DOI] [PubMed] [Google Scholar]
- 28.Wu G, Hao L, Han Z, Gao S, Latham KE, Pardo-Manuel de Villena F, et al. Maternal transmission ratio distortion at the mouse Om locus results From meiotic drive at the second meiotic division. Genetics. 2005;170(1):327–34. 10.1534/genetics.104.039479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Helleu Q, Gérard PR, Dubruille R, Ogereau D, Prud’homme B, Loppin B, et al. Rapid evolution of a Y-chromosome heterochromatin protein underlies sex chromosome meiotic drive. Proceedings of the National Academy of Sciences. 2016;113(15):4110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akera T, Chmátal L, Trimm E, Yang K, Aonbangkhen C, Chenoweth DM, et al. Spindle asymmetry drives non-Mendelian chromosome segregation. Science. 2017;358(6363):668–72. 10.1126/science.aan0092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu X, Zhao Z, Zheng X, Zhou J, Kong W, Wang P, et al. A selfish genetic element confers non-Mendelian inheritance in rice. Science. 2018;360(6393):1130–2. 10.1126/science.aar4279 [DOI] [PubMed] [Google Scholar]
- 32.Shen R, Wang L, Liu X, Wu J, Jin W, Zhao X, et al. Genomic structural variation-mediated allelic suppression causes hybrid male sterility in rice. Nature Communications. 2017;8(1):1310 10.1038/s41467-017-01400-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie Y, Niu B, Long Y, Li G, Tang J, Zhang Y, et al. Suppression or knockout of SaF/SaM overcomes the Sa-mediated hybrid male sterility in rice. Journal of integrative plant biology. 2017;59(9):669–79. 10.1111/jipb.12564 [DOI] [PubMed] [Google Scholar]
- 34.Tao Y, Araripe L, Kingan SB, Ke Y, Xiao H, Hartl DL. A sex-ratio meiotic drive system in Drosophila simulans. II: An X-linked distorter. PLOS Biology. 2007;5(11):e293 10.1371/journal.pbio.0050293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tao Y, Hartl DL, Laurie CC. Sex-ratio segregation distortion associated with reproductive isolation in Drosophila. Proceedings of the National Academy of Sciences. 2001;98(23):13183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tao Y, Masly JP, Araripe L, Ke Y, Hartl DL. A sex-ratio meiotic drive system in Drosophila simulans. I: An autosomal suppressor. PLOS Biology. 2007;5(11):e292 10.1371/journal.pbio.0050292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin C-J, Hu F, Dubruille R, Vedanayagam J, Wen J, Smibert P, et al. The hpRNA/RNAi pathway is essential to resolve intragenomic conflict in the Drosophila male germline. Developmental Cell. 2018;46(3):316–26.e5. 10.1016/j.devcel.2018.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.López Hernández JF, Zanders SE. Veni, vidi, vici: the success of wtf meiotic drivers in fission yeast. Yeast.1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eickbush MT, Young, JM and Zanders, SE. Killer meiotic drive and dynamic evolution of the wtf gene family. bioRxiv. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bowen NJ, Jordan IK, Epstein JA, Wood V, Levin HL. Retrotransposons and their recognition of pol II promoters: a comprehensive survey of the transposable elements from the complete genome sequence of Schizosaccharomyces pombe. Genome Res. 2003;13(9):1984–97. 10.1101/gr.1191603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuang Z, Boeke JD, Canzar S. The dynamic landscape of fission yeast meiosis alternative-splice isoforms. Genome Res. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rhind N, Chen Z, Yassour M, Thompson DA, Haas BJ, Habib N, et al. Comparative functional genomics of the fission yeasts. Science. 2011;332(6032):930–6. 10.1126/science.1203357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith GR. Genetic analysis of meiotic recombination in Schizosaccharomyces pombe. Methods Mol Biol. 2009;557:65–76. 10.1007/978-1-59745-527-5_6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fantes PA, Hoffman CS. A brief history of Schizosaccharomyces pombe research: A perspective over the past 70 years. Genetics. 2016;203(2):621–9. 10.1534/genetics.116.189407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeffares DC, Rallis C, Rieux A, Speed D, Převorovský M, Mourier T, et al. The genomic and phenotypic diversity of Schizosaccharomyces pombe. Nature Genetics. 2015;47:235 10.1038/ng.3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farlow A, Long H, Arnoux S, Sung W, Doak TG, Nordborg M, et al. The spontaneous mutation rate in the fission yeast Schizosaccharomyces pombe. Genetics. 2015;201(2):737–44. 10.1534/genetics.115.177329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mizuguchi T, Taneja N, Matsuda E, Belton J-M, FitzGerald P, Dekker J, et al. Shelterin components mediate genome reorganization in response to replication stress. Proceedings of the National Academy of Sciences. 2017;114(21):5479–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dawe RK, Cande WZ. Induction of centromeric activity in maize by suppressor of meiotic drive 1. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(16):8512–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jaenike J. Sex chromosome meiotic drive. Annual Review of Ecology and Systematics. 2001;32(1):25–49. [Google Scholar]
- 50.Hurst LD, Pomiankowski A. Causes of sex ratio bias may account for unisexual sterility in hybrids: a new explanation of Haldane's rule and related phenomena. Genetics. 1991;128(4):841–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frank SA. Haldane’s rule: A defense of the meiotic drive theory. Evolution. 1991;45(7):1714–7. 10.1111/j.1558-5646.1991.tb02678.x [DOI] [PubMed] [Google Scholar]
- 52.Phadnis N, Orr HA. A single gene causes both male sterility and segregation distortion in Drosophila hybrids. Science. 2009;323(5912):376–9. 10.1126/science.1163934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goldstein AL, McCusker JH. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast. 1999;15(14):1541–53. 10.1002/(SICI)1097-0061(199910)15:14<1541::AID-YEA476>3.0.CO;2-K [DOI] [PubMed] [Google Scholar]
- 54.Longtine MS, McKenzie Iii A, Demarini DJ, Shah NG, Wach A, Brachat A, et al. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14(10):953–61. 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U [DOI] [PubMed] [Google Scholar]
- 55.Chen J, Smoyer CJ, Slaughter BD, Unruh JR, Jaspersen SL. The SUN protein Mps3 controls Ndc1 distribution and function on the nuclear membrane. The Journal of Cell Biology. 2014;204(4):523–39. 10.1083/jcb.201307043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shaner NC, Campbell RE, Steinbach PA, Giepmans BNG, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nature Biotechnology. 2004;22:1567 10.1038/nbt1037 [DOI] [PubMed] [Google Scholar]
- 57.Sonnhammer EL, von Heijne G, Krogh A. A hidden Markov model for predicting transmembrane helices in protein sequences. Proceedings International Conference on Intelligent Systems for Molecular Biology. 1998;6:175–82. [PubMed] [Google Scholar]
- 58.Fernández-Álvarez A, Bez C, O'Toole Eileen T, Morphew M, Cooper JP. Mitotic nuclear envelope breakdown and spindle nucleation are controlled by interphase contacts between centromeres and the nuclear envelope. Developmental Cell. 2016;39(5):544–59. 10.1016/j.devcel.2016.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wood V, Harris MA, McDowall MD, Rutherford K, Vaughan BW, Staines DM, et al. PomBase: a comprehensive online resource for fission yeast. Nucleic acids research. 2012;40(Database issue):D695–9. 10.1093/nar/gkr853 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cartoons of the driving-mosaic chromosomes. % transmission refers to transmission of the mosaic chromosome into spores of mosaic/Sk heterozygotes homozygous for rec12Δ. rec12Δ mutants fail to initiate meiotic recombination, so chromosomes get transmitted whole through meiosis. Sp DNA is depicted in blue, Sk DNA in red. The numbers under the chromosomes are the breakpoints between the Sp and Sk regions. These data were published in [7]. All driving chromosomes show the Sp DNA region between 1,397,380 and 1,810,659 which includes Sp wtf13. All driving chromosomes also share Sk DNA between 252,650–763,460 and after 1,810,859. The second region includes the wtf18 locus, so all the driving chromosomes have Sk wtf18.
(TIF)
Sk allele names are depicted in red. Sp allele names are depicted in blue. (A) Alignment of the long isoforms of Sk Wtf4 and Sp Wtf13. Sk Wtf4 and Sp Wtf13 share 78% amino acid identity (left). Both genes have two transcriptional and translational start sites (right). (B) Alignment of the long isoform of Sp Wtf13 and Sk Wtf13 (83% amino acid identity) (left). Sk wtf13 has only one transcriptional and translational start site (right). (C) Alignment of the proteins encoded by the Sp wtf18 allele present in the reference genome and the Sk wtf18 allele (97% identity) (left). Both wtf18 genes have one transcriptional and translational start site (right). (D) Alignment of the Wtf18 protein from Sk and the Wtf18-2 protein from Sp. Sk Wtf18 and Sp Wtf18-2 share 98% amino acid identity. M’s represent the translational start sites.
(TIF)
Alignment of long-read RNA sequencing data from Kuang et al to Sp wtf13 (A) and Sp wtf18 (B) [41]. Our annotation of the genes is shown on top (grey) and the PomBase annotation is shown below (blue). M’s represent the translational start sites. In red and orange, are the meiotic transcripts sequenced by [41]. Only transcripts with 11 reads or more are depicted. If the transcript had more than 50 reads, the transcript is shown in red. The data were visualized using IGV (http://www.broadinstitute.org/igv). Exons are depicted as thick boxes with white arrows, introns as thin lines with blue arrows, and the UTRs as thin lines. No transcripts with more than 11 reads were reported at six hours for Sp wtf18. The PomBase gene model for Sp wtf13 and wtf18 is different than the one suggested by the RNA sequencing data [59]. Our annotation predictions are consistent with those of [8] which were made computationally. We use the gene models suggested by the data (grey).
(TIF)
Representative image (top) and quantification (bottom) of PI stained tetrads from the indicated diploids. (A) Sp wtf13/ade6+ (n = 58 4-spore asci). (B) empty vector/ade6+ (n = 79 4-spore asci). (C) Sp wtf13antidote/ade6+ (n = 123 4-spore asci). (D) Sp wtf18-2/ade6+ (n = 116 4-spore asci). The images were smoothed using Gaussian blur. The scale bar represents 3μm. The pattern of spore death produced by Sp wtf13/ade6+, Sp wtf13antidote/ade6+ and Sp wtf18-2/ade6+ diploids were all significantly different from that of the empty vector/ade6+ control diploids (G-test, p-value< 0.0001).
(TIF)
Sk allele names are depicted in red. Sp allele names are depicted in blue. Alignment of Sp Wtf13 (long isoform), Sp Wtf18, Sp Wtf18-2 and Sk Wtf18.
(TIF)
Allele transmission and fertility of Sk diploids with the indicated Sp wtf13 and Sp wtf18 mutant alleles integrated at the ade6 marker locus. Cartoons depicting the mutations made at the C-termini of the proteins are shown. Alleles were followed using drug resistance markers (kanMX4 or hphMX6). Diploids 1, 6 and 7 are repeated from Fig 2. Diploids 6, 7, 24 and 25 were compared to diploid 1 to detect suppression of the drive phenotype of Sp wtf13 and differences in fertility as measured by viable spore yield. Diploids 27–31 were compared to diploid 26 as control to detect if these alleles could suppress the drive phenotype of Sp wtf13repΔ. * indicates p-value of < 0.05 (G-test [allele transmission] and Wilcoxon test [fertility]). Fertility was normalized to the empty-vector control (Fig 2, diploid 10) and reported as percent. More than 200 viable haploid spores were scored for each cross. Spores that inherited both markers and were thus hygromycinR Ade+ or hygromycinR geneticinR were excluded from the analyses. Raw data can be found in S1 and S2 Tables. The yellow boxes are the repeat units found at the C-terminus. The wtf18-2mut1 allele is wtf18-2 (D366N). The Sp wtf18-2mut2 allele is Sp wtf18-2 (G370E, M372T, D373E, V374A). For the Sp wtf18+rep allele, the first repeat unit (LGNAFGG) was inserted between residues 332 and 333. In both Sp wtf13repΔ and wtf18-2repΔ, the first repeat unit was deleted. The wtf18 allele from Sk is shown in red (contains three repeats units; the first yellow box is depicted with a brown line to indicate a different residue in the first unit compared to wtf13repΔ, see S5 Fig).
(TIF)
Using two different double heterozygotes, we showed that Sp wtf18 from the reference genome is unable to suppress drive from Sp wtf13. The first double heterozygotes (top) included wtf13+ wtf18+ on one haplotype and wtf13Δ wtf18-2+ on the other. In these diploids, the wtf13Δ spores are expected to also inherit the wtf18-2 suppressor 72% of the time due to linkage (28 cM) between the loci. The second diploids (bottom) we tested had wtf13+ wtf18-2+ on one haplotype and wtf13Δ wtf18+ on the other. In these diploids, the wtf13Δ spores are expected to inherit the wtf18 allele 72% of the time. The table shows the expected results if both alleles of wtf18 are suppressors and if only wtf18-2 is a suppressor. The expected results are based on the assumption that the suppressor only rescues the spores which inherit the suppressor (demonstrated in Fig 4B). The observed results fit the model in which only wtf18-2 is a suppressor of wtf13. The wtf13Δ allele was followed using the hphMX6 drug resistance cassette.
(TIF)
Example of the localization of Wtf13-YFP (green). Propidium iodide (PI) was used to detect spores that have lost membrane integrity. Sp wtf13-YFP transgene was integrated at lys4 in Sk. The drive phenotype of this allele is indistinguishable from the untagged Sp wtf13 allele (Data in S1 Table, compare diploid 34 to diploid 1). This allele exhibit the same localization as the weaker Sp wtf13-YFP allele shown in Fig 5B. The images were smoothed using Gaussian blur. The scale bar represents 3μm.
(TIF)
(A) Linear unmixing (see Materials and Methods) of the representative image presented in Fig 5C is shown. mCh-wtf18-2 was integrated at lys4 in Sk. (B) Linear unmixing of the representative image in Fig 6B is shown. The transgene constructs were both integrated at lys4 in opposite haplotypes. (C) Linear unmixing of the representative image in Fig 6D is shown. wtf13-YFP was integrated at ade6 in Sk. mCh-wtf18-2 was integrated at lys4 in Sk. The autofluorescence images are shown with the same intensity as their respective channels. The brightness and contrast were adjusted differently for images A, B and C and the images were smoothed using Gaussian blur. Scale bar represents 3μm.
(TIF)
(A) The cartooned diploid was generated by integrating Sp wtf13-YFP at ade6 and Sp mCh-wtf18-2 at lys4 in Sk. The ade6 and lys4 loci are unlinked, thus the alleles will segregate randomly. This diploid will generate: parental ditype (PD), non-parental ditype (NPD) and tetratype (TT) tetrads. Due to random assortment, we expect PD, NPD and TT at a 1:1:4 ratio. Consistent with this, we determined that there were 15% PD, 17% NPD and 68% TT tetrads (See Material and Methods). (B) Examples of the localization of mCh-Wtf18-2 (magenta) and Wtf13-YFP (green) in representatives of the three classes of tetrads are shown. The brightness and contrast were adjusted differently for each image and images were smoothed using Gaussian blur. The scale bar represents 3μm.
(TIF)
Examples of the localization of mCh-Wtf18-2 (magenta) and Wtf13-YFP (green) in non-parental ditype tetrads from diploids heterozygous for Sp mCh-wtf18-2 and Sp wtf13-YFP. The images were processed to remove autofluorescence using linear unmixing, scaled to the same size, and smoothed using Gaussian blur. The brightness and contrast were adjusted differently for each image. The NPD tetrads from Fig 6D and S9C Fig as well as S10B Fig are also represented in this Supplemental Figure for easy comparison. The scale bar represents 2μm.
(TIF)
Plots show the probabilities of inside, outside and transmembrane helices in (A) Wtf13antidote, (B) Wtf18-2, (C) Wtf18, (D) Wtf13repΔ, (E) Wtf18-2repΔ and (F) Wtf18+rep. Outside is depicted in cyan, inside in magenta and the transmembrane helices in black. At the top of every plot there is the prediction of the transmembrane protein topology.
(TIF)
Each of the horizontal lines represents the relevant genotype and allele transmission of the indicated diploid. The first column represents the diploid number, which matches the numbers in Fig 2 and Fig 3. In columns 2–5, the strain number (SZY) and relevant genotype of the haploid parent strains used to determine the allele transmission at the drive locus (ade6 or lys4). Sp alleles are labeled in blue. Sk alleles are labeled in red. Columns 6 and 7 indicate which phenotypes were followed at the control locus (ura4) and the number of progeny that showed the indicated phenotype. Columns 9 and 10 indicate the phenotypes that were followed at the drive loci (ade6 or lys4) and the number of haploid progeny that exhibited the indicated phenotype. Some of the progeny inherited both markers from the parent strains. The number of those progeny (geneticinR Ade+, hygromycinR Ade+ or hygromycinR geneticinR) is presented in column 11 and the percent of the progeny with this phenotype is shown in column 12. Column 13 shows the fraction of the haploid progeny that inherited the genotype of allele 1. Column 14 shows the fraction of the haploid progeny that inherited the genotype of allele 2. Column 15 shows the total progeny assayed excluding progeny that inherited both markers. Column 16 shows the total progeny including progeny that inherited both markers. Column 17 shows the total number of diploids assayed. Last column shows the p-value calculated by comparing diploids 1–9, 21–23 to control diploid 10 using a G-test. Diploids 11–13, 24 and 25 were compared to diploid 1 using a G-test. Diploids 27–31 were compared to control diploid 26.
(PDF)
Each of the rows represents the diploid assayed, which matches the diploid number in Figs 2, 3 and 4. The numbers underneath the diploid number are SZY numbers of the haploid parent strains. All the viable spore yield values are shown for each diploid. Diploids 1–9, 11, 12, 13, 21, 22–31 were normalized to control diploid 10. Diploids 14–19 were normalized to diploid 20. To determine any fertility defect in Fig 2, we compared diploids 1–9, 21, 22 and 23 to control diploid 10. To test if the wtf18 alleles rescued the fertility phenotype caused by the wtf13 driver, we compared diploid 6, 7, 11, 12 and 13 from Fig 3, and diploids 24 and 25 from S6 Fig to diploid 1. In S6 Fig, diploids 27–31 were compared to diploid 26 as control. Diploids in Fig 4 were all compared to diploid 20. We calculated the p-value for each diploid using the Wilcoxon test.
(PDF)
Each of the horizontal lines represents the relevant genotype and allele transmission of the indicated diploid. The first column represents the diploid number, which matches the numbers in Fig 4. In columns 2–5, the strain number (SZY) and relevant genotype of the haploid parent strains used to determine the allele transmission at the Sp wtf13 or Sp wtf18-2 loci. Columns 6 and 7 indicate which phenotypes were followed at the control locus (ura4) and the number of progeny that showed the indicated phenotype. Columns 9 and 10 indicate the phenotypes that were followed at the Sp wtf13 or Sp wtf18-2 loci (bolded) and the number of haploid progeny that exhibited the indicated phenotype. Column 11 shows the fraction of the haploid progeny that inherited the genotype of allele 1. Column 12 shows the fraction of the haploid progeny that inherited the genotype of allele 2. Column 13 shows the total progeny assayed. Column 14 shows the total number of diploids assayed. Last column shows the p-value calculated by comparing diploids 16 and 17 to control diploid 14, and diploids 18 and 19 to control diploid 15. P-values were calculated using a G-test.
(PDF)
Each of the horizontal lines represents the relevant genotype and allele transmission of the indicated diploid. The first column shows the Fig where these data were represented, or the diploid number referred to in the text. In columns 2–5, the strain number (SZY) and relevant genotype of the haploid parent strains used to determine the allele transmission are shown. Diploids with the indicated genotype will generate four different types of spores (column 6–9). Columns 6 and 7 are the parental classes and columns 8 and 9 are the recombinant classes. Column 10 shows the total progeny assayed. Column 11 shows the total number of diploids assayed.
(PDF)
(PDF)
(PDF)
Notes: The ura4-X allele in SZY562 and SZY581 listed in the table is either ura4-D18 or ura4-294. Sp wtf13 (M1X, M12X) is the Sp wtf13 poison allele. Sp wtf18-2 (D366N) is the Sp wtf18-2mut1 allele used in S6 Fig. The Sp wtf18-2 (G370E, M372T, D373E, V374A) allele is Sp wtf18-2mut2 in S6 Fig. The Sp wtf18-2 (p.333-339Δ) allele contains the first repeat unit deleted. Sp wtf18 (G332_I333insLGNAFGG) is the wtf18+rep allele in which we inserted the first repeat unit between residues 332 and 333. Sp wtf18-2 (D366N, D366_I367insN) is the Sp wtf18-2D366NN allele used in Fig 3. Sp wtf13 (p.343-349Δ) is an allele containing the deletion of the first repeat unit. We are listing the mutations in the protein. Sp wtf13 (358A>T, 359T>A, 360G>C) is the Sp wtf13 antidote allele. We are listing the mutations in the nucleotide sequence.
(PDF)
Data Availability Statement
Original data underlying this manuscript can be accessed from the Stowers Original Data Repository at http://www.stowers.org/research/publications/libpb-1270.