Abstract
Love is one of our most powerful emotions, inspiring some of the greatest art, literature and conquests of human history. Although aspects of love are surely unique to our species, human romantic relationships are displays of a mating system characterized by pair bonding, likely built on ancient foundational neural mechanisms governing individual recognition, social reward, territorial behaviour and maternal nurturing. Studies in monogamous prairie voles and mice have revealed precise neural mechanisms regulating processes essential for the pairbond. Here, we discuss current viewpoints on the biology underlying pair bond formation, its maintenance and associated behaviours from neural and evolutionary perspectives.
Love is a powerful emotion that has been a substantial driving force behind many aspects of culture and society from the beginning of humanity. In fact, the evolution of striatal neurochemistry promoting pair bonding in early hominids may have preceded the expansion of cerebral cortical complexity and the emergence of language1. Nonetheless, the richness and complexity of romantic bonds lead some to conclude that the nature of love will forever evade rigorous scientific understanding. However, if we consider that love is an emergent property of several complex cognitive processes, each of which has their evolutionary origins in more general neurobiological processes present in model organisms, then we can begin to understand some of the constituents of love using modern neurobiology2. Indeed, recent investigations into the neurobiology of pair bonding in monogamous species have provided remarkable insights into the biology of social behaviour. Here, we review recent neurobiological studies in monogamous voles, as well as studies in mice, on constituent neural processes of social bonding to propose a dynamic neural circuit-level model of the pair bond — the evolutionary antecedent of romantic love.
Pair bonding
Pair bonding is a term used in biology and behavioural sciences to describe a strong social relationship between individuals in a breeding pair in monogamous species. Social monogamy is a mating system that is characterized by partners sharing territory, biparental care and preferential mating but not sexual exclusivity. By contrast, partners from sexually monogamous species mate only with each other. Sexual monogamy is rare in nature, and although social monogamy is more common, the frequency of species displaying this mating system is remarkably diverse across vertebrate taxa. In fish, amphibians and reptiles, pair bonding is rarely observed, with a few exceptions, including shinglebacks (Tiliqua rugosa)3 and the Caribbean cleaning goby (Elacatinus evelynae)4. In birds, however, up to 90% of all species display some form of monogamy5, and within some genera, such as those including geese, swans and eagles, lifelong pair bonds are common. A recent study comprising most known mammalian species showed that 9% of mammals are socially monogamous6. Human beings have a strong propensity to form pair bonds, and social monogamy has been observed in nearly all human societies. Cross-cultural data show that stable pair-bonded relationships have several positive health implications, including lower mortality7. Insecure attachment and conflict in romantic relationships are associated with depression8, lowered immune function9 and poor cardiovascular health10.
Regarding the neurobiology of pair bonding, most of our knowledge today comes from studies in voles11–15. In the socially monogamous prairie vole (Microtus ochrogaster), males and females form long-lasting pair bonds, and both sexes take care of offspring16. In both male and female prairie voles, mating facilitates the development of the pair bond, which can be assessed in the laboratory using the partner preference test17. Pair-bonded animals prefer to spend time in close contact with their partner rather than with a novel stimulus animal. Partner-directed affiliative behaviour in prairie voles is regulated by neural mechanisms involving several identified brain regions and is thought to be the result of synaptic plasticity linking the neural encoding of sociosensory information from the partner with reward, thereby increasing the reinforcing value of the mate relative to other social stimuli18. This plasticity is governed by circuit activation in the context of several neuromodulators during social engagement. The neuropeptides oxytocin (OT) and arginine vasopressin (AVP) and the neurotransmitter dopamine (DA) have been shown to play critical roles in the circuitry regulating bonding in prairie voles14. Here, we first describe the evidence of their involvement in pair bond formation. We then propose a model of pair bonding based on a synthesis of the literature on these molecules, the circuits involved and the neural processes required for the development and subsequent maintenance of a pair bond.
Oxytocin.
OT is a nine-amino-acid peptide that modulates several social behaviours, including parental nurturing19, individual discrimination20, empathy-related behaviours21 and social bonding14,22. OT is synthesized in the paraventricular nucleus (PVN) and supraoptic nucleus (SON) of the hypothalamus. Hypothalamic OT neurons project to the posterior pituitary, where OT is released into the periphery, and throughout the forebrain, targeting regions rich in OT receptors (OXTRs)23–26. OT released into the periphery promotes uterine contractions during labour and milk ejections during lactation23. Centrally released OT is critical for the onset of maternal responsiveness and mother-infant bonding19,27. These OT-dependent, evolutionary ancient maternal instincts are present in all mammals and may be the evolutionary origin of pair bonding18 as well as empathy-related consoling behaviours beyond the context of parenting21,28 (BOX 1).
Box 1 |. Consoling behaviour in prairie voles.
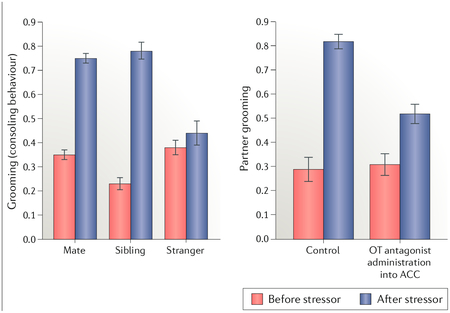
Consolation is a comforting behavioural response directed towards a distressed party. In humans, consolation is common and considered an external manifestation of empathy. in addition to humans, consolation has been observed in several different species, including great apes, elephants and wolves. until recently, empathy-related traits were thought to require advanced cognition and therefore had not been studied extensively in rodents, with a few exceptions28. A recent study showed that prairie voles display consoling behaviour, indicated by increased licking and grooming, towards stressed conspecifics (see the figure, left panel)21. This effect was specific for familiar individuals, as no consoling of stressed strangers was observed. these novel findings suggest that consoling is evolutionarily ancient and does not require higher cognitive function. Consoling behaviour is also related to social structure, as non-monogamous meadow voles did not display this trait. the empathic capacity of prairie voles seems to go beyond consoling, as it was observed that individuals match the fear response and anxiety-related outcomes, including corticosterone increase, of their stressed cagemates.
By adopting an immediate early gene approach, it was further shown that the anterior cingulate cortex (ACC), a brain region implicated in human empathy, was differentially activated when individuals interacted with stressed cagemates compared with unstressed cagemates21. interestingly, in prairie voles, the ACC abundantly expresses oxytocin receptors (OXTRs), and it was further shown that infusion of an OXTR antagonist (OTA) in this region blocks the consoling behaviour. the fact that consoling seems to be regulated by the oxytocin (OT) system points to the intriguing possibility that this behaviour, similarly to our evolutionary model of how the brain OT system came to facilitate pair bonding, represents a neurobiological redirection of maternal instincts towards adults. Figure adapted with permission from REF.21, Science/AAAS
Intracerebroventricular (ICV) infusion of OT facilitates partner preference formation after a brief cohabitation in the absence of mating in male and female prairie voles17,29. Conversely, ICV infusion of an OXTR antagonist (OTA) prevents mating-induced partner preferences in prairie voles of both sexes30,31. An early study in closely related vole species showed that prairie voles have higher densities of OXTR in the nucleus accumbens (NAc), medial prefrontal cortex (mPFC) and amygdala compared with the non-monogamous sister species montane (Microtus montanus) and meadow (Microtus pennsylvanicus) voles32, suggesting that these are neuroanatomical sites of OT action in pair-bonding behaviour (FIG. 1 a). Indeed, blocking OXTR in the NAc or mPFC (but not the adjacent caudate-putamen) prevents mating-induced partner preference formation33. Additionally, viral-vector-mediated RNAi knockdown of OXTR in the NAc inhibits partner preference formation in female prairie voles34, while increasing OXTR density in the NAc facilitates it35, providing direct evidence that NAc OXTR expression can substantially influence pair-bonding behaviour. The effect of OXTR in the amygdala on pair bonding has yet to be explored.
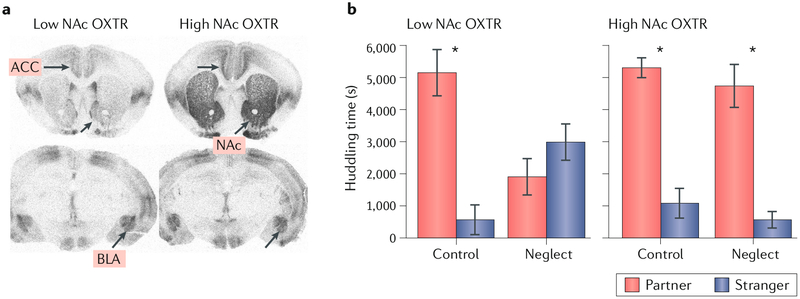
Fig. 1 |. Individual variation in oxytocin receptor expression in the nucleus accumbens confers resilience to neonatal neglect.
, a | The distribution of and variation in oxytocin receptors (OXTRs) in rostral (top row) and caudal (bottom row) brain regions of two prairie voles. Note the robust individual variation in OXTR density in the nucleus accumbens (NAc), with less variation in other areas, such as the basolateral amygdala (BLA). The density of OXTR in the NAc is robustly predicted by genetic variation in non-coding regions of the Oxtr gene, b | High NAc OXTR density confers resilience to a neglect paradigm involving neonatal social isolation. The histograms show that when animals with low NAc OXTR density are exposed to neonatal neglect, they then fail as adults to form a preference for spending time with their cohabiting partner (huddling time) compared with a nove ‘stranger’; control animals with low NAc OXTR expression that have not experienced neonatal neglect display strong partner preferences. By contrast, the huddling time preferences of adult voles with high NAc OXTR with regard to their partners are unaffected by neonatal neglect. ACC, anterior cingulate cortex. Part b adapted from REF39, CC-BY-4.0.
There is remarkable individual variability in the density of OXTR in the NAc and caudate-putamen among prairie voles, with very little variation in other brain regions36 (FIG. 1 a). Variation in OXTR in the NAc in prairie voles is associated with social phenotypes including alloparental behaviour37 and individual differences in male monogamy-related behaviour in naturalistic settings38. Furthermore, variation in NAc OXTR density confers resilience or susceptibility to the effect of daily neonatal social isolation on the ability to form partner preferences later in life39 (Box 2; fig. 1b). The density of OXTR in the NAc is under strong genetic control. A set of single nucleotide polymorphisms (SNPs) in the prairie vole OXTR gene (Oxtr) explains more than three-quarters of the variation in OXTR density in the NAc, is strongly associated with OXTR mRNA levels in this region and enables prediction of pair-bonding behaviour in males36. These results suggest that small changes in the DNA sequence have robust effects on brain phenotype and behaviour simply by changing the expression pattern of receptors that respond to neuromodulators.
Box 2 |. OXTR and resilience to early adversity.
By combining concepts drawn from behavioural ecology and psychology, Belsky et al. developed an evolutionary theory of human socialization predicting how a stressful rearing environment during childhood results in subsequent unstable pair bonds in adulthood126. This theory has become popular and is currently supported by empirical studies in humans showing associations between low parental investment and subsequent accelerated reproductive development127–129 and between childhood family climate and pair-bonding-related outcomes130.
Intriguingly, animal studies show a similar picture and suggest that the association between early-life experiences and adult pair-bonding behaviour involves neuropeptide circuitry underlying social behaviour, including the oxytocin (OT) system. In rats and voles, early-life maternal separation results in social deficits in adulthood, an effect that can be alleviated by supplemental tactile stimulation. Touch is associated with both central and peripheral OT release in rats, and licking and grooming as well as injections of OT in rat pups result in increased maternal behaviour in adulthood19,39. Furthermore, manipulating OT receptor (OXTR) expression in the nucleus accumbens (NAc) at weaning affects adult alloparental and pair-bonding behaviour in prairie voles35. NAc OXTR expression in female prairie voles is also associated with resiliency to neonatal isolation (FIG. 1b). Accumbal OXTR density shows remarkable individual variation in prairie voles, largely explained by sequence variation in the Oxtr gene36, and appears to be robust against environmental influences. However, the effect of neonatal isolation on adult pair-bonding behaviour interacts with the variation in Oxtr, as females with high OXTR density in the NAc are resilient to this early-life challenge39, representing a case of gene-by-environment interaction and suggesting a developmental role of OXTR signalling in the NAc in adult social behaviour. These results are consistent with several studies in humans demonstrating that variation in the OXTR gene interacts with early-life adversity to influence psychiatric outcomes131–133.
Vasopressin.
AVP is a neuropeptide with a structure similar to that of OT that has been shown to be of importance for social recognition, territorial scent marking and aggressive behaviour40. In male prairie voles, pair-bonding behaviour is facilitated by brain AVP and prevented by central injections of an AVP la receptor (AVPR1A) antagonist41,42. As with OXTR, the neuroanatomical distribution of AVPR1A differs considerably among vole species with different mating strategies. For example, prairie voles display relatively high AVPR1A density in the ventral pallidum (VP), the major output of the NAc and reward centre, compared with montane and meadow voles41. Blocking AVPR1A in the VP43 or reducing the expression of AVPR1A in this region using viral-vector-mediated RNAi knockdown44 inhibits partner preference formation in male prairie voles. Remarkably, increasing the density of AVPR1A in the VP of meadow voles via viral-vector-mediated gene transfer makes these voles more social and display prairie vole-like pair-bonding behaviour45. AVPR1A signalling in the lateral septum (LS) is also essential for pair bonding46, possibly facilitating processes related to social recognition47,48. An intrinsic AVP system in the olfactory bulb also facilitates social recognition by modulating information processing by olfactory bulb neurons, but its contribution to pair bonding has not been investigated49.
Pair-bonded male prairie voles display mate-guarding behaviour manifested as selective aggression towards male and female conspecifics, reminiscent of territorial behaviour displayed by many mammals. AVP is essential for mate guarding, and this behaviour is regulated in part by AVPR1A signalling in the anterior hypothalamus50. In fact, blocking AVPR1A signalling in the brain of male prairie voles disrupts both pair bonding and mate guarding41,51,52. The role of AVP in both selective aggression and pair bonding suggests that, similar to how OT-dependent aspects of pair bonding appear to have evolved via tweaking of the maternal nurturing circuits, the AVP-dependent properties of male pair-bonding behaviour are derived from the integration of ancient neural mechanisms governing territorial behaviour, social recognition and reward53. Indeed, in both cases, these behaviours emerged through evolutionary changes in neuropeptide receptor distribution in the brain.
Studies in voles have also provided intriguing insights into the genetic regulation of the AVP system. While the coding region of the gene encoding AVPR1A (Avprla) is 99% homologous between prairie and meadow voles54, there is an ~500-base-pair-long repeat sequence (microsatellite) in the prairie vole promoter region that is absent in meadow voles. Variation in this microsatellite is associated with AVPR1A density in the LS and the probability of forming a partner preference in male prairie voles55. In addition to microsatellite variation, SNPs in the prairie vole Avprla strongly predict AVPR1A density in the retrosplenial cortex, a brain region implicated in spatial navigation, and are associated with sexual fidelity in male prairie voles studied under naturalistic conditions56. For a detailed description of how the AVP system is involved in monogamous behaviour in the wild and particularly how memory circuits and spatial ability relate to social behaviour and mating tactics, see a recent review by Alexander Ophir57.
Dopamine.
DA plays a critical role in reward, reinforcement learning and addition. Acting in the NAc, DA facilitates the association of sensory stimuli with reward, for example, during sex, and motivates behaviour on the basis of the reinforcement53. Mesolimbic DA plays a major role in pair-bonding behaviour and interacts with the OT and AVP systems to facilitate partner preference formation. Mating results in DA release in the NAc in male and female prairie voles, and partner preference is prevented by infusion of a nonspecific DA antagonist into this region14. Activation of DA receptors in NAc facilitates partner preference formation in the absence of mating. Despite these nonspecific effects, the two DA receptor subtypes have opposing roles in regulating pair bonding. Activation of DA D2-type receptors (D2R) in the NAc stimulates, while activating DA Dl- type receptors (DIR) prevents, pair bond formation in prairie voles58.
Accumbal DA signalling not only is important for partner preference formation but also plays a role in pair bond maintenance58. Sexually naive male prairie voles show low levels of aggression towards strangers. After 2 weeks of cohabitation with a female, however, intense selective aggression towards both male and female novel conspecifics develops. This behavioural change is thought to represent a mechanism for maintaining an established bond by rejecting new potential partners and preventing cuckoldry via mate guarding. Interestingly, this change in behaviour is regulated by changes in DA receptor density in the striatum. Males experiencing 2 weeks of cohabitation with a partner have a 60% increased expression of DIR in the NAc compared with control animals cohabitating with a same-sex sibling, while D2R expression remains unchanged58. Furthermore, blocking DIR in the NAc prevents the selective aggression displayed by cohabitated males. The DIR-mediated increase in selective aggression in both males and females is regulated by the κ-opioid receptor in the NAc59,60. Interestingly, the μ-opioid system in the striatum also plays a role in pair bond formation61,62, drawing interesting molecular parallels between pair bonding and addiction63.
The shift in the DIR and D2R balance in favour of DIR during pair bonding is remarkably similar to DA receptor plasticity in drug addiction63. All known drugs of abuse cause DA release in the NAc64, and chronic exposure to cocaine, for example, results in simultaneous increased D1R and decreased D2R signalling in this region63,65. These data suggest that the mechanism underlying the intense selective interest in a mating partner in socially monogamous animals overlaps functionally with comparable monomania in drug addiction. In further support of this theory, daily administration of amphetamine to sexually naive male prairie voles results in an upregulation of D1R and prevents these animals from forming pair bonds, similarly to how pair-bonded males reject novel females66. This effect is reversed by blocking D1R in the NAc66. Conversely, the increased expression of D1R during pair bond formation decreases the rewarding effects of amphetamines67. While pair bonding and addiction involve similar neural mechanisms, there are also important differences68,69. The consequences of drug addiction are almost exclusively negative, while romantic bonds can have a profoundly positive impact on quality of life.
Other systems.
While the roles of OT, AVP and DA in pair bonding have been the most extensively investigated, other neurochemical systems likely play a role as well. For example, serotonin interacts with the OT system to mediate social reward70 and thus likely plays a role in pair bonding. Indeed, a serotonin 1A receptor antagonist reduced affiliative behaviour in pair-bonded monogamous titi monkeys71. μ-opioid signalling in the striatum is necessary for the development of pair bonds61,62. In voles, κ-opioid receptors in the NAc mediate pair bond maintenance59, and the opioid and DA systems interact in this region to maintain bonds60.
The stress axis can also influence pair bonding. Infusion of corticotropin-releasing factor (CRF) into the NAc facilitates partner preference formation in male prairie voles72. Corticosterone has sexually dimorphic effects on pair bonding in prairie voles; decreasing circulating corticosterone facilitates partner preference formation in females73, while increasing corticosterone facilitates pair bonding in males74.
A neural circuit model of pair bonding
While the roles of OT, AVP, DA and the NAc in regulating pair-bonding behaviour have been well documented, the precise neural mechanisms leading to selective affiliation between partners have yet to be determined. FIGURE 2 shows a hypothetical neural model of pair bond formation in prairie voles, focusing on selective processes that we hope will guide future research. This model synthesizes direct experimental evidence from voles and results from studies in mice describing the neural mechanisms underlying behavioural processes presumed to be essential for pair bonding. Our model is primarily focused on dynamic neural processes involving OT because of recent advances in mechanistic research implicating this peptide.
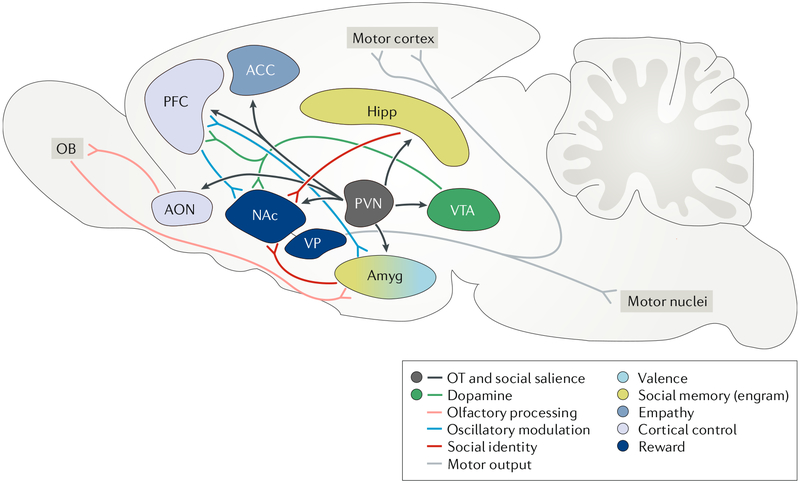
Fig. 2 |. A neural model of pair bond formation.
During mating, the olfactory bulb (OB) processes olfactory information representing the identity of the partner, and oxytocin (OT)-sensitive neurons in the anterior olfactory nucleus (AON) improve thesignal-to-noise ratio of the OB output. Neural traces enabling individual discrimination are conveyed to the amygdala (Amyg), which forms a social memory of the partner and assigns valence to the cues. Social engrams, groups of neurons activated by an individual, form in the hippocampus (Hipp) and possibly in the Amyg and project to the nucleus accumbens (NAc). The ventral teg mental area (VTA) releases dopamine in the NAc and prefrontal cortex (PFC), activating the reward system. OT released from paraventricular nucleus (PVN) neurons acts across the networkto improve the salience and flow of social information across the network. The PFC coordinates oscillations of the NAc and possibly the Amyg to facilitate the flow of social information and synaptic plasticity, linking the neural representation of partner identity with reward. Partner cues become inherently rewarding, and NAc activation disinhibits the ventral pallidum (VP), leading to the initiation of partner-directed behaviours via motor output. OT signalling in the anterior cingulate cortex (ACC) drives consoling behaviour towards the distressed partner.
Social interaction (and, more robustly, mating) stimulates DA release in terminal fields of ventral tegmental area (VTA) neurons in the NAc14 as well as OT input from the PVN to multiple nodes of a social salience network75 proposed to be involved in partner preference formation in prairie voles, including the anterior olfactory nucleus (AON), amygdala, hippocampus, mPFC and NAc. The mechanisms of pair bond formation are hypothesized to include two different plasticity processes: the formation of a distinct neural representation of the partner, allowing for partner recognition, and a persistent attraction to the partner that continues after mating, leading to a partner preference. In the sections below, we discuss several processes that enable these plasticity processes to occur and culminate in a selective pair bond with a partner.
Sensory information processing.
Fine-tuned extraction of high-resolution sensory information from conspecifics during social interactions is essential to social recognition76 and is an important component of pair-bonding behaviour. The brain of the individual forming the bond must perceive the mate not only as an opposite sex conspecific but also as a unique individual. Olfaction is the primary sensory modality engaged by rodents during social contact, and odours are used for individual discrimination. OT plays a role in the early steps of olfactory information refinement. The processing of olfactory signals to facilitate social recognition in rodents is dependent on the main olfactory bulb (MOB)77. Initial processing of odour cues from the olfactory sensory neurons takes place in the MOB by projection neurons (mitral and tufted cells) modulated by interneuron networks made up primarily of granule cells78, which receive dense glutamatergic input from the AON79. The AON expresses high levels of OXTRs in rodents80–82 and receives projections from OT neurons in the PVN25, and blocking OXTR in the AON impairs social recognition in mice78. Interestingly, OXTR expression in the AON of female prairie voles is regulated by oestradiol, and virgin females exposed to male chemosignals show increased oestradiol and OXTR in the AON82, perhaps priming them for bonding. OT acting in the AON facilitates top-down recruitment of interneurons within the MOB to increase the overall inhibitory tone, improving odorant coding and facilitating social recognition by improving the signal-to-noise ratio78 (FIG. 3a). Information transmission by neurons is often a noisy stochastic process in which true signals can be masked by baseline firing rates, similar to how high levels of static generated by a radio can obscure auditory information from an incoming signal. Comparable to how adjusting a radio receiver to closely match the carrier frequency will enhance the signal over that of a static relationship, OT improves the fidelity of olfactory signals processed in the MOB by lowering the baseline firing of mitral and tufted cells and increasing their peak odour responses.
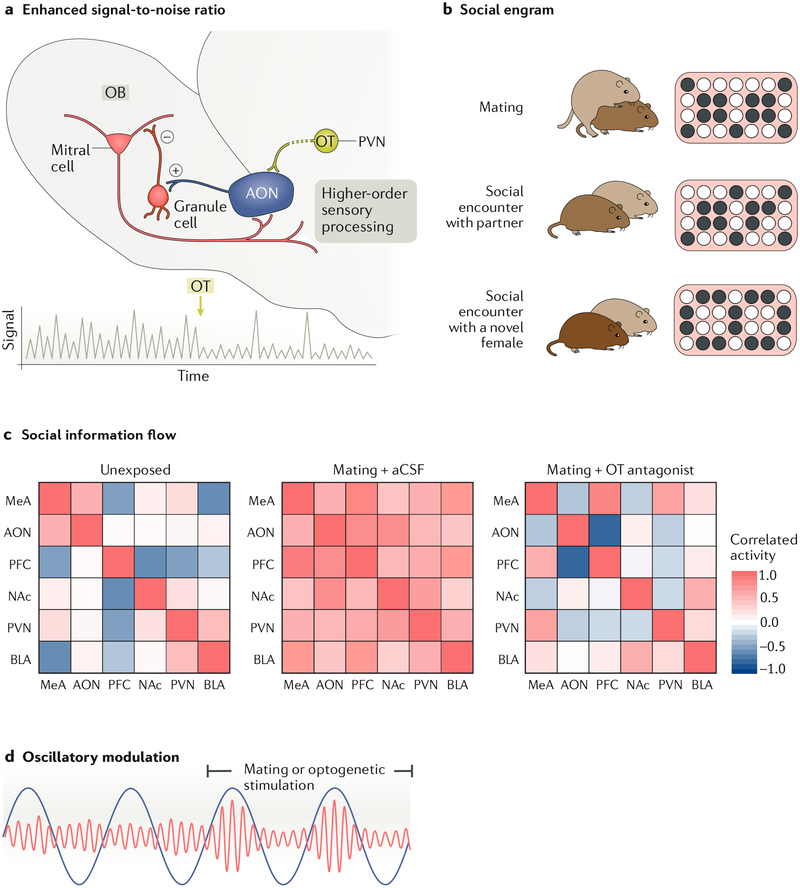
Fig. 3 |. Schematics illustrating selected processes proposed to be involved in pair bonding on the basis of our model.
a | Oxytocin (OT) improves the signai-to-noise ratio during the detection of social signals in the olfactory bulb (OB). OT released from terminals of neurons in the paraventricular nucleus (PVN) increases the excitatory drive of excitatory neurons (indicated by the plus sign) in the cortical anterior olfactory nucleus (AON), which activates inhibitory granule cells (indicated by the minus sign) in the OB, thereby reducing noise produced by the mitral projection neurons. The trace below illustrates the increase in the signal-to-noise ratio in mitral cells, which then project to higher-order sensory processing centres, such as the amygdala, b | Social engrams (populations of neurons that respond collectively to an individual) have been identified in the hippocampus and project to the nucleus accumbens (NAc). The schematic illustrates social engrams in the male brain (right), with circles representing neurons within a brain region and black circles specifically representing neurons activated by different social encounters. Note the high similarity in activity patterns during mating with the partner (top) and later social encounter with the partner (centre) but the different pattern seen during exposure to a novel female (bottom), c | Fleat maps showing correlated activity between brain regions in the social neural network (red is more correlated). The left panelshows little coordinated activity in a male’s brain when placed alone in a home cage (unexposed). Mating (under control conditions with infusion of artificial cerebrospinal fluid (aCSF)) results in robust coordinated activity across the network (middle heat map), but this coordinated activity is diminished when OT antagonists are infused into the brain, d | Schematic illustrating howtheta oscillatory activity from the medial prefrontal cortex (PFC) (blue line) entrains gamma oscillations within the NAc (red line), particularly during mating. We propose that the rhythmically controlled peaks in the accumbens gamma amplitude are moments of maximal sensitivity to neural input. If the PFC similarly controls oscillations of areas conveying social information, such as engrams (sender), the signal can be coordinated to reach the NAc at a peak of sensitivity, leading to synaptic plasticity. BLA, basolateral amygdala; MeA, medial amygdala. Part a adapted with permission from REF.78, Elsevier. Part c adapted with permission from REF.31, Elsevier.
OT not only is implicated in olfactory transduction but also plays a role in other sensory systems, suggesting that OT has a general function in the processing of sociosensory information. For example, OT can act in the mouse auditory cortex to promote maternal behaviour by improving the cortical response to infant distress calls83. This effect is driven by a synaptic mechanism characterized by balancing the magnitude and timing of inhibition and excitation to improve neuronal transmission.
Salience and social recognition.
Olfactory and other sociosensory information representing the identity of the partner is relayed to the amygdala. The amygdala is a collection of parallel circuits processing and responding to multiple aspects of salient stimuli84 and encoding valence85. In rodents, the medial amygdala (MeA) receives direct input from the vomeronasal system via the accessory olfactory bulb86 and both direct and indirect input from the MOB87. Therefore, the MeA is anatomically positioned to facilitate the integration of sensory information from a mating partner with emotional salience and convey important cues to the other parts of the brain either directly or via glutamatergic projections to the basolateral amygdala (BLA), which encodes the valence of stimuli18,85. The MeA shows strong OXTR expression and receives OT innervation from the PVN26,88. OT-deficient mice display deficits in social recognition, despite retaining fully functioning odorant discrimination of non-social cues20. This selective social recognition deficit can be rescued by site-specific infusion of OT into the MeA88. In both male and female prairie voles, cohabitation and social interaction with a mating partner activate the MeA, as indicated by elevated FOS expression compared with that of control animals89,90. Lesions of the MeA in male prairie voles disrupt social recognition of a mating partner, as well as the expression of aífiliative behaviours91.
Long-term social recognition is mediated by OT-dependent synaptic plasticity in the MeA92, in line with a growing amount of data showing that OT regulates plasticity mechanisms in several different brain regions93–95. Considering that salient sensory information received and processed by the MeA (and other parts of the amygdala) is later transmitted to mesocorticolimbic targets regulating selective social attachments, it is possible that OT, acting on OXTR, facilitates partner-specific memory during pair bond formation by strengthening synapses in the amygdala to allow sociosensory information from the mating partner to be more strongly relayed to the NAc, VP18 and hippocampus85.
The hippocampus and social memory.
Although the amygdala has been implicated in OT-dependent social memory, the hippocampus has long been recognized as the primary site of memory processing. The hippocampus receives sensory information from the olfactory systems via the MeA, BLA and LS85,86 and shares reciprocal connections with the mPFC96. Interestingly, in both rodents and non-human primates, multiple subregions of the hippocampus express OXTR97, and OXTR signalling in the hippocampus enhances social discrimination in mice98,99. Hippocampal synaptic plasticity mechanisms relevant for social memory storage are strongly regulated by the activation of OXTRs. The fidelity of social information transmission signals in the hippocampus is improved by OT, which increases the firing rate of fast-spiking interneurons100. This modulation acts to suppress the background firing rate of CA1 pyramidal neurons and ultimately increases the signal-to-noise ratio, similar to the above-described role of OT in olfactory processing. In mice, social recognition is dependent on CA1 pyramidal cells of the ventral hippocampus, and inhibition of these cells disrupts social discrimination performance101. Ventral CA1 pyramidal neurons activated during social exposure to a specific mouse are preferentially reactivated by re-exposure to that same mouse compared with novel stimuli102. Ventral CA1 cells show strong projections to multiple nodes of our model, including the BLA and NAc, and projections to the NAc are essential for social discrimination in mice101. These data suggest that social engrams, a collection of cells whose combined activation encodes the memory trace of the sensory stimuli of the partner (FIG. 3b), exist in the hippocampus and possibly amygdala and project to the NAc. We propose that the essence of the pair bond is synaptic plasticity, which strengthens neural communication between partner-specific social engrams and the NAc; hence, the partner cues become inherently rewarding outside of a sexual context. This plasticity is facilitated by OT, AVP and DA.
Oxytocin and dopamine in the striatum.
How do OT and DA interact to make salient sensory information, such as a partner engram, rewarding and facilitate a persistent pair bond lasting beyond the immediately rewarding properties of sex? Mating stimulates VTA neurons to release DA in the NAc and mPFC. Sex also results in the PVN releasing OT into the NAc26. OT from the PVN released into the VTA increases DA release in the NAc and gates social reward103. The NAc is a critical site for the integration of saliency from the amygdala, context and social identity from the hippocampus, and goal-directed information from the mPFC to ultimately guide behavioural output via disinhibition of the VP104. While sex robustly engages the OT and DA systems, positive social interactions, for example, grooming or social touch, may stimulate OT and DA systems as well because partner preferences can form after prolonged social interactions without mating105.
Studies show that the interaction between DA and OT in the NAc is essential for pair-bonding behaviour in both male and female prairie voles. Pharmacologically blocking either OXTRs or D2Rs in the NAc shell prevents mating-induced pair bond formation106. Furthermore, microinjections of either OT or DA into the NAc shell facilitate pair bond formation, but the effect of OT is blocked by a D2R antagonist, and conversely, the influence of DA is dependent on intact OXTR signalling106. Thus, pair bond formation requires simultaneous and coordinated activation of both OXTRs and D2Rs in the NAc shell, and recent research suggests that OXTRs form heterodimers with D2Rs on medium spiny neurons (MSNs), causing an increase in affinity of DA to D2Rs when OT is concurrently bound to an OXTR107. DA action on D2Rs depresses NAc GABAergic MSNs108, which in turn is proposed to result in disinhibition of the VP, allowing excitatory input elicited by mating partner stimuli from the BLA, as well as AVP input proposed to originate from the MeA43, to activate the VP18. However, whether there exists a strengthened engram of the partner in the NAc or whether OT and DA interact to strengthen synapses from partner engrams in other regions, such as the BLA or hippocampus, remains to be determined. Whether OXTRs are expressed in D1R–, D2R–or both neuron types within the NAc is also unknown.
Although human OT studies should be interpreted with some caution109, evidence suggests that in pair-bonded humans, OT enhances the neural response of the NAc specifically to partner cues. In a functional MRI study, men in monogamous relationships were shown photographs of either their partner or other equally attractive women after being given intranasal OT or a placebo and then asked to rate the attractiveness of the women in the photographs. Men rated their wife as more attractive after receiving OT than when receiving the placebo. OT did not change their rating of the other women. In addition, OT substantially increased the NAc blood-oxygen-level-dependent (BOLD) signal in the men when they viewed the image of their partner but not other women110. Interestingly, OXTR mRNA is expressed in the human NAc111, which is not the case for non-monogamous rhesus macaques97, and OXTR mRNA and OT fibres are present in the human cortex112.
Oxytocin and the flow of social information.
OT seems to be involved in all nodes included in our hypothetical neural model of pair bonding (FIG. 2). Although the exact function of OT differs depending on the brain region and the specific neural processes involved, we argue that OT plays a general role in facilitating the flow of high-integrity social information across multiple forebrain regions, regardless of whether this is achieved by increasing the signal-to-noise ratio, altering the excitatory-to-inhibitory balance or other processes. Thus, OT can be considered the ‘grease’ of the social brain. In line with this idea, we recently showed that blocking OT signalling in the brain not only prevents partner preference formation but also has a strong influence on brain network coordination during mating in male prairie voles31. Mating results in a strong and relatively uniform increase in correlated FOS expression across the pair-bonding model network in vehicle control males, a pattern that is strongly disrupted in OTA-injected males31 (FIG. 3c).
Dynamic medial prefrontal cortex modulation of the nucleus accumbens.
We recently showed that pair bond formation in female prairie voles is associated with changes in local field potential functional connectivity and cross-frequency coupling between the mPFC, which is involved in executive function, and the NAc113. Mating specifically altered both the temporally local and sustained post-mating mPFC-to-NAc circuit activation in a way that predicted subsequent huddling behaviour. That is, animals whose mPFC theta (4–6 Hz) oscillation most strongly modulated the amplitude of NAc gamma (80 Hz) oscillations had a shorter latency to begin huddling with the partner (FIG. 3d). Furthermore, optogenetically mimicking mating-induced neural activity by stimulating the theta-frequency activity of mPFC terminals in the NAc when a female is in close proximity to a caged male accelerates partner preference formation113.
This mating-enhanced rhythmic action of mPFC on NAc may engage oscillatory-based plasticity mechanisms, allowing the NAc to respond more strongly to neural representations of partner cues (engrams) from the BLA or hippocampus during mating and upon future encounters. Furthermore, it is possible that the mPFC drives synchronized oscillatory activity across the mPFC–NAc–BLA circuit to improve plasticity mechanisms strengthening the neural link between social recognition and reward. Such orchestration of rhythmic oscillation in the BLA and NAc by the mPFC could result in temporal synchrony of the sender signal and receiver sensitivity, thereby maximizing communication and subsequent synaptic plasticity. We hypothesize that the individual variation in the strength of the mPFC modulation of NAc gamma oscillations (and, consequently, individuals’ inclination to start engaging in affiliative behaviour) is at least partly explained by variability in NAc OXTRs, which is, as noted above, under strong genetic control36 (FIG. 1). In support of this hypothesis, we have shown that site-specific blocking of OXTRs in the NAc disrupts coordinated activity between the NAc and mPFC as well as other regions, including the BLA75.
Ventral pallidum.
As noted above, D2R activation in the NAc shell results in disinhibition of the VP. The VP acts as a relay nucleus from the NAc and modulates motor output in response to rewarding stimuli through projections to the thalamus and motor nuclei114; in the context of pair bonding, this modulation ultimately results in the expression of affiliative behaviour towards a mating partner. The disinhibited VP can in turn be strongly activated by partner-stimuli-induced input from the amygdala or other areas; these synapses may be strengthened as a result of sociosexual stimulation of OT, AVP and DA released during pair bond formation. Once this formation is completed, stimulus characteristics of the partner can then continuously activate the VP over long periods of time, creating an enduring pair bond18. However, the nature of the output of the VP that transforms behaviour such that the bonded animals increase proximity to their corresponding partner remains a mystery.
Oxytocin and pair bond maintenance
The notion that pair bond formation is a consequence of partner cues becoming inherently rewarding is appealing, but anyone who has been in a long-term relationship realizes that the rewarding properties of a pair bond fluctuate over time and that the motivating force behind maintaining a bond is not always positive. There is evidence that an interaction between the CRF system and OT may play a role in maintaining the bond through negative reinforcement (FIG. 4). Male and female prairie voles that have pair-bonded with a partner and then separated from their partner display increased passive coping behaviour in behavioural tests used to assess depressive-like behaviour, reminiscent of grieving or bereavement115–117. Individuals who have been separated from their pair-bonded partner display increased immobility in the forced swim test (FST) and tail suspension test, increased adrenal gland mass and activation of the stress axis. Males separated from their sibling do not show this social-loss-induced depressive behaviour or stress activation. Separation from a pair-bonded partner also results in heightened sensitivity to painful stimuli118.
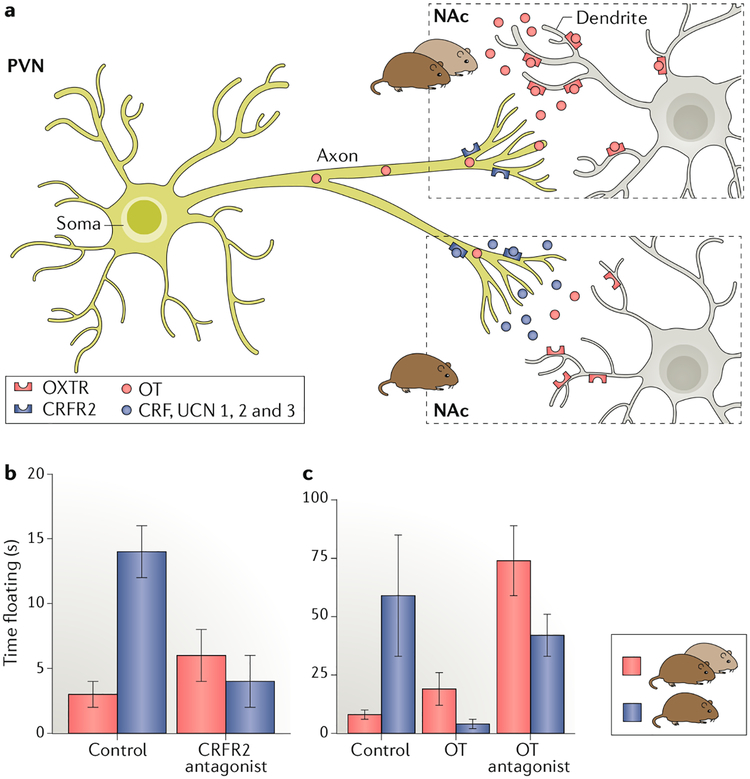
Fig. 4 |. The corticotropin-releasing factor system and oxytocin interact to maintain the pair bond.
, a | Effect of loss of the partner on the corticotropin-reieasing factor (CRF) and oxytocin (OT) signalling in the nucleus accumbens (NAc). Separation from the partner results in decreased OT synthesis in the paraventricular nucleus (PVN) and a reduction of OT receptors (OXTRs) in the NAc. Upon separation from the partner, CRF or the related urocortins (UCN 1,2 and 3) are released in the NAc, binding to CRF type 2 receptors (CRFR2) on OT terminals and inhibiting OT release. Thus, separation from the partner leads to decreased OT signalling through multiple mechanisms, b |The forced swim test is a behavioural assay in which an increased amount of time spent passively floating indicates a depressive-like state. Male voles paired with their partner spent little time floating, but this time increased upon separation from their partner. This social-loss-induced behaviour was eliminated by local infusion of the CRFR2 antagonist into the NAc.c | Infusion of OT into the NAc also eliminates the increase in passive floating in the forced swim test. Infusion of the OT antagonist into the NAc increases the floating behaviour in males even when they remain paired. Thus, the withdrawal of OT signalling following separation may maintain bonds by creating an aversive emotionalstate. Part b adapted from REF.115, Springer Nature Limited. Part c adapted with permission from REF120, Elsevier.
CRF mRNA expression is increased during pair bonding, and the CRF system has been strongly implicated in depression. Furthermore, both type 1 (CRFR1) and type 2 (CRFR2) CRF receptors are involved in the response of male prairie voles to the loss of a bonded partner because blocking either receptor reduces immobility in the FST115. Site-specific infusion of a CRFR2 antagonist into the NAc rescued the effect of partner loss, while a CRFR2 agonist in the same region in non-separated males increased depressive-like behaviour in the FST (FIG. 4b). Furthermore, the OT and CRF systems are linked, as indicated by high colocalization of CRFR2s on OT neurons in the hypothalamus119 as well as on OT fibres projecting to the NAc120, and CRFR2 activation in the NAc suppresses OT release, whereas blocking CRFR2 stimulates OT release120. Interestingly, OT infusion into the NAc eliminates partner loss adversity, and blocking OXTR in this brain region mimics the effect of partner loss120 (FIG. 4c). Consistent with these findings, site-specific knockdown of OXTR in the NAc using RNAi resulted in increased immobility in the FST in non-separated animals120.
Collectively, these studies suggest that mating and pair bonding prime the CRF system by increasing CRF synthesis, similar to loading a gun. Separation from the partner pulls the trigger and stimulates CRFR2 activation in the NAc, which suppresses OT release. At the same time, OT synthesis in the PVN and OXTRs in the NAc is reduced (FIG. 4a), culminating in multiple hits on the OT system, which ultimately leads to a negative affect120. We propose that this CRFR2-mediated social-loss-induced withdrawal of OT in the NAc leads to an aversive experience that ultimately motivates a wandering individual to reunite with its partner, maintaining the bond using mechanisms similar to those that drive a drug addict to use drugs of abuse63. Thus, building and maintaining a pair bond both involve complementary positive and negative reinforcement acting in a complementary ‘yin and yang’ manner116,121.
Conclusions
We propose that pair bond formation is essentially the result of neural plasticity that links the neural encoding of partner cues with the brain’s reward system such that a partner becomes persistently and inherently reinforced, leading to selective affiliative behaviour. In developing this model, we draw on studies directly related to partner preference formation in prairie voles as well as novel studies in mice related to social information processing, social engrams and reinforcement learning. We propose that positive reinforcement facilitates the formation of a pair bond, while negative reinforcement plays a role in maintaining the bond. Our model should not be taken as fact but rather as inspiration to rigorously test our ideas and build upon them. This is an unprecedented time in neuroscience in which we can now monitor the activity of ensembles of neurons using calcium indicators and optogenetically or chemogenetically manipulate specific neuronal populations on the basis of their neurochemical phenotype of prior activity. Genome-editing techniques such as CRISPR-Cas9 enable performing targeted genetic manipulations in non-traditional species, including voles. These tools should allow us to understand how a partner is uniquely represented in the brain through social engrams and their projections. We will learn more precisely how communication between social engrams and reward regions changes through plasticity mechanisms during pair bond formation. We can monitor, in high temporal resolution, the coherence and cross-frequency coupling between nodes of a social salience network that may affect synaptic plasticity. Along the way, we will gain insights into other social processes with implications for empathy and intra-species bonding22 and even perhaps improve treatments for autism122–125. We will also gain better understanding of the genetic and neural bases of individual variation in behaviour.
We propose that pair bonding is the evolutionary antecedent of romantic love and that the pair bond is an essential element of romantic love. The pair bond is the innate, subconscious motivating force forging a strong attraction between partners. Romantic love is the emergent property of the integration of the pair bond mechanisms described here, the ability to translate multimodal sensory information from our partner into the mental language of romance, the ability to reflect on past interactions with our partner and the ability to contemplate our future life journey together, abilities that are enabled by the cortical complexity that is unique to humans. While we may remain far from a satisfying neural explanation for the mysteries of romantic relationships, there are strong conceptual and neurobiological foundations for bringing to bear the powerful circuit-level neurobiological approaches necessary to move the field closer to understanding why we love.
Hominids.
Humans and their ancestors following separation from the Pan clade.
Sociosensory information.
Any form of sensory information (for example, olfactory, visual, tactile and auditory) perceived from a social source, typically a conspecific.
Alloparental behavior.
Parental nurturing behavior (for example, retrieving, licking and grooming) exhibited towards a non-descendent infant or juvenile.
Odorant coding.
The transduction of odours into distinct neural signals in the olfactory bulb and downstream pathways, which enables an organism to distinguish complex odours and associate an odour or its source with reinforcers.
Acknowledgements
Preparation of this manuscript was supported by US National Institutes of Health (NIH) grants R01MH096983, R01MH112788 and 1P50MH100023 to L.J.Y. and P51OD11132 to the Yerkes National Primate Research Center (YNPRC). The authors thank K. Inoue for his contribution to the manuscript.
Reviewer information
Nature Reviews Neuroscience thanks A. Bonci, O. Bosch and R. Froemke for their contribution to the peer review of this work.
Footnotes
Competing interests
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Raghanti MA et al. A neurochemical hypothesis for the origin of hominids. Proc. Natl Acad. Sci. USA 115, E1108–E1116 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young LJ Being human: love: neuroscience reveals all. Nature 457, 148 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Bull CM Monogamy in lizards. Behav. Processes 51, 7–20 (2000). [DOI] [PubMed] [Google Scholar]
- 4.Whiteman EA & Cote IM Monogamy in marine fishes. Biol. Rev. Camb. Philos. Soc 79, 351–375 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Dunn PO, Whittingham LA & Pitcher TE Mating systems, sperm competition, and the evolution of sexual dimorphism in birds. Evolution 55, 161–175 (2001). [DOI] [PubMed] [Google Scholar]
- 6.Lukas D & Clutton-Brock TH The evolution of social monogamy in mammals. Science 341, 526–530 (2013). [DOI] [PubMed] [Google Scholar]
- 7.House JS, Landis KR & Umberson D Social relationships and health. Science 241, 540–545 (1988). [DOI] [PubMed] [Google Scholar]
- 8.Bifulco A, Moran PM, Ball C & Bernazzani O Adult attachment style. I: Its relationship to clinical depression. Soc. Psychiatry Psychiatr. Epidemiol 37, 50–59 (2002). [DOI] [PubMed] [Google Scholar]
- 9.Kiecolt-Glaser JK et al. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Arch. Gen. Psychiatry 62, 1 377–1384 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Orth-Gomer K et al. Marital stress worsens prognosis in women with coronary heart disease: The Stockholm Female Coronary Risk Study. JAMA 284, 3008–3014 (2000). [DOI] [PubMed] [Google Scholar]
- 11.Johnson ZV & Young LJ Neurobiological mechanisms of social attachment and pair bonding. Curr. Opin. Behav. Sci 3, 38–44 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGraw LA & Young LJ The prairie vole: an emerging model organism for understanding the social brain. Trends Neurosci 33, 103–109 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross HE & Young LJ Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front. Neuroendocrinol 30, 534–547 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young LJ & Wang Z The neurobiology of pair bonding. Nat. Neurosci 7, 1048–1054 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Young KA, Gobrogge KL, Liu Y & Wang Z The neurobiology of pair bonding: insights from a socially monogamous rodent. Front. Neuroendocrinol 32, 53–69 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahern TH, Hammock EA & Young LJ Parental division of labor, coordination, and the effects of family structure on parenting in monogamous prairie voles (Microtus ochrogaster). Dev. Psychobiol 53, 118–131 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams JR, Insel TR, Harbaugh CR & Carter CS Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster). J. Neuroendocrinol 6, 247–250 (1994). [DOI] [PubMed] [Google Scholar]
- 18.Numan M & Young LJ Neural mechanisms of mother–infant bonding and pair bonding: similarities, differences, and broader implications. Horm. Behav 77, 98–112 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rilling JK & Young LJ The biology of mammalian parenting and its effect on offspring social development. Science 345, 771–776 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferguson JN et al. Social amnesia in mice lacking the oxytocin gene. Nat. Genet 25, 284–288 (2000). [DOI] [PubMed] [Google Scholar]
- 21.Burkett JP et al. Oxytocin-dependent consolation behavior in rodents. Science 351, 375–378 (2016).This study demonstrates that consoling behaviour in prairie voles displays multiple qualities that are characteristic of empathy and is regulated by OT receptors in the anterior cingulate cortex.
- 22.Nagasawa M et al. Social evolution. Oxytocin-gaze positive loop and the coevolution of human-dog bonds. Science 348, 333–336 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Burbach P, Young LJ & Russell J in Knobilan Neill’s Physiology of Reproduction (ed. Neill JD) 3055–3127 (Elsevier, 2006). [Google Scholar]
- 24.Johnson ZV & Young LJ Oxytocin and vasopressin neural networks: implications for social behavioral diversity and translational neuroscience. Neurosci. Biobehav. Rev 76, 87–98 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knobloch HS et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 73, 553–566 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Ross HE et al. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience 162, 892–903 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dobolyi A, Cservenak M & Young LJ Thalamic integration of social stimuli regulating parental behavior and the oxytocin system. Front. Neuroendocrinol https://doi.org/S0091-3022(18)30050-5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Waal FBM & Preston SD Mammalian empathy: behavioural manifestations and neural basis. Nat. Rev. Neurosci 18, 498–509 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Cho MM, DeVries AC, Williams JR & Carter CS The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster). Behav. Neurosci 113, 1071–1079 (1999). [DOI] [PubMed] [Google Scholar]
- 30.Insel TR & Hulihan TJ A gender-specific mechanism for pair bonding: oxytocin and partner preference formation in monogamous voles. Behav. Neurosci 109, 782–789 (1995). [DOI] [PubMed] [Google Scholar]
- 31.Johnson ZV et al. Central oxytocin receptors mediate mating-induced partner preferences and enhance correlated activation across forebrain nuclei in male prairie voles. Horm. Behav 79, 8–17 (2016).This paper demonstrates that OT signalling throughout the brain coordinates activity in many nodes of the pair-bonding network, supporting the view that OT facilitates the flow of information across the brain, acting as the ‘grease’ of the social brain.
- 32.Insel TR & Shapiro LE Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc. Natl Acad. Sci. USA 89, 5981–5985 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young LJ, Lim MM, Gingrich B & Insel TR Cellular mechanisms of social attachment. Horm. Behav 40, 133–138 (2001). [DOI] [PubMed] [Google Scholar]
- 34.Keebaugh AC, Barrett CE, Laprairie JL, Jenkins JJ & Young LJ RNAi knockdown of oxytocin receptor in the nucleus accumbens inhibits social attachment and parental care in monogamous female prairie voles. Soc. Neurosci 10, 561–570 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keebaugh AC & Young LJ Increasing oxytocin receptor expression in the nucleus accumbens of pre-pubertal female prairie voles enhances alloparental responsiveness and partner preference formation as adults. Horm. Behav 60, 498–504 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King LB, Walum H, Inoue K, Eyrich NW & Young LJ Variation in the oxytocin receptor gene predicts brain region-specific expression and social attachment. Biol. Psychiatry 80, 160–169 (2016).This paper demonstrates that the remarkable individual variation in OXTR expression in the striatum is largely determined by a set of SNPs in non-coding regions of the prairie vole Oxtr gene.
- 37.Olazabal DE & Young LJ Oxytocin receptors in the nucleus accumbens facilitate “spontaneous” maternal behavior in adult female prairie voles. Neuroscience 141, 559–568 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Ophir AG, Gessel A, Zheng DJ & Phelps SM Oxytocin receptor density is associated with male mating tactics and social monogamy. Horm. Behav 61, 445–453 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barrett CE, Arambula SE & Young LJ The oxytocin system promotes resilience to the effects of neonatal isolation on adult social attachment in female prairie voles. Transl Psychiatry 5, e606 (2015).This paper shows that daily neonatal social isolations disrupt adult pair bond formation and that those animals with many OXTRs in the NAc are resilient to the effects of this model of neglect.
- 40.Albers HE The regulation of social recognition, social communication and aggression: vasopressin in the social behavior neural network. Horm. Behav 61, 283–292 (2012). [DOI] [PubMed] [Google Scholar]
- 41.Nair HP & Young LJ Vasopressin and pair-bond formation: genes to brain to behavior. Physiology 21, 146–152 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Donaldson ZR, Spiegel L & Young LJ Central vasopressin V1a receptor activation is independently necessary for both partner preference formation and expression in socially monogamous male prairie voles. Behav. Neurosci 124, 159–163 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lim MM & Young LJ Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience 125, 35–45 (2004). [DOI] [PubMed] [Google Scholar]
- 44.Barrett CE et al. Variation in vasopressin receptor (Avpr1a) expression creates diversity in behaviors related to monogamy in prairie voles. Horm. Behav 63, 518–526 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lim MM et al. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature 429, 754–757 (2004).This paper demonstrates that species differences in vasopressin receptor expression in the VP mediate species differences in pair bonding by showing that overexpressing the prairie vole vasopressin receptor in the VP of meadow voles causes them to form partner preferences.
- 46.Liu Y, Curtis JT & Wang ZX Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster). Behav. Neurosci 115, 910–919 (2001). [DOI] [PubMed] [Google Scholar]
- 47.Everts HG & Koolhaas JM Lateral septal vasopressin in rats: role in social and object recognition? Brain Res 760, 1–7 (1997). [DOI] [PubMed] [Google Scholar]
- 48.Bielsky IF, Hu SB, Ren X, Terwilliger EF & Young LJ The V1 a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron 47, 503–513 (2005). [DOI] [PubMed] [Google Scholar]
- 49.Tobin VA et al. An intrinsic vasopressin system in the olfactory bulb is involved in social recognition. Nature 464, 413–417(2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gobrogge KL, Liu Y, Young LJ & Wang Z Anterior hypothalamic vasopressin regulates pair-bonding and drug-induced aggression in a monogamous rodent. Proc. Natl Acad Sci. USA 106, 19144–19149 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winslow JT, Hastings N, Carter CS, Harbaugh CR & Insel TR A role for central vasopressin in pair bonding in monogamous prairie voles. Nature 365, 545–548(1993). [DOI] [PubMed] [Google Scholar]
- 52.Young LJ, Winslow JI, Nilsen R & Insel TR Species differences in V, a receptor gene expression in monogamous and nonmonogamous voles: behavioral consequences. Behav. Neurosci Ill, 599–605 (1997). [DOI] [PubMed] [Google Scholar]
- 53.Young L & Alexander B The Chemistry Between Us: Love, Sex, and the Science of Attraction (Current, 2012).
- 54.Young LJ, Nilsen R, Waymire KG, MacGregor GR & Insel TR Increased affiliative response to vasopressin in mice expressing the V1 a receptor from a monogamous vole. Nature 400, 766–768 (1999). [DOI] [PubMed] [Google Scholar]
- 55.Hammock EA & Young LJ Microsatellite instability generates diversity in brain and sociobehavioral traits. Science 308, 1630–1634 (2005). [DOI] [PubMed] [Google Scholar]
- 56.Okhovat M, Berrio A, Wallace G, Ophir AG & Phelps SM Sexual fidelity trade-offë promote regulatory variation in the prairie vole brain. Science 350, 1371–1374 (2015).This study demonstrates that sequence variation in the vasopressin receptor gene influences expression in a brain region involved in spatial memory and influences sexual fidelity in a naturalistic setting.
- 57.Ophir AG Navigating monogamy: nonapeptide sensitivity in a memory neural circuit may shape social behavior and mating decisions. Front. Neurosci 11, 397(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aragona BJ et al. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat. Neurosci 9, 133–139 (2006).This paper demonstrates that the emergence of selective aggression following pair bonding is mediated by a change in the ratio of D1 Rs to D2Rs in the NAc.
- 59.Resendez SL, Kuhnmuench M, Krzywosinski T & Aragona BJ kappa-Opioid receptors within the nucleus accumbens shell mediate pair bond maintenance. J. Neurosci 32, 6771–6784 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Resendez SL et al. Dopamine and opioid systems interact within the nucleus accumbens to maintain monogamous pair bonds. eLife 5, e 15325 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burkett JP, Spiegel LL, Inoue K, Murphy AZ & Young LJ Activation of mu-opioid receptors in the dorsal striatum is necessary for adult social attachment in monogamous prairie voles. Neuropsychopharmacology 36,2200–2210 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Resendez SL et al. mu-Opioid receptors within subregions of the striatum mediate pair bond formation through parallel yet distinct reward mechanisms. J. Neurosci 33,9140–9149 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burkett JP & Young LJ The behavioral, anatomical and pharmacological parallels between social attachment, love and addiction. Psychopharmacol. (Berl.) 224, 1–26(2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Di Chiara G et al. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology 47 (Suppl. 1), 227–241 (2004). [DOI] [PubMed] [Google Scholar]
- 65.Volkow ND, Fowler JS, Wang GJ, Baler R & Telang F Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology 56 (Suppl. 1), 3–8 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Y et al. Nucleus accumbens dopamine mediates amphetamine-induced impairment of social bonding in a monogamous rodent species. Proc. Natl Acad. Sci. USA 107, 1217–1222 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Y, Young KA, Curtis JI, Aragona BJ & Wang Z Social bonding decreases the rewarding properties of amphetamine through a dopamine D1 receptor-mediated mechanism. J. Neurosci 31, 7960–7966 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burkett J & Young L Love and addiction: an uneasy marriage? A response to “The devil is in the differences”. Psychopharmacology 224, 31–32 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hostetler CM & Ryabinin AE Love and addiction: the devil is in the differences: a commentary on “the behavioral, anatomical and pharmacological parallels between social attachment, love and addiction”. Psychopharmacology 224, 27–29; discussion 31–32 (2012). [DOI] [PubMed] [Google Scholar]
- 70.Dolen G, Darvishzadeh A, Huang KW & Malenka RC Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501, 179–184 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Larke RH, Maninger N, Ragen BJ, Mendoza SP & Bales KL Serotonin 1A agonism decreases affiliative behavior in pair-bonded titi monkeys. Horm. Behav 86, 71–77 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lim MM et al. CRF receptors in the nucleus accumbens modulate partner preference in prairie voles. Horm. Behav 51, 508–515 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.DeVries AC, DeVries MB, Taymans S & Carter CS Modulation of pair bonding in female prairie voles (Microtus ochrogaster) by corticosterone. Proc. Natl Acad. Sci. USA 92, 7744–7748 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.DeVries AC, DeVries MB, Taymans SE & Carter CS Stress has sexually dimorphic effects on pair bonding in prairie voles. Proc. Natl Acad. Sci. USA 93, 11980–11984 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johnson ZV, Walum H, Xiao Y, Riefkohl PC & Young LJ Oxytocin receptors modulate a social salience neural network in male prairie voles. Horm. Behav 87, 16–24 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brennan PA & Kendrick KM Mammalian social odours: attraction and individual recognition. Phil. Trans. R. Soc. B 361, 2061–2078 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sanchez-Andrade G & Kendrick KM The main olfactory system and social learning in mammals. Behav. Brain Res 200, 323–335 (2009). [DOI] [PubMed] [Google Scholar]
- 78.Oettl LL et al. Oxytocin enhances social recognition by modulating cortical control of early olfactory processing. Neuron 90, 609–621 (2016).This study shows that in mice, OT acting in the cortical AON increases the excitatory drive of inhibitory neurons in the olfactory bulb to effectively increase the signal-to-noise ratio of olfactory output, thereby facilitating individual discrimination.
- 79.Balu R, Pressler RT & Strowbridge BW Multiple modes of synaptic excitation of olfactory bulb granule cells. J. Neurosci 27, 5621–5632 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Insel T, L. J. Y, Witt D & Crews D Gonadal steroids have paradoxical effects on brain oxytocin receptors. J. Neuroendo 5, 619–628 (1993). [DOI] [PubMed] [Google Scholar]
- 81.Tribollet E, Barberis C, Jard S, Dubois-Dauphin M & Dreifuss JJ Localization and pharmacological characterization of high affinity binding sites for vasopressin and oxytocin in the rat brain by light microscopic autoradiography. Brain Res 442, 105–118 (1988). [DOI] [PubMed] [Google Scholar]
- 82.Witt DM, Carter CS & Lnsel TR Oxytocin receptor binding in female prairie voles: endogenous and exogenous oestradiol stimulation. J. Neuroendocrinol 3, 155–161 (1991). [DOI] [PubMed] [Google Scholar]
- 83.Marlin BJ et al. Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature 520, 499–504 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Janak PH & Tye KM From circuits to behaviour in the amygdala. Nature 517, 284–292 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Beyeler A et al. Organization of valence-encoding and projection-defined neurons in the basolateral amygdala. Cell Rep 22, 905–918 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bielsky IF & Young LJ Oxytocin, vasopressin, and social recognition in mammals. Peptides 25, 1565–1574 (2004). [DOI] [PubMed] [Google Scholar]
- 87.Pro-Sistiaga P et al. Convergence of olfactory and vomeronasal projections in the rat basal telencephalon. J. Comp. Neurol 504, 346–362 (2007). [DOI] [PubMed] [Google Scholar]
- 88.Ferguson JN, Aldag JM, Insel TR & Young LJ Oxytocin in the medial amygdala is essential for social recognition in the mouse. J. Neurosci 21, 8278–8285 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cushing BS, Mogekwu N, Le WW, Hoffman GE & Carter CS Cohabitation induced Fos immunoreactivity in the monogamous prairie vole. Brain Res 965, 203–211 (2003). [DOI] [PubMed] [Google Scholar]
- 90.Northcutt KV & Lonstein JS Social contact elicits immediate-early gene expression in dopaminergic cells of the male prairie vole extended olfactory amygdala. Neuroscience 163, 9–22 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kirkpatrick B, Carter CS, Newman SW & Insel TR Axon-sparing lesions of the medial nucleus of the amygdala decrease affiliative behaviors in the prairie vole (Microtus ochrogaster): behavioral and anatomical specificity. Behav. Neurosci 108, 501–513 (1994). [DOI] [PubMed] [Google Scholar]
- 92.Gur R, Tendler A & Wagner S Long-term social recognition memory is mediated by oxytocin-dependent synaptic plasticity in the medial amygdala. Biol. Psychiatry 76, 377–386 (2014). [DOI] [PubMed] [Google Scholar]
- 93.Fang LY, Quan RD & Kaba H Oxytocin facilitates the induction of long-term potentiation in the accessory olfactory bulb. Neurosci. Lett 438, 133–137 (2008). [DOI] [PubMed] [Google Scholar]
- 94.Lin YT, Huang CC & Hsu KS Oxytocin promotes long-term potentiation by enhancing epidermal growth factor receptor-mediated local translation of protein kinase Mzeta. J. Neurosci 32, 15476–15488 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tomizawa K et al. Oxytocin improves long-lasting spatial memory during motherhood through Map kinase cascade. Nat. Neurosci 6, 384–390 (2003). [DOI] [PubMed] [Google Scholar]
- 96.Vertes RP Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience 142, 1–20 (2006). [DOI] [PubMed] [Google Scholar]
- 97.Freeman SM & Young LJ Comparative perspectives on oxytocin and vasopressin receptor research in rodents and primates: translational implications. J. Neuroendocrinol https://doi.org/10.1111/jne.12382 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Raam T, McAvoy KM, Besnard A, Veenema AH & Sahay A Hippocampal oxytocin receptors are necessary for discrimination of social stimuli. Nat. Commun 8, 2001 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lin YT et al. Conditional deletion of hippocampal CA2/CA3a oxytocin receptors impairs the persistence of long-term social recognition memory in mice. J. Neurosci 38, 1218–1231 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Owen SF et al. Oxytocin enhances hippocampal spike transmission by modulating fast-spiking interneurons. Nature 500, 458–462 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Okuyama T, Kitamura T, Roy DS, Itohara S & Tonegawa S Ventral CA1 neurons store social memory. Science 353, 1536–1541 (2016).This study demonstrates the existence of a social engram, or a collection of neurons whose firing represents the identity of another individual, in the ventral hippocampus that projects to the NAc.
- 102.Tonegawa S, Liu X, Ramirez S & Redondo R Memory engram cells have come of age. Neuron 87, 918–931 (2015). [DOI] [PubMed] [Google Scholar]
- 103.Hung LW et al. Gating of social reward by oxytocin in the ventral tegmental area. Science 357, 1406–1411 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Love TM Oxytocin, motivation and the role of dopamine. Pharmacol. Biochem. Behav 119, 49–60 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Williams J, Catania K & Carter C Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Horm. Beh 26, 339–349 (1992). [DOI] [PubMed] [Google Scholar]
- 106.Liu Y & Wang ZX Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience 121, 537–544 (2003). [DOI] [PubMed] [Google Scholar]
- 107.Romero-Fernandez W, Borroto-Escuela DO, Agnati LF & Fuxe K Evidence for the existence of dopamine D2-oxytocin receptor heteromers in the ventral and dorsal striatum with facilitatory receptor-receptor interactions. Mol. Psychiatry 18, 849–850 (2013). [DOI] [PubMed] [Google Scholar]
- 108.Humphries MD & Prescott TJ The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Prog. Neurobiol 90, 385–417 (2010). [DOI] [PubMed] [Google Scholar]
- 109.Walum H, Waldman ID & Young LJ Statistical and methodological considerations for the interpretation of intranasal oxytocin studies. Biol. Psychiatry 79, 251–257 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Scheele D et al. Oxytocin enhances brain reward system responses in men viewing the face of their female partner. Proc. Natl Acad. Sci. USA 110, 20308–20313 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bethlehem RAI et al. Intranasal oxytocin enhances intrinsic corticostriatal functional connectivity in women. TranslPsychiatry 7, e1099 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rogers CN et al. Oxytocin- and arginine vasopressin-containing fibers in the cortex of humans, chimpanzees, and rhesus macaques. Am. J. Primatol https://doi.org/10.1002/ajp.22875 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Amadei EA et al. Dynamic corticostriatal activity biases social bonding in monogamous female prairie voles. Nature 546, 297–301 (2017).This study demonstrates that modulation of NAc gamma oscillations by mPFC theta oscillations predicts the onset of affiliative huddling after mating and causally facilitates partner preference formation.
- 114.Smith KS, Tindell AJ, Aldridge JW & Berridge KC Ventral pallidum roles in rewand and motivation. Behav Brain Res 196, 155–167 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bosch OJ, Nair HP, Ahern TH, Neumann ID & Young LJ The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacology 34, 1406–1415 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pohl TI, Young LJ & Bosch OJ Lost connections: oxytocin and the neural, physiological, and behavioral consequences of disrupted relationships. Int. J. Psychophysiol https://doi.org/S0167-8760(17)30446-4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bosch OJ, Pohl TT, Neumann ID & Young LJ Abandoned prairie vole mothers show normal maternal care but altered emotionality: Potential influence of the brain corticotropin-releasing factor system. Behav. Brain Res 341, 114–121 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Osako Y et al. Partner loss in monogamous rodents: modulation of pain and emotional behavior in male prairie voles. Psychosom. Med 80, 62–68 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dabrowska J et al. Neuroanatomical evidence for reciprocal regulation of the corticotrophin-releasing factor and oxytocin systems in the hypothalamus and the bed nucleus of the stria terminalis of the rat: implications for balancing stress and affect. Psychoneuroendocrinology 36, 1312–1326 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bosch OJ et al. Oxytocin in the nucleus accumbens shell reverses CRFR2-evoked passive stress-coping after partner loss in monogamous male prairie voles. Psychoneuroendocrinology 64, 66–78 (2016).This study suggests that the emergence of depressive-like behaviour following social loss is mediated by CRFR2 activation in the NAc, which then reduces OT signalling, thereby maintaining the pair bond by inducing an aversive state following separation from the partner.
- 121.Resendez SL & Aragona BJ Aversive motivation and the maintenance of monogamous pair bonding. Rev. Neurosci 24, 51–60 (2013). [DOI] [PubMed] [Google Scholar]
- 122.Young LJ & Barrett CE Neuroscience. Can oxytocin treat autism? Science 347, 825–826 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Modi ME et al. Melanocortin receptor agonists facilitate oxytocin-dependent partner preference formation in the prairie vole. Neuropsychopharmacology 40, 1856–1865 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Modi ME & Young LJ D-cycloserine facilitates socially reinforced learning in an animal model relevant to autism spectrum disorders. Biol. Psychiatry 70, 298–304 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Young LJ, Pitkow LJ & Ferguson JN Neuropeptides and social behavior: animal models relevant to autism. Mol. Psychiatry 7, S38–S39 (2002). [DOI] [PubMed] [Google Scholar]
- 126.Belsky J, Steinberg L & Draper P Childhood experience, interpersonal development, and reproductive strategy: and evolutionary theory of socialization. Child Dev 62, 647–670 (1991). [DOI] [PubMed] [Google Scholar]
- 127.Belsky J et al. Family rearing antecedents of pubertal timing. Child Dev 78, 1302–1321 (2007). [DOI] [PubMed] [Google Scholar]
- 128.Bogaert AF Menarche and father absence in a national probability sample. J. Biosoc. Sci 40, 623–636 (2008). [DOI] [PubMed] [Google Scholar]
- 129.Ellis BJ & Essex MJ Family environments, adrenarche, and sexual maturation: a longitudinal test of a life history model. Child Dev 78, 1799–1817 (2007). [DOI] [PubMed] [Google Scholar]
- 130.Pesonen AK et al. Reproductive traits following a parent-child separation trauma during childhood: a natural experiment during World War II. Am. J. Hum. Biol 20, 345–351 (2008). [DOI] [PubMed] [Google Scholar]
- 131.Bradley B et al. Association between childhood maltreatment and adult emotional dysregulation in a low-income, urban, African American sample: moderation by oxytocin receptor gene. Dev. Psychopathol 23, 439–452 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.McQuaid RJ, McInnis OA, Stead JD, Matheson K & Anisman H A paradoxical association of an oxytocin receptor gene polymorphism: early-life adversity and vulnerability to depression. Front. Neurosci 7, 128 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Myers AJ et al. Variation in the oxytocin receptor gene is associated with increased risk for anxiety, stress and depression in individuals with a history of exposure to early life stress. J. Psychiatr. Res 59, 93–100 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]