Summary
An increasing body of literature supports a role for neutrophils as players in the orchestration of adaptive immunity. During acute and chronic inflammatory conditions, neutrophils rapidly migrate not only to sites of inflammation, but also to draining lymph nodes and spleen, where they engage bidirectional interactions with B‐ and T‐lymphocyte subsets. Accordingly, a relevant role of neutrophils in modulating B‐cell responses under homeostatic conditions has recently emerged. Moreover, specialized immunoregulatory properties towards B or T cells acquired by distinct neutrophil populations, originating under pathological conditions, have been consistently described. In this article, we summarize the most recent data from human studies and murine models on the ability of neutrophils to modulate adaptive immune responses under physiological and pathological conditions and the mechanisms behind these processes.
Keywords: B cells, neutrophils, T cells
Abbreviations
- APCs
antigen‐presenting cells
- APRIL
A proliferation‐inducing ligand
- ARG‐1
arginase 1
- BAFF
B‐cell‐activating factor of the tumour necrosis factor family
- BM
bone marrow
- DCs
dendritic cells
- Fo
follicular
- G‐CSF
granulocyte colony‐stimulating factor
- IFN‐α
interferon‐α
- IgM
immunoglobulin M
- IL‐21
interleukin‐21
- iΝΚT cells
invariant natural killer T cells
- LDGs
low‐density granulocytes
- LDNs
low‐density neutrophils
- mAb
monoclonal antibody
- MHC‐II
major histocompatibility complex class II
- MZ
marginal zone
- NBH
B cell‐helper neutrophils
- NETs
neutrophil extracellular traps
- PD‐L1
programmed death‐ligand 1
- PTX3
pentraxin 3
- RA
rheumatoid arthritis
- ROS
reactive oxygen species
- SLE
systemic lupus erythematosus
- TANs
tumour‐associated neutrophils
- TGF‐β1
transforming growth factor β 1
- Th
T helper type
- TI
T‐cell‐independent
- Treg
regulatory T
Introduction
Neutrophils are classically considered as the first line of defence against infection.1 Hence, the main functions of these cells have long been thought to be limited to the consequent initiation and amplification of the inflammatory response.1 However, this dogmatic view of neutrophils has been challenged by accumulating evidence supporting a function of these cells not only in the initiation, but also in the modulation of both innate and adaptive immune responses.2, 3 In fact, besides their classical bactericidal activities, neutrophils display an array of complex biological functions, including cytokine production,4 antigen presentation,5 release of exosomes6 and neutrophil extracellular traps (NETs; extracellular fibres, primarily composed of DNA filaments complexed with granular antimicrobial peptides),7 expression/release of immunomodulatory molecules [e.g. reactive oxygen species (ROS), programmed death‐ligand 1 (PD‐L1), Arginase 1 (ARG‐1)],8, 9, 10 through which they can activate or down‐regulate innate and adaptive immune cells. Current studies have also revealed that neutrophils can no longer be considered as belonging to a homogeneous population of cells.9, 10, 11, 12, 13 Hence, specialized immunoregulatory neutrophil populations, which have either undergone a separate maturation/differentiation process or have been specifically instructed by the microenvironment to acquire distinct functions, have been recently described in both humans and mice.9, 10, 11, 12, 13 Furthermore, the discovery that neutrophils can populate the spleen and lymph nodes, under both homeostatic and inflammatory conditions, has strongly reinforced the concept that these cells, in a similar way to dendritic cells (DCs) and macrophages, can deliver signals that drive not only innate but also adaptive immune responses.14, 15 In this context, it is important to highlight that neutrophils can modulate adaptive B‐cell and T‐cell responses, both directly and indirectly.16, 17 The latter type of modulation occurs, for example, through the ability of neutrophils to facilitate or inhibit the functions of monocytes and DCs, including their differentiation to professional antigen‐presenting cells (APCs), as extensively reviewed elsewhere.18, 19
Overall, the concept that neutrophils can initiate, amplify and/or suppress adaptive immune effector responses by establishing direct bidirectional crosstalk with adaptive immune cells has gained a lot of attention in the past few years. The recent studies that are reviewed in this work, which describe neutrophil‐centred crosstalk with adaptive immune cells occurring in humans, as well as in a variety of experimental animal models, have not only corroborated the existence of these interactions, but also better clarified their potential physiopathological significance.
Neutrophils and B cells
An important advance demonstrating that human neutrophils may directly modulate B‐cell responses comes from observations performed in vitro on the capacity of human neutrophils to produce cytokines that are crucial for B‐cell survival, maturation and differentiation, such as B‐cell‐activating factor of the tumour necrosis factor family (BAFF)20, 21 and A Proliferation‐Inducing Ligand (APRIL).22 These observations were then substantiated by the discovery of populations of neutrophils that, under steady‐state, colonize the perifollicular area of the human (as well as mouse and rhesus macaque) spleen and display B‐cell‐helper properties.14 These neutrophil populations were defined as B‐cell‐helper neutrophils (NBH), and shown to specifically enhance, likely due to their selective localization in the marginal zone (MZ), T‐cell‐independent antibody responses by MZ B cells.14 Compared with circulating neutrophils, NBH cells were shown to secrete more B‐cell‐stimulating/attracting factors, such as BAFF, APRIL, CD40L, interleukin‐21 (IL‐21) and CXCL12, as well as to produce more NETs.14 By contrast, T‐cell‐dependent responses of follicular B cells were shown not to be affected by human splenic NBH.14 The fact that steady‐state titres of serum immunoglobulins to T‐cell‐independent antigens were found to be reduced in patients with severe congenital neutropenias, strongly supported the potential role of neutrophils in sustaining MZ B‐cell responses under homeostatic conditions.14 Interestingly, the B‐cell‐helper properties of human NBH were then shown to be driven by splenic innate lymphoid cell‐derived granulocyte–macrophage colony‐stimulating factor,23 unveiling the existence of an innate cell network within lymphoid organs, directly involved in sustaining humoral responses under homeostatic conditions. Although these data on human splenic neutrophils have generated some controversies,24 evidence of the capacity of neutrophils to specifically interact with MZ B cells not only under homeostatic, but also during responses to immunization or infections, has been reported in mice.25, 26, 27 For example, it has been shown that Pentraxin 3 represents another important mediator through which splenic murine neutrophils promote both homeostatic and post‐immune antibody responses to T‐cell‐independent antigen by MZ B cells.25 Such an observation has further strengthened the view of neutrophils as important mediators of innate‐like antibody production. Advance in the field has been recently provided by cutting‐edge imaging technology to track the dynamic behaviour of various splenic neutrophil populations during the acute phases of Streptococcus pneumoniae infection in mice.27 This work has revealed the existence of a population of splenic neutrophils that is resident within the red pulp and is involved in pathogen clearance. An additional population of blood neutrophils was instead shown to infiltrate the MZ area of the spleen between 24 and 48 hr after S. pneumoniae infection, and to be instructed, by the microenvironment, to differentiate into NBH sustaining T‐cell‐independent antibody production by MZ B cells.27, 28 Future studies are needed to clarify whether the resident splenic NBH neutrophils described by Puga et al.14 display similar or different features compared with the newly recruited splenic NBH neutrophils described by Deniset et al.27 to populate the MZ area in response to infection.28 The acquisition of B‐cell‐helper activity toward MZ B cells by neutrophils has been shown to represent a fundamental mechanism for the induction of long‐term protective immunity in a murine model of antiviral monoclonal immunotherapies.29 Accordingly, in mice infected by a murine leukaemia virus (FrCasE), and treated with an antiviral neutralizing IgG2a monoclonal antibody (mAb 667, recognizing the retroviral envelope glycoprotein), neutrophils were proved to be crucial to sustain the enhanced serum concentration of antiviral IgGs, but not IgM, as well as the differentiation of splenic MZ and bone marrow (BM) plasma cells observed in response to the antiviral mAb treatment.29 These functions seemed to be mediated by the capacity of the antiviral mAb treatment to induce strong B‐cell‐helper properties, including an enhanced expression of BAFF and lymphotoxin‐α in splenic neutrophils.29 These observations uncover a novel role for neutrophils as crucial actors to achieve optimized mAb‐induced protective immunity (vaccine‐like effects).
It remains controversial whether neutrophils directly interact also with follicular B cells, in addition to MZ B cells. For a long time, neutrophils were thought to be excluded from the B‐cell follicles, for example after a bacterial challenge.15, 30 However, recent studies have suggested that neutrophils can actually be recruited to B‐cell follicles when proper inflammatory signals are present. For example, human splenic neutrophils were shown to lose their selective perifollicular topography, and to extensively infiltrate the follicular mantle and germinal centre areas of splenic follicles, under systemic inflammatory or infectious disorders.14 Similarly, in the past few years, several studies performed in immunized or infected mice, or even in healthy elderly mice, have demonstrated that neutrophils can actually accumulate in the B‐cell zones as a consequence of the disruption of the splenic microanatomy and lymph node structure.15, 31, 32 For instance, a significant neutrophil influx was observed in the B‐cell area of draining lymph nodes after 7 days post‐immunization in a model of adjuvant‐induced emergency granulopoiesis in neutropenic mice.31 The recruited neutrophils have been shown to secrete BAFF, in a granulocyte colony‐stimulating factor (G‐CSF)‐dependent manner, and to support accelerated plasma cell generation.31 However, whether neutrophils establish direct interaction with follicular B cells, or are instead interacting with MZ B cells that have also migrated within the follicular B‐cell area as a consequence of the lymphoid organ microanatomy disruption, has not been clarified in this study.31 Whatever the case is, an important role for neutrophil‐derived signals in sustaining B‐cell functions has been extensively demonstrated under several pathological settings, including autoimmunity and cancer.33, 34
Regarding autoimmune diseases, abnormalities of various neutrophil functions that may contribute to the generation of autoreactive B‐cell clones, including the already mentioned altered production of BAFF,21, 26, 35, 36, 37, 38 APRIL37 and IL‐6,39 have been described to occur in both humans and mice. In this context, it is worth mentioning the paper by Palanichamy et al.,37 in which a higher expression of interferon‐α (IFN‐α), APRIL and BAFF by the mature fraction of neutrophils present in BM has been proposed to contribute to the dysregulated B‐cell ontogeny and selection observed in both patients with systemic lupus erythematosus (SLE) and NZM lupus‐prone mice.37 Recently, BAFF production by splenic neutrophils has also been proposed to sustain the differentiation of long‐lived splenic plasma cells in lupus‐prone mice receiving anti‐CD20 antibody treatment,40 suggesting a potential contribution for BAFF‐producing neutrophils to the lack of response to B‐cell depletion therapy observed in certain autoimmune patients.41 The mechanism catching most of the attention concerning the role of neutrophils in autoimmune diseases is the production of NETs.7, 42, 43 NETs contain proteins that can function as major autoantigenic targets in rheumatological diseases, including double‐stranded DNA and histones in SLE, myeloperoxidase and proteinase 3 in anti‐neutrophil cytoplasmic antibody‐associated vasculitis, and citrullinated protein, including vimentin and enolase, as well as histones, in rheumatoid arthritis.7, 42, 43 Interestingly, specialized pro‐inflammatory neutrophil populations, displaying an enhanced capacity to produce NETs and inflammatory cytokines, have been identified within the mononuclear cell fraction from the peripheral blood of patients with autoimmune diseases, including SLE,44, 45 psoriasis,46 rheumatoid arthritis47 and anti‐neutrophil cytoplasmic antibody‐associated vasculitis.48 These neutrophil populations have been defined as low‐density granulocytes, either due to their altered buoyancy properties,11 or to distinguish them from immunosuppressive low‐density neutrophil (LDN) populations, also known as granulocytic‐myeloid‐derived suppressor cells (PMN‐MDSCs), identified instead within the mononuclear cell fraction of patients with cancer, infection and other inflammatory diseases9, 10 (see ‘Neutrophils and T cells’ section below). Besides functioning as a source of autoantigens and pro‐inflammatory molecules/cytokines, NETs have also been reported to mediate the pathological vicious crosstalk between neutrophils and several DC subsets (e.g. plasmacytoid DCs), in turn driving the production of IFN‐α in SLE,49, 50 psoriasis,51 type 1 diabetes,52 autoimmune vasculitis53 and, more recently, Wiskott‐Aldrich syndrome.38 Interestingly, NETs have also been shown to directly activate human memory B cells, by accessing their endosomal compartments and triggering Toll‐like receptor‐9 activation.54 This novel mechanism seems to be particularly relevant for SLE patients, in whom NETs have been shown to drive the production of antigen‐specific memory B cells, in turn leading to the production of pathogenic autoantibodies.54 Similarly, IL‐17A‐containing NETs have been proposed to drive the differentiation of antigen‐specific memory B cells into long‐lived plasma cells in a mouse model of chronic humoral response induced by venom of Thalassophryne nattereri.55
As far as cancer is concerned, there is currently evidence supporting a role of neutrophils in the differentiation of neoplastic B cells. For example, human neutrophils have been proposed to play a role in the pathogenesis of B‐cell lymphomas, in particular through the production of APRIL.56 More recently, the same research group have identified tumour cell‐derived CXCL8 as the crucial mediator to recruit APRIL‐expressing human neutrophils into diffuse large B‐cell lymphoma lesions.57 The acquisition of an accentuated B‐cell helper phenotype by splenic neutrophils, including an elevated expression of BAFF and APRIL, has also been reported as a mechanism through which neutrophils can support B‐cell chronic lymphocytic leukaemia development in mice.58 Interestingly, a role of NETs, and their interaction with CD5+ B cells, has also been proposed as a pathological mechanism promoting the transition from autoimmunity to lymphoma in a mouse model of B‐cell chronic lymphocytic leukaemia.59 Finally, cell–cell interaction between CD11b and intercellular adhesion molecule 1 expressed, respectively, by neutrophils and B cells, has been reported to be involved in the protection of neoplastic B cells against cytotoxic anticancer therapies.60
Despite the extensive literature supporting a role of neutrophils in driving exaggerated B‐cell responses that can lead to autoimmune and neoplastic disease development, it is important to highlight that evidence that neutrophils may inhibit B‐cell responses is also emerging. For example, neutrophils have been proposed to inhibit IgA production by B cells in a mouse model of sublingual immunization,61 or to inhibit germinal centre B‐cell formation during the early stages of murine lupus development.62 Similarly, during the early phases of the humoral response after local Staphylococcus aureus immunization and infection, murine neutrophils have been shown to establish F‐actin‐mediated intercellular contacts with B cells within the lymph nodes, and in turn suppress antibody production via transforming growth factor ‐β 1 production.30 Evidence that human neutrophils can inhibit B‐cell responses also exists. Indeed, populations of LDNs/PMN‐MDSCs isolated from healthy individuals were shown to induce B‐cell suppression through cell contact‐dependent mechanisms, release of mediators such as ARG‐1, nitric oxide and ROS, and/or induction of cell death.63 Although intriguing, this observation needs to be further clarified as LDNs/PMN‐MDSCs are generally not present in healthy individuals, so they may represent subpopulations of activated neutrophils generated by some technical artefacts.9, 10 Curiously, B cells have been proposed to induce neutrophil apoptosis and to regulate aged neutrophil clearance within the lung in mice,64 suggesting the existence of reciprocal interactions between neutrophils and B cells that can be crucial in modulating their survival and functions under both homeostatic and inflammatory conditions.
Neutrophils and T cells
As extensively discussed in previous reviews,2, 16, 17, 65, 66 neutrophils and T cells are known to reciprocally influence their effector functions through contact‐dependent mechanisms, chemokine/cytokine production, or NET release. Neutrophils can positively/negatively modulate the functions of a variety of T‐cell subsets, including subpopulations of CD4+ αβ T cells [e.g. T helper type 1 (Th1) cells, Th17 cells, Th2 cells and T regulatory (Treg) cells], CD8+ αβ T cells and γδ T cells, either in vitro or in vivo, in both humans and mice.2, 16, 17, 65, 66 However, an open issue in the field is whether the capacity to influence T‐cell functions is exerted by pre‐existing neutrophils, that under specific circumstances acquire immunomodulatory properties, or by discrete neutrophil populations that emerge under physiopathological settings and that become specialized in either promoting or inhibiting T‐cell responses.5, 9, 10
In this context, it is well known that human and mouse neutrophils can acquire APC properties, in vitro and in vivo.5, 67 Importantly, neutrophils have been shown to function not only as APC for CD4+ T cells, but also to cross‐present antigens to CD8+ T cells.17, 68, 69 More recent findings have further extended our knowledge on the APC‐like properties displayed by neutrophils. For example, a potential role of neutrophils in priming vaccine responses has been shown.70, 71, 72 In this context, it has been shown, for the first time, that human neutrophils are able to present antigens to autologous antigen‐specific memory CD4+ T cells in a major histocompatibility complex class II (MHC‐II; HLA‐DR) ‐dependent fashion.71 Importantly, under the same experimental conditions, neutrophils were unable to induce antigen‐specific responses by naive T cells,71 suggesting that neutrophils belong to the so‐called ‘atypical’ APCs.73 These observations were further corroborated by showing that neutrophils sorted from vaccine‐draining lymph nodes of rhesus macaques were able to present vaccine antigen to autologous antigen‐specific memory CD4+ T cells ex vivo.71 Finally, in an additional study performed in rhesus macaques, it has been shown that neutrophils can participate to the adjuvant‐driven innate immune activation that leads to priming of vaccine responses.72 Neutrophils were recently shown to function as APCs also in a mouse model of acute graft‐versus‐host disease induced by conditioning‐induced damage of the intestinal tract.74 In this study, neutrophils were shown to migrate from the ileum to the mesenteric lymph nodes, to therein co‐localize with T cells, and to finally present antigen on MHC‐II, therefore proving for their participation to alloantigen presentation.74 In line with the concept that neutrophils may play an important role in allergic late‐phase reactions,16 neutrophils isolated from the peripheral blood of birch pollen‐allergic donors were shown to induce proliferative and cytokine responses by allergen‐specific effector T cells.75
While the studies reported above describe APC‐like functions acquired by peripheral neutrophils, populations of so‐called ‘neutrophil–DC hybrids’, expressing markers of both neutrophils and DCs, have also been obtained from in vitro differentiation of murine and human neutrophil precursors,76, 77 as well as isolated in vivo.76, 77, 78, 79 These neutrophil–DC hybrids retain intrinsic functional abilities of neutrophils (including the capacity to capture exogenous material, extrude neutrophil extracellular traps and kill bacteria), but also exhibit several DC properties (including dendritic morphology, podosome formation and presentation of various forms of foreign protein antigens to naive CD4+ T cells).76 Given these unique features, a potential role of neutrophil–DC hybrids as potent effectors in anticancer immunity in humans,77 and antifungal defence in mice,79 has been recently proposed. In the former case, neutrophil–DC hybrids have been described as a population of tumour‐associated neutrophils (TANs) that infiltrate the tumour tissue in early‐stage lung cancer.77 These cells were shown to efficiently present antigen and trigger antitumour responses from memory CD8+ and CD4+ T cells.77 These findings, which are in apparent contrast with the general concept that TANs negatively modulate T‐cell responses and promote tumour progression (refs 9, 80 and see below), have been recently supported by another study in which TANs isolated from individuals with colorectal cancer were shown to enhance CD8+ T‐cell responses,81 therefore suggesting that the immunomodulatory functions of TANs might depend on the tumour stage and type.
Extensive demonstrations of the capacity to acquire immunosuppressive properties towards CD4+ and CD8+ T cells by circulating and/or tissue‐derived neutrophil populations in humans and mice also exist.8, 9, 10, 82, 83 In humans, the populations of circulating immunosuppressive neutrophils described to date are heterogeneous.8, 9, 10 In fact, some populations of mature suppressive neutrophils have been identified within either normal density neutrophils (that sediment on top of the red cell fraction after blood centrifugation over density gradients) or the whole leucocytes (obtained after red cell lysis of whole blood).8, 9, 10 However, immunosuppressive LDNs/PMN‐MDSCs represent the population of human immunosuppressive neutrophils that has gained most of the attention in the field.9, 10 These cells have been originally identified within the mononuclear cell fraction of patients with several types of solid tumours or haematological malignancies,9, 10 but more recently they have also been found in a broad variety of acute and chronic inflammatory disease conditions, including infection with human immunodeficiency virus type 1,84, 85 hepatitis B virus,86 or sepsis.87, 88 Interestingly, immunosuppressive LDNs/PMN‐MDSCs have also been described in conditions in which an altered T‐cell tolerance is present, such as in pregnancy and breastfeeding,89, 90, 91, 92 in neonates,93, 94, 95, 96 or in healthy volunteers receiving G‐CSF for stem cell mobilization.97, 98, 99 As immunosuppressive LDNs/PMN‐MDSCs are composed by a mixture of immature and mature neutrophils, an open question is whether the suppressive cells correspond to the immature neutrophils or to the subpopulation of mature ones, which often display features of ‘activated/degranulated’ cells.9, 10 In this context, two recent studies,99, 100 have shown that the most suppressive subset of human LDNs/PMN‐MDSCs belong to the mature neutrophil population, at least in healthy volunteers receiving G‐CSF for stem cell mobilization,99 or in head and neck cancer patients,100 respectively. By contrast, immature neutrophils have been reported to either promote,99 or modestly suppress,100 T‐cell proliferation, indicating that they can display disease‐specific functional plasticity.
Another crucial issue is that the definition of ‘immunosuppressive’ must rely on the demonstrated capacity to suppress T‐cell responses (such as proliferation and/or IFN‐γ production) by neutrophils.8, 9, 10 The inhibition of T‐cell responses by human immunosuppressive neutrophils primarily occurs through an overproduction of ARG‐1, ROS,8, 9, 10 or via PD‐L1‐dependent interactions.85, 101, 102, 103 Polarization of T‐helper cell responses towards an anti‐inflammatory phenotype (expansion of Th2 and Treg cells and inhibition of Th1 cells) has also been proposed as an immunosuppressive mechanism used by human LDNs/PMN‐MDSCs.95, 104, 105 In this context, considering the general lack of knowledge on the ability to polarize T‐helper cell responses by normal neutrophils, it is interesting that LDNs/PMN‐MDSCs present in patients with SLE were shown to promote, and not to inhibit, Th17 differentiation in an ARG‐1‐dependent fashion.106 The latter observation suggests the existence of preferential crosstalk occurring between neutrophil and T‐cell populations depending on the disease type. Among the recently suggested mechanisms of T‐cell suppression by human neutrophils, it is worth mentioning a study in which membrane‐coated microvesicles released by apoptotic neutrophils, but not apoptotic neutrophils themselves, were shown to suppress the proliferation of a subset of CD25− CD127+ T cells by down‐regulating IL‐2 and IL‐2 receptor expression and signalling.107 The existence of such a variety of immunosuppressive mechanisms might be related to the many different types of immunosuppressive neutrophil populations, conditioned by external factors. Cytokines such as IFN‐γ 101 and granulocyte–macrophage colony‐stimulating factor,108 or the direct contact with mesenchymal stem cells109 or tumour cells,103, 110 have all been proposed to induce a suppressive phenotype in neutrophils from healthy donors. In this context, evidence that immunosuppressive neutrophil populations are present in the spleen,14 placenta,91 airways,111 or in tumours,112, 113 already exists, but our understanding of the relationship between circulating and tissue neutrophils is still in its infancy. It is important to remark that, considering the very low number of circulating and tissue‐derived immunosuppressive neutrophil populations that can be isolated for functional assays, the identification of specific markers identifying pure populations of immunosuppressive neutrophils would definitely help advance the field. Among the candidate markers, lectin‐like oxidized low‐density lipoprotein receptor‐1 has been recently proposed to distinguish immunosuppressive PMN‐MDSCs from normal neutrophils in blood and tissues from cancer patients,113, 114 as well as in blood from infants.96 However, these findings require further validation across more laboratories.
Granulocytic‐myeloid derived suppressor cells and TANs exerting immunosuppressive properties towards T cells have been isolated also from the BM, spleen and tumour tissues of mice. The phenotypic and functional features of these suppressive neutrophil populations have been extensively reviewed elsewhere.80, 83 Murine PMN‐MDSCs have been shown to suppress T‐cell function mostly through ROS production80, 83 or, as shown more recently, via PD‐L1 expression,115 or S100A9‐mediated up‐regulation of prostaglandin E2.96 Similar to human suppressive neutrophils, our knowledge on the specific features of murine PMN‐MDSCs is limited by the lack of specific markers that distinguish them from normal neutrophils. As far as murine TANs, there is a general consensus in the field that, similar to the macrophage M1/M2 polarization, TANs can also switch from an antitumorigenic N1 phenotype, evident during the early phases of tumour growth, to a pro‐tumorigenic N2 phenotype acquired during tumour progression.80, 116 The ability of N2 TANs to inhibit the CD8+ T‐cell antitumoral immune response is one of the main mechanisms through which these cells have been shown to favour tumour progression.80, 117, 118 In this context, TANs isolated from different models of murine cancer have been shown to promote immunosuppression by strongly inducing CD8+ T‐cell apoptosis via tumour necrosis factor‐α and nitric oxide‐dependent mechanisms.118
Finally, it is worth mentioning two papers showing, for the first time, the existence of heterogeneous LDN/PMN‐MDSCs within the peripheral blood of mice119 and rhesus macaques,120 pointing to the possibility of performing phenotypical and functional comparisons of LDNs/PMN‐MDSCs across different species. In mice, immunosuppressive LDNs/PMN‐MDSCs were shown to be generated during tumour progression.119 Interestingly, transforming growth factor‐β 1 seems the main factor involved in both N2 polarization of TANs and conversion of normal density neutrophils into immunosuppressive LDNs.116, 119 In rhesus macaques, immunosuppressive LDNs/PMN‐MDSCs were shown to be constitutively present and to increase after vaccination.120 Notably, the mature CD33+, but not the immature CD33−, neutrophil component was shown to display ARG‐1‐dependent immunosuppressive properties,120 hence, in a similar way to human suppressive LDNs/PMN‐MDSCs.
Neutrophils and Treg cells
The existence of direct crosstalk between neutrophils and Treg cells has been suggested mostly for human neutrophils.17 More recently, evidence that this crosstalk can occur also in vivo, in different murine disease models, has been reported.121, 122, 123 For instance, CCL17 production by murine TANs has been shown to contribute to tumour growth by recruiting Treg cells within the tumour tissue.121 The presence of a murine neutrophil population modulating Treg cell infiltration through the production of anti‐inflammatory lipoxin A4 has also been described to occur within the ocular tissue, and shown to prevent dry‐eye pathogenesis.122 Interestingly, both human and murine neutrophils exposed to pregnancy hormones progesterone and estriol were shown to promote the induction of a population of CD4+ Treg cells (displaying a GARP+ CD127lo FOXP3+ phenotype and producing IL‐10, IL‐17 and vascular endothelial growth factor) through transfer of apoptotic neutrophil‐derived proteins, including forkhead box protein 1, to T cells.123 Finally, the observation that neutrophil depletion in pregnant mice leads to abnormal fetal–maternal unit and embryo development, accompanied by significantly attenuated neutrophil‐induced T‐cell numbers in draining lymph nodes, suggests that the induction of this T‐cell populations by neutrophils could be a crucial mechanism for maternal–fetal tolerance.123
Neutrophils and γδ T cells
Controversial observations have been reported on the crosstalk occurring between neutrophils and γδ T cells in both humans and mice.17, 66 Human neutrophils were shown to either stimulate γδ T cells,124 or negatively modulate γδ T‐cell activation,125, 126 mostly through the release of serine proteases or ROS production. Recently, immunosuppressive LDNs/PMN‐MDSCs were shown to suppress γδ T‐cell function.127 However, these data need to be further verified since LDNs/PMN‐MDSCs were unexpectedly isolated from healthy donors.127 Also, mouse neutrophils were initially shown to inhibit γδ T‐cell functions in a model of Cryptococcus neoformans infection,128 whereas a more recent paper shows that mouse neutrophils can actually sustain IL‐17 production by γδ T cells in the lung, by way of IL‐1β secretion during the initial phase of pneumococcal infection.129 Evidence supporting the capacity of murine neutrophils to inhibit the proliferation and IL‐17 production by γδ T cells through ROS production also comes from studies performed in tumour‐bearing mice130 and a mouse model of psoriasis (SC, DB and PS, unpublished observations). These apparently controversial results might be explained by the different populations of neutrophils or type of functional assays used in the different studies. Additional studies are therefore required to better clarify the regulatory role of human and mouse neutrophils towards γδ T cells. On the other hand, evidence supporting an important role of γδ T cells in promoting the recruitment of neutrophils within inflammatory tissues, including tumours, in both humans and mice, is also continuously growing.66, 112, 117, 131
Neutrophils and invariant NKT cells
Neutrophils have been shown to impair invariant natural killer T (iNKT) cell functions through cell–cell contact‐dependent mechanisms, in both humans and mice.17 Studies performed in different mouse models of inflammatory diseases have also reported the capacity of both neutrophils and iNKT cells to reciprocally modulate their recruitment within inflamed tissues.132, 133, 134 Finally, a more recent study has instead proposed a novel regulatory loop occurring between neutrophils, iNKT cells and B cells during IL‐18‐driven inflammation, ultimately restraining the formation of self‐reactive antibodies during sterile inflammation.26 Accordingly, neutrophils were shown to induce up‐regulation of the death‐receptor ligand, Fas ligand, in iNKT cells, via CD1d cognate interactions. Fas ligand expression by iNKT cells has been, in turn, demonstrated to be crucial to restrict the expansion of harmful autoreactive B‐cell responses.26
Concluding remarks
The data described in this article are schematically depicted in Fig. 1. A major issue that remains to be clarified in the field is whether the interactions occurring between neutrophils and adaptive immune cells are mediated by newly generated immunoregulatory neutrophil populations, or by pre‐existing neutrophils conditioned by the specific disease and acquiring distinct immunoregulatory phenotypes. Future studies taking advantage of technologies, such as intravital microscopy or next‐generation sequencing, will extend our knowledge on the immunoregulatory role of neutrophils in adaptive immunity. Altogether, these discoveries may allow us to envisage effective therapeutic interventions targeted at disease‐specific pathological populations/subsets of neutrophils.
Figure 1.
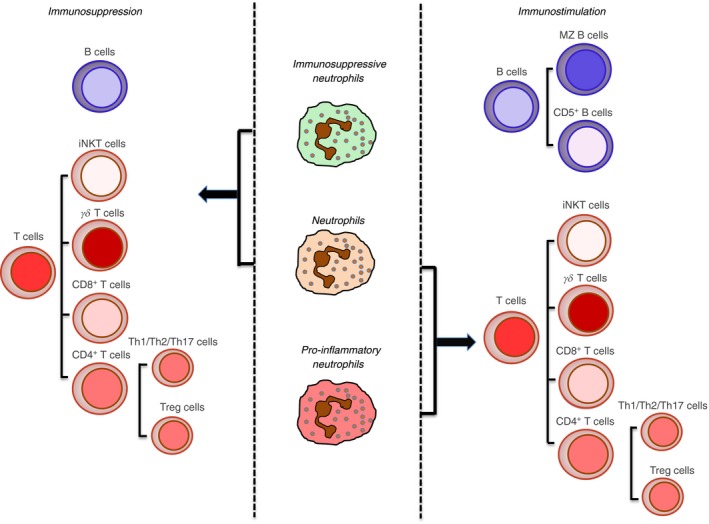
Crosstalk between neutrophils with adaptive immune cells. The cartoon displays the adaptive immune cell types with which human/mouse normal or immunosuppressive/proinflammatory neutrophils establish stimulatory/inhibitory interactions, based on the current literature. Specifically: B cells, including marginal zone (MZ) B cells and CD5+ B cells; T cells, including invariant natural killer T (iNKT) cells, γδ T cells, CD8+ T cells, CD4+ T helper type 1 (Th1) cells, Th17 cells; Th2 cells and T regulatory (Treg) cells.
Disclosure
The authors declare no competing financial interest.
Author contribution
SC, DB, MAC and PS wrote the review.
Acknowledgements
This work was supported by grants from Associazione Italiana per la Ricerca sul Cancro (AIRC, IG20339 to M.A.C.), Ministero dell'Istruzione, dell'Università e della Ricerca (PRIN 2015YYKPNN to M.A.C.) and kindly backed by COST (European Cooperation in Science and Technology) and the COST Action BM1404 Mye‐EUNITER http://www.mye-euniter.euCOST is part of the EU Framework Programme Horizon 2020.
References
- 1. Scapini P, Tamasia N, Pucillo C, Cassatella MA. Granulocytes and mast cells In: Paul W, ed. Fundamental Immunology, 7th edn Philadelphia, PA: Wolters Kluwer‐Lippincott Williams & Wilkins, 2013: 468–86. [Google Scholar]
- 2. Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol 2011; 11:519–31. [DOI] [PubMed] [Google Scholar]
- 3. Nicolas‐Avila JA, Adrover JM, Hidalgo A. Neutrophils in homeostasis, immunity, and cancer. Immunity 2017; 46:15–28. [DOI] [PubMed] [Google Scholar]
- 4. Tamassia N, Bianchetto‐Aguilera F, Arruda‐Silva F, Gardiman E, Gasperini S, Calzetti F et al Cytokine production by human neutrophils: revisiting the “dark side of the moon”. Eur J Clin Invest 2018; doi: 10.1111/eci.12952 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 5. Lin A, Lore K. Granulocytes: new members of the antigen‐presenting cell family. Front Immunol 2017; 8:1781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dalli J, Montero‐Melendez T, Norling LV, Yin X, Hinds C, Haskard D et al Heterogeneity in neutrophil microparticles reveals distinct proteome and functional properties. Mol Cell Proteomics 2013; 12:2205–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol 2018; 18:134–47. [DOI] [PubMed] [Google Scholar]
- 8. Pillay J, Tak T, Kamp VM, Koenderman L. Immune suppression by neutrophils and granulocytic myeloid‐derived suppressor cells: similarities and differences. Cell Mol Life Sci 2013; 70:3813–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moses K, Brandau S. Human neutrophils: their role in cancer and relation to myeloid‐derived suppressor cells. Semin Immunol 2016; 28:187–96. [DOI] [PubMed] [Google Scholar]
- 10. Scapini P, Marini O, Tecchio C, Cassatella MA. Human neutrophils in the saga of cellular heterogeneity: insights and open questions. Immunol Rev 2016; 273:48–60. [DOI] [PubMed] [Google Scholar]
- 11. Carmona‐Rivera C, Kaplan MJ. Low‐density granulocytes: a distinct class of neutrophils in systemic autoimmunity. Semin Immunopathol 2013; 35:455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Christoffersson G, Phillipson M. The neutrophil: one cell on many missions or many cells with different agendas? Cell Tissue Res 2018; 371:415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hellebrekers P, Vrisekoop N, Koenderman L. Neutrophil phenotypes in health and disease. Eur J Clin Invest 2018; doi: 10.1111/eci.12943 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Puga I, Cols M, Barra CM, He B, Cassis L, Gentile M et al B cell‐helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol 2011; 13:170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hampton HR, Chtanova T. The lymph node neutrophil. Semin Immunol 2016; 28:129–36. [DOI] [PubMed] [Google Scholar]
- 16. Mocsai A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med 2013; 210:1283–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scapini P, Cassatella MA. Social networking of human neutrophils within the immune system. Blood 2014; 124:710–9. [DOI] [PubMed] [Google Scholar]
- 18. Breedveld A, Groot Kormelink T, van Egmond M, de Jong EC. Granulocytes as modulators of dendritic cell function. J Leukoc Biol 2017; 102:1003–16. [DOI] [PubMed] [Google Scholar]
- 19. Prame Kumar K, Nicholls AJ, Wong CHY. Partners in crime: neutrophils and monocytes/macrophages in inflammation and disease. Cell Tissue Res 2018; 371:551–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scapini P, Nardelli B, Nadali G, Calzetti F, Pizzolo G, Montecucco C et al G‐CSF‐stimulated neutrophils are a prominent source of functional BLyS. J Exp Med 2003; 197:297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Scapini P, Bazzoni F, Cassatella MA. Regulation of B‐cell‐activating factor (BAFF)/B lymphocyte stimulator (BLyS) expression in human neutrophils. Immunol Lett 2008; 116:1–6. [DOI] [PubMed] [Google Scholar]
- 22. Huard B, McKee T, Bosshard C, Durual S, Matthes T, Myit S et al APRIL secreted by neutrophils binds to heparan sulfate proteoglycans to create plasma cell niches in human mucosa. J Clin Invest 2008; 118:2887–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Magri G, Miyajima M, Bascones S, Mortha A, Puga I, Cassis L et al Innate lymphoid cells integrate stromal and immunological signals to enhance antibody production by splenic marginal zone B cells. Nat Immunol 2014; 15:354–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nagelkerke SQ, aan de Kerk DJ, Jansen MH, van den Berg TK, Kuijpers TW. Failure to detect functional neutrophil B helper cells in the human spleen. PLoS ONE 2014; 9:e88377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chorny A, Casas‐Recasens S, Sintes J, Shan M, Polentarutti N, Garcia‐Escudero R et al The soluble pattern recognition receptor PTX3 links humoral innate and adaptive immune responses by helping marginal zone B cells. J Exp Med 2016; 213:2167–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hagglof T, Sedimbi SK, Yates JL, Parsa R, Salas BH, Harris RA et al Neutrophils license iNKT cells to regulate self‐reactive mouse B cell responses. Nat Immunol 2016; 17:1407–14. [DOI] [PubMed] [Google Scholar]
- 27. Deniset JF, Surewaard BG, Lee WY, Kubes P. Splenic Ly6G(high) mature and Ly6G(int) immature neutrophils contribute to eradication of S. pneumoniae . J Exp Med 2017; 214:1333–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scapini P, Cassatella MA. Location in the spleen dictates the function of murine neutrophils. J Exp Med 2017; 214:1207–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Naranjo‐Gomez M, Lambour J, Piechaczyk M, Pelegrin M. Neutrophils are essential for induction of vaccine‐like effects by antiviral monoclonal antibody immunotherapies. JCI Insight 2018; 3:e97339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kamenyeva O, Boularan C, Kabat J, Cheung GY, Cicala C, Yeh AJ et al Neutrophil recruitment to lymph nodes limits local humoral response to Staphylococcus aureus . PLoS Pathog 2015; 11:e1004827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Parsa R, Lund H, Georgoudaki AM, Zhang XM, Ortlieb Guerreiro‐Cacais A, Grommisch D et al BAFF‐secreting neutrophils drive plasma cell responses during emergency granulopoiesis. J Exp Med 2016; 213:1537–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tomay F, Wells K, Duong L, Tsu JW, Dye DE, Radley‐Crabb HG et al Aged neutrophils accumulate in lymphoid tissues from healthy elderly mice and infiltrate T‐ and B‐cell zones. Immunol Cell Biol 2018; 96:831–40. [DOI] [PubMed] [Google Scholar]
- 33. Roosnek E, Burjanadze M, Dietrich PY, Matthes T, Passweg J, Huard B. Tumors that look for their springtime in APRIL. Crit Rev Oncol Hematol 2009; 72:91–7. [DOI] [PubMed] [Google Scholar]
- 34. Cerutti A, Puga I, Magri G. The B cell helper side of neutrophils. J Leukoc Biol 2013; 94:677–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Holden NJ, Williams JM, Morgan MD, Challa A, Gordon J, Pepper RJ et al ANCA‐stimulated neutrophils release BLyS and promote B cell survival: a clinically relevant cellular process. Ann Rheum Dis 2011; 70:2229–33. [DOI] [PubMed] [Google Scholar]
- 36. Coquery CM, Wade NS, Loo WM, Kinchen JM, Cox KM, Jiang C et al Neutrophils contribute to excess serum BAFF levels and promote CD4+ T cell and B cell responses in lupus‐prone mice. PLoS ONE 2014; 9:e102284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Palanichamy A, Bauer JW, Yalavarthi S, Meednu N, Barnard J, Owen T et al Neutrophil‐mediated IFN activation in the bone marrow alters B cell development in human and murine systemic lupus erythematosus. J Immunol 2014; 192:906–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cervantes‐Luevano KE, Caronni N, Castiello MC, Fontana E, Piperno GM, Naseem A et al Neutrophils drive type I interferon production and autoantibodies in patients with Wiskott‐Aldrich syndrome. J Allergy Clin Immunol 2018; doi: 10.1016/j.jaci.2017.11.063 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zimmermann M, Arruda‐Silva F, Bianchetto‐Aguilera F, Finotti G, Calzetti F, Scapini P et al IFNα enhances the production of IL‐6 by human neutrophils activated via TLR8. Sci Rep 2016; 6:19674–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thai LH, Le Gallou S, Robbins A, Crickx E, Fadeev T, Zhou Z et al BAFF and CD4+ T cells are major survival factors for long‐lived splenic plasma cells in a B‐cell‐depletion context. Blood 2018; 131:1545–55. [DOI] [PubMed] [Google Scholar]
- 41. Musette P, Bouaziz JD. B cell modulation strategies in autoimmune diseases: new concepts. Front Immunol 2018; 9:622–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gupta S, Kaplan MJ. The role of neutrophils and NETosis in autoimmune and renal diseases. Nat Rev Nephrol 2016; 12:402–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee KH, Kronbichler A, Park DD, Park Y, Moon H, Kim H et al Neutrophil extracellular traps (NETs) in autoimmune diseases: a comprehensive review. Autoimmun Rev 2017; 16:1160–73. [DOI] [PubMed] [Google Scholar]
- 44. Denny MF, Yalavarthi S, Zhao W, Thacker SG, Anderson M, Sandy AR et al A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J Immunol 2010; 184:3284–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carlucci PM, Purmalek MM, Dey AK, Temesgen‐Oyelakin Y, Sakhardande S, Joshi AA et al Neutrophil subsets and their gene signature associate with vascular inflammation and coronary atherosclerosis in lupus. JCI Insight 2018; 3:e99276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lin AM, Rubin CJ, Khandpur R, Wang JY, Riblett M, Yalavarthi S et al Mast cells and neutrophils release IL‐17 through extracellular trap formation in psoriasis. J Immunol 2011; 187:490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wright HL, Makki FA, Moots RJ, Edwards SW. Low‐density granulocytes: functionally distinct, immature neutrophils in rheumatoid arthritis with altered properties and defective TNF signalling. J Leukoc Biol 2017; 101:599–611. [DOI] [PubMed] [Google Scholar]
- 48. Grayson PC, Carmona‐Rivera C, Xu L, Lim N, Gao Z, Asare AL et al Neutrophil‐related gene expression and low‐density granulocytes associated with disease activity and response to treatment in antineutrophil cytoplasmic antibody‐associated vasculitis. Arthritis Rheumatol 2015; 67:1922–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Garcia‐Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z et al Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med 2011; 3:73ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J et al Neutrophils activate plasmacytoid dendritic cells by releasing self‐DNA‐peptide complexes in systemic lupus erythematosus. Sci Transl Med 2011; 3:73ra19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B et al Plasmacytoid dendritic cells sense self‐DNA coupled with antimicrobial peptide. Nature 2007; 449:564–9. [DOI] [PubMed] [Google Scholar]
- 52. Diana J, Simoni Y, Furio L, Beaudoin L, Agerberth B, Barrat F et al Crosstalk between neutrophils, B‐1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nat Med 2013; 19:65–73. [DOI] [PubMed] [Google Scholar]
- 53. Kessenbrock K, Krumbholz M, Schonermarck U, Back W, Gross WL, Werb Z et al Netting neutrophils in autoimmune small‐vessel vasculitis. Nat Med 2009; 15:623–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gestermann N, Di Domizio J, Lande R, Demaria O, Frasca L, Feldmeyer L et al Netting neutrophils activate autoreactive B cells in Lupus. J Immunol 2018; 200:3364–71. [DOI] [PubMed] [Google Scholar]
- 55. Grund LZ, Novaski I, Quesniaux VF, Ryffel B, Lopes‐Ferreira M, Lima C. Neutrophils releasing IL‐17A into NETs are essential to plasma cell differentiation in inflamed tissue dependent on IL‐1R. Autoimmunity 2017; 50:86–101. [DOI] [PubMed] [Google Scholar]
- 56. Schwaller J, Schneider P, Mhawech‐Fauceglia P, McKee T, Myit S, Matthes T et al Neutrophil‐derived APRIL concentrated in tumor lesions by proteoglycans correlates with human B‐cell lymphoma aggressiveness. Blood 2007; 109:331–8. [DOI] [PubMed] [Google Scholar]
- 57. Manfroi B, McKee T, Mayol JF, Tabruyn S, Moret S, Villiers C et al CXCL‐8/IL8 produced by diffuse large B‐cell lymphomas recruits neutrophils expressing a proliferation‐inducing ligand APRIL. Cancer Res 2017; 77:1097–107. [DOI] [PubMed] [Google Scholar]
- 58. Gatjen M, Brand F, Grau M, Gerlach K, Kettritz R, Westermann J et al Splenic marginal zone granulocytes acquire an accentuated neutrophil B‐cell helper phenotype in chronic lymphocytic leukemia. Cancer Res 2016; 76:5253–65. [DOI] [PubMed] [Google Scholar]
- 59. Sangaletti S, Tripodo C, Portararo P, Dugo M, Vitali C, Botti L et al Stromal niche communalities underscore the contribution of the matricellular protein SPARC to B‐cell development and lymphoid malignancies. Oncoimmunology 2014; 3:e28989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hirz T, Matera EL, Chettab K, Jordheim LP, Mathe D, Evesque A et al Neutrophils protect lymphoma cells against cytotoxic and targeted therapies through CD11b/ICAM‐1 binding. Oncotarget 2017; 8:72818–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jee J, Bonnegarde‐Bernard A, Duverger A, Iwakura Y, Cormet‐Boyaka E, Martin TL et al Neutrophils negatively regulate induction of mucosal IgA responses after sublingual immunization. Mucosal Immunol 2015; 8:735–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bird AK, Chang M, Barnard J, Goldman BI, Meednu N, Rangel‐Moreno J et al Neutrophils slow disease progression in murine lupus via modulation of autoreactive germinal centers. J Immunol 2017; 199:458–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lelis FJN, Jaufmann J, Singh A, Fromm K, Teschner AC, Poschel S et al Myeloid‐derived suppressor cells modulate B‐cell responses. Immunol Lett 2017; 188:108–15. [DOI] [PubMed] [Google Scholar]
- 64. Kim JH, Podstawka J, Lou Y, Li L, Lee EKS, Divangahi M et al Aged polymorphonuclear leukocytes cause fibrotic interstitial lung disease in the absence of regulation by B cells. Nat Immunol 2018; 19:192–201. [DOI] [PubMed] [Google Scholar]
- 65. Muller I, Munder M, Kropf P, Hansch GM. Polymorphonuclear neutrophils and T lymphocytes: strange bedfellows or brothers in arms? Trends Immunol 2009; 30:522–30. [DOI] [PubMed] [Google Scholar]
- 66. Kalyan S, Kabelitz D. When neutrophils meet T cells: beginnings of a tumultuous relationship with underappreciated potential. Eur J Immunol 2014; 44:627–33. [DOI] [PubMed] [Google Scholar]
- 67. Takashima A, Yao Y. Neutrophil plasticity: acquisition of phenotype and functionality of antigen‐presenting cell. J Leukoc Biol 2015; 98:489–96. [DOI] [PubMed] [Google Scholar]
- 68. Beauvillain C, Delneste Y, Scotet M, Peres A, Gascan H, Guermonprez P et al Neutrophils efficiently cross‐prime naive T cells in vivo . Blood 2007; 110:2965–73. [DOI] [PubMed] [Google Scholar]
- 69. Davey MS, Morgan MP, Liuzzi AR, Tyler CJ, Khan MWA, Szakmany T et al Microbe‐specific unconventional T cells induce human neutrophil differentiation into antigen cross‐presenting cells. J Immunol 2014; 193:3704–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Trentini MM, de Oliveira FM, Kipnis A, Junqueira‐Kipnis AP. The role of neutrophils in the induction of specific Th1 and Th17 during vaccination against tuberculosis. Front Microbiol 2016; 7:898–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Vono M, Lin A, Norrby‐Teglund A, Koup RA, Liang F, Lore K. Neutrophils acquire the capacity for antigen presentation to memory CD4+ T cells in vitro and ex vivo . Blood 2017; 129:1991–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Liang F, Lindgren G, Sandgren KJ, Thompson EA, Francica JR, Seubert A et al Vaccine priming is restricted to draining lymph nodes and controlled by adjuvant‐mediated antigen uptake. Sci Transl Med 2017; 9:eaal2094. [DOI] [PubMed] [Google Scholar]
- 73. Cassatella MA. Human mature neutrophils as atypical APC. Blood 2017; 129:1895–6. [DOI] [PubMed] [Google Scholar]
- 74. Hulsdunker J, Ottmuller KJ, Neeff HP, Koyama M, Gao Z, Thomas OS et al Neutrophils provide cellular communication between ileum and mesenteric lymph nodes at graft‐versus‐host disease onset. Blood 2018; 131:1858–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Polak D, Hafner C, Briza P, Kitzmuller C, Elbe‐Burger A, Samadi N et al A novel role for neutrophils in IgE‐mediated allergy: evidence for antigen‐presentation in late‐phase reactions. J Allergy Clin Immunol 2018; doi: 10.1016/j.jaci.2018.06.005 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Geng S, Matsushima H, Okamoto T, Yao Y, Lu R, Page K et al Emergence, origin, and function of neutrophil‐dendritic cell hybrids in experimentally induced inflammatory lesions in mice. Blood 2013; 121:1690–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Singhal S, Bhojnagarwala PS, O'Brien S, Moon EK, Garfall AL, Rao AS et al Origin and role of a subset of tumor‐associated neutrophils with antigen‐presenting cell features in early‐stage human lung cancer. Cancer Cell 2016; 30:120–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Matsushima H, Geng S, Lu R, Okamoto T, Yao Y, Mayuzumi N et al Neutrophil differentiation into a unique hybrid population exhibiting dual phenotype and functionality of neutrophils and dendritic cells. Blood 2013; 121:1677–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Fites JS, Gui M, Kernien JF, Negoro P, Dagher Z, Sykes DB et al An unappreciated role for neutrophil‐DC hybrids in immunity to invasive fungal infections. PLoS Pathog 2018; 14:e1007073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Shaul ME, Fridlender ZG. Cancer related circulating and tumor‐associated neutrophils ‐ subtypes, sources and function. FEBS J 2018; doi: 10.1111/febs.14524 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 81. Governa V, Trella E, Mele V, Tornillo L, Amicarella F, Cremonesi E et al The interplay between neutrophils and CD8+ T cells improves survival in human colorectal cancer. Clin Cancer Res 2017; 23:3847–58. [DOI] [PubMed] [Google Scholar]
- 82. Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF et al Recommendations for myeloid‐derived suppressor cell nomenclature and characterization standards. Nat Commun 2016; 7:12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Veglia F, Perego M, Gabrilovich D. Myeloid‐derived suppressor cells coming of age. Nat Immunol 2018; 19:108–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Cloke T, Munder M, Bergin P, Herath S, Modolell M, Taylor G et al Phenotypic alteration of neutrophils in the blood of HIV seropositive patients. PLoS ONE 2013; 8:e72034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bowers NL, Helton ES, Huijbregts RP, Goepfert PA, Heath SL, Hel Z. Immune suppression by neutrophils in HIV‐1 infection: role of PD‐L1/PD‐1 pathway. PLoS Pathog 2014; 10:e1003993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Pallett LJ, Gill US, Quaglia A, Sinclair LV, Jover‐Cobos M, Schurich A et al Metabolic regulation of hepatitis B immunopathology by myeloid‐derived suppressor cells. Nat Med 2015; 21:591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Darcy CJ, Minigo G, Piera KA, Davis JS, McNeil YR, Chen Y et al Neutrophils with myeloid derived suppressor function deplete arginine and constrain T cell function in septic shock patients. Crit Care 2014; 18:R163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Janols H, Bergenfelz C, Allaoui R, Larsson AM, Ryden L, Bjornsson S et al A high frequency of MDSCs in sepsis patients, with the granulocytic subtype dominating in gram‐positive cases. J Leukoc Biol 2014; 96:685–93. [DOI] [PubMed] [Google Scholar]
- 89. Kostlin N, Kugel H, Spring B, Leiber A, Marme A, Henes M et al Granulocytic myeloid derived suppressor cells expand in human pregnancy and modulate T‐cell responses. Eur J Immunol 2014; 44:2582–91. [DOI] [PubMed] [Google Scholar]
- 90. Ssemaganda A, Kindinger L, Bergin P, Nielsen L, Mpendo J, Ssetaala A et al Characterization of neutrophil subsets in healthy human pregnancies. PLoS ONE 2014; 9:e85696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kostlin N, Hofstadter K, Ostermeir AL, Spring B, Leiber A, Haen S et al Granulocytic myeloid‐derived suppressor cells accumulate in human placenta and polarize toward a Th2 phenotype. J Immunol 2016; 196:1132–45. [DOI] [PubMed] [Google Scholar]
- 92. Kostlin N, Schoetensack C, Schwarz J, Spring B, Marme A, Goelz R et al Granulocytic myeloid‐derived suppressor cells (GR‐MDSC) in breast milk (BM); GR‐MDSC accumulate in human BM and modulate T‐cell and monocyte function. Front Immunol 2018; 9:1098–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Rieber N, Gille C, Kostlin N, Schafer I, Spring B, Ost M et al Neutrophilic myeloid‐derived suppressor cells in cord blood modulate innate and adaptive immune responses. Clin Exp Immunol 2013; 174:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Gervassi A, Lejarcegui N, Dross S, Jacobson A, Itaya G, Kidzeru E et al Myeloid derived suppressor cells are present at high frequency in neonates and suppress in vitro T cell responses. PLoS ONE 2014; 9:e107816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kostlin N, Vogelmann M, Spring B, Schwarz J, Feucht J, Hartel C et al Granulocytic myeloid‐derived suppressor cells from human cord blood modulate T‐helper cell response towards an anti‐inflammatory phenotype. Immunology 2017; 152:89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. He YM, Li X, Perego M, Nefedova Y, Kossenkov AV, Jensen EA et al Transitory presence of myeloid‐derived suppressor cells in neonates is critical for control of inflammation. Nat Med 2018; 24:224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Vasconcelos ZF, Santos BM, Costa ES, Lima M, Tabak DG, Bouzas LF et al T‐lymphocyte function from peripheral blood stem‐cell donors is inhibited by activated granulocytes. Cytotherapy 2003; 5:336–45. [DOI] [PubMed] [Google Scholar]
- 98. Luyckx A, Schouppe E, Rutgeerts O, Lenaerts C, Fevery S, Devos T et al G‐CSF stem cell mobilization in human donors induces polymorphonuclear and mononuclear myeloid‐derived suppressor cells. Clin Immunol 2012; 143:83–7. [DOI] [PubMed] [Google Scholar]
- 99. Marini O, Costa S, Bevilacqua D, Calzetti F, Tamassia N, Spina C et al Mature CD10+ and immature CD10– neutrophils present in G‐CSF‐treated donors display opposite effects on T cells. Blood 2017; 129:1343–56. [DOI] [PubMed] [Google Scholar]
- 100. Lang S, Bruderek K, Kaspar C, Hoing B, Kanaan O, Dominas N et al Clinical relevance and suppressive capacity of human MDSC subsets. Clin Cancer Res 2018; 24:4834–4844. [DOI] [PubMed] [Google Scholar]
- 101. de Kleijn S, Langereis JD, Leentjens J, Kox M, Netea MG, Koenderman L et al IFN‐γ‐stimulated neutrophils suppress lymphocyte proliferation through expression of PD‐L1. PLoS ONE 2013; 8:e72249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Buddhisa S, Rinchai D, Ato M, Bancroft GJ, Lertmemongkolchai G. Programmed death ligand 1 on Burkholderia pseudomallei‐infected human polymorphonuclear neutrophils impairs T cell functions. J Immunol 2015; 194:4413–21. [DOI] [PubMed] [Google Scholar]
- 103. He G, Zhang H, Zhou J, Wang B, Chen Y, Kong Y et al Peritumoural neutrophils negatively regulate adaptive immunity via the PD‐L1/PD‐1 signalling pathway in hepatocellular carcinoma. J Exp Clin Cancer Res 2015; 34:141–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Eruslanov E, Neuberger M, Daurkin I, Perrin GQ, Algood C, Dahm P et al Circulating and tumor‐infiltrating myeloid cell subsets in patients with bladder cancer. Int J Cancer 2012; 130:1109–19. [DOI] [PubMed] [Google Scholar]
- 105. Vollbrecht T, Stirner R, Tufman A, Roider J, Huber RM, Bogner JR et al Chronic progressive HIV‐1 infection is associated with elevated levels of myeloid‐derived suppressor cells. AIDS 2012; 26:F31–7. [DOI] [PubMed] [Google Scholar]
- 106. Wu H, Zhen Y, Ma Z, Li H, Yu J, Xu ZG et al Arginase‐1‐dependent promotion of TH17 differentiation and disease progression by MDSCs in systemic lupus erythematosus. Sci Transl Med 2016; 8:331ra40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Shen G, Krienke S, Schiller P, Niessen A, Neu S, Eckstein V et al Microvesicles released by apoptotic human neutrophils suppress proliferation and IL‐2/IL‐2 receptor expression of resting T helper cells. Eur J Immunol 2017; 47:900–10. [DOI] [PubMed] [Google Scholar]
- 108. Khanna S, Graef S, Mussai F, Thomas A, Wali N, Yenidunya BG et al Tumor‐derived GM‐CSF promotes granulocyte immunosuppression in Mesothelioma patients. Clin Cancer Res 2018; 24:2859–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Giallongo C, Tibullo D, Parrinello NL, La Cava P, Di Rosa M, Bramanti V et al Granulocyte‐like myeloid derived suppressor cells (G‐MDSC) are increased in multiple myeloma and are driven by dysfunctional mesenchymal stem cells (MSC). Oncotarget 2016; 7:85764–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Alvarez KLF, Beldi M, Sarmanho F, Rossetti RAM, Silveira CRF, Mota GR et al Local and systemic immunomodulatory mechanisms triggered by Human Papillomavirus transformed cells: a potential role for G‐CSF and neutrophils. Sci Rep 2017; 7:9002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ingersoll SA, Laval J, Forrest OA, Preininger M, Brown MR, Arafat D et al Mature cystic fibrosis airway neutrophils suppress T cell function: evidence for a role of arginase 1 but not programmed death‐ligand 1. J Immunol 2015; 194:5520–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wu P, Wu D, Ni C, Ye J, Chen W, Hu G et al γδ T17 cells promote the accumulation and expansion of myeloid‐derived suppressor cells in human colorectal cancer. Immunity 2014; 40:785–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Condamine T, Dominguez GA, Youn JI, Kossenkov AV, Mony S, Alicea‐Torres K et al Lectin‐type oxidized LDL receptor‐1 distinguishes population of human polymorphonuclear myeloid‐derived suppressor cells in cancer patients. Sci Immunol 2016; doi: 10.1126/sciimmunol.aaf8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Nan J, Xing YF, Hu B, Tang JX, Dong HM, He YM et al Endoplasmic reticulum stress induced LOX‐1+ CD15+ polymorphonuclear myeloid‐derived suppressor cells in hepatocellular carcinoma. Immunology 2018; 154:144–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Langereis JD, Pickkers P, de Kleijn S, Gerretsen J, de Jonge MI, Kox M. Spleen‐derived IFN‐γ induces generation of PD‐L1+‐suppressive neutrophils during endotoxemia. J Leukoc Biol 2017; 102:1401–9. [DOI] [PubMed] [Google Scholar]
- 116. Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L et al Polarization of tumor‐associated neutrophil phenotype by TGF‐β: “N1” versus “N2” TAN. Cancer Cell 2009; 16:183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS et al IL‐17‐producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 2015; 522:345–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Michaeli J, Shaul ME, Mishalian I, Hovav AH, Levy L, Zolotriov L et al Tumor‐associated neutrophils induce apoptosis of non‐activated CD8 T‐cells in a TNFα and NO‐dependent mechanism, promoting a tumor‐supportive environment. Oncoimmunology 2017; 6:e1356965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Sagiv JY, Michaeli J, Assi S, Mishalian I, Kisos H, Levy L et al Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep 2015; 10:562–73. [DOI] [PubMed] [Google Scholar]
- 120. Lin A, Liang F, Thompson EA, Vono M, Ols S, Lindgren G et al Rhesus macaque myeloid‐derived suppressor cells demonstrate T cell inhibitory functions and are transiently increased after vaccination. J Immunol 2018; 200:286–94. [DOI] [PubMed] [Google Scholar]
- 121. Mishalian I, Bayuh R, Eruslanov E, Michaeli J, Levy L, Zolotarov L et al Neutrophils recruit regulatory T‐cells into tumors via secretion of CCL17 – a new mechanism of impaired antitumor immunity. Int J Cancer 2014; 135:1178–86. [DOI] [PubMed] [Google Scholar]
- 122. Gao Y, Min K, Zhang Y, Su J, Greenwood M, Gronert K. Female‐specific downregulation of tissue polymorphonuclear neutrophils drives impaired regulatory T cell and amplified effector T cell responses in autoimmune dry eye disease. J Immunol 2015; 195:3086–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Nadkarni S, Smith J, Sferruzzi‐Perri AN, Ledwozyw A, Kishore M, Haas R et al Neutrophils induce proangiogenic T cells with a regulatory phenotype in pregnancy. Proc Natl Acad Sci USA 2016; 113:E8415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Towstyka NY, Shiromizu CM, Keitelman I, Sabbione F, Salamone GV, Geffner JR et al Modulation of γδ T‐cell activation by neutrophil elastase. Immunology 2018; 153:225–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Fazio J, Kalyan S, Wesch D, Kabelitz D. Inhibition of human γδ T cell proliferation and effector functions by neutrophil serine proteases. Scand J Immunol 2014; 80:381–9. [DOI] [PubMed] [Google Scholar]
- 126. Sabbione F, Gabelloni ML, Ernst G, Gori MS, Salamone G, Oleastro M et al Neutrophils suppress γδ T‐cell function. Eur J Immunol 2014; 44:819–30. [DOI] [PubMed] [Google Scholar]
- 127. Sacchi A, Tumino N, Sabatini A, Cimini E, Casetti R, Bordoni V et al Myeloid‐derived suppressor cells specifically suppress IFN‐γ production and antitumor cytotoxic activity of Vδ2 T cells. Front Immunol 2018; 9:1271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Wozniak KL, Kolls JK, Wormley FL Jr. Depletion of neutrophils in a protective model of pulmonary cryptococcosis results in increased IL‐17A production by γδ T cells. BMC Immunol 2012; 13:65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Hassane M, Demon D, Soulard D, Fontaine J, Keller LE, Patin EC et al Neutrophilic NLRP3 inflammasome‐dependent IL‐1β secretion regulates the γδ T17 cell response in respiratory bacterial infections. Mucosal Immunol 2017; 10:1056–68. [DOI] [PubMed] [Google Scholar]
- 130. Mensurado S, Rei M, Lanca T, Ioannou M, Goncalves‐Sousa N, Kubo H et al Tumor‐associated neutrophils suppress pro‐tumoral IL‐17+ γδ T cells through induction of oxidative stress. PLoS Biol 2018; 16:e2004990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Jiang X, Park CO, Geddes Sweeney J, Yoo MJ, Gaide O, Kupper TS. Dermal γδ T cells do not freely re‐circulate out of skin and produce IL‐17 to promote neutrophil infiltration during primary contact hypersensitivity. PLoS ONE 2017; 12:e0169397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Thanabalasuriar A, Neupane AS, Wang J, Krummel MF, Kubes P. iNKT cell emigration out of the lung vasculature requires neutrophils and monocyte‐derived dendritic cells in inflammation. Cell Rep 2016; 16:3260–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Huang E, Liu R, Lu Z, Liu J, Liu X, Zhang D et al NKT cells mediate the recruitment of neutrophils by stimulating epithelial chemokine secretion during colitis. Biochem Biophys Res Commun 2016; 474:252–8. [DOI] [PubMed] [Google Scholar]
- 134. Ligocki AJ, Niederkorn JY. Natural killer T cells contribute to neutrophil recruitment and ocular tissue damage in a model of intraocular tumor rejection. Invest Ophthalmol Vis Sci 2016; 57:813–23. [DOI] [PMC free article] [PubMed] [Google Scholar]


