Summary
B7 family members and their receptors play a central role in the regulation of T‐cell responses through T‐cell co‐stimulation and co‐inhibition pathways that constitute attractive targets for the development of immunotherapeutic drugs. In this study, we report that VSIG‐3/IGSF11 is a ligand of B7 family member VISTA/PD‐1H and inhibits human T‐cell functions through a novel VSIG‐3/VISTA pathway. An extensive functional ELISA binding screening assay reveals that VSIG‐3 binds to the new B7 family member VISTA but does not interact with other known members of the B7 family. Under the same experimental conditions, we did not observe any significant interaction between VSIG‐8 and VISTA. In addition, VSIG‐3 inhibits human T‐cell proliferation in the presence of T‐cell receptor signaling. Furthermore, VSIG‐3 significantly reduces cytokine and chemokine production by human T cells including IFN‐γ, IL‐2, IL‐17, CCL5/Rantes, CCL3/MIP‐1α, and CXCL11/I‐TAC. Anti‐VISTA neutralization antibodies attenuate the binding of VSIG‐3 and VISTA, as well as VSIG‐3‐induced T‐cell inhibition. Hence, we have identified a novel ligand for VISTA that is able to inhibit human T‐cell proliferation and cytokine production. This unique VSIG‐3/VISTA co‐inhibitory pathway may provide new strategies for the treatment of human cancers, autoimmune disorders, infection, and transplant rejection and may aid in the design of better vaccines.
Keywords: B7 family, immune checkpoints, immunotherapy, VISTA/PD‐1H, VSIG‐3/IGSF11
Abbreviations
- APCs
antigen‐presenting cells
- BTN
butyrophilin
- CTLA‐4
cytotoxic T‐lymphocyte antigen 4
- ELISA
enzyme‐linked immunosorbent assay
- IFN‐γ
interferon‐γ
- IGSF11
immunoglobulin superfamily member 11
- IL‐2
interleukin‐2
- PBMCs
peripheral blood mononuclear cells
- PBS
phosphate‐buffered saline
- PD‐1H
programmed cell death protein 1 homolog
- RT‐PCR
reverse transcription polymerase chain reaction
- SLAM
signaling lymphocytic activation molecule
- SPR
surface plasmon resonance
- VISTA
the V domain‐containing immunoglobulin suppressor of T‐cell activation
- VSIG‐3
V‐Set and immunoglobulin domain containing 3
Introduction
Critical modulators of the immune system, also referred to as immune checkpoint regulators, generate co‐stimulation or co‐inhibition of T‐cell responses.1 Checkpoint blockages can generate potent anti‐tumor responses by enabling the immune system to seek and destroy cancer cells. Members of the B7 family have emerged as important checkpoint regulators, and recently the United States Food and Drug Administration has approved the use of three antibodies, Ipilimumab (anti‐CTLA‐4, Bristol‐Myers Squibb, New York, NY), nivolumab (anti‐PD‐1, Bristol‐Myers Squibb, New York, NY), and pembrolizumab (anti‐PD‐1, Merck, Kenilworth, New Jersey), that block the function of cytotoxic T‐lymphocyte antigen‐4 (CTLA‐4) or programmed cell death protein 1 (PD‐1) for cancer immunotherapy.2, 3 There is an urgent need for more agents to enter clinical use.
The V domain‐containing immunoglobulin suppressor of T‐cell activation (VISTA), also termed Differentiation of Embryonic Stem Cells 1 (Dies1), Gi24 and PD‐1 homolog (PD‐1H), is a 55 000 to 65 000 molecular weight type I immunoglobulin membrane protein with the extracellular domain homologous to PD‐L1. VISTA is highly expressed on mature CD11b high myeloid‐derived antigen‐presenting cells (APCs) and to a lesser extent on CD4+, CD8+, and regulatory T cells, and is also found on tumor‐infiltrating lymphocytes.4 It has been demonstrated that VISTA is a co‐inhibitory receptor on CD4+ T cells or a co‐inhibitory ligand for T cells. In vitro, VISTA–immunoglobulin fusion protein (VISTA‐Ig) inhibits T‐cell activation, proliferation, and the production of cytokines, such as interleukin‐2 (IL‐2) and interferon‐γ (IFN‐γ), during anti‐CD3 activation, suggesting that VISTA may potentially function as a co‐inhibitory ligand for T cells.5 Another group reported that VISTA−/− CD4+ T cells showed stronger antigen‐specific proliferation and cytokine production than wild‐type CD4+ T cells, which supports the thesis that VISTA functions as an inhibitory receptor on CD4+ T cells.6 The analysis of both VISTA‐Ig and genetic ablation suggests that VISTA is a negative checkpoint regulator of T‐cell activation. The binding partners of VISTA that mediate these effects may be potential targets for therapeutic intervention. However, to date, they have remained unclear.
V‐Set and Immunoglobulin domain containing 3 (VSIG‐3), also known as immunoglobulin superfamily member 11 (IGSF11) and brain‐ and testis‐specific immunoglobulin superfamily (BT‐IgSF), was originally identified as a member of the immunoglobulin superfamily, and mediates homophilic adhesion in a calcium‐independent manner.7 VSIG‐3 expression is elevated in colorectal cancers and hepatocellular carcinomas as well as intestinal‐type gastric cancers. Suppression of VSIG‐3 by small interfering RNA (siRNA) retarded the growth of gastric cancer cells, suggesting that VSIG‐3 is a good candidate for gastric cancer immunotherapy.8 A recent study showed that VSIG‐3 regulates synaptic transmission and plasticity through interaction with the post‐synaptic scaffolding protein PSD‐95 and AMPA glutamate receptors (AMPARs).9 However, the biological functions and binding partners of VSIG‐3 outside the brain have not yet been identified.
We report that VSIG‐3 is a novel ligand for VISTA and that the engagement of VSIG‐3 with VISTA on activated T cells inhibits T‐cell proliferation as well as cytokine and chemokine production. The co‐inhibitory functions of VSIG‐3 on activated T cells, combined with the highly elevated expression of VSIG‐3 in colorectal cancers, hepatocellular carcinomas, and intestinal‐type gastric cancers suggest that the blockage of the VSIG‐3/VISTA pathway represents a new cancer immunotherapeutic strategy.
Materials and Methods
Peripheral blood mononuclear cell preparation and cell culture
Human blood from healthy adult donors was purchased from New York Biological, Southampton, NY. Peripheral blood mononuclear cells (PBMCs) were isolated by centrifugation on Ficoll‐paque Plus (d = 1·077 g/ml) (GE Healthcare, Pittsburgh, PA, USA), at 900 g for 25 min at 18–20°. Cells were then washed three times in phosphate‐buffered saline (PBS) at 400 g for 15 min and re‐suspended in Iscove's modified Dulbecco's medium (Sigma, St. Louis, MO) with l‐glutamine, supplemented with 10% heat inactivated human serum (Sigma). PBMCs were cultured at 37° in 5% CO2.
Functional enzyme‐linked immunosorbent binding assay
The extracellular domains of recombinant proteins used in this study were produced by R&D Systems.
For the functional enzyme‐linked immunosorbent assay (ELISA) binding screening assay, recombinant proteins (2 μg/ml) were immobilized on 96‐well ELISA plates by incubation at 2–8° for 24 hr. Control wells did not have immobilized proteins. Then, ELISA plates were blocked with 1% bovine serum albumin (BSA) ‐PBS at room temperature for 2 hr. Bait proteins, biotinylated human VSIG‐3 or VISTA (2 μg/ml), were subsequently added into each well of ELISA plates and incubated at room temperature for 2 hr. Binding of VSIG‐3 or VISTA was detected by adding streptavidin‐horseradish peroxidase (HRP) followed by substrate color reagents (R&D Systems, Minneapolis, MN).
For the VISTA and VSIG‐3 functional ELISA binding assay, recombinant human VISTA proteins (2 μg/ml) were immobilized on 96‐well ELISA plates by incubation at 2–8° for 24 hr. Then, ELISA plates were blocked with 1% BSA‐PBS at room temperature for 2 hr. Biotinylated human VSIG‐3, or VSIG‐8 proteins at the indicated concentrations were subsequently added into each well of ELISA plates and incubated at room temperature for 2 hr.
T‐cell proliferation assay
For anti‐CD3‐induced T‐cell proliferation, 1 μg/ml anti‐human CD3 (R&D Systems) was pre‐coated in the 96‐well plates overnight at 2–8°. Then human VSIG‐3 IgG1Fc fusion protein (VSIG‐3 IgG1Fc) or control human IgG1Fc at the indicated concentrations was immobilized for 3 hr at 37° in the wells. Human CD3+ T cells were purified from PBMCs by negative selection using MagCellect Human CD3+ T Cell Isolation Kit (R&D Systems) according to the manufacturer's instructions, and added into each well at 2 × 105 per well and cultured for the indicated time. Cell proliferation was assessed by a fluorometric assay using the redox‐sensitive dye Alamar Blue (Resazurin) (R&D Systems).
For the carboxyfluorescein succinimidyl ester (CSFE) (Thermo Fisher Scientific, Waltham, MA) labeled T‐cell proliferation assay, CSFE‐labeled T cells were incubated with plate‐bound anti‐human CD3 (1 μg/ml), VSIG‐3 IgG1Fc (10 μg/ml), or control IgG1Fc (10 μg/ml) for the indicated time and stained with human CD3 epsilon phycoerythrin‐conjugated antibody (R&D Systems) for flow cytometry analysis.
Cytokine secretion assay and cytokine measurement
For the in vitro plate‐bound VSIG‐3 assay, 1 μg/ml anti‐human CD3 (R&D Systems) was pre‐coated in 96‐well plates overnight at 2–8°. Then human VSIG‐3 IgG1Fc or control human IgG1Fc at the indicated concentrations was immobilized for 3 hr at 37° in the wells. Human PBMCs or purified T cells were cultured in the wells of a 96‐well plate in the present plate‐bound anti‐CD3 and either human VSIG‐3 IgG1Fc or control human IgG1Fc at the indicated concentrations for 24–96 hr. Cell‐free culture supernatants were harvested for cytokine and chemokine measurement.
For the in vitro Baf/3‐VSIG‐3 assay, Baf/3 cells were transduced with retrovirus expressing human VSIG‐3‐Enhanced green fluorescent protein (EGFP) or EGFP in the presence of 10 μg/ml polybrene (Sigma‐Aldrich, St. Louis, MO), and VSIG‐3 expression on the cell surface was confirmed by flow cytometry analysis. Baf/3‐VSIG‐3 or Baf/3 cells were pretreated with 100 μg/ml mitomycin C (Tocris, Biotechne, Minneapolis, MN) at 37° for 1 hr, and then co‐incubated with human PBMCs at a 1 : 5 ratio in the presence of plate‐bound anti‐human CD3 (1 μg/ml, R&D Systems, Minneapolis, MN) for 24 hr. Cell‐free culture supernatants were harvested for cytokine measurement.
Cytokine secretion profile in the cell‐free culture supernatants was measured using a Proteome Profiler Human XL Cytokine Array Kit, which measures 105 human cytokines, chemokines, and acute‐phase proteins (R&D Systems). Human IFN‐γ, IL‐2, IL‐17, CCL5/Rantes, CCL3/MIP‐1, and CXCL11/I‐TAC were also measured using Quantikine ELISA Kits according to the manufacturer's instructions (R&D Systems). Human VISTA/PD‐1H neutralization antibody (sheep anti‐human VISTA, Catalog # AF7126) and human VISTA IgG1 Fc (Catalog # 7126‐B7) used for the cytokine secretion neutralization assay was obtained from R&D Systems.
Flow cytometry analysis
For cell‐surface VISTA staining, cells were incubated with mouse anti‐human VISTA/PD‐1H Alexa Fluor 488‐conjugated antibody (R&D Systems, Catalog # FAB71261G, Clone # 730804) and mouse anti‐human CD4 Alexa Fluor 405‐conjugated antibody (R&D Systems, Catalog # FAB3791V). For cell surface VSIG‐3 staining, cells were incubated with mouse anti‐human VSIG‐3 Alexa Fluor 647‐conjugated antibody (R&D Systems, Clone # 774208) for 30–45 min at 2–8°, washed with buffer, and analyzed by flow cytometry using a BD LSRFortessa (BD Biosciences, San Jose, CA).
Co‐immunoprecipitation and immunoblotting
Cytoplasmic extracts of PBMCs were prepared by lysis in ice‐cold Pierce IP Lysis Buffer (Thermo Fisher Scientific) with Halt proteinase inhibitor (Thermo Fisher Scientific) in 100 × 106 PBMC/100 μl buffer. Nuclei and insoluble cell debris were removed by centrifugation at 14 000 g for 10 min at 2–8°. Immunoprecipitation was performed using Dynabeads M‐280 Streptavidin kit (Thermo Fisher Scientific) according to the manufacturer's instructions. Briefly, biotinylated VSIG‐3, VSIG‐3 extracellular domain (ECD) V−type immunoglobulin−like domain [amino acids (aa) 23–136] and C−type immunoglobulin−like domain (aa144–241) Fc fusion proteins were incubated with Dynabeads M‐280 streptavidin magnetic beads for 30 min at room temperature on a rotary mixer. After removing unbound proteins from the supernatant, the Dynabeads‐VSIG‐3 protein complex was added into PBMC lysates and incubated at room temperature for 30 min. Next, the VSIG‐3 protein‐binding partner protein complex was collected by eluting the Dynabeads M‐280 Streptavidin magnetic beads with elution buffer. Co‐immunoprecipitated proteins were subjected to 4–20% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane (Millipore, Burlington, MA). Immunoblotting was performed with anti‐human VISTA or isotype control monoclonal antibodies (R&D Systems). Proteins were visualized by enhanced chemiluminescence using HRP‐conjugated goat anti‐mouse IgG (R&D Systems) and Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific).
VISTA siRNA nucleofection
Human CD3+ T cells were transfected with human VISTA siRNA (Catalog # AM16708, siRNA ID 258598, Thermo Fisher Scientific) or Silencer™ negative control siRNA (Catalog # 4390843, Thermo Fisher Scientific, 20 μg siRNA per 10 × 106 T cells) using the Amaxa Lonza nucleofector system and Nucleofector™ Kits for Human T Cells (Lonza, Basel, Switzerland). After nucleofection, T cells were transferred to media in a 24‐well plate and cultured overnight. Nucleofected cells were used for the cytokine secretion assay.
Real‐time reverse transcription–polymerase chain reaction
Total RNA from human PBMCS was isolated using the Aurum™ Total RNA Mini Kit (BioRad, Hercules, CA). Ten nanograms of total RNA was subjected to two‐step reverse transcription–polymerase chain reaction (RT‐PCR) using iScript™ Advanced cDNA Synthesis Kit (BioRad) and SsoAdvanced™ Universal SYBR® Green Supermix (BioRad). Real‐time SYBR Green PCR analysis was performed for human IL‐17 (PrimePCR SYBR Green Assay: IL‐17, Human, BioRad) and GADPH (PrimePCR SYBR Green Assay: GADPH, Human, BioRad). Relative quantification was performed using the CFX Connect™ Real‐Time PCR Detection System (BioRad). The IL‐17 transcript levels were normalized to GADPH transcript levels from the same preparations of cDNA.
Statistical analysis
Student's t‐test was used for statistical analysis, and P values reflect comparison with the control sample. P values < 0·05 were considered statistically significant.
Results
Identification of VSIG3 as a binding partner for VISTA
To identify VISTA interacting partners, we screened VISTA binding with B7, butyrophilin (BTN), signaling lymphocytic activation molecule (SLAM), and V‐set and immunoglobulin domain‐containing protein (VSIG) family members using a functional ELISA binding assay. Under our experimental conditions, we did not find any evidence for VISTA protein binding to known B7 family members. However, we identified VSIG‐3 as a binding candidate of VISTA. No significant binding was seen between VISTA and other VSIG family members, including VSIG‐8. Mature human VSIG3 consists of a 219‐aa extracellular domain (ECD), a 21‐aa transmembrane segment, and a 169‐aa cytoplasmic domain. The ECD contains one N−terminal V−type immunoglobulin−like domain followed by one C−type immunoglobulin−like domain. We generated truncated recombinant human VSIG‐3 ECD V−type immunoglobulin−like domain (aa 23–136) and C−type immunoglobulin−like domain (aa 144–241) Fc fusion proteins. Similar to the full‐length ECD, both V−type immunoglobulin−like domain and C−type immunoglobulin−like domain of VSIG‐3 Fc fusion proteins bound to human VISTA protein in a functional ELISA binding assay (data not shown).
To assess the strength of interaction between VISTA and VSIG‐3, VISTA IgG1Fc fusion protein (VISTA IgG1Fc, 2 μg/ml in a 100‐μl volume) was coated on the wells of the ELISA plate. After blocking of the wells with 1% BSA, varying amounts of VSIG‐3 protein labeled with biotin were added, and the binding was detected with streptavidin‐HRP. As shown in Fig. 1(a), VSIG‐3 bound VISTA with an apparent K d of 3·4 nm (~0·25 μg/ml). Under this assay condition, there was no significant binding between VSIG‐8 and VISTA. Surface plasmon resonance (SPR) analysis on a BiaCore T‐200 of the VSIG‐3/VISTA protein complex suggests biphasic association and dissociation kinetics (see Supplementary material, Fig. S1). To further confirm the specific interaction between VISTA and VSIG‐3, we carried out functional ELISA binding assays in the presence of sheep or mouse anti‐human VISTA antibodies. Inclusion of anti‐human VISTA antibodies completely blocked this interaction, suggesting that VSIG‐3 is a novel binding partner for B7 family member VISTA (Fig. 1b).
Figure 1.
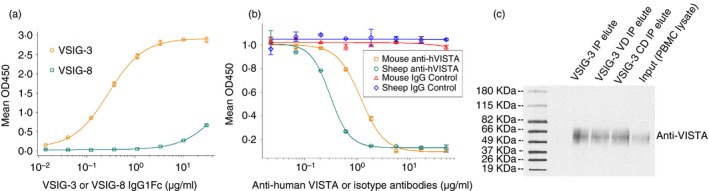
VSIG‐3 binds to VISTA. (a) Recombinant human VSIG‐3 protein specifically binds to recombinant VISTA in a functional ELISA binding assay. When VISTA IgG1Fc was immobilized at 2 μg/ml, 100 μl/well, the concentration of VSIG‐3 IgG1Fc that produces 50% of the optimal binding response was approximately 0·25 μg/ml (3·4 nm). The non‐specific binding is subtracted and in all cases was < 2% of the total signal. (b) Anti‐human VISTA antibodies block the binding of VSIG‐3 and VISTA. VSIG‐3 IgG1Fc proteins were immobilized at 2 μg/ml; 1 μg/ml VISTA IgG1Fc biotinylated proteins were pretreated with indicated concentration of anti‐human VISTA antibodies or isotype controls, then added into VSIG‐3‐coated ELISA plates. (c) Co‐immunoprecipitation of protein extracts from peripheral blood mononuclear cells with Dynabeads M‐280 Streptavidin magnetic beads coupled to biotinylated VSIG‐3, VSIG‐3 ECD V−type immunoglobulin−like domain (VSIG‐3 VD) and C−type immunoglobulin−like domain (VSIG‐3 CD) Fc fusion proteins were analyzed by SDS–PAGE followed by immune blot using anti‐human VISTA antibody. Results are representative of three independent experiments.
To evaluate the interaction of VSIG‐3 with VISTA on a biological level, we performed a co‐immunoprecipitation experiment. Biotinylated VSIG‐3, VSIG‐3 ECD V−type immunoglobulin−like domain (aa 23–136) and C−type immunoglobulin−like domain (aa 144–241) Fc fusion proteins were incubated with human PBMC lysates. Biotinylated VSIG‐3/VISTA protein complexes were recovered on magnetic beads coupled with streptavidin and analyzed by Western blotting with anti‐human VISTA. Consistent with our functional ELISA binding assay, VSIG‐3 full‐length, V‐type immunoglobulin‐like domain and C‐type immunoglobulin‐like domain Fc fusion proteins bind to cell‐membrane‐bound VISTA (Fig. 1c). This result indicates that VSIG‐3/VISTA interaction is biologically relevant and may represent a new independent pathway related to the classic B7 family.
VSIG3 as a ligand of VISTA inhibits anti‐CD3‐induced cytokine and chemokine production from human PBMCs
Previous studies by Northern blotting indicated that the VSIG‐3 gene is expressed in the testis and ovary and at lower levels in the brain, kidney, and skeletal muscles. In addition, semi‐quantitative RT‐PCR analysis suggested that some gastric cancer cells and hepatoma cells over‐expressed VSIG‐3.8 Human VSIG‐3 contains 409 aa and has an N‐terminal signal peptide, a 219‐aa ectodomain composed of IgV‐IgC domains, a transmembrane region, and a 169‐aa cytoplasmic tail (Fig. 2). Homology search via various databases shows that VSIG‐3 shares significant homology with the B7 family and has varying levels of amino acid identity with human B7‐1 (26%), B7‐2 (26%), B7‐H1/PD‐L1 (24%), B7‐H2/ICOS‐L (43%), B7‐DC/PD‐L2 (23%), B7‐H3 (24%), B7‐H4 (24%), and B7‐H7/HHLA2 (27%). B7‐1, the founding member of the B7 family, only shares 13–21% homology with other human B7 molecules.
Figure 2.
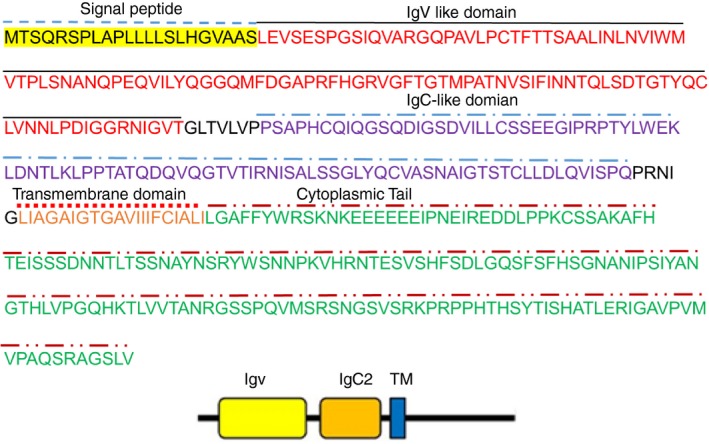
The predicted structure of human VSIG‐3. Signal peptide, IgV‐like and IgC‐like domains, transmembrane region, and the cytoplasmic tail of human VSIG‐3 protein are indicated.
The biological functions of VSIG‐3 are still unknown. In an effort to discover the potential functions of VSIG‐3, we treated human PBMCs with plate‐coated VSIG‐3‐IgG1Fc in the presence of anti‐CD3 antibody to mimic T‐cell receptor ligation. The cytokine and chemokine profile in the cell culture supernatant was determined using the Proteome Profiler™ Human Cytokine Array Kit. Interestingly, immobilized VSIG‐3 IgG1Fc significantly inhibited anti‐CD3‐induced IL‐17, CCL5/Rantes, CCL3/MIP‐1α, and CXCL11/I‐TAC secretion from human PBMCs (Fig. 3a).
Figure 3.
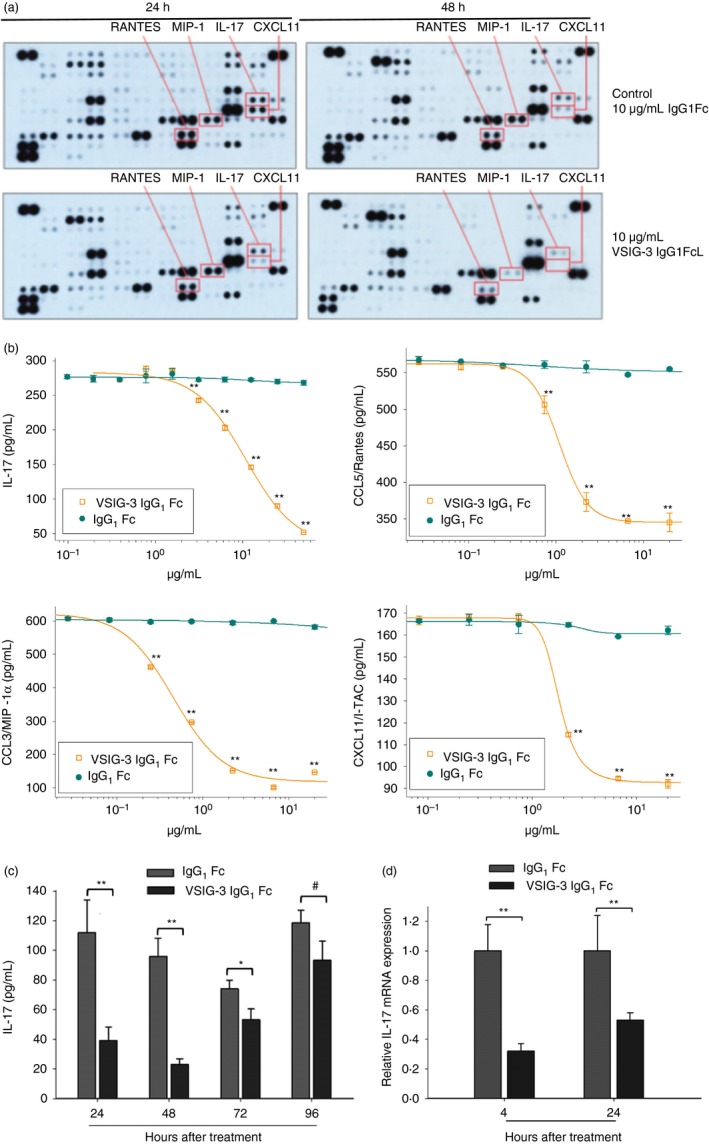
Immobilized VSIG‐3 protein inhibited cytokine and chemokine production from anti‐CD3 activated T cells in peripheral blood mononuclear cells (PBMCs). (a) Human PBMCs were treated with a combination of plate‐bound anti‐CD3 (1 μg/ml) and either plate‐bound VSIG‐3 IgG1Fc (10 μg/ml) or control IgG1Fc (10 μg/ml) for 24 or 48 hr. The cytokine levels of the supernatants were measured using the Proteome Profiler™ Human Cytokine Array Kit. (b) Array results were further confirmed by measuring individual cytokines using Quantikine® ELISA kits. VSIG‐3 significantly reduced production of interleukin‐17 (IL‐17), CCL5/Rantes, CCL3/MIP‐1α, and CXCL11/I‐TAC from anti‐CD3 activated PBMCs in a dose‐dependent manner. (c) The level of anti‐CD3‐induced IL‐17 secretion from PBMCs at different time‐points after VSIG‐3 treatment was measured by ELISA. (d) The mRNA expression level for IL‐17 in VSIG‐3 IgG1Fc or control IgG1Fc treated PBMCs was assessed by real‐time PCR, **P < 0·01, *P < 0·05, # P > 0·05 compared with the IgG1Fc controls. Results are representative of three independent experiments.
To confirm the cytokine array data, PBMCs were treated with varying concentrations of plate‐coated VSIG‐3 IgG1Fc in the presence of anti‐CD3 antibody. Interleukin‐17, CCL5/Rantes, CCL3/MIP‐1α, and CXCL11/I‐TAC secretion in cell culture supernatant was measured using Quantikine® ELISA kits. Consistent with our cytokine array data, VSIG‐3 significantly reduced the production of anti‐CD3‐induced IL‐17, CCL5/Rantes, CCL3/MIP‐1α, and CXCL11/I‐TAC from human PBMCs in a dose‐dependent manner (Fig. 3b). Furthermore, VSIG‐3 inhibited anti‐CD3‐induced IL‐17 secretion on PBMCs from 24 to 72 hr following VSIG‐3 treatment. Robust inhibitory effect on IL‐17 secretion was seen at 24 and 48 hr after VSIG‐3 treatment (Fig. 3c). In correlation with IL‐17 protein expression levels, VSIG‐3 also significantly inhibited anti‐CD3‐induced IL‐17 mRNA expression on PBMCs (Fig. 3d). The reduced secretion of IL‐17, which plays a critical role in the immune response to infection and the reduced levels of CCL5/Rantes, CCL3/MIP‐1α, and CXCL11/I‐TAC, which are important for the infiltration of T cells, monocytes, dendritic cells, and macrophages into tissues, suggests that VSIG‐3 may have an important role in negative regulation of the immune system.
To confirm that VSIG‐3 expressed on cell surface can inhibit T‐cell activation such as immobilized VSIG‐3 IgG1 Fc, Baf/3 cells were transduced with retrovirus expressing VSIG‐3. The cell surface expression of VSIG‐3 was confirmed by anti‐human VSIG‐3 staining and flow cytometry analysis. As shown in Fig. 4(a), over 95% of Baf‐3‐VSIG‐3 cells expressed VSIG‐3 on the cell surface. Baf/3‐VGIS‐3 or Baf/3 cells treated with Mitomycin C were co‐cultured with human PBMCs in the presence of anti‐human CD3. Co‐culture of Baf/3‐VSIG‐3 cells with PBMCs produced less IFN‐γ (353 ± 12 pg/ml versus 805 ± 25 pg/ml) and IL‐17 (205 ± 9 pg/ml versus 323 ± 14 pg/ml) cytokines compared with co‐culture of Baf/3 cells with PBMCs (Fig. 4b,c), suggesting that VSIG‐3 expressed on the cell surface can suppress the T‐cell‐mediated immune response.
Figure 4.
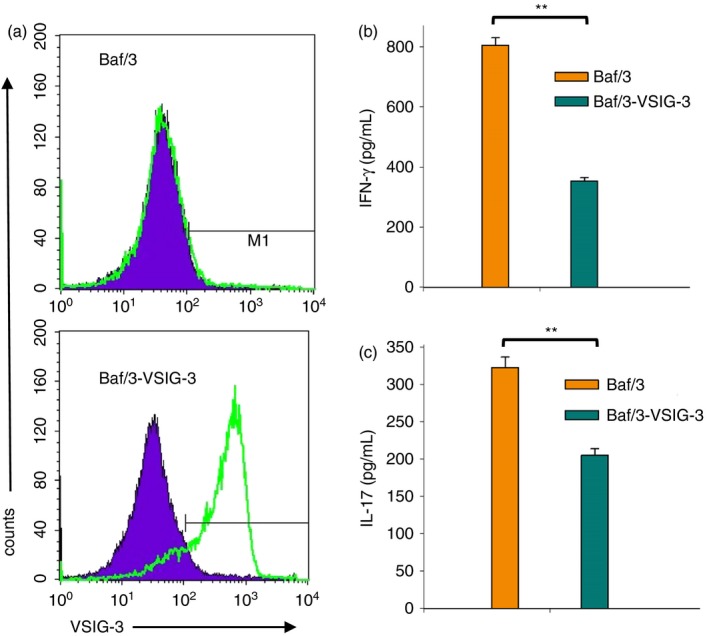
VSIG‐3 expressed on cell surface suppressed cytokine production from anti‐CD3 activated T cells in peripheral blood mononuclear cells (PBMCs). Mitomycin C pretreated Baf/3‐VSIG‐3 or Baf/3 cells were co‐cultured with human PBMCs in the presence of 1 μg/ml anti‐human CD3 for 24 hr. The interferon‐γ (IFN‐γ) and interleukin‐17 (IL‐17) level in the cell culture supernatant was measured using Quantikine® ELISA kits.
To further evaluate whether the VSIG‐3/VISTA interaction mediates the VSIG‐3 immunosuppressive effect, we treated human PBMCs with plate‐coated VSIG‐3‐IgG1Fc in the presence of anti‐CD3 antibody and various concentrations of human recombinant VISTA‐IgG1Fc. Soluble VISTA‐IgG1Fc protein pretreatment abrogated VSIG‐3‐induced IL‐17 and CCL5/Rantes inhibition in a dose‐dependent manner (Fig. 5a,b), indicating that the effect of VSIG‐3 on CCL5/Rantes inhibition is through the VSIG‐3/VISTA pathway. Furthermore, we incubated anti‐human VISTA antibody pretreated human PBMCs with plate‐coated VSIG‐3‐IgG1Fc in the presence of anti‐CD3 antibody. The anti‐human VISTA neutralization antibody pretreatment significantly attenuated VSIG‐3‐induced IL‐17 inhibition in a dose‐dependent manner (Fig. 5c). Taken together, these data support our suggestion that VSIG‐3 is a ligand of VISTA, and the VSIG‐3/VISTA interaction has profound co‐inhibitory functions on human T‐cell responses.
Figure 5.
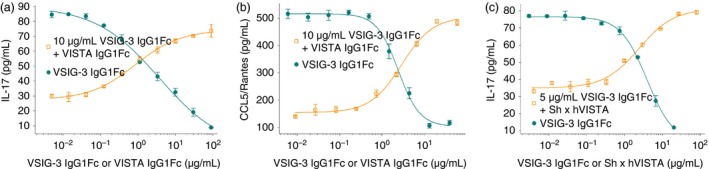
VSIG‐3 is a specific ligand of VISTA. (a, b) The extracellular domain of VISTA protein attenuates VSIG‐3 functions. Pretreatment of immobilized VSIG‐3 IgG1Fc (10 μg/ml) with the indicated concentrations of VISTA IgG1Fc for 1 hr significantly attenuated the ability of VSIG‐3 to inhibit interleukin‐17 (IL‐17) and CCL5/Rantes secretion from anti‐CD3‐activated peripheral blood mononuclear cells (PBMCs). (c) Anti‐VISTA antibody attenuates VSIG‐3 inhibitory functions. Human PBMCs were treated with immobilized anti‐CD3 (1 μg/ml) and various concentrations of VSIG‐3 IgG1Fc, or 5 μg/ml of VSIG‐3 IgG1Fc plus indicated concentrations of sheep anti‐human VISTA antibody or sheep IgG as a control. VSIG‐3 significantly inhibited anti‐CD3‐induced IL‐17 production in a dose‐dependent manner. Anti‐human VISTA antibody attenuated the inhibitory ability of VSIG‐3 on IL‐17 secretion from anti‐CD3‐activated PBMCs. Sheep IgG control did not alter the inhibitory effect of VSIG‐3 on IL‐17 (data not shown). Results are representative of three independent experiments.
VSIG‐3 acts as an inhibitory ligand on human T cells
Previous studies observed substantial elevation of VISTA on both CD4+ and CD8+ T cells after T‐cell activation.6 As a result, we set forth to determine whether the effects of VSIG‐3 on PBMCs are directly targeted to T cells. To answer this question, we isolated human CD3+ T cells from PBMCs and treated them with plate‐coated VSIG‐3 IgG1Fc in the presence of anti‐CD3 antibody. As expected, VSIG3 inhibited anti‐CD3‐induced IL‐2, IFN‐γ, IL‐17, CCL5/Rantes, CCL3/MIP‐1α, and CXCL11/I‐TAC production on human T cells in a dose‐dependent manner (Fig. 6a,b). Next, human T‐cell proliferation was assessed with a fluorometric assay using the redox‐sensitive dye Alamar Blue (Resazurin). As shown in Figure 6(c), VSIG‐3 inhibited anti‐CD3‐induced human CD3+ T‐cell proliferation in a dose‐dependent manner. Moreover, VSIG‐3 mediated inhibition of T‐cell proliferation was further confirmed by measuring CFSE fluorescence intensity with gating on CD3+ cells. Consistent with Alamar Blue (Resazurin) proliferation results, CD3+ T cells proliferated strongly in the presence of plate‐bound anti‐CD3 and control IgG1Fc, with > 40% (72 hr) or 50% (96 hr) CD3+ cell dividing. In contrast, T cells proliferated weakly in the presence of plate‐bound anti‐CD3 and VSIG‐3 IgG1Fc, with < 25% (72 hr) or 35% (96 hr) CD3+ cells dividing (Fig. 6d). The results from these assays on T cells suggest that VSIG‐3 acts as an inhibitory ligand, and directly inhibits human T‐cell activation.
Figure 6.
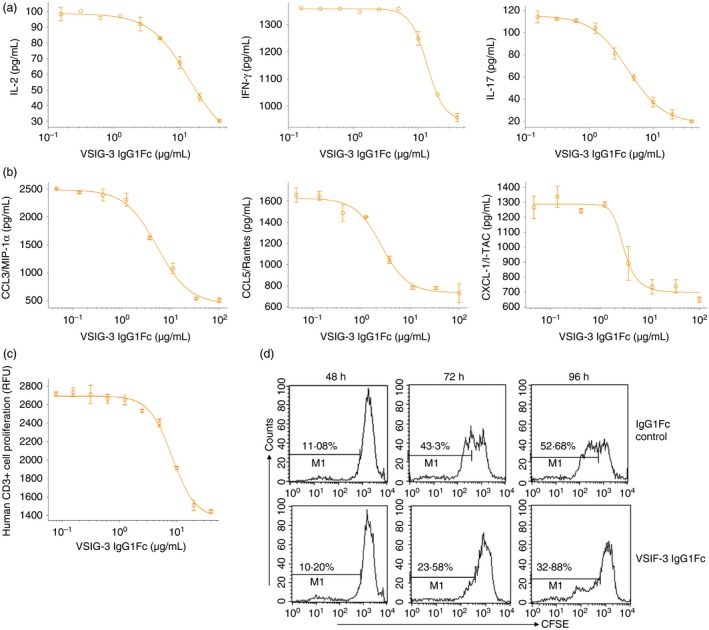
VSIG‐3 negatively regulated human T‐cell activation. (a, b) VSIG‐3 inhibits anti‐CD3‐induced interleukin‐2 (IL‐2), interferon‐γ (IFN‐γ), IL‐17, CCL5/Rantes, CCL3/MIP‐1α, and CXCL11/I‐TAC production on human CD3+ T cells in a dose‐dependent manner. Human CD3+ T cells were isolated from peripheral blood mononuclear cells (PBMCs) using a MagCellect human CD3+ T‐cell isolation kit. Human T cells were then incubated with immobilized anti‐human CD3 monoclonal antibody (1 μg/ml) and the indicated concentrations of VSIG‐3 IgG1Fc or IgG1Fc for 24 hr. IL‐2, IFN‐γ, and IL‐17 secretion into the cell culture supernatant was measured using the human IL‐2, IFN‐γ, IL‐17 CCL5/Rantes, CCL3/MIP‐1α, and CXCL11/I‐TAC Quantikine ELISA kits. (c) VSIG‐3 inhibits anti‐CD3‐induced human CD3+ T‐cell proliferation in a dose‐dependent manner. Human T cells were incubated with immobilized anti‐human CD3 (1 μg/ml) and the indicated concentrations of VSIG‐3 IgG1Fc or IgG1Fc for 72 hr. Cell proliferation was assessed by a fluorometric assay using the redox‐sensitive dye Alamar Blue (Resazurin). IgG1Fc controls did not alter anti‐CD3‐induced IL‐2, IFN‐γ, IL‐17 secretion, and cell proliferation on CD3+ T cells (data not shown). (d) CFSE‐labeled T cells were treated with a combination of plate‐bound anti‐CD3 (1 μg/ml) and either plate‐bound VSIG‐3 IgG1Fc (10 μg/ml) or control IgG1Fc (10 μg/ml) for the indicated hours. T cells were stained with anti‐CD3 and analyzed by flow cytometry. Results are representative of three independent experiments.
VSIG‐3/VISTA interaction delivers a negative co‐inhibitory signal to T cells
To investigate whether VSIG‐3/VISTA interaction is involved in T‐cell activation, we knockdown VISTA expression on T cells using VISTA siRNA. VISTA siRNA and negative control siRNA transfected T cells were treated with 1 μg/ml plate‐bound anti‐human CD3, 10 μg/ml VSIG‐3 IgG1Fc, or IgG1Fc proteins. After 24 hr of treatment, cells were harvested for testing VISTA expression to verify VISTA siRNA transfection. Cell‐free culture supernatants were collected to measure cytokine production. As expected, VISTA expression on VISTA siRNA‐transfected T cells was significantly reduced (Fig. 7a). The expression level of VISTA on T cells was correlated with the inhibitory effect of VSIG‐3 on cytokine secretion from T cells, as VSIG‐3 showed stronger IFN‐γ (66·4% versus 33·4%), IL‐2 (42·1% versus 20·4%), and IL‐17 (67·1% versus 34·3%) inhibition on negative control siRNA‐transfected T cells compared with VISTA siRNA‐transfected T cells (Fig. 7b). This result suggests that VISTA acts as a receptor for VSIG‐3 to deliver a negative signal to inhibit T‐cell activation.
Figure 7.
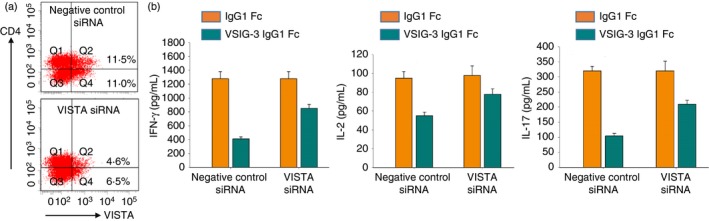
VSIG‐3/VISTA interaction inhibited interferon‐γ (IFN‐γ), interleukin‐17 (IL‐17), and IL‐2 production on human T cells. Human CD3+ T cells were isolated from peripheral blood mononuclear cells (PBMCs) using a MagCellect human CD3+ T cell isolation kit and transfected with VISTA small interfering RNA (siRNA) or negative control siRNA, then incubated with plate‐bound anti‐human CD3 (1 μg/ml), VSIG‐3 IgG1Fc (10 μg/ml), or IgG1Fc (10 μg/ml) proteins for 24 hr. (a) Cells were stained with human VISTA Alexa Fluor 488‐conjugated antibody, and human CD4 Alexa Fluor 405‐conjugated Antibody, or isotype control antibodies to assess VISTA expression. (b) The level of IFN‐γ, IL‐17, and IL‐2 secretion from T cells was assessed using the human IFN‐γ, IL‐17, and IL‐2 Quantikine ELISA kits.
Discussion
We have discovered that VSIG3 is a novel ligand for VISTA and inhibits human T cells in vitro, whereas its in vivo role remains to be validated in the future. VSIG‐3 and VISTA interaction may represent a new independent T‐cell co‐inhibitory pathway.
VSIG‐3 was originally cloned as a gene from both human and mouse that encodes a new member of the immunoglobulin superfamily that expresses preferentially in both the brain and testis and is up‐regulated in intestinal‐type gastric cancer.10 The expression of VSIG‐3 on normal immune cells and lymph tissues has not been reported. VSIG‐3 was predicted to function as an adhesion molecule based on homology and structural similarity with the tight junction protein endothelial cell‐selective adhesion molecule (ESAM),10 and it was subsequently shown to be a homophilic cell adhesion molecule.7 Recently, regulation of excitatory synaptic transmission and plasticity functions was suggested for VSIG‐3 through its interactions with the postsynaptic scaffolding protein PSD‐95 and AMPARs. Lately VSIG‐3 as a new binding partner for VISTA was identified using a cell surface displayed library, and confirmed by both fluorescence‐activated cell sorting and SPR assays.11 However, the biological significance of VSIG‐3 and VISTA interaction is still unknown. In this study, we show that in addition to its well‐established functions as an adhesion molecule, VSIG‐3 regulates T‐cell functions in vitro through its association with VISTA.
The expression and functions of VISTA are still poorly understood. It has been suggested by Wang et al. that VISTA is expressed on dendritic cells or tumor cells and acts as a ligand to inhibit T‐cell responses through an unknown receptor on CD4+ T cells.12 Flies et al. proposed an alternative explanation from Wang et al. stating that VISTA, which is constitutively expressed on CD4+ T cells, can be further up‐regulated and acts as a receptor on CD4+ T cells to transmit a negative signal.6 Therefore, VISTA functions as an immune checkpoint protein that suppresses T‐cell activation. It is possible that VISTA may have dual functionality as both a receptor and a ligand, and the unknown VISTA binding partner(s) may function as an inhibitory ligand on APCs and tumor cells or as a receptor on T cells. In this study, we provide three lines of evidence to suggest specific binding interaction of VSIG‐3 with VISTA as shown in Fig. 1: (i) the association of the ectodomains of VSIG‐3 and VISTA in the nanomolar range, (ii) the ability of anti‐VISTA antibodies to abolish the binding interaction of VSIG‐3 and VISTA, (iii) the co‐immunoprecipitation of VISTA with the ectodomain of VSIG‐3 in PBMCs. Although this work was in progress, Yang et al. identified binding of VSIG‐3 to VISTA by SPR and reported a K d value in the μm range.11 We have repeated the experiment by Yang et al. several times in a BiaCore T200. Typical data are shown in the Supplementary material (Fig. S1). Our data qualitatively agree with the previously reported SPR data. Both the association and dissociation phases are biphasic. A rapid initial association is followed by a slower association process and conversely the dissociation reaction shows a rapid initial phase followed by a much slower reaction. The slopes of the fast phases of the sensograms are too steep for reliable curve fitting and hindered quantitative interpretation. Inspection of the slow dissociation phase of the sensogram indicates that the half‐life of the slowly decaying complex is ~5 min. The data suggest that the origin of the signal in the functional ELISA binding assay (Fig. 1a), which gives an apparent K d ~3·4 nm, is due to a kinetically trapped and quite stable VSIG‐3/VISTA complex.
To assess the biological effects of VSIG‐3 on T cells, we treated human PBMCs or purified T cells with plate‐bound anti‐CD3 and VSIG‐3 IgG1Fc and measured T‐cell proliferation as well as cytokine expression profiles. Using cytokine arrays we examined the expression levels of cytokine and chemokine from anti‐CD3‐activated T cells in PBMCs and in purified T cells. The results from the cytokine array assay, which are for profiling purposes, were confirmed with ELISAs. In both approaches, the expression levels of CCL5/Rantes, CCL3/MIP‐1α, CXCL‐11/I‐TAC, IL‐17, IFN‐γ, and IL‐2 were decreased by VSIG‐3. We did not find differences in cytokine expression levels in the absence of anti‐CD3 activation following VSIG‐3 treatment. Our data suggest that the decrease of cytokine expression levels is due to an effect of VSIG‐3 on anti‐CD3‐activated T cells. VSIG‐3 also inhibited the proliferation of anti‐CD3‐activated T cells in a dose‐dependent manner (Fig. 6c). It should be noted that the decrease in cytokine secretion is most obvious at 24 and 48 hr after treatment, whereas the decrease of T‐cell numbers is observed after 72 hr of treatment (Fig. 6d). This indicates that the decrease in cytokine secretion is not directly related to the inhibition of T‐cell proliferation by VSIG‐3. Furthermore, CCL5/Rantes, CCL3/MIP‐1α, and CXCL‐11/I‐TAC are T helper type 1 (Th1) ‐attracting chemokines and preferentially attract CCR5+ Th1 cells,13 whereas IFN‐γ, IL‐17, and IL‐2 support Th1 and Th17 cell immune responses.14 The up‐regulation of VSIG‐3 in gastric cancers and hepatocellular carcinomas combined with its immune inhibitory functions suggests that VSIG‐3 could be involved in one of the mechanisms that these cancers use to inactivate the host's immune system.
The anti‐VISTA antibody and ectodomain of VISTA protein attenuate the effect of VSIG‐3 on activated T cells (Fig. 5). However, knockdown of the VISTA receptor on CD3+ cells using siRNA only shows that the expression level of VISTA on T cells correlates with the inhibitory effect of VSIG‐3 on cytokine secretion from T cells (Fig. 7). These results suggest that VISTA is a receptor for VSIG‐3, and involvement of receptors other than VISTA in VSIG‐3 signaling requires further investigation.
VSIG‐3 not only functions as a negative regulator of human T cells by inhibition of T‐cell proliferation and cytokine secretion but also negatively regulates the secretion of CCL5/Rantes, CCL3/MIP‐1α, and CXCL‐11/I‐TAC chemokines in human PBMCs and T cells (Figs 2 and 6). Our results suggest that VSIG‐3 may inhibit the infiltration of Th1 cells into the tumor tissues. Th1 cells are the primary T‐helper cell subset involved in anti‐tumor responses. They have been associated with anti‐tumor responses in mouse models, achieved in part by their secretion of IFN‐γ, which has multiple functions in the immune system's ability to control the growth of tumors or even eliminate them. Notably, this occurs through the recruitment and activation of cells of the innate immune system and enhancement of the production of anti‐tumor chemokines.15 Mouse VSIG‐3 binds the ectodomain of mouse VISTA and preliminary experiments with mouse splenic T cells (data not shown), which suggests that mouse VSIG‐3 is a modulator of the mouse immune system. Hence, it appears that VSIG‐3, in addition to its functions as an adhesion molecule, may have also evolved as an immune modulator in animals. The up‐regulation of VSIG‐3 in gastric cancers and hepatocellular carcinomas in combination with our findings demonstrating the inhibitory effects on T‐cell activation suggest that VSIG‐3 participates in the immunosuppressive environment of these cancers, and VSIG‐3/VISTA could make a potential target for immunotherapies. The expression of VSIG‐3 on APCs and in lymph tissues is not defined. Knowledge about the expression profile of VSIG‐3 on immune cells may allow the design of experiments to address the physiological significance of VSIG‐3 in the regulation of a normal immune response. The experimental studies are underway in our laboratory.
In summary, we describe a new VSIG‐3/VISTA T‐cell co‐inhibitory pathway (Fig. 8). The monoclonal antibodies against PD‐1 are currently used for cancer treatment. Further studies on the VSIG‐3/VISTA pathway may shed light on how to modulate this pathway as a novel immunotherapeutic strategy against human cancers, as well as autoimmune and infectious diseases.
Figure 8.
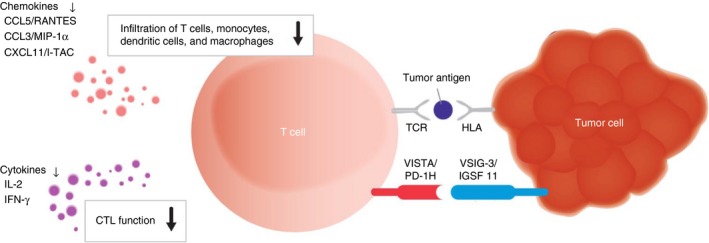
Illustration of VISG3/VISTA interaction. VSIG‐3/VISTA interaction delivers a negative co‐inhibitory signal to T cells.
Disclosures
The authors have no commercial or financial conflict of interest.
Supporting information
Figure S1. VSIG3 associates with VISTA in a biphasic binding reaction. VISTA was immobilized on a CM5 chip at 4000 RU, similar conditions to those in Yang et al.15 Data were collected at 25° using a flow rate of 30 µl/min. The VSIG‐3 concentrations for obtaining the sensograms were 3·0, 1·5, 0·75, 0·375, and 0·187 µm. The buffer was PBS, 0·05% Tween‐20.
Appendix S1. Biphasic reaction kinetics for the association and dissociation reaction of the VSIG‐3/VISTA interaction.
Acknowledgements
We thank the personnel of the Molecular Biology, Cell Culture, and Antibody Development Departments for their support and production of the recombinant proteins and antibodies. We thank Zumari Chatham in the Marketing Department for helping us to edit the figures. We would also like to thank Frank Mortari and Tim Manning for manuscript editing.
Contributor Information
Jinghua Wang, Email: jinghua.wang@bio-techne.com.
Vassilios Kalabokis, Email: Vassili.Kalabokis@bio-techne.com.
References
- 1. Pitt JM, Vétizou M, Daillère R, Roberti MP, Yamazaki T, Routy B et al Resistance mechanisms to immune‐checkpoint blockade in cancer: tumor‐intrinsic and ‐extrinsic factors. Immunity 2016; 44:1255–69. [DOI] [PubMed] [Google Scholar]
- 2. Larkin J, Chiarion‐Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD et al Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ma W, Gilligan BM, Yuan J, Li T. Current status and perspectives in translational biomarker research for PD‐1/PD‐L1 immune checkpoint blockade therapy. J Hematol Oncol. 2016; 9:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lines JL, Sempere LF, Broughton T, Wang L, Noelle R. VISTA is a novel broad‐spectrum negative checkpoint regulator for cancer immunotherapy. Cancer Immunol Res 2014; 2:510–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Le Mercier I, Chen W, Lines JL, Day M, Li J, Sergent P et al VISTA regulates the development of protective antitumor immunity. Cancer Res 2014; 74:1933–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Flies DB, Han X, Higuchi T, Zheng L, Sun J, Ye JJ et al Coinhibitory receptor PD‐1H preferentially suppresses CD4⁺ T cell‐mediated immunity. J Clin Invest. 2014; 124:1966–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harada H, Suzu S, Hayashi Y, Okada S. BT‐IgSF, a novel immunoglobulin superfamily protein, functions as a cell adhesion molecule. J Cell Physiol 2005; 204:919–26. [DOI] [PubMed] [Google Scholar]
- 8. Watanabe T, Suda T, Tsunoda T, Uchida N, Ura K, Kato T et al Identification of immunoglobulin superfamily 11 (IGSF11) as a novel target for cancer immunotherapy of gastrointestinal and hepatocellular carcinomas. Cancer Sci 2005; 96:498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jang S, Oh D, Lee Y, Hosy E, Shin H, van Riesen C et al Synaptic adhesion molecule IgSF11 regulates synaptic transmission and plasticity. Nat Neurosci 2016; 19:84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Katoh M, Katoh M. IGSF11 gene, frequently up‐regulated in intestinal‐type gastric cancer, encodes adhesion molecule homologous to CXADR, FLJ22415 and ESAM. Int J Oncol 2003; 23:525–31. [PubMed] [Google Scholar]
- 11. Yang W, Padkjær SB, Wang J, Sun Z, Shan B, Yang L et al Construction of a versatile expression library for all human single‐pass transmembrane proteins for receptor pairings by high throughput screening. J Biotechnol 2017; 260:18–30. [DOI] [PubMed] [Google Scholar]
- 12. Wang L, Rubinstein R, Lines JL, Wasiuk A, Ahonen C, Guo Y et al VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med 2011; 208:577–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lebre MC, Burwell T, Vieira PL, Lora J, Coyle AJ, Kapsenberg ML et al Differential expression of inflammatory chemokines by Th1‐ and Th2‐cell promoting dendritic cells: a role for different mature dendritic cell populations in attracting appropriate effector cells to peripheral sites of inflammation. Immunol Cell Biol 2005; 83:525–35. [DOI] [PubMed] [Google Scholar]
- 14. Holley MM, Kielian T. Th1 and Th17 cells regulate innate immune responses and bacterial clearance during central nervous system infection. J Immunol 2012; 188:1360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Terhune J, Berk E, Czerniecki BJ. Dendritic cell‐induced Th1 and Th17 cell differentiation for cancer therapy. Vaccines (Basel). 2013; 1:527–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. VSIG3 associates with VISTA in a biphasic binding reaction. VISTA was immobilized on a CM5 chip at 4000 RU, similar conditions to those in Yang et al.15 Data were collected at 25° using a flow rate of 30 µl/min. The VSIG‐3 concentrations for obtaining the sensograms were 3·0, 1·5, 0·75, 0·375, and 0·187 µm. The buffer was PBS, 0·05% Tween‐20.
Appendix S1. Biphasic reaction kinetics for the association and dissociation reaction of the VSIG‐3/VISTA interaction.


