Summary
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS) affecting young people and leading to demyelination and neurodegeneration. The disease is clearly more common in women, in whom incidence has been rising. Gender differences include: earlier disease onset and more frequent relapses in women; and faster progression and worse outcomes in men. Hormone‐related physiological conditions in women such as puberty, pregnancy, puerperium, and menopause also exert significant influence both on disease prevalence as well as on outcomes. Hormonal and/or genetic factors are therefore believed to be involved in regulating the course of disease. In this review, we discuss clinical evidence for the impact of sex hormones (estrogens, progesterone, prolactin, and testosterone) on MS and attempt to elucidate the hormonal and immunological mechanisms potentially underlying these changes. We also review current knowledge on the relationship between sex hormones and resident CNS cells and provide new insights in the context of MS. Understanding these molecular mechanisms may contribute to the development of new and safer treatments for both men and women.
Keywords: gender, multiple sclerosis, pregnancy, sex hormones
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS) affecting young people and leading to demyelination and neurodegeneration. Although its etiology is not clear, autoimmunity, influenced by environmental and/or genetic factors, is known to play a major role in disease pathogenesis.
Clearly more common in women, prevalence in females has been rising, causing an increased gender bias with a current female to male ratio of 3 : 1.1 Important gender differences in progression and inflammatory activity of disease have been observed. Women experience more frequent relapses in relapsing–remitting (RRMS) forms, whereas men accumulate disability faster, reach disability milestones more rapidly, and show poorer recovery after initial disease relapse.2
Hormonal and/or genetic factors are therefore presumably involved in regulating the course of the disease, and sex hormones such as estrogens, progesterone, prolactin, and androgens probably play a role in these complex mechanisms. Indeed, different hormone‐related physiological conditions in women, such as puberty, pregnancy, puerperium, and menopause, significantly impact the frequency and course of disease.
In this review, we discuss clinical evidence of the impact of hormonal factors in MS and attempt to elucidate the complex hormonal and immunological mechanisms potentially underlying these effects. Understanding these molecular mechanisms may contribute to the development of new and safer treatments for both men and women.
Clinical observations
Differences in prevalence and clinical characteristics
Prevalence differences between genders occur in most autoimmune disorders, with women generally more frequently affected than men. In MS, gender prevalence bias may be increasing. In the 1980s, female to male prevalence was approximately 2 : 13 but recent reports indicate levels up to 3 : 1.1, 4, 5 This trend is noted primarily in RRMS forms and has been noted not only in western societies but also in Iran6 and Latin America.7 Gender bias seems to be associated with a latitudinal gradient, as more significant increases are observed in northern latitudes, worldwide.8 Given that changes in MS prevalence have occurred in a short time, they are most likely related to a combination of behavioral and/or environmental factors together with epigenetic mechanisms.
Over the past 50 years, lifestyle changes in women related to smoking habits, use of birth control, diet, obesity, and sunlight exposure could explain incidence differences. Also, women today tend to have fewer children, at an older age. Interestingly, there is evidence to suggest an association between parity and MS risk in women.9 In one study, greater number of offspring was linked to reduced risk of first clinical demyelinating event; this, in combination with older maternal age at childbirth, may help to explain the current increase in MS prevalence in women.9
Both MS symptoms as well as their severity also appear to differ between men and women, with women experiencing more frequent relapses,10 developing more inflammatory lesions on magnetic resonance imaging (MRI) and presenting earlier onset.11, 12 Men, on the other hand, show faster progression and worse outcomes13 with more cerebellar involvement, cognition impairment, gray matter atrophy,14 and T1 lesions.15 Clinical MS phenotypes have been recently reported to differ with race/ethnicity, for example Hispanics and African Americans are at risk of a more severe early disease course.16 Nevertheless, most of the sexual dimorphism findings have been replicated in non‐western societies.17, 18 The main differences found between sexes are summarized in Fig. 1.
Figure 1.
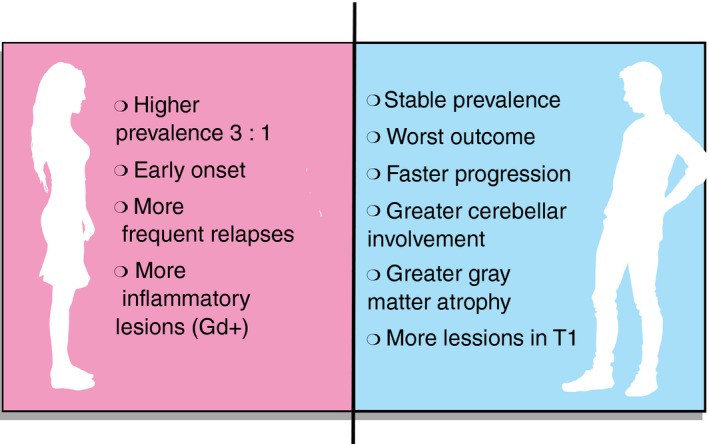
Main differences in course of multiple sclerosis between women and men.
Recently, an association was reported between gender identity disorders and MS risk,19 in which adjusted relative risk of MS in men with gender identity disorders was significantly increased, suggesting the influence of feminizing hormones or low testosterone levels on risk, providing further evidence of the importance of sex hormones in the pathophysiology of the disease. Intriguingly, gender differences like the ones described are not observed in women with late‐onset MS (diagnosed over 50 years of age), suggesting that complex hormonal mechanisms related to menopause or aging might be at play (see section on Menopause).
Menarche and puberty
Gender bias is not seen in MS before puberty20 but there is evidence that age at menarche may be related to age of disease onset,21 further linking sex hormones to MS risk during childhood and adolescence.
Menarche is currently occurring 1–4 months sooner among European women born since 1935 for each decade monitored, probably due to changes in nutrition, hygiene, and general health conditions in western populations.22
This finding coincides with an increase in MS incidence and several studies have now shown that early menarche is associated with increased risk of MS and earlier onset of disease.21, 23, 24 No differences were found in age of puberty between males with MS and controls. Conversely, age at menarche among women with MS was generally lower than in women not developing the disease, suggesting an inverse association between age at menarche and MS risk. For each year of age that menarche is delayed, MS risk falls 13%.21
In addition, puberty affects the course of MS, as relapse rates increase in young girls during the peri‐menarcheal period.25 There also seems to be association between older age at menarche and reduced risk of reaching the EDSS 6 milestone in progressive MS.26
It is important to note that higher body weight has been linked to earlier onset of puberty in girls. Hormonal pathways activated during puberty and in obese individuals impact the immune system, with potentially different effects on MS triggers. A role for adiposity‐related inflammatory mechanisms is also supported by reports of elevated fat tissue markers in MS such as leptin.27, 28
Pregnancy
Effect on MS relapses
Evidence for an effect of pregnancy on MS activity comes from studies reporting 70% decrease in relapse rates during the third trimester compared with pre‐pregnancy levels, and increased relapse rates 3–6 months after delivery, to levels almost three times higher than pre‐pregnancy ones.29
Normal pregnancy induces physiological changes including elevated cardiac output, increased lipid levels with weight gain, as well as changes in estriol, progesterone, prolactin, α‐fetoprotein, leptin, glucocorticoids, and insulin growth factor. Early during pregnancy, immune tolerance develops in order to protect the fetus against rejection, through regulatory T (Treg) cell elevation, reduction in T helper cells (Th)1/Th17 activity and increase in Th2 activity. Total number of natural killer (NK) cells falls, although the proportion of CD56bright NK cells increases in late pregnancy.30, 31
In the third trimester and during early postpartum, interleukin‐12 (IL‐12) production increases threefold and tumor necrosis factor‐α (TNF‐α) levels drop 40% compared with late postpartum values. A shift to a Th2 profile with increased IL‐10 and decreased CXCR3 expression by CD4+ and CD8+ cells occurs, with increased expression of the chemokine receptor CXCR4 and the mRNA IL‐10/interferon‐γ (IFN‐γ) ratio.30 In addition, there is elevation of proteins of the heat‐shock family with immunomodulatory capacity, as well as of tolerance‐promoting molecules, including HLA‐G, CD200, Fas ligand, α‐fetoprotein, and indoleamine 2,3‐dioxygenase.31
Additional mechanisms that may play a role in MS amelioration during pregnancy include: production of IFN‐τ by human placenta trophoblast cells32 (structurally similar to IFN‐β), and elevation of estrogen and progesterone levels, which can induce changes in the IFN‐γ promoter region, and in T‐bet, FoxP3, programmed cell death protein 1, and cytotoxic T‐lymphocyte antigen 4 transcription factors. These hormones also exert profound effects on B cells, increasing expression of different genes such as cd22, shp‐1, bcl‐2, and vcam‐1.33
Human chorionic gonadotropin (hCG) is a glycoprotein hormone synthesized by syncytiotrophoblast cells immediately after embryonic implantation, inducing profound down‐regulation of maternal cellular immunity against trophoblastic paternal antigens. There is increasing evidence indicating that hCG alters dendritic cell activity through up‐regulation of indoleamine 2,3‐dioxygenase, reducing T‐cell activation and cytokine production, as well as stimulating Treg cell recruitment to the fetal–maternal interface. These changes are critical in promoting maternal tolerance, and may also ultimately influence immune responses in pregnant women with MS.34 Furthermore, up to 95% of CD19+ CD24hi CD27+ regulatory B cells also express the hCG (LH/hCGR) receptor. Addition of human recombinant hCG to isolated CD19+ B cells in vitro induces strong production of IL‐10, a potent anti‐inflammatory cytokine.35 Based on these findings, investigators believe that hCG may drive an expansion of IL‐10‐producing regulatory B cells during normal pregnancy, so controlling undesired immune activation (which would otherwise jeopardize fetal well‐being), with the added effect of contributing to control of MS during pregnancy.36
Little is known about the effects on MS of microchimerism (bidirectional trafficking of cells between mother and fetus), but it may play a role in disease pathogenesis. Of interest, risk of disability does not increase in women with MS having children with more than one partner, as would be the case if the immune response to paternal genes was harmful.37
Effect of pregnancy on disability
Effects of pregnancy on disability levels in MS remain controversial. Given the beneficial effect of pregnancy on relapse rates and on inflammatory changes, it could afford protection against relapse‐related disability. In a cohort of 2466 patients followed for 10 years, more pregnancies were independently associated with lower disability scores.38
However, although some studies show that women with MS who have been pregnant have less progression of disability39 and propose that these effects might even be cumulative since multiparous women seem to have better outcomes,26 other studies have shown no effect on permanent disability scores.40, 41, 42
Importantly, such observational studies are prone to selection bias given the fact that rigorous matching of groups at baseline is impossible. For example, young women with severe disease at onset are less likely to become pregnant.
Taken together, currently available data do not provide convincing evidence that there is a significant beneficial effect of pregnancy on long‐term disability, although it does seem clear that pregnancy does not increase risk of secondary progression.43
Figure 2 summarizes the most important hormonal and immune mechanisms observed during pregnancy.
Figure 2.
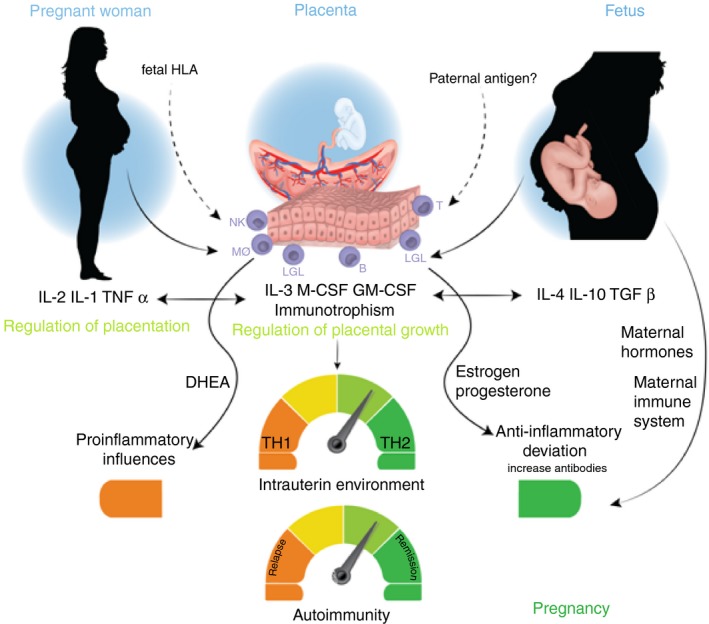
During pregnancy, influences from the placenta, the mother and the fetus condition placentation, regulating placental growth predisposing regulation of a Th2‐like anti‐inflammatory immune response. During this period, it is possible to observe a marked increase in the levels of estrogens, progesterone, glucocorticoids, and activated vitamin D coming from both the mother and the placenta. Taken together, these factors decrease maternal cellular immunity, evidenced by decreased number of multiple sclerosis exacerbations during pregnancy, particularly during the third trimester. B, B cells; DHEA, dehydroepiandrosterone; GM‐CSF, granulocyte–macrophage colony‐stimulating factor; IFN, interferon; LGL, large granular lymphocytes; M‐CSF, macrophage colony‐stimulating factor; MS, multiple sclerosis; MΦ, macrophages; NK, NK cells; T, T cells.
Puerperium
During puerperium, a clear increase in relapse rates has been observed.44 Reasons for increased disease activity postpartum include abrupt drop in estrogen, progesterone, and glucocorticoids levels and rapid normalization of immune function to pre‐pregnancy conditions. In fact, after delivery the situation can even be attributed to what has been defined as an immune reconstitution inflammatory syndrome‐like phenomenon, secondary to rebound of pro‐inflammatory cell types and functions as a consequence of fetal and placental delivery. Concurrently, the rise of hormones involved in breastfeeding mechanisms activated at this time may also play a role in increasing disease activity.
Figure 3 summarizes the most important puerperal changes in hormones and immune mechanisms.
Figure 3.
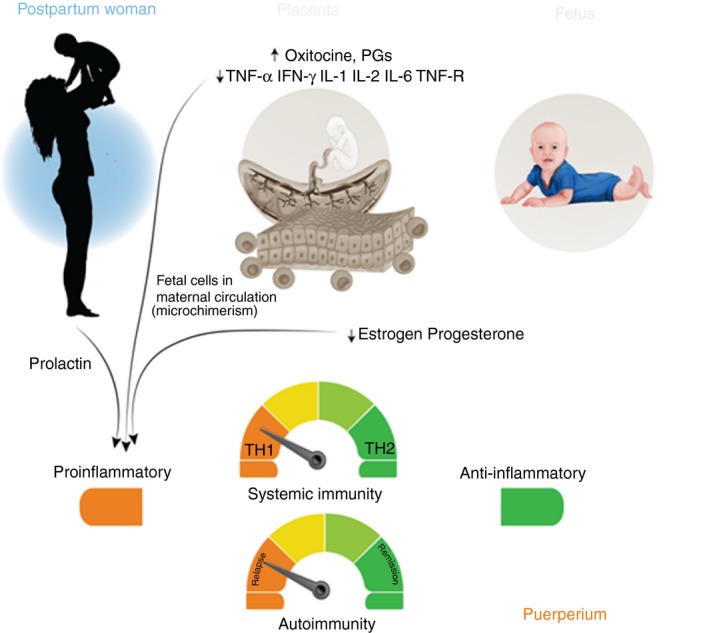
After childbirth, the protective influence of the placenta and the fetus is lost, and a pro‐inflammatory intrauterine environment prevails, determined mainly by maternal factors. A fall in the levels of estrogen, progesterone, glucocorticoids, and activated vitamin D is observed. Together these factors may condition an increase in the number of exacerbations observed during the postpartum period. PGs, Prostaglandins.
The most reliable clinical predictors of postpartum relapse are: number of relapses during the year before pregnancy, number of relapses during pregnancy, and disability score at pregnancy onset.40, 44 Patients with MS should plan carefully for pregnancy, striking a balance between the desire to become a parent and that of maintaining disease quiescence.
Breastfeeding
Prolactin (PRL), oxytocin, progesterone, and glucocorticoids all increase during breastfeeding. As will be discussed below, the role of PRL in MS pathogenesis remains unclear. It has been shown to have beneficial effects through protection against excitotoxicity and by enhancing remyelination.45 By contrast, prolactin levels were found to be higher in patients with MS compared with controls, which seems to promote B‐cell autoreactivity.46 Furthermore, hyperprolactinemia may be associated with clinical relapses in MS, especially among patients with hypothalamic lesions and/or optic neuritis.47 Controversy also exists regarding the role of breastfeeding in MS: while some authors suggest that breastfeeding protects against relapses48, 49, 50 and that women who nurse infants longer may be at lower subsequent risk of developing MS;51 others indicate that lactation does not suppress the disease52 nor have any effect on relapses.53 Again, these studies are prone to selection bias because mothers with more active disease before pregnancy are less likely to breastfeed or will do so for shorter periods in order to restart disease‐modifying therapies (DMT) sooner. In fact, a recent meta‐ analysis showed that women who breastfed were significantly less likely to use DMTs before conception, and had fewer relapses during pregnancy.54
Overall, no clear evidence exists that breastfeeding provides sufficient protection from relapses to warrant not restarting DMTs shortly after giving birth, in women with active relapsing disease before pregnancy. Individual patients and their clinicians must weigh their decision to breastfeed on a case‐by‐case basis.
Fertility treatments
There are no clear data to indicate that women experiencing MS suffer impaired fertility.55 Because general infertility rates are 10–20% for couples in western countries,56 infertility in women with MS may represent co‐occurrence, such that some may seek fertility treatments. Assisted reproductive technology (ART) has generated a rise in the number of live births.56 This generally involves the use of hormone therapy including: gonadotropin‐releasing hormone (GnRH) agonists or antagonists, follicle‐stimulating hormone (FSH), luteinizing hormone (LH), hCG, and/or progesterone to induce ovulation or to assist implantation.
Several studies have reported an increase in annualized relapse rates after ART use, especially in women treated with GnRH agonists, or when fertilization treatments failed.57, 58, 59, 60, 61 Mechanisms proposed include: cessation of DMT, stressful events associated with infertility, and immunological changes induced by GnRH, as well as augmented immune cell migration across the blood–brain barrier.60 It would seem prudent to recommend caution when women with MS undergo ART and monitor carefully for intervention as necessary.
Oral contraceptives
Because oral contraceptives (OCs) contain estrogens (or estrogen receptor regulators) and progestogens, their influence on MS has been investigated, although most studies did not specify OC combinations or type, suggesting that differences in hormones and dosage are likely.
Regarding risk of developing MS with OC use, some studies failed to find an association,62, 63 whereas others showed short‐term reduction in risk of MS.64 In opposition to these findings, a recent study on OC use in women with MS was associated with slightly increased risk of MS and clinically isolated syndrome (CIS).65
Concern over whether OCs could affect disease symptoms, activity and/or progression has also been expressed. In fact, one study reported that OC use was associated with a higher risk of reaching EDSS 6,26 even though most epidemiological evidence has not found a negative effect on MS and some studies even suggest a positive effect, not only on symptoms66 but also on MRI activity (when used in combination with IFN‐β1a)67 and on progression of disability.68
Menopause
Little information is available concerning possible influences of menopause and hormone replacement therapy (HRT) on the course of MS.
Any effects of menopausal hormone changes per se, may be confounded by age‐related changes in MS disease activity and co‐morbidities. Monitoring of patients who developed MS after the age of 50 suggested similar rates of disease progression for both sexes, with men experiencing more rapid progression if disease onset occurred between 18 and 49 years.69
In the past, several studies have reported worsening of MS symptoms at menopause.70 More recently, however, improvement in MS symptoms and quality of life (QOL) was found in postmenopausal MS patients receiving HRT,71 although it was not clear whether hormone replacement use improved physical QOL, or reflected the fact that women with better physical QOL were likely to receive age‐appropriate medical care, including HRT.
Worsening of MS after menopause suggests that gonadal steroid deprivation occurring at this time may be causally linked to neurodegeneration, as has been observed in a recent study in which ovarian aging, measured according to anti‐Müllerian hormone (AMH) levels, was associated with gray matter volume loss as well as with higher degree of disability in women with MS, independent of chronological age and disease duration.72 Some studies have demonstrated lower serum AMH levels in women with MS of childbearing age,73 but others only found low AMH levels in patients with higher disease activity.74
In summary, MS disability after menopause seems to worsen. Nevertheless, it should be pointed out that most studies on this issue do not include cohorts of men as a comparison group, which would be necessary to distinguish the effects of ageing per se from those linked to loss of reproductive function. Accelerated ovarian aging may represent one of several factors influencing MS.
Effects of the female reproductive cycle on MS are summarized in Fig. 4.
Figure 4.
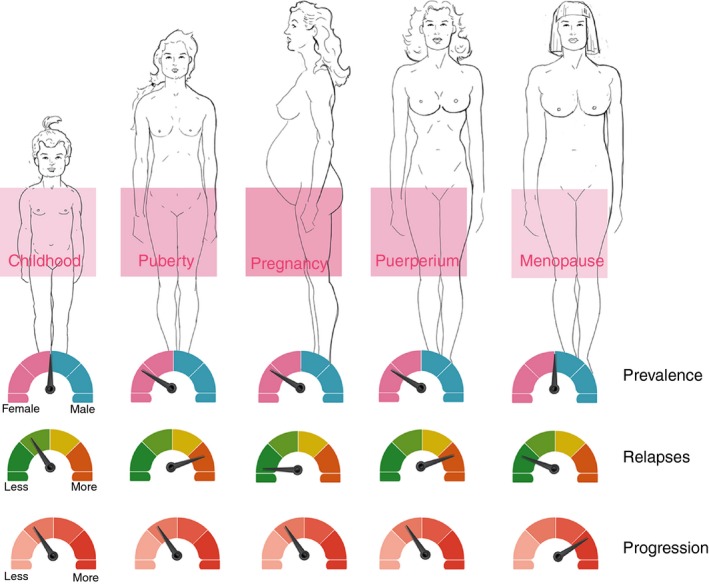
Effects of the female reproductive cycle on multiple sclerosis. Before puberty, multiple sclerosis (MS) prevalence is similar between boys and girls. After menarche, rates in girls are three times higher. During pregnancy, a decrease in relapse rates of approximately 70% is observed in the third trimester. After delivery, relapse rates increase to nearly three times higher than pre‐pregnancy levels during the first 3–6 months. Recent evidence suggests worsening of MS symptoms at menopause, and probably increased disease progression.
Sex hormones
Because of the presence of hormone receptors on immune cells, sex hormones, such as estrogens, progesterone, androgens, and prolactin, can influence different aspects of immune system function, potentially affecting risk, activity, and progression of MS. Sex hormone effects will vary depending not only on their concentration but also on the type of target cell and the receptor subtype expressed on a given cell type. Hence, understanding how gender impacts autoimmunity in general and MS in particular, requires elucidation of complex interactions occurring between sex hormones, sex chromosomes, and immune response genes. In the following section, we will discuss current knowledge and new insights into the relationship of sex hormones and the immune system, as well as with resident CNS cells, in the context of MS.
Estrogens
Endogenous estrogens produced in female mammals include estrone (E1), 17β‐estradiol (E2) and estriol (E3, produced only in pregnancy). E2 is the predominant form in premenopausal women. Estrogen receptors (ERs) exist in two forms: ERα (NR3A1) and ERβ (NR3A2). These receptors act as ligand‐activated transcription factors and, therefore, directly regulate a broad range of estrogen‐responsive genes present in all cells of the innate and adaptive immune system.33 The main effects induced by estrogens in the cells of the immune system are summarized in Table 1.
Table 1.
Main effects regulated by estrogens in immune system cells
| Immune cell | Estrogen effect |
|---|---|
| Neutrophils |
|
| Macrophages |
|
| Dendritic cells |
|
| Th1 cells |
|
| Th2 cells |
|
| Treg cells |
|
| B cells |
|
CINC, Cytokine‐induced neutrophil chemoattractant; CCL, CC chemokine ligand; CTLA‐4, Cytotoxic T‐Lymphocyte Antigen 4; CXCL, CXC chemokine ligand; DCs, Dendritic cells; FoxP3, forkhead box P3; IFN, Interferon; IL, Interleukin; iNOS, Inducible nitric oxide synthase; PD‐1, Program Cell Death Protein‐1; TGF, Tumor growth factor; TNF, Tumor necrosis factor
E2 exerts mainly anti‐inflammatory effects by inhibiting production and signaling of pro‐inflammatory cytokines, such as TNF‐α, IL‐1 and IL‐6, as well as inhibiting NK cell activation, and by inducing expression of anti‐inflammatory cytokines such as IL‐4, IL‐10, favoring a Th2 phenotype75 and transforming growth factor‐β (TGF‐β) expression, and activating Treg cells.76
However, at lower concentrations (equivalent to pre‐ovulatory menstrual cycle levels) E2 stimulates TNF‐α, IFN‐γ, IL‐1 production, and NK cell activity, probably promoting a pro‐inflammatory environment.77 This may explain observations in which lymphocytes from non‐pregnant female mice produced higher concentrations of IFN‐γ in response to antigen stimulation78 or virus infection,79 than those from male mice. Enhancement of IFN‐γ secretion may be involved in the well‐known susceptibility of females to autoimmune disease induction in several experimental animal models, including experimental autoimmune encephalomyelitis (EAE)80 and be the reason behind the greater female prevalence of autoimmune diseases in humans.
As early as 1994, ovariectomy was reported as causing earlier EAE onset.81 Conversely long‐term treatment with por‐estrous levels of E2 delayed it, with E3 treatment (to por‐estrous levels) postponing disease onset even longer. Five times higher doses of E2, similar to those seen during pregnancy, were required to obtain the same effects as the low E3 dose.81 Although E3‐treated mice had higher serum levels of anti‐MBP‐IgG1 antibodies, E3 reduced EAE severity, and caused antigen‐specific T lymphocytes to produce greater amounts of IL‐10.82 Likewise, estrogen administered before immunization delayed the onset of symptoms and reduced disease activity.83 These beneficial effects are principally driven by ERα‐dependent mechanisms resulting in decreased production of autoantigen‐specific pro‐inflammatory molecules (IFN‐γ, TNF‐α, IL‐17, inducible nitric oxide synthase, and monocyte chemoattractant protein 1) and stimulation of the Th2 anti‐inflammatory pathway (IL‐4, IL‐10, and TGF‐β) which in turn leads to inhibition of inflammation and demyelination. Treatment with the ERβ agonist diarylpropionitrile on the other hand, confers clinical protection late during EAE, which is associated with reduced demyelination, preserved axon numbers, and increased functional remyelination.84 Furthermore, ERβ‐specific synthetic ligands as well as endogenous sterols other than 17β‐estradiol regulate ERβ transrepression activities, potently inhibiting the transcriptional activation of inflammatory response genes in microglia and astrocytes.85 Recently, ERβ ligands were found to have neuroprotective effects in EAE by acting on CD11c+ myeloid dendritic cells and macrophages. In addition, treatment increased mature oligodendrocyte lineage cell numbers, an effect that disappeared once treatment was removed.86
Phase I and phase II clinical trials using E3 as well as other sex hormone combinations have been conducted. One study in 10 women with RRMS treated with oral E3 (8 mg/day for 6 months) in an attempt to recapitulate the beneficial effects of pregnancy, found a significant decrease in delayed‐type hypersensitivity responses to tetanus toxoid, a decrease in IFN‐γ levels of peripheral blood mononuclear cells, and fewer and smaller gadolinium‐enhancing lesions on monthly brain MRI, compared with pretreatment baseline conditions. When E3 treatment was discontinued, enhancing lesions increased to pretreatment levels and when E3 treatment was reinstituted, enhancing lesions again decreased significantly.87, 88
This open‐label pilot trial provided the basis for a randomized, placebo‐controlled, double‐blind, phase II trial of E3 combined with glatiramer acetate in women with RRMS. The trial enrolled 164 patients (83 in the E3‐treated group and 81 in the placebo group). After 24 months, confirmed relapse rate and time to confirmed relapse were lower in the E3‐treated group, in which fatigue also improved significantly.89 No differences were observed, however, on MRI in numbers of gadolinium‐enhancing lesions, T2 lesions, or total brain volume.
Overall, a paradox exists with respect to estrogen‐induced immunomodulatory effects in MS. On the one hand, they suppress inflammation in EAE as well as in other autoimmune disease models. On the other hand, they can generate pro‐inflammatory effects in chronic autoimmune diseases. Which is the case, will depend on different variables such as: (i) cell types involved during different phases of disease, (ii) target organ and specific microenvironment, (iii) reproductive status of the woman with the condition, (iv) estrogen concentration, and (v) ER expression levels.75
Progesterone
Progesterone belongs to the progestogen steroid hormone family, named for its pro‐gestational effect mediated through the progesterone receptor (PR) which is part of the nuclear hormone receptor superfamily of transcription factors. PRs are widely distributed on immune cells, including: granulocytes, NK cells, dendritic cells, T cells and B cells.
In general, progesterone switches the immune response from pro‐inflammatory to anti‐inflammatory, favoring Treg cell differentiation and promoting down‐regulation of IFN‐γ production by NK cells, and glucocorticoid‐mediated thymocyte apoptosis. Progesterone also mediates reduction of nitric oxide production and expression of toll‐like receptors by macrophages, and promotes Th2 differentiation in vitro as well as expression of co‐stimulatory molecules such as CD80, CD86, and CD40, and MHC II.90, 91 In C57BL/6 mice, progesterone therapy causes reduction in the severity of EAE induced by myelin oligodendrocyte glycoprotein (MOG). Whereas estrogens need to be administered before EAE induction, progesterone treatment started as late as 2 weeks after MOG immunization still exerts beneficial effects. Interestingly, progesterone treatment administered to ovariectomized rats before EAE induction worsened brain inflammation and neurological symptoms, indicating the protective effects of progesterone probably require additional gonadal factors. The protection involves suppression of IL‐2 and IL‐17 as well as release of IL‐23 by dendritic cells, a critical inducer of Th17 differentiation in EAE and probably in MS.92
Both progesterone and its synthetic analog (Nestorone) promote myelin repair and reduce neuroinflammation in the cuprizone model in a dose‐dependent fashion. Likewise, a progesterone derivative, allopregnanolone, showed neuroprotective effects with an additional influence on neuronal signaling,93 decreasing axonal damage and restoring myelin gene expression by oligodendrocytes, which protected against injury.94
Progesterone is known to increase proliferation and maturation of oligodendrocyte progenitor cells to mature oligodendrocytes, attenuating microglia and astrocyte activation and proliferation.95 In male rats, early treatment with progesterone after complete spinal cord transection stimulated oligodendrocyte progenitor cell proliferation. Prolonged progesterone treatment promoted myelin protein synthesis, and after spinal cord contusion reduced secondary damage, preserving white matter, and improving motor outcomes.96, 97
Based on these findings, a multicenter, randomized, double‐blind, placebo‐controlled clinical trial was performed with the aim of preventing MS relapses postpartum by administering high doses of progestin, in combination with estradiol for endometrial protection. Treatment was given immediately after delivery and continued for 3 months postpartum. Unfortunately, results showed no protective effect of sexual steroids on relapse rate after delivery or on development of new lesions on MRI.98, 99
Androgens
Androgens are steroid hormones commonly associated with the development of masculine characteristics, testis formation, spermatogenesis, growth of muscle mass, and bone maturation. They include testosterone, the majority of which (98%) becomes irreversibly converted to the active metabolite 5α‐dihydrotestosterone. The 5α‐dihydrotestosterone binds with higher affinity than testosterone to the androgen receptor (AR), which is expressed at different levels in leukocytes, regulating transcription of target genes.100 When evaluating testosterone effects, it should be kept in mind that some could be mediated via the ER pathway, as testosterone can be converted into E1 or E2 by the enzyme aromatase. Moreover, evidence suggests that testosterone metabolites can also activate ERs directly.101
Androgen levels are higher in males, and autoimmune disease incidence is generally lower. For this reason, androgens are thought to play a positive role in the development and function of the innate immune response, inhibiting the adaptive immune system and thus, to some extent, protect against autoimmunity.
Effects of androgens on immune function include a shift from Th1 to Th2 phenotype, based on increased production of IL‐5 and IL‐10 and decreased pro‐inflammatory cytokines including IFN‐γ, TNF‐α, and IL‐17. Testosterone also reduces lymphocyte proliferation and differentiation and may suppress immunoglobulin production. Supra‐physiological doses of testosterone may also inhibit cytotoxic NK cell activity.102, 103
Similar changes were seen after in vivo testosterone treatment of SJL mice with EAE. In this strain, endogenous testosterone was protective, an effect linked to androgen‐mediated Th2 bias, as suggested by the IFN‐γ/IL‐10 ratio.104 Likewise, androgen treatment of naive T‐cell receptor transgenic T cells resulted in a decrease of the IFN‐γ/IL‐10 ratio, due both to reduction of IFN‐γ and increase of IL‐10.105
In the thymus, the autoimmune regulator (Aire) gene enforces T‐cell self‐tolerance, in part by promoting tissue‐specific antigen expression in medullary thymic epithelial cells. In addition to effects on the peripheral immune system, androgens also up‐regulate Aire‐mediated thymic tolerance, protecting against autoimmunity. Androgens recruit AR to Aire promoter regions, subsequently enhancing Aire transcription. In mice and humans, thymic Aire expression is higher in males than females. In an MS mouse model, androgen administration as well as male gender protected against autoimmunity in an Aire‐dependent manner, indicating control of intrathymic Aire‐mediated tolerance mechanisms by androgens, which helped to explain gender‐related differences observed in MS.106
Several studies describe a possible neuroprotective effect of testosterone, as it can cross the blood–brain barrier and directly influence neuronal cells. It has also been shown that androgens protect spinal cord neurons in culture from glutamate toxicity107 and can induce neuronal differentiation and increase neuritic outgrowth. Testosterone also protects neuronal cell lines from oxidative stress108 increasing the expression of neurotrophic factors such as brain‐derived neurotrophic factor (BDNF). In male mice with EAE, administration of testosterone reduced microglial activation and restored synaptic excitatory transmission as well as presynaptic and postsynaptic protein levels in the hippocampus.109
In line with these findings, testosterone levels measured in men with MS at different ages generally support the idea that reduced testosterone is a risk factor for disease.103 Based on epidemiological studies, it could be hypothesized that the later age of onset of MS in men, relative to women, may result from the age‐related decline in protective testosterone levels.17 In men with MS, a relative androgen deficiency is noted. Low levels of testosterone were correlated with EDSS severity and were predictive of decreases in cognitive function.110 In women with MS, testosterone levels may be lower than in controls and correlate negatively with disability and radiologic markers.111
In one pilot study, 10 men with MS were treated with 10 g of testosterone gel (100 mg) in a cross‐over design (6‐month observation followed by 12 months of treatment). Treatment resulted in significant slowing of brain atrophy measured from monthly MRI, as well as improvement in cognitive testing. However, no significant effects of treatment were observed on gadolinium‐enhancing lesions.112 Testosterone also significantly reduced delayed‐type hypersensitivity skin recall responses and induced a shift in peripheral lymphocyte composition, by decreasing CD4+ T cells and increasing NK cells. In addition, peripheral blood mononuclear cells collected during treatment produced significantly more BDNF, platelet‐derived growth factor‐BB, and TGF‐β 1. Interleukin‐2 production on the other hand was decreased. These findings are consistent with an immunomodulatory as well as a potentially neuroprotective effect of testosterone treatment in MS.113
Prolactin
As previously mentioned, lack of consensus related to breastfeeding has renewed interest in the effects of PRL on MS.49, 53 Prolactin is secreted predominantly by the anterior pituitary and is regulated by both inhibitory and stimulatory inputs from hypothalamic dopamine and tirotropin‐releasing hormones, respectively. Interestingly, PRL is also secreted by extra‐pituitary tissues including decidual, mammary, and epithelial cells as well as by monocytes and B and T lymphocytes.114
The PRL receptor (PRLR) belongs to the cytokine receptor superfamily, which includes receptors for IL‐2, IL‐6, granulocyte–macrophage colony‐stimulating factor, and leptin. PRLR is found on monocytes, T and B lymphocytes, and other cells of the immune system.47 In addition to its primary role in mammary gland development and in initiation and maintenance of lactation, PRL has a number of physiological roles including immune system modulation and neuroregeneration.114, 115, 116
The main effects of PRL on the immune system are summarized in Table 2.
Table 2.
Prolactin effects on cells of the immune system
| Immune cell | Prolactin effect |
|---|---|
| Macrophages |
|
| Granulocytes |
|
| Natural killers cells |
|
| Dendritic cells |
|
| B cells |
|
| T cells |
|
BAFF, B‐cell activating factor; Bcl‐2, B‐cell lymphoma 2; iNOS, induced nitric oxide synthase; IP‐10, interferon‐γ‐induced protein; IRF‐1, interferon regulatory factor‐1; LFA‐1, lymphocyte function‐associated antigen‐1; MCP‐1, monocyte chemoattractant protein‐1; MIP‐1α, macrophage inflammatory protein‐1α; Trp 63, transformation‐related protein 63; VLA‐4, very late antigen‐4.
Evidence of an association between hyperprolactinemia and MS is conflicting. In some studies PRL was found to be higher in patients with MS but without a link to disease duration or activity.117, 118 Other studies, however, detected increased PRL levels in MS patients during relapses119 or found a positive correlation between plasma PRL levels and white matter volume.120 Elevated PRL levels are found more often in premenopausal women than in men.121, 122 Prolactin elevation in MS patients may be due to non‐specific hypothalamic–pituitary–adrenal axis dysregulation, secondary to demyelination or neurodegeneration, or to higher prevalence of, or enhanced sensitivity to, psychosocial stress.47, 121
In the EAE model, low‐dose PRL administered either prophylactically or therapeutically did not exacerbate the course of disease, whereas high doses did so significantly.47 Prolactin suppression using the dopamine agonist bromocriptine on the other hand dampened both humoral and cellular immunity. In vivo pretreatment with bromocriptine inhibited in vitro lymphocyte proliferative responses of the spleen after stimulation with concanavalin A. Bromocriptine therapy remained protective even when initiated 1 week after initial immunization and was also effective in suppression of late disease.123
Therefore, although PRL has been classically considered a detrimental factor in MS, recent reports point to important neuroprotective and remyelinating properties of the hormone, encouraging a reinterpretation of the influence it may have on MS.45 Indeed, PRL has been shown to mediate proliferation of oligodendrocyte precursor cells during pregnancy and to promote myelin repair,115 increase expression of functional survival motor neuron protein in a mouse model of spinal muscular atrophy,124 stimulate precursor cells in the adult mouse hippocampus,125 and mediate neuroprotection against excitotoxicity via its receptor.126 Lastly, in a model of retinal degeneration, PRL limited photoreceptor apoptosis, gliosis, and changes in neurotropin expression, preserving ocular photoresponse. Moreover, retinas from PRLR‐deficient mice exhibited photoresponse dysfunction and gliosis correlating with decreased levels of retinal basic fibroblast growth factor (bFGF), glial cell‐derived neurotrophic factor (GDNF), and BDNF.127
In conclusion, PRL may exert dual and opposing effects in MS, which result from a delicate balance between protection of CNS tissue and stimulation of the immune system. Although several studies have investigated the association between PRL and MS in humans, limitations in design preclude establishing a clear causal relationship.47 From a therapeutic perspective, great caution must be taken when trying to manipulate PRL levels in MS.
As described above, extensive research has been devoted to the role of sex hormones in MS to explain the increased susceptibility of females over males, but increased rate of disability progression of males over females. However, direct effects of sex chromosomes and indirect effects of sex chromosomes (mediated by sex hormones) on the immune system and the neurodegeneration phenomena observed in MS should also be considered.128 Development of different mouse core genotypes backcrossed onto the SJL background,129 has permitted the separation of effects of sex chromosomes from effects of sex hormones. Using these models, it has been possible to demonstrate that sex chromosomes have a direct effect in the induction of immune responses in adoptive EAE, which is unlikely to be mediated by different effects of different sex hormones.129, 130 Likewise, the use of bone marrow chimeras has allowed demonstration that sex chromosomes can affect the CNS response to a given immune‐mediated injury, resulting in more axonal and neuronal loss and greater demyelination.131
Interaction between hormonal and environmental factors
As mentioned in the Introduction the exact reason for the increased incidence of MS in women is unknown, nevertheless the change occurred too fast to be attributable to genetic causes alone,4 suggesting environmental factors that are sexually dimorphic are more commonly encountered in one sex than the other could intervene. Several possibilities have been proposed including oral contraceptive use, parity, increased numbers of women smoking, changes in lifestyle or in workforce, and dietary habits.
The gut is the largest immune organ in the body exposed daily to environmental factors that maintains a tolerant state. Recent reports on the influence of gut microbes on gender‐based autoimmunity suggest that both bacteria and sex hormones interact directly to regulate disease fate in genetically susceptible individuals. Gender bias in specific pathogen‐free mice occurs more often in female mice and can be reversed by transfer of gut microbiota from male mice, suggesting that an interaction between sex hormones and gut microbes may influence autoimmunity by triggering inflammatory or tolerogenic effects.132
Estrogens influence the microbiome through mechanisms involving increasing epithelial thickness, mucus secretion, and glycogen levels. Female mice are more resistant to gut injury than males, and inhibition of androgens with flutamide in male mice reduces gut injury. Estrogen‐like compounds may also promote proliferation and growth of certain types of bacteria.133 On the other hand, estrogens are metabolized by microbes secreting β‐glucuronidase, an enzyme that deconjugates estrogen, allowing the ‘active’ metabolite to enter the bloodstream and bind to estrogen receptors.134
Treatment with pregnancy levels of estrogen induces changes in the composition and diversity of the gut microbiota, associated with protection against EAE.135 It was shown that while EAE led to dysbiosis in mice, pretreatment with estrogen reduced these changes and increased regulatory B‐cell and anti‐inflammatory macrophage numbers in mesenteric lymph nodes and in the spinal cord.135
Vitamin D (D3) is an environmental factor that may be involved in the development of autoimmunity. Sun protection behavior, which has increased in recent decades, has significantly reduced the hours of light exposure in the population at large. Interestingly, D3 has shown greater immunomodulatory effects in women than in men with MS, with the inverse correlation between UV ray exposure and MS risk being several times stronger in women.136
Investigations have shown that D3 significantly inhibits EAE in female mice, but not in male mice, and ovariectomy abrogates the protective effect, suggesting a link between female hormones and D3 metabolism.137 Further, D3 stimulated E2 synthesis, and E2 inhibited D3 breakdown, improving D3 responsiveness. Moreover, both molecules synergistically decreased pathogenic Th1 and Th17 cells and increased Treg cells. D3 and E2 also interact with female D3 receptor (VDR) ‐expressing CD4+ T cells to promote peripheral self‐tolerance.138 In addition, in MS patients D3 acts more strongly on female lymphocyte inhibition of T‐cell proliferation, reducing the number of IFN‐γ‐ and IL‐17‐producing cells and increasing the number IL‐10‐secreting ones. Interestingly, E2 reproduced these effects on self‐reactive T cells and macrophages from male subjects, suggesting a functional synergy between 1,25‐dihydroxyvitamin D3 and 17β‐estradiol, mediated through ERα.139 In correlation with these immunomodulatory changes, women with MS had fewer CYP24A1 transcripts encoding the D3‐inactivating enzyme, and presented greater binding and internalization of DBP, the serum glycoprotein that reversibly binds and transports vitamin D3 and its metabolites to autoreactive CD4+ T cells and macrophages.139 Overall, these findings suggest that females inactivate D3 more slowly, allowing it to accumulate, so triggering more potent anti‐inflammatory effects. Of note, the ER antagonist ICI 182780 reversed E2 effects on CYP24A1 transcripts and DBP uptake, demonstrating that the effects were ER‐signaling dependent.139
Other environmental candidates have been thought to play a role in autoimmune disease risk, such as infectious agents and chemicals, including compounds commonly found in cosmetics, organic solvents, and pesticides. As exposure to these elements varies between men and women, this might explain some of the gender differences observed in MS prevalence.
Further studies are needed to better understand the cross talk between sex hormones and environmental factors, which could open new avenues of research to identify particular molecules promoting regulation of the immune response.
Conclusions and future perspectives
The observed increase in gender ratio suggests gender‐specific associations between environmental and/or gene–environment interactions, and susceptibility to MS. Hence, some environmental factors such as D3 or the microbiome may in turn affect endogenous sex hormone levels, which then alter hormonal interaction with MS susceptibility genes.
Difficulties in fully replicating the protective effects of pregnancy in MS during clinical trials using sex hormones89, 98, 99, 140 underscore just how difficult it is to attribute gender effects in MS to a single biological factor. Furthermore, the effect of sex hormones during the aging process has not been well studied, and consequently the impact of both variables on the immune response during the course of MS is severely limited. Future clinical and preclinical studies should consider these variables in detail. Similarly, the development of suitable animal models could provide relevant information. Current clinical observations reflect the complex interplay of genetic, epigenetic, hormonal, and environmental factors present in MS. Increasing use of bioinformatic approaches, in large and clinically well‐characterized cohorts, will help to unravel the molecular mechanisms involved and may identify new pathways for therapies that can be targeted in more sex‐specific ways, to halt autoimmune attacks and even promote neuroprotection and repair in the CNS.
Author contributions
MCY contributed to the conception and design of the manuscript, drafted the original manuscript, designed the figures, and provided important intellectual contributions. JC contributed to the conception and design of the manuscript, drafted the original, designed the figures, revised the draft and provided important intellectual contributions.
Disclosures
JC is a board member of Merck‐Serono Argentina, Novartis Argentina, Genzyme LATAM, Genzyme global, Biogen‐Idec LATAM, and Merck‐Serono LATAM. He is part of the Steering Committee for the clinical trials of Ofatumumab (Novartis Global). Dr. Correale has received reimbursement for developing educational presentations for Merck‐Serono Argentina, Merck‐Serono LATAM, Biogen‐Idec Argentina, Genzyme Argentina, Novartis Argentina, Novartis LATAM, Novartis Global, and TEVA Argentina as well as professional travel/accommodations stipends. MCY has received reimbursement for developing educational presentations and for travel/accommodations stipends from Merck‐Serono Argentina, Biogen‐Idec Argentina, Genzyme Argentina, Bayer Inc, Novartis Argentina, and TEVA‐Tuteur Argentina.
Acknowledgements
We are grateful for the collaboration of Dr. Ismael Calandri for the preparation of the figures. This study was supported by an internal grant from the Dr. Raul Carrea Institute for Neurological Research, FLENI.
Contributor Information
María C. Ysrraelit, Email: mcysrraelit@fleni.org.ar
Jorge Correale, Email: jorge.correale@gmail.com.
References
- 1. Koch‐Henriksen N, Sørensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol 2010; 9:520–32. [DOI] [PubMed] [Google Scholar]
- 2. Ribbons KA, McElduff P, Boz C, Trojano M, Izquierdo G, Duquette P et al Male sex is independently associated with faster disability accumulation in relapse‐onset MS but not in primary progressive MS. PLoS One 2015; 10:e0122686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Confavreux C, Aimard G, Devic M. Course and prognosis of multiple sclerosis assessed by the computerized data processing of 349 patients. Brain 1980; 103:281–300. [DOI] [PubMed] [Google Scholar]
- 4. Orton S‐M, Herrera BM, Yee IM, Valdar W, Ramagopalan SV, Sadovnick AD et al Sex ratio of multiple sclerosis in Canada: a longitudinal study. Lancet Neurol 2006; 5:932–6. [DOI] [PubMed] [Google Scholar]
- 5. Ramagopalan SV, Byrnes JK, Orton S‐MS‐M, Dyment DA, Guimond C, Yee IM et al Sex ratio of multiple sclerosis and clinical phenotype. Eur J Neurol 2010; 17:634–7. [DOI] [PubMed] [Google Scholar]
- 6. Maghzi A, Ghazavi H, Ahsan M, Etemadifar M, Mousavi S, Khorvash F, et al Increasing female preponderance of multiple sclerosis in Isfahan, Iran: a population‐based study. Mult Scler 2010; 16:359–61. [DOI] [PubMed] [Google Scholar]
- 7. Rojas JI, Patrucco L, MIguez J, Sinay V, Cassara FP, Cáceres F et al Gender ratio trends over time in multiple sclerosis patients from Argentina. J Clin Neurosci 2017; 38:84–6. [DOI] [PubMed] [Google Scholar]
- 8. Trojano M, Lucchese G, Graziano G, Taylor BV, Simpson S, Lepore V et al Geographical variations in sex ratio trends over time in multiple sclerosis. PLoS One 2012; 7:e48078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ponsonby AL, Lucas RM, Van Der Mei IA, Dear K, Valery PC, Pender MP et al Offspring number, pregnancy, and risk of a first clinical demyelinating event the ausimmune study. Neurology 2012; 78:867–74. [DOI] [PubMed] [Google Scholar]
- 10. Kalincik T, Vivek V, Jokubaitis V, Lechner‐Scott J, Trojano M, Izquierdo G et al Sex as a determinant of relapse incidence and progressive course of multiple sclerosis. Brain 2013; 136:3609–17. [DOI] [PubMed] [Google Scholar]
- 11. Pozzilli C, Tomassini V, Marinelli F, Paolillo A, Gasperini C, Bastianello S. “Gender gap” in multiple sclerosis: magnetic resonance imaging evidence. Eur J Neurol 2003; 10:95–7. [DOI] [PubMed] [Google Scholar]
- 12. Confavreux C, Vukusic S. Age at disability milestones in multiple sclerosis. Brain 2006; 129:595–605. [DOI] [PubMed] [Google Scholar]
- 13. Confavreux C, Vukusic S, Adeleine P. Early clinical predictors and progression of irreversible disability in multiple sclerosis: an amnesic process. Brain 2003; 126:770–82. [DOI] [PubMed] [Google Scholar]
- 14. Schoonheim MM, Popescu V, Rueda Lopes FC, Wiebenga OT, Vrenken H, Douw L et al Subcortical atrophy and cognition: sex effects in multiple sclerosis. Neurology 2012; 79:1754–61. [DOI] [PubMed] [Google Scholar]
- 15. Antulov R, Weinstock‐Guttman B, Cox JL, Hussein S, Durfee J, Caiola C et al Gender‐related differences in MS: a study of conventional and nonconventional MRI measures. Mult Scler 2009; 15:345–54. [DOI] [PubMed] [Google Scholar]
- 16. Rivas‐Rodríguez E, Amezcua L. Ethnic considerations and multiple sclerosis disease variability in the United States. Neurol Clin 2018; 36:151–62. [DOI] [PubMed] [Google Scholar]
- 17. Bove R, Chitnis T. Sexual disparities in the incidence and course of MS. Clin Immunol 2013; 149:201–10. [DOI] [PubMed] [Google Scholar]
- 18. Negrotto L, Correale J. Evolution of multiple sclerosis prevalence and phenotype in Latin America. Mult Scler Relat Disord 2018; 22:97–102. [DOI] [PubMed] [Google Scholar]
- 19. Pakpoor J, Wotton CJ, Schmierer K, Giovannoni G, Goldacre MJ. Gender identity disorders and multiple sclerosis risk: a national record‐linkage study. Mult Scler 2016; 22:1759–62. [DOI] [PubMed] [Google Scholar]
- 20. Chitnis T. Role of puberty in multiple sclerosis risk and course. Clin Immunol 2013; 149:192–200. [DOI] [PubMed] [Google Scholar]
- 21. Sloka JS, Pryse‐Phillips WEM, Stefanelli M. The relation between menarche and the age of first symptoms in a multiple sclerosis cohort. Mult Scler 2006; 12:333–9. [DOI] [PubMed] [Google Scholar]
- 22. Onland‐Moret NC, Peeters PHM, van Gils CH, Clavel‐Chapelon F, Key T, Tjønneland A et al Age at menarche in relation to adult height: the EPIC study. Am J Epidemiol 2005; 162:623–32. [DOI] [PubMed] [Google Scholar]
- 23. Bove R, Chua AS, Xia Z, Chibnik L, De Jager PL, Chitnis T. Complex relation of HLA‐DRB1*1501, age at menarche, and age at multiple sclerosis onset. Neurol Genet 2016; 2:e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ramagopalan SV, Valdar W, Criscuoli M, DeLuca GC, Dyment DA, Orton S‐M et al Age of puberty and the risk of multiple sclerosis: a population based study. Eur J Neurol 2009; 16:342–7. [DOI] [PubMed] [Google Scholar]
- 25. Lulu S, Graves J, Waubant E. Menarche increases relapse risk in pediatric multiple sclerosis. Mult Scler 2016; 22:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. D'Hooghe MB, Haentjens P, Nagels G, D'Hooghe T, De Keyser J. Menarche, oral contraceptives, pregnancy and progression of disability in relapsing onset and progressive onset multiple sclerosis. J Neurol 2012; 259:855–61. [DOI] [PubMed] [Google Scholar]
- 27. Matarese G, Carrieri PB, Montella S, De Rosa V, La Cava A. Leptin as a metabolic link to multiple sclerosis. Nat Rev Neurol 2010; 6:455–61. [DOI] [PubMed] [Google Scholar]
- 28. Chitnis T, Graves J, Weinstock‐Guttman B, Belman A, Olsen C, Misra M et al Distinct effects of obesity and puberty on risk and age at onset of pediatric MS. Ann Clin Transl Neurol 2016; 3:897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Confavreux C, Hutchinson M, Hours MM, Cortinovis‐Tourniaire P, Moreau T. Rate of pregnancy‐related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N Engl J Med 1998; 339:285–91. [DOI] [PubMed] [Google Scholar]
- 30. Shuster EA. Hormonal influences in multiple sclerosis. Curr Top Microbiol Immunol 2008; 318:267–311. [DOI] [PubMed] [Google Scholar]
- 31. Airas L. Hormonal and gender‐related immune changes in multiple sclerosis. Acta Neurol Scand 2015; 132:62–70. [DOI] [PubMed] [Google Scholar]
- 32. Martal JL, Chêne NM, Huynh LP, L'Haridon RM, Reinaud PB, Guillomot MW et al IFN‐τ: a novel subtype I IFN1. Structural characteristics, non‐ubiquitous expression, structure‐function relationships, a pregnancy hormonal embryonic signal and cross‐species therapeutic potentialities. Biochimie 1998; 80:755–77. [DOI] [PubMed] [Google Scholar]
- 33. Khan D, Ansar Ahmed S. The immune system is a natural target for estrogen action: opposing effects of estrogen in two prototypical autoimmune diseases. Front Immunol 2015; 6:635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bansal AS, Bora SA, Saso S, Smith JR, Johnson MR, Thum M‐Y. Mechanism of human chorionic gonadotrophin‐mediated immunomodulation in pregnancy. Expert Rev Clin Immunol 2012; 8:747–53. [DOI] [PubMed] [Google Scholar]
- 35. Rolle L, Memarzadeh Tehran M, Morell‐García A, Raeva Y, Schumacher A, Hartig R et al Cutting edge: IL‐10‐producing regulatory B cells in early human pregnancy. Am J Reprod Immunol 2013; 70:448–53. [DOI] [PubMed] [Google Scholar]
- 36. Muzzio D, Zygmunt M, Jensen F. The role of pregnancy‐associated hormones in the development and function of regulatory B cells. Front Endocrinol (Lausanne) 2014; 5:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Basso O, Campi R, Frydenberg M, Koch‐Henriksen N, Brønnum‐Hansen H, Olsen J. Multiple sclerosis in women having children by multiple partners. A population‐based study in Denmark. Mult Scler 2004; 10:621–5. [DOI] [PubMed] [Google Scholar]
- 38. Jokubaitis VG, Spelman T, Kalincik T, Lorscheider J, Havrdova E, Horakova D et al Predictors of long‐term disability accrual in relapse‐onset multiple sclerosis. Ann Neurol 2016; 80:89–100. [DOI] [PubMed] [Google Scholar]
- 39. D'Hooghe MB, Nagels G, Uitdehaag BMJ. Long‐term effects of childbirth in MS. J Neurol Neurosurg Psychiatry 2010; 81:38–41. [DOI] [PubMed] [Google Scholar]
- 40. Vukusic S, Hutchinson M, Hours M, Moreau T, Cortinovis‐Tourniaire P, Adeleine P et al Pregnancy and multiple sclerosis (the PRIMS study): clinical predictors of post‐partum relapse. Brain 2004; 127:1353–60. [DOI] [PubMed] [Google Scholar]
- 41. Weinshenker BG, Rice GP, Noseworthy JH, Carriere W, Baskerville J, Ebers GC. The natural history of multiple sclerosis: a geographically based study. 3. Multivariate analysis of predictive factors and models of outcome. Brain 1991; 114:1045–56. [DOI] [PubMed] [Google Scholar]
- 42. D'Amico E, Leone C, Patti F. Offspring number does not influence reaching the disability's milestones in multiple sclerosis: a seven‐year follow‐up study. Int J Mol Sci 2016; 17:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Koch M, Uyttenboogaart M, Heersema D, Steen C, De Keyser J. Parity and secondary progression in multiple sclerosis. J Neurol Neurosurg Psychiatry 2009; 80:676–8. [DOI] [PubMed] [Google Scholar]
- 44. Finkelsztejn A, Brooks JBB, Paschoal FM, Fragoso YD. What can we really tell women with multiple sclerosis regarding pregnancy? A systematic review and meta‐analysis of the literature. BJOG 2011; 118:790–7. [DOI] [PubMed] [Google Scholar]
- 45. Costanza M, Pedotti R. Prolactin: friend or foe in central nervous system autoimmune inflammation? Int J Mol Sci 2016; 17:E2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Correale J, Farez MF, Ysrraelit MC. Role of prolactin in B cell regulation in multiple sclerosis. J Neuroimmunol 2014; 269:76–86. [DOI] [PubMed] [Google Scholar]
- 47. Zhornitsky S, Yong VW, Weiss S, Metz LM, Correale J, Farez MF et al Prolactin in multiple sclerosis. Mult Scler 2013; 19:15–23. [DOI] [PubMed] [Google Scholar]
- 48. Langer‐Gould A, Beaber BE. Effects of pregnancy and breastfeeding on the multiple sclerosis disease course. Clin Immunol 2013; 149:244–50. [DOI] [PubMed] [Google Scholar]
- 49. Hellwig K, Rockhoff M, Herbstritt S, Borisow N, Haghikia A, Elias‐Hamp B et al Exclusive breastfeeding and the effect on postpartum multiple sclerosis relapses. JAMA Neurol 2015; 72:1132. [DOI] [PubMed] [Google Scholar]
- 50. Langer‐Gould A, Huang SM, Gupta R, Leimpeter AD, Greenwood E, Albers KB et al Exclusive breastfeeding and the risk of postpartum relapses in women with multiple sclerosis. Arch Neurol 2009; 66:958–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Langer‐Gould A, Smith JB, Hellwig K, Gonzales E, Haraszti S, Koebnick C et al Breastfeeding, ovulatory years, and risk of multiple sclerosis. Neurology 2017; 89:563–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Airas L, Jalkanen A, Alanen A, Pirttila T, Marttila RJ. Breast‐feeding, postpartum and prepregnancy disease activity in multiple sclerosis. Neurology 2010; 75:474–6. [DOI] [PubMed] [Google Scholar]
- 53. Portaccio E, Ghezzi A, Hakiki B, Martinelli V, Moiola L, Patti F et al Breastfeeding is not related to postpartum relapses in multiple sclerosis. Neurology 2011; 77:145–50. [DOI] [PubMed] [Google Scholar]
- 54. Pakpoor J, Disanto G, Lacey MV, Hellwig K, Giovannoni G, Ramagopalan SV. Breastfeeding and multiple sclerosis relapses: a meta‐analysis. J Neurol 2012; 259:2246–8. [DOI] [PubMed] [Google Scholar]
- 55. Cavalla P, Rovei V, Masera S, Vercellino M, Massobrio M, Mutani R et al Fertility in patients with multiple sclerosis: current knowledge and future perspectives. Neurol Sci 2006; 27:231–9. [DOI] [PubMed] [Google Scholar]
- 56. Sutcliffe AG, Ludwig M. Outcome of assisted reproduction. Lancet 2007; 370:351–9. [DOI] [PubMed] [Google Scholar]
- 57. Laplaud D‐A, Lefrère F, Leray E, Barrière P, Wiertlewski S. Augmentation du risque de poussée de sclérose en plaques après stimulation ovarienne pour fécondation in vitro . Gynécol Obstet Fertil 2007; 35:1047–50. [DOI] [PubMed] [Google Scholar]
- 58. Hellwig K, Schimrigk S, Beste C, Müller T, Gold R. Increase in relapse rate during assisted reproduction technique in patients with multiple sclerosis. Eur Neurol 2009; 61:65–8. [DOI] [PubMed] [Google Scholar]
- 59. Michel L, Foucher Y, Vukusic S, Confavreux C, de Sèze J, Brassat D et al Increased risk of multiple sclerosis relapse after in vitro fertilisation. J Neurol Neurosurg Psychiatry 2012; 83:796–802. [DOI] [PubMed] [Google Scholar]
- 60. Correale J, Farez MFMF, Ysrraelit MCMC. Increase in multiple sclerosis activity after assisted reproduction technology. Ann Neurol 2012; 72:682–94. [DOI] [PubMed] [Google Scholar]
- 61. Hellwig K, Correale J. Artificial reproductive techniques in multiple sclerosis. Clin Immunol 2013; 149:219–24. [DOI] [PubMed] [Google Scholar]
- 62. Thorogood M, Hannaford PC. The influence of oral contraceptives on the risk of multiple sclerosis. Br J Obstet Gynaecol 1998; 105:1296–9. [DOI] [PubMed] [Google Scholar]
- 63. Hernán MA, Hohol MJ, Olek MJ, Spiegelman D, Ascherio A. Oral contraceptives and the incidence of multiple sclerosis. Neurology 2000; 55:848–54. [DOI] [PubMed] [Google Scholar]
- 64. Alonso A, Jick SS, Olek MJ, Ascherio A, Jick H, Hernán MA. Recent use of oral contraceptives and the risk of multiple sclerosis. Arch Neurol 2005; 62:1362–5. [DOI] [PubMed] [Google Scholar]
- 65. Hellwig K, Chen LH, Stancyzk FZ, Langer‐Gould AM. Oral contraceptives and multiple sclerosis/clinically isolated syndrome susceptibility. PLoS One 2016; 11:e0149094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kempe P, Hammar M, Brynhildsen J. Symptoms of multiple sclerosis during use of combined hormonal contraception. Eur J Obstet Gynecol Reprod Biol 2015; 193:1–4. [DOI] [PubMed] [Google Scholar]
- 67. Pozzilli C, De Giglio L, Barletta VT, Marinelli F, De Angelis F, Gallo V et al Oral contraceptives combined with interferon b in multiple sclerosis. Neurol Neuroimmunol NeuroInflamm 2015; 2:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zapata LB, Oduyebo T, Whiteman MK, Houtchens MK, Marchbanks PA, Curtis KM. Contraceptive use among women with multiple sclerosis: a systematic review. Contraception 2016; 94:612–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Miller DH, Fazekas F, Montalban X, Reingold SC, Trojano M. Pregnancy, sex and hormonal factors in multiple sclerosis. Mult Scler 2014; 20:527–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bove R, Healy BC, Secor E, Vaughan T, Katic B, Chitnis T et al Patients report worse MS symptoms after menopause: findings from an online cohort. Mult Scler Relat Disord 2015; 4:18–24. [DOI] [PubMed] [Google Scholar]
- 71. Bove R, White CC, Fitzgerald KC, Chitnis T, Chibnik L, Ascherio A et al Hormone therapy use and physical quality of life in postmenopausal women with multiple sclerosis. Neurology 2016; 87:1457–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Graves JS, Henry RG, Cree BACC, Lambert‐Messerlian G, Greenblatt RM, Waubant E et al Ovarian aging is associated with gray matter volume and disability in women with MS. Neurology 2017; 90:e254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Thöne J, Kollar S, Nousome D, Ellrichmann G, Kleiter I, Gold R et al Serum anti‐Müllerian hormone levels in reproductive‐age women with relapsing‐remitting multiple sclerosis. Mult Scler 2015; 21:41–7. [DOI] [PubMed] [Google Scholar]
- 74. Sepúlveda M, Ros C, Martínez‐Lapiscina EH, Solà‐Valls N, Hervàs M, Llufriu S et al Pituitary‐ovary axis and ovarian reserve in fertile women with multiple sclerosis: a pilot study. Mult Scler 2016; 22:564–8. [DOI] [PubMed] [Google Scholar]
- 75. Straub RH. The complex role of estrogens in inflammation. Endocr Rev 2007; 28:521–74. [DOI] [PubMed] [Google Scholar]
- 76. Prieto A, Díaz D, Barcenilla H, Castrillo C, Monserrat J, Merino AG et al Increased spontaneous ex vivo apoptosis and subset alterations in peripheral blood T cells from patients with multiple sclerosis. J Clin Immunol 2006; 26:101–12. [DOI] [PubMed] [Google Scholar]
- 77. Gilmore W, Weiner LP, Correale J. Effect of estradiol on cytokine secretion by proteolipid protein‐specific T cell clones isolated from multiple sclerosis patients and normal control subjects. J Immunol 1997; 158:446–51. [PubMed] [Google Scholar]
- 78. Huygen K, Palfliet K. Strain variation in interferon gamma production of BCG‐sensitized mice challenged with PPD II. Importance of one major autosomal locus and additional sexual influences. Cell Immunol 1984; 85:75–81. [DOI] [PubMed] [Google Scholar]
- 79. McFarland HI, Bigley NJ. Sex‐dependent, early cytokine production by NK‐like spleen cells following infection with the D variant of encephalomyocarditis virus (EMCV‐D). Viral Immunol 1989; 2:205–14. [DOI] [PubMed] [Google Scholar]
- 80. Ansar Ahmed S, Penhale WJ, Talal N. Sex hormones, immune responses, and autoimmune diseases. Mechanisms of sex hormone action. Am J Pathol 1985; 121:531–51. [PMC free article] [PubMed] [Google Scholar]
- 81. Jansson L, Olsson T, Holmdahl R. Estrogen induces a potent suppression of experimental autoimmune encephalomyelitis and collagen‐induced arthritis in mice. J Neuroimmunol 1994; 53:203–7. [DOI] [PubMed] [Google Scholar]
- 82. Kim S, Liva SM, Dalal MA, Verity MA, Voskuhl RR. Estriol ameliorates autoimmune demyelinating disease: implications for multiple sclerosis. Neurology 1999; 52:1230–8. [DOI] [PubMed] [Google Scholar]
- 83. Bebo BF, Fyfe‐Johnson A, Adlard K, Beam AG, Vandenbark AA, Offner H. Low‐dose estrogen therapy ameliorates experimental autoimmune encephalomyelitis in two different inbred mouse strains. J Immunol 2001; 166:2080–9. [DOI] [PubMed] [Google Scholar]
- 84. Voskuhl RR, Gold SM. Sex‐related factors in multiple sclerosis susceptibility and progression. Nat Rev Neurol 2012; 8:255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Saijo K, Collier JG, Li AC, Katzenellenbogen JA, Glass CK. An ADIOL‐ERβ‐CtBP transrepression pathway negatively regulates microglia‐mediated inflammation. Cell 2011; 145:584–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kim RY, Mangu D, Hoffman AS, Kavosh R, Jung E, Itoh N et al Oestrogen receptor β ligand acts on CD11c+ cells to mediate protection in experimental autoimmune encephalomyelitis. Brain 2018; 141:132–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sicotte NL, Liva SM, Klutch R, Pfeiffer P, Bouvier S, Odesa S et al Treatment of multiple sclerosis with the pregnancy hormone estriol. Ann Neurol 2002; 52:421–8. [DOI] [PubMed] [Google Scholar]
- 88. Soldan SS, Alvarez Retuerto AI, Sicotte NL, Voskuhl RR. Immune modulation in multiple sclerosis patients treated with the pregnancy hormone estriol. J Immunol 2003; 171:6267–74. [DOI] [PubMed] [Google Scholar]
- 89. Voskuhl RR, Wang H, Wu TCJ, Sicotte NL, Nakamura K, Kurth F et al Estriol combined with glatiramer acetate for women with relapsing‐remitting multiple sclerosis: a randomised, placebo‐controlled, phase 2 trial. Lancet Neurol 2016; 15:35–46. [DOI] [PubMed] [Google Scholar]
- 90. Enninga EAL, Nevala WK, Creedon DJ, Markovic SN, Holtan SG. Fetal sex‐based differences in maternal hormones, angiogenic factors, and immune mediators during pregnancy and the postpartum period. Am J Reprod Immunol 2015; 73:251–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Arruvito L, Giulianelli S, Flores AC, Paladino N, Barboza M, Lanari C et al NK cells expressing a progesterone receptor are susceptible to progesterone‐induced apoptosis. J Immunol 2008; 180:5746–53. [DOI] [PubMed] [Google Scholar]
- 92. Hughes GC. Progesterone and autoimmune disease. Autoimmun Rev 2012; 11:A502–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ramien C, Taenzer A, Lupu A, Heckmann N, Engler JB, Patas K et al Sex effects on inflammatory and neurodegenerative processes in multiple sclerosis. Neurosci Biobehav Rev 2016; 67:137–46. [DOI] [PubMed] [Google Scholar]
- 94. Noorbakhsh F, Baker GB, Power C. Allopregnanolone and neuroinflammation: a focus on multiple sclerosis. Front Cell Neurosci 2014; 8:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Labombarda F, González S, Lima A, Roig P, Guennoun R, Schumacher M et al Progesterone attenuates astro‐ and microgliosis and enhances oligodendrocyte differentiation following spinal cord injury. Exp Neurol 2011; 231:135–46. [DOI] [PubMed] [Google Scholar]
- 96. Garcia‐Ovejero D, González S, Paniagua‐Torija B, Lima A, Molina‐Holgado E, De Nicola AF et al Progesterone reduces secondary damage, preserves white matter, and improves locomotor outcome after spinal cord contusion. J Neurotrauma 2014; 31:857–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Guennoun R, Labombarda F, Gonzalez Deniselle MC, Liere P, De Nicola AF, Schumacher M. Progesterone and allopregnanolone in the central nervous system: response to injury and implication for neuroprotection. J Steroid Biochem Mol Biol 2015; 146:48–61. [DOI] [PubMed] [Google Scholar]
- 98. Vukusic S, El‐Etr M, Ionescu I, Cotton F, Merle H, Cornu C, et al The POPARTMUS French‐Italian multicentric trial of post partum progestin and estriol in multiple sclerosis: final results. Mult Scler 2012; 18(4 Suppl. 1):45.21865412 [Google Scholar]
- 99. Durand‐Dubief F, Ionescu I, Bracoud L, Cotton F, Merle H, Cornu C et al E‐EM. The POPARTMUS French‐Italian multicentric trial of postpartum progestin and estradiol in multiple sclerosis: MRI findings. Mult Scler 2014; 20:95. [Google Scholar]
- 100. Penning TM, Bauman DR, Jin Y, Rizner TL. Identification of the molecular switch that regulates access of 5α‐DHT to the androgen receptor. Mol Cell Endocrinol 2007; 265–266:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Balthazart J, Foidart A, Absil P, Harada N. Effects of testosterone and its metabolites on aromatase‐immunoreactive cells in the quail brain: relationship with the activation of male reproductive behavior. J Steroid Biochem Mol Biol 1996; 56:185–200. [DOI] [PubMed] [Google Scholar]
- 102. Gold SM, Voskuhl RR. Estrogen and testosterone therapies in multiple sclerosis. Prog Brain Res 2009; 175:239–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Chitnis T. The role of testosterone in MS risk and course. Mult Scler 2018; 24:36–41. [DOI] [PubMed] [Google Scholar]
- 104. Dalal M, Kim S, Voskuhl RR. Testosterone therapy ameliorates experimental autoimmune encephalomyelitis and induces a T helper 2 bias in the autoantigen‐specific T lymphocyte response. J Immunol 1997; 159:3–6. [PubMed] [Google Scholar]
- 105. Bebo BF, Schuster JC, Vandenbark AA, Offner H. Androgens alter the cytokine profile and reduce encephalitogenicity of myelin‐reactive T cells. J Immunol 1999; 162:35–40. [PubMed] [Google Scholar]
- 106. Zhu M‐L, Bakhru P, Conley B, Nelson JS, Free M, Martin A et al Sex bias in CNS autoimmune disease mediated by androgen control of autoimmune regulator. Nat Commun 2016; 7:11350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ogata T, Nakamura Y, Tsuji K, Shibata T, Kataoka K. Steroid hormones protect spinal cord neurons from glutamate toxicity. Neuroscience 1993; 55:445–9. [DOI] [PubMed] [Google Scholar]
- 108. Chisu V, Manca P, Lepore G, Gadau S, Zedda M, Farina V. Testosterone induces neuroprotection from oxidative stress. Effects on catalase activity and 3‐nitro‐l‐tyrosine incorporation into α‐tubulin in a mouse neuroblastoma cell line. Arch Ital Biol 2006; 144:63–73. [PubMed] [Google Scholar]
- 109. Ziehn MO, Avedisian AA, Dervin SM, Umeda EA, O'Dell TJ, Voskuhl RR. Therapeutic testosterone administration preserves excitatory synaptic transmission in the hippocampus during autoimmune demyelinating disease. J Neurosci 2012; 32:12312–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Bove R, Musallam A, Healy BC, Raghavan K, Glanz BI, Bakshi R et al Low testosterone is associated with disability in men with multiple sclerosis. Mult Scler 2014; 20:1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Tomassini V, Onesti E, Mainero C, Giugni E, Paolillo A, Salvetti M et al Sex hormones modulate brain damage in multiple sclerosis: MRI evidence. J Neurol Neurosurg Psychiatry 2005; 76:272–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Sicotte NL, Giesser BS, Tandon V, Klutch R, Steiner B, Drain AE et al Testosterone treatment in multiple sclerosis. Arch Neurol 2007; 64:683. [DOI] [PubMed] [Google Scholar]
- 113. Gold SM, Chalifoux S, Giesser BS, Voskuhl RR. Immune modulation and increased neurotrophic factor production in multiple sclerosis patients treated with testosterone. J Neuroinflammation 2008; 5:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev 2000; 80:1523–631. [DOI] [PubMed] [Google Scholar]
- 115. Gregg C, Shikar V, Larsen P, Mak G, Chojnacki A, Yong VW et al White matter plasticity and enhanced remyelination in the maternal CNS. J Neurosci 2007; 27:1812–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Chikanza IC. Prolactin and neuroimmunomodulation: in vitro and in vivo observations. Ann N Y Acad Sci 1999; 876:119–30. [DOI] [PubMed] [Google Scholar]
- 117. Markianos M, Koutsis G, Evangelopoulos ME, Mandellos D, Sfagos C. Serum and cerebrospinal fluid prolactin levels in male and female patients with clinically‐isolated syndrome or relapsing‐remitting multiple sclerosis. J Neuroendocrinol 2010; 22:503–8. [DOI] [PubMed] [Google Scholar]
- 118. Wei W, Liu L, Cheng Z‐L, Hu B. Increased plasma/serum levels of prolactin in multiple sclerosis: a meta‐analysis. Postgrad Med 2017; 129:605–10. [DOI] [PubMed] [Google Scholar]
- 119. Moshirzadeh S, Ghareghozli K, Harandi AA, Pakdaman H. Serum prolactin level in patients with relapsing–remitting multiple sclerosis during relapse. J Clin Neurosci 2012; 19:622–3. [DOI] [PubMed] [Google Scholar]
- 120. De Giglio L, Marinelli F, Prosperini L, Contessa GM, Gurreri F, Piattella MC et al Relationship between prolactin plasma levels and white matter volume in women with multiple sclerosis. Mediators Inflamm 2015; 2015:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Grinsted L, Heltberg A, Hagen C, Djursing H. Serum sex hormone and gonadotropin concentrations in premenopausal women with multiple sclerosis. J Intern Med 1989; 226:241–4. [DOI] [PubMed] [Google Scholar]
- 122. Lombardi G, Celso M, Bartelli M, Cilotti A, Del Popolo G. Female sexual dysfunction and hormonal status in multiple sclerosis patients. J Sex Med 2011; 8:1138–46. [DOI] [PubMed] [Google Scholar]
- 123. Riskind PN, Massacesi L, Doolittle TH, Hauser SL. The role of prolactin in autoimmune demyelination: suppression of experimental allergic encephalomyelitis by bromocriptine. Ann Neurol 1991; 29:542–7. [DOI] [PubMed] [Google Scholar]
- 124. Farooq F, Molina FA, Hadwen J, MacKenzie D, Witherspoon L, Osmond M et al Prolactin increases SMN expression and survival in a mouse model of severe spinal muscular atrophy via the STAT5 pathway. J Clin Invest 2011; 121:3042–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Walker TL, Vukovic J, Koudijs MM, Blackmore DG, Mackay EW, Sykes AM et al Prolactin stimulates precursor cells in the adult mouse hippocampus. PLoS One 2012; 7:e44371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Vergara‐Castañeda E, Grattan DR, Pasantes‐Morales H, Pérez‐Domínguez M, Cabrera‐Reyes EA, Morales T et al Prolactin mediates neuroprotection against excitotoxicity in primary cell cultures of hippocampal neurons via its receptor. Brain Res 2016; 1636:193–9. [DOI] [PubMed] [Google Scholar]
- 127. Arnold E, Thebault S, Baeza‐Cruz G, Arredondo Zamarripa D, Adan N, Quintanar‐Stephano A et al The hormone prolactin is a novel, endogenous trophic factor able to regulate reactive glia and to limit retinal degeneration. J Neurosci 2014; 34:1868–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Voskuhl RR, Sawalha AH, Itoh Y. Sex chromosome contributions to sex differences in multiple sclerosis susceptibility and progression. Mult Scler 2018; 24:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Voskuhl RR, Pitchekian‐Halabi H, Mackenzie‐Graham A, McFarland HF, Raine CS. Gender differences in autoimmune demyelination in the mouse: implications for multiple sclerosis. Ann Neurol 1996; 39:724–33. [DOI] [PubMed] [Google Scholar]
- 130. Golden LC, Voskuhl R. The importance of studying sex differences in disease: the example of multiple sclerosis. J Neurosci Res 2017; 95:633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Du S, Itoh N, Askarinam S, Hill H, Arnold AP, Voskuhl RR. XY sex chromosome complement, compared with XX, in the CNS confers greater neurodegeneration during experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA 2014; 111:2806–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Gomez A, Luckey D, Taneja V. The gut microbiome in autoimmunity: sex matters. Clin Immunol 2015; 159:154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Baker JM, Al‐Nakkash L, Herbst‐Kralovetz MM. Estrogen–gut microbiome axis: physiological and clinical implications. Maturitas 2017; 103:45–53. [DOI] [PubMed] [Google Scholar]
- 134. Chen KL, Madak‐Erdogan Z. Estrogen and microbiota crosstalk: should we pay attention? Trends Endocrinol Metab 2016; 27:752–5. [DOI] [PubMed] [Google Scholar]
- 135. Benedek G, Zhang J, Nguyen H, Kent G, Seifert HA, Davin S et al Estrogen protection against EAE modulates the microbiota and mucosal‐associated regulatory cells. J Neuroimmunol 2017; 310:51–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Orton S‐M, Wald L, Confavreux C, Vukusic S, Krohn JP, Ramagopalan SV et al Association of UV radiation with multiple sclerosis prevalence and sex ratio in France. Neurology 2011; 76:425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Nashold FE, Spach KM, Spanier JA, Hayes CE. Estrogen controls vitamin D3‐mediated resistance to experimental autoimmune encephalomyelitis by controlling vitamin D3 metabolism and receptor expression. J Immunol 2009; 183:3672–81. [DOI] [PubMed] [Google Scholar]
- 138. Spanier JA, Nashold FE, Mayne CG, Nelson CD, Hayes CE. Vitamin D and estrogen synergy in Vdr‐expressing CD4+ T cells is essential to induce Helios+ FoxP3+ T cells and prevent autoimmune demyelinating disease. J Neuroimmunol 2015; 286:48–58. [DOI] [PubMed] [Google Scholar]
- 139. Correale J, Ysrraelit MCC, Gaitan MII. Gender differences in 1,25 dihydroxyvitamin D3 immunomodulatory effects in multiple sclerosis patients and healthy subjects. J Immunol 2010; 185:4948–58. [DOI] [PubMed] [Google Scholar]
- 140. Langer‐Gould A. Sex hormones and multiple sclerosis: another informative failure. Lancet Neurol 2016; 15:22–3. [DOI] [PubMed] [Google Scholar]


