Summary
Pemphigus foliaceus (PF) is a blistering autoimmune skin disease rare in most of the world but endemic in certain regions of Brazil. PF is characterized by the detachment of epidermal cells and the presence of autoantibodies against desmoglein 1. In previous studies, we have shown that genetic polymorphisms and variable expression levels of certain leucocyte receptor complex (LRC) genes were associated with PF. However, the role of the LRC on PF susceptibility remained to be investigated. Here, we analysed 527 tag single nucleotide polymorphisms (SNPs) distributed within the 1·5 Mb LRC. After quality control, a total of 176 SNPs were analysed in 229 patients with PF and 194 controls. Three SNPs were associated with differential susceptibility to PF. The intergenic variant rs465169 [odds ratio (OR) = 1·50; P = 0·004] is located in a region that might regulate several immune‐related genes, including VSTM1,LILRB1/2,LAIR1/2,LILRA3/4 and LENG8. The rs35336528 (OR = 3·44; P = 0·009) and rs1865097 (OR = 0·57; P = 0·005) SNPs in LENG8 and FCAR genes, respectively, were also associated with PF. Moreover, we found four haplotypes with SNPs within the KIR3DL2/3,LAIR2 and LILRB1 genes associated with PF (P < 0·05), which corroborate previously reported associations. Thus, our results confirm the importance of the LRC for differential susceptibility to PF and reveal new markers that might influence expression levels of several LRC genes, as well as candidates for further functional studies.
Keywords: 19q13.42, FCAR, LENG8, LRC, pemphigus foliaceus, rs465169
Abbreviations
- AIRE
autoimmune regulator
- ATF‐3
cyclic AMP‐dependent transcription factor 3
- CACNG6
calcium voltage‐gated channel auxiliary subunit gamma 6
- CD33
myeloid differentiation antigen 33
- CI
confidence interval
- DSG1
desmoglein 1
- eQTL
expression quantitative trait loci
- FCAR
Fc fragment of IgA receptor
- GRCh37/hg19
genome reference consortium human build 37
- HLA
human leucocyte antigen
- HWE
Hardy–Weinberg equilibrium
- IgSF
immunoglobulin superfamily
- KIR
killer‐cell immunoglobulin‐like receptor
- LAIR
leucocyte‐associated immunoglobulin‐like receptor
- LD
linkage disequilibrium
- LENG8‐AS1
LENG8 natural antisense RNA 1
- LENG8
leucocyte receptor cluster member 8
- LILR
leucocyte immunoglobulin‐like receptor
- LRC
leucocyte receptor complex
- MAF
minor allele frequency
- MAF
musculoaponeurotic fibrosarcoma
- NALP12
NLR family pyrin domain containing 12
- NF‐κB
nuclear factor kappa B transcription factor
- NKG7
natural killer cell granule protein 7
- OR
odds ratio
- PF
pemphigus foliaceus
- r2
correlation of linkage disequilibrium (LD) analysis
- SNP
single nucleotide polymorphism
- Th2
T helper 2
- VSTM1
V‐set transmembrane domain containing 1
- ZNF331
zinc finger protein 331
Introduction
Pemphigus is a group of blistering autoimmune skin diseases characterized by keratinocyte detachment (acantholysis), which results in flaccid blisters, and by the presence of autoantibodies against desmosomal cadherins. In pemphigus foliaceus (PF), the blisters are observed at superficial layers of the epidermis (granular and corneal layers) and the major autoantigen is desmoglein 1 (DSG1).1, 2 The rare sporadic form of PF occurs across the globe; however, this disease is endemic in Central and Southeastern Brazil, where it is popularly known as fogo selvagem (which means ‘wild fire’). In some areas, the disease reaches the astonishing prevalence of 1·5% to 3%,3 the highest ever reported for an autoimmune disease. Even so, it still is a neglected disease and represents a significant public health challenge. Endemic PF has been also reported in Tunisia4, 5 and in other South American countries, such as Colombia, Peru and Bolivia.6
Both endemic and sporadic PF exhibit similar clinical, immunological and histological characteristics.7 PF is a complex disease triggered by ill‐defined environmental factors. It has been suggested that salivary antigens inoculated by hematophagous insects (such as Simulium nigrimanum) may trigger endemic PF in Brazil by inducing cross‐reactive autoantibody responses against DSG1 in genetically susceptible individuals.8, 9 Nevertheless, the involvement of salivary proteins of the insects or of infectious triggers is a hypothesis that remains to be further investigated.10
Variants of numerous genes influence susceptibility to PF. The first genetic associations reported were variants within the human leucocyte antigen (HLA) genes, with strong associations with alleles of HLA‐DRB1 and HLA‐DQB1.11, 12, 13 Furthermore, our group has demonstrated several other associations of PF with genes involved in immune responses.14, 15, 16, 17, 18, 19, 20
The focus of this study is to analyze variants within the leucocyte receptor complex (LRC) searching for associations with PF. The LRC is located at the 19q13.42 genomic region and contains more than 45 genes, including some immunoglobulin superfamily (IgSF) receptor genes: the polymorphic killer‐cell immunoglobulin‐like receptors (KIR), leucocyte immunoglobulin‐like receptors (LILR), leucocyte‐associated immunoglobulin‐like receptors (LAIR),21 among others. Further in the LRC centromeric region, in the so‐called extended LRC, there are additional genes that encode receptors expressed in several immune system cells.22, 23
Previously, we identified the LRC as an important region for pemphigus susceptibility, with strong associations for polymorphisms in KIR,24 LAIR1 and LAIR2.25 The presence of three or more activating KIR and some KIR‐HLA combinations were associated with PF,24 and the presence of both KIR3DL2*001 and the ligand HLA‐A3 or HLA‐A11 increases susceptibility to PF.26 Our group also performed a genome‐wide expression profiling with over 50 000 probes that revealed that genes in 19q13 (CD33, NALP12, NKG7, LILRB2) were differentially expressed in CD4+ lymphocytes when comparing patients and controls, as well as between different clinical forms.27 Together, these results indicate that variation within the LRC region is relevant for PF susceptibility.
We hypothesized that variants within the 19q13.42 region impact the expression or the structure of coding and non‐coding LRC gene products, with functional consequences that might influence susceptibility to pemphigus. We used genotype data of over 520 single nucleotide polymorphisms (SNPs) in this region and performed a case‐control association study, and we integrated our results with functional genomics data from public databases to reveal the most likely candidate variants.
Materials and methods
Patient and control population samples
Two‐hundred and twenty‐nine unrelated patients with a PF diagnosis based on clinical, histopathological and/or immunohistochemical criteria were recruited at hospitals from endemic areas as described in Cipolla et al.18 The control sample encompassed 194 unrelated individuals with no known history of autoimmune diseases who lived in endemic areas. Both the patient and the control samples were of predominantly European ancestry. All individuals signed informed consent. This study was performed according to Brazilian federal laws, and approved by the Human Research Ethics Committee of the Federal University of Paraná.
Microarray genotyping
DNA was obtained from peripheral blood and extracted by phenol–chloroform–isoamyl alcohol protocol.28 Genotyping was performed by microarray DNA Human Infinium® CoreExome‐24 Beadchip (Illumina®, San Diego, California, EUA), according to manufacturer instructions.
Selection of 19q13.42 region markers and statistical analysis
From microarray DNA data, we extracted 527 SNPs within the 19q13.42 region (chr19: 54 000 000–55 400 000, GRCh37/hg19), considering ZNF331 and FCAR as the LRC flanking genes. After standard quality controls,29 we filtered by minor allele frequency higher than 2% (MAF > 0·02) and by SNPs in Hardy–Weinberg equilibrium (HWE) in the control sample (P > 0·01), and then performed the case‐control study with the 176 remaining SNPs (Table S1).
Association analysis was performed by logistic regression with the two‐first components from principal component analysis as covariant to correct for possible population stratification. Because the effect of genetic variants on disease susceptibility may not differ between the heterozygote and the homozygote genotypes, we also applied the dominant and recessive models of the less frequent allele in the population (the minor allele), in addition to the standard additive model. Linkage disequilibrium (LD), odds ratio (OR) and 95% confidence interval (CI) were calculated, and the HWE was analysed with PLINK software v1.9.30 The P‐value 0·01 was considered as the significance limit for the additive, dominant and recessive models. For the haplotype model association analysis, haplotypes with a frequency higher than 5% in patients or controls were considered, and P = 0·05 was adopted as the significance limit.
In silico analysis
The SNP and gene annotations were performed with UCSC31 and Ensembl32 genome browsers. To visualize the SNP distribution along chromosome 19, the additive and dominant association results were evaluated by the LocusZoom33 online tool. We evaluated LD between SNPs analysed in our study with the merged European sample (CEU, TSI, FIN, GBR, IBS) from the 1000 Genomes project using SNiPA34 and LDLink.35 The possible structural and regulatory impacts of the genetic variants and their expression quantitative trait loci (eQTL) effects were observed with SNiPA,34 HaploReg,36 RegulomeDB37 online tools and GTEx Portal38 database.
Results
SNPs within the LRC are associated with pemphigus
We found two SNPs (rs465169 and rs35336528) associated with PF in the additive and dominant models, and a third SNP, rs1865097, associated in the dominant model (Fig. 1; Table 1). Allele and genotype frequencies are presented in Table S2.
Figure 1.
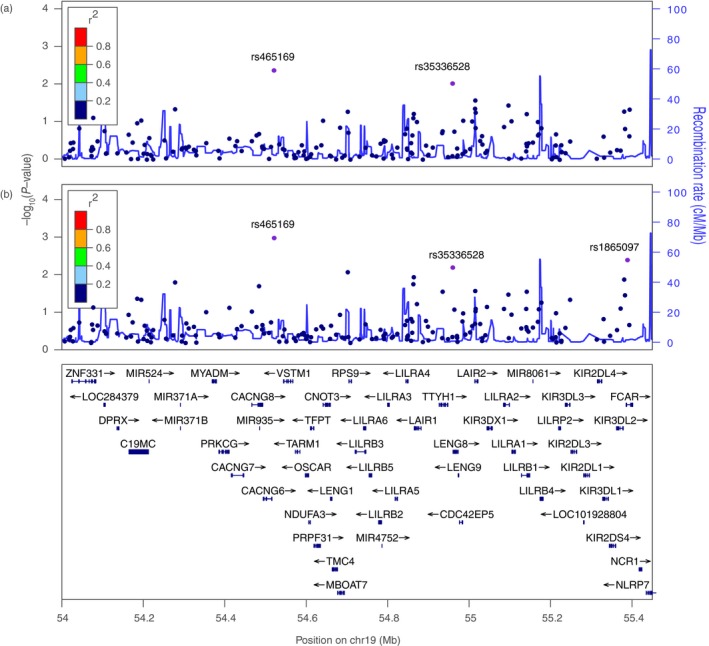
Result of the association analysis according to the additive (a) and dominant (b) models between 176 single nucleotide polymorphisms (SNPs) and pemphigus foliaceus (PF), showing the distribution of SNPs and gene localization along the chromosome 19q13.4 genomic region (GRCh37/hg19). The purple dots are SNPs associated with PF. Mb, megabase (106 bp). Adapted from LocusZoom online tool.33
Table 1.
SNPs associated with PF
| Model | OR | 95% CI | P | Gene | |
|---|---|---|---|---|---|
| rs465169 | Additive | 1·50 | 1·14–1·98 | 0·004 | Intergenic |
| Dominant | 1·97 | 1·30–2·99 | 0·001 | ||
| Recessive | 1·43 | 0·88–2·34 | 0·151 | ||
| rs35336528 | Additive | 3·39 | 1·37–8·41 | 0·008 | LENG8 |
| Dominant | 3·44 | 1·37–8·66 | 0·009 | ||
| Recessive | NC | NC | NC | ||
| rs1865097 | Additive | 0·74 | 0·55–0·99 | 0·046 | FCAR |
| Dominant | 0·57 | 0·38–0·85 | 0·005 | ||
| Recessive | 1·04 | 0·57–1·91 | 0·891 |
CI, confidence interval; NC, not calculated because of the low frequency of genotype GG; OR, odds ratio.
In bold P‐value < 10−2.
The dominant model refers to a dominant effect of the minor alleles: rs465169 A, rs35336528 G, rs1865097 A.
The minor alleles of the rs465169 and rs35336528 SNPs were associated with increased susceptibility to PF (Table 1). The intergenic SNP rs465169 is located between the calcium voltage‐gated channel auxiliary subunit gamma 6 (CACNG6) and V‐set transmembrane domain containing 1 (VSTM1) genes (Fig. 1), and the rs35336528 SNP is in exon 11 of the leucocyte receptor cluster member 8 (LENG8) gene. The minor allele A of the intronic rs1865097 SNP in the FCAR (Fc fragment of IgA receptor) gene was associated with decreased susceptibility to PF (Table 1).31, 32
Although those SNPs are in the same chromosomal region, there was no LD among these three SNPs (r 2 ≈ 0) in the studied population. However, strong LD among these SNPs and approximately 15 others (r 2 > 0·9) was observed in European populations from the 1000 genomes project (Table S3). Therefore, the three SNPs (rs465169, rs35336528, rs1865097) are useful tag SNPs for genetic variation along genomic segments of 4, 19·5 and 2·6 kb, respectively.
Significant associations with LILR and KIR haplotypes
In order to explore the possible effect of cis combinations of SNPs on PF susceptibility, we performed a haplotype association analysis. We found four haplotypes with variants in KIR family genes, LAIR2 and LILRB1 genes, and the LILRP2 pseudogene associated with PF. None of them included SNPs individually associated with PF; however, for several of them a borderline P‐value was seen (0·1 > P > 0·01). The SNPs, gene annotation and haplotype frequencies in patients with PF and controls are described in Table 2.
Table 2.
Four haplotypes are associated with increased or decreased susceptibility of PF
| Haplotype | SNP 1 | SNP 2 | SNP 3 | Haplotype | PF F (%) | CTR F (%) | OR | P |
|---|---|---|---|---|---|---|---|---|
| H1 | rs1325158 | rs4806527 | rs2075731 | TGC | 6·3 | 2·5 | 2·56 | 0·023 |
| LILRP2 downstream | LILRP2 downstream | KIR3DL3 | ||||||
| H2 | rs4806768 | rs61737870 | TC | 36·6 | 44·3 | 0·73 | 0·030 | |
| LAIR2 | LAIR2 | |||||||
| H3 | rs1654644 | rs11665986 | TT | 23·2 | 18·3 | 1·46 | 0·037 | |
| KIR3DL2 | FCAR upstream | |||||||
| H4 | rs10423364 | rs1061680 | rs61739173 | ACA | 5·2 | 8·1 | 0·58 | 0·041 |
| LILRB1 upstream | LILRB1 | LILRB1 |
CTR, controls samples; F, frequency; H, haplotype; OR, odds ratio; PF, pemphigus foliaceus patients; SNP, single nucleotide polymorphism.
The haplotypes H1 and H3 were associated with increased risk to PF (OR = 2·56 and OR = 1·46, respectively; P < 0·05), and are nearby the KIR family genes. The haplotype TGC (H1) was composed of three SNPs; two (rs1325158 and rs4806527) are located downstream of the LILRP2 pseudogene and one (rs2075731) is within exon 3 of KIR3DL3 gene. The haplotype TT (H3) was composed of the rs1654644 SNP in intron 6 of KIR3DL2 gene and rs11665986 SNP upstream of FCAR gene. Although the SNPs from the two associated haplotypes (H1 and H3) did not show strong LD with each other (r 2 < 0·2), strong LD (r 2 > 0·9) was seen between H1 and 16 SNPs, and between H3 and 17 SNPs in European populations from the 1000 genomes project (Tables S4 and S6). Thus, those SNPs can be considered tag SNPs for genetic variation along genomic segments of 1·7 and 11 kb (H1) and 61 kb (H3). Despite the short distance (7 kb) between KIR and FCAR genes, the SNPs of H3 (rs1654644‐rs11665986) were not in LD with the FCAR SNP rs1865097 individually associated with PF (r 2 < 0·1; Europeans of the 1000 genomes project).
We found two other haplotypes (H2 and H4) associated with PF (OR = 0·73 and OR = 0·58, respectively; P < 0·05). They are located in LAIR2 and LILRB1 genes, respectively. The haplotype TC (H2) was composed of the rs4806768 T allele in intron 2 and rs61737870 C allele in exon 3 of the LAIR2 gene, which were not in LD (r 2 < 0·2). These H2 SNPs were in strong LD (r 2 > 0·9) with, respectively, six and 11 other SNPs within LAIR2 in European populations from the 1000 genomes project (Table S5). Lastly, the haplotype ACA (H4) was composed of rs10423364 upstream of LILRB1 and two SNPs (rs1061680 and rs61739173) in exons 6 and 8 of the LILRB1 gene, respectively. We identified strong LD between rs10423364 and rs1061680 SNPs (r 2 = 0·87), while the other SNP pairs of the same haplotype were not in LD (r 2 < 0·2) in our dataset. Moreover, those SNPs (rs10423364, rs1061680, rs61739173) were in strong LD (r 2 > 0·9) with, respectively, 26, 11 and three other SNPs within LILRB1, in European populations from the 1000 genomes project (Table S7). Thus, the haplotypes H2 and H4 SNPs tag genetic variation of the LAIR2 and LILRB1 genes along genomic segments of 5 and 26 kb, respectively (Tables S5 and S7).
Variants associated with PF might influence expression levels of LRC genes
In order to understand the possible regulatory impact of the SNPs associated with PF, we performed in silico analysis using several public databases and tools (see ‘ In silico analysis’). We found several variants within transcription factor binding sites (MAF, NK‐kB, AIRE, among others), regulatory motifs or sites of chromatin modification that could impact the expression of a variety of LRC genes (Tables S3–S7). Although the mechanisms that could explain the impact of the associated alleles or haplotypes on gene expression levels remain unknown, the H3 haplotype (rs1654644 T‐ rs11665986 T) and the rs10423364 G allele in the H4 haplotype upregulate, respectively, the KIR3DL1/2, KIR2DS4 and LILRB1 genes in whole blood, according to GTEx Portal38 (Figs S1 and S2).
Discussion
Previous findings demonstrated associations of PF with variants in the LAIR2 and KIR genes, and with expression of several LRC genes.24, 25, 26, 27 Here, we describe three variants associated with PF (intergenic rs465169, rs35336528 in LENG8 and rs1865097 in FCAR) that have never been associated with any disease. One possible reason is that genome‐wide association studies (GWAS) usually exhibit low SNP density in this region. This is caused by the numerous sequence repeats and homologous genes with high sequence similarity that impose technical difficulties for genotyping. Moreover, uncommon variation in extension occurs as a consequence of variable numbers of LRC genes among haplotypes. Because stringent P‐values are needed to avoid false‐positives in such studies (usually P < 5 × 10−8 or P < 5 × 10−7), many statistically weak associations that may be relevant for disease are missed in GWAS. Additionally, despite involvement of LRC gene products in immune responses, variants within the LRC were rarely analysed as candidates for autoimmune and other complex diseases. Our results confirm that the LRC is a hot spot for pemphigus susceptibility and may help to reveal still unknown molecular mechanisms involved in pathogenesis. On the basis of these results, we suggest that the LRC should be investigated in other diseases that involve alterations of immune responses.
The rs465169 SNP is an intergenic variant located within a regulatory region. The risk allele rs465169 A can potentially amplify the binding of the transcript factor avian musculoaponeurotic fibrosarcoma oncogene homologue (MAF), which can activate or repress transcription depending on the binding site and the protein‐binding partner39 (Table S3). Although the impact of the rs465169 variants (A or G allele) on interaction between MAF and its DNA binding site remains unclear, this protein is considered a key regulatory factor of epidermal differentiation in upper layers in non‐pathological conditions.40, 41 Therefore, association of this SNP with a skin disease characterized by detachment of the upper skin layers could have a plausible biological explanation. In addition, MAF is involved in T‐cell apoptosis as well as activation of IL‐4 expression in T helper 2 (Th2) cells. IL‐4 is cytokine that participates in B‐cell activation and production of IgG1.42
Furthermore, the rs465169 SNP might influence the regulation of several LRC genes: VSTM1, NLRP12, LAIR1, LILRA3, LILRA5, LILRA6, LILRB2, LENG8, according to online tools (Table S3). We have previously shown that the expression levels of LILRB2, LILRA5, LAIR1, VSTM1 and NLRP12 were increased in patients with PF when compared with controls.27 Therefore, our results suggest rs465169 as a strong candidate for functional validation.
In LENG8 gene, the variant rs35336528 G was associated with 3·4 times increased risk of PF. It is a missense variant that results in an isoleucine to valine replacement in LENG8. This protein is potentially involved in glycosylated protein recognition and RNA transport.23, 43
Apart from the amino acid change caused by this SNP, rs35336528 is located within a regulatory region. The presence of the rs35336528 G allele may alter the cyclic AMP‐dependent transcription factor (ATF3) regulatory motif. ATF3 is known for repressing transcription by stabilizing the binding of inhibitory cofactors to promoters, while its isoform 2 activates transcription by sequestering inhibitory cofactors.44 In silico analysis also evidenced that the region where rs35336528 is located exhibits numerous chromatin state and histone modifications in foreskin fibroblasts cells. Besides, rs35336528 is in strong LD with other SNPs that might regulate KIR, LILR and other LRC genes (r 2 > 0·8; Table S3). Moreover, in silico analysis indicated that the rs35336528 SNP has a cis‐eQTL effect on the LENG8 natural antisense RNA 1, LENG8‐AS1. Indeed, antisense long non‐coding RNAs (lncRNAs) potentially regulate the expression of their sense counterparts.45 Both LENG8 and LENG8‐AS1 appear to be highly expressed in lymphocytes and skin tissue in non‐pathogenic conditions.38, 46 Thus, it would be worth checking if rs35336528 in fact impacts expression of LENG8, of its antisense RNA, as well as of other LRC genes, and if it is a functional SNP influencing pemphigus pathogenesis.
The intronic variant rs1865097 in the FCAR gene could influence FCAR, CTB‐61M7.2 and KIR gene expression. According to GTEx Portal,38 the protective genotype rs1865097 A/A increases FCAR expression levels. FCAR (also named CD89) encodes a receptor for the Fc region of IgA (FcαRI) and acts as a regulator of anti‐ and pro‐inflammatory responses mediated by IgA.47, 48 IgA‐FcαRI interactions can play a harmful role in inflammatory and autoimmune skin‐blistering diseases, including linear IgA bullous disease, dermatitis herpetiformis and IgA pemphigus. IgA autoantibodies are rarely detected in serum and skin of patients with PF; however, FcαRI signals can be induced independently of IgA, by interaction with pentaxins.48 Additionally, rs1865097 is in strong LD with numerous SNPs that may regulate KIR genes, which have been previously associated with PF.24, 26
We found four haplotypes associated with PF that contain variants in KIR, LAIR2 and LILRB1 genes, and in flanking regions of the LILRP2 pseudogene and the FCAR gene. Despite the fact that none of those SNPs was associated with PF individually, low P‐values (0·1 > P > 0·01) were observed for several of them. It is reasonable to suppose that certain combinations of SNPs exhibit a stronger phenotypic effect than each variant individually, because of additive interactions or of LD between the haplotype and a causal variant of another locus. Moreover, SNPs may not be associated individually due to epistatic interaction with other polymorphisms. It will be necessary to deeply explore that genomic region and to perform functional analyses, searching for the reasons for these associations.
The KIR haplotype rs1654644‐rs11665986 TT (H3) increases the KIR3DL1, KIR3DL2 and KIR2DS4 expression levels in whole blood, according to the GTEx Portal38 (Fig. S1). In addition, SNPs from H1 (rs4806527) and H3 (rs1654644, rs11665986) are in strong LD with other variants that can potentially influence the expression of the inhibitory KIR2DL1, KIR2DL3/4, KIR3DL1/3, the activating KIR2DS4, as well as FCAR (Tables S4 and S6). These findings corroborate the role of KIR genes in pemphigus recognized by a former study of our group, which demonstrated that the presence of three or more activating KIR genes protect from PF (OR = 0·49; P = 0·003).24 Additionally, the non‐synonymous variant at position 1190 (rs3745902 T) of the inhibitory KIR3DL2 marks low expression levels and was associated with protection against PF.26 Here we observed that the KIR3DL2 low‐expression‐associated variant rs3745902 26 is in moderate LD (r 2 = 0·51, D′ = 0·83; European populations from 1000 genomes project) with rs1654644 SNP from H3, which was also associated with PF.
LAIR‐2 (leucocyte‐associated immunoglobulin‐like receptor 2, also known as CD306) is a secreted receptor that competes with the homologous membrane‐bound LAIR‐1 by binding the same collagenous ligands.49 The disruption of the inhibition of immune responses by LAIR‐1 can lead to loss of self‐tolerance in a variety of autoimmune diseases, such as systemic lupus erythematosus50, 51 and rheumatoid arthritis.52, 53 We observed that rs4806768 SNP from H2 is in strong LD with rs2277974 (r 2 = 0·97; Table S5); a SNP within a LAIR2 haplotype previously associated with PF and with differential expression levels.25 The rs4806768 T allele occurs in cis with rs2277974 T allele, previously associated with LAIR2 lower expression levels. Interestingly, the rs4806768 SNP alters a binding motif for the autoimmune regulator (AIRE) protein. AIRE is an important regulator of central tolerance that interacts with histones and chromatin, inducing the expression of a wide array of otherwise tissue‐restricted self‐antigens genes in the thymus. AIRE can inactivate CD4+ T cells and change the TCR repertoire and functions of peripheral CD8+ regulatory T cells.54, 55 Thus, the H2 haplotype that is associated with lower risk of PF includes a regulatory motif and marks LAIR2 expression, and therefore should be explored in forthcoming studies.
This is the first study that shows association of polymorphisms of LILRB1 with PF. LILRB1 (also named ILT2, LIR1, MIR7, CD85, CD85J) is a highly polymorphic member of the LILR gene family.56, 57 It encodes the inhibitory receptor leucocyte immunoglobulin‐like receptor subfamily B member 1 (LIR‐1, LILRB1) whose ligands are HLA class I molecules.58, 59, 60
As shown, the LILRB1 haplotype (H4) rs10423364‐rs1061680‐rs61739173 ACA was associated with decreased susceptibility to PF. The rs10423364 G allele that increases the LILRB1 expression levels in whole blood (Fig. S2) is in strong LD (r 2 > 0·98) with haplotype AGG (rs1004443‐rs3760860‐rs3760861) in the LILRB1 promoter (Table S7). These three promoter SNPs are in absolute LD and form two haplotypes denoted AGG and GAA. The AGG haplotype was associated with lower LILRB1 expression on B and T lymphocytes and monocytes.56 Interestingly, the same AGG haplotype was associated with an increased number of NK cells that express LILRB1 and with higher LILRB1 expression levels in NK cells.57 The rs10423364 SNP is within a binding site for the nuclear factor kappa B (NF‐κB), whose pleiotropic function can be stimulated by immune, inflammation and apoptosis signals.61, 62, 63 Moreover, the rs10423364 is in strong LD with SNPs that may regulate several other LRC genes, such as NLRP12, LAIR1, LAIR2, LILRA4, LILRP2 (r 2 ≥ 0·99; Table S7). The missense rs1061680 C allele that occurs in LD with rs10423364 A results in a threonine at amino acid position 142 and is associated with low expression of LILRB1 on the NK cell surface.57 Thus, although the observed association of PF with the ACA LILRB1 haplotype H4 may be explained by differential expression levels of LILRB1 (and other LRC genes), the results of previous work about the specific effect of polymorphisms indicate that LILRB1 expression varies among cell types, so the functional impact of the H4 polymorphisms should be clarified in future studies.
Conclusion
Our results indicate an important role of the LRC genes in PF etiology. It is interesting to notice that most of the variants associated with PF are potentially involved in gene regulatory mechanisms and differential expression levels. We found four unsuspected genes or regulatory elements whose variants are associated with differential susceptibility to PF: an intergenic regulatory region, LENG8, FCAR and LILRB1. Altogether, our results provide clear‐cut evidence that variants of the 19q13.42 region are involved in pathogenesis of pemphigus, possibly due to their effect on regulation of gene expression. This agrees with results of a genome‐wide gene expression profiling that revealed several LRC genes differentially expressed in patients with PF compared with controls.27 Although our focus was on genetic variation, we discussed several possible consequences of the polymorphisms that should be further evaluated in functional studies.
Author contributions
TDJF, MLPE, DM designed the study; MLPE provided the samples; DGA generated the data; TDJF and RCA analysed the data; TDJF, DGA and MLPE drafted the manuscript. All authors contributed with ideas and critically reviewed this manuscript.
Disclosures
The authors declare no conflicts of interest.
Supporting information
Figure S1. The expression quantitative trait (eQTL) effect of haplotype 3 SNPs on KIR (killer‐cell immunoglobulin like receptor) genes expression in whole blood.
Figure S2. The expression quantitative trait (eQTL) effect of the rs10423364 genotypes on leucocyte immunoglobulin like receptor B1 (LILRB1) gene expression in whole blood.
Table S1. List of 176 SNPs included in association analysis for endemic PF.
Table S2. Allele and genotype frequencies of SNPs associated with PF among patients and controls.
Table S3. Predicted regulatory effects of the SNPs rs465169, rs35336528 and rs1865097, and of the SNPs in strong LD with them (r 2 ≥ 0·8).
Table S4. Predicted regulatory effects of SNPs rs1325158, rs4806527 and rs2075731 from haplotype H1 associated with PF, and of SNPs in strong LD (r 2 ≥ 0·8) with them.
Table S5. Predicted regulatory effects of SNPs rs4806768 and rs61737870 from haplotype H2 associated with PF, and of SNPs in strong LD (r 2 ≥ 0·8) with them.
Table S6. Predicted regulatory effects of SNPs rs61739173 and rs11665986 from haplotype H3 associated with PF, and of SNPs in strong LD (r 2 ≥ 0·8) with them.
Table S7. Predicted regulatory effects of SNPs rs10423364, rs1061680 and rs61739173 from haplotype H4 associated with PF, and of SNPs in strong LD (r 2 ≥ 0·8) with them.
Acknowledgements
The authors thank the patients and healthy controls for volunteering for this study. The authors also thank our laboratory colleagues for their friendship and support. The authors would like to thank Kirsten M. Anderson for kindly reviewing this manuscript. This study was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Programa de Apoio a Núcleos de Excelência (PRONEX), Fundação Araucária.
References
- 1. Mahoney MG, Wang Z, Rothenberger K, Koch PJ, Amagai M, Stanley JR. Explanations for the clinical and microscopic localization of lesions in pemphigus foliaceus and vulgaris. J Clin Invest 1999; 103:461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arteaga LA, Prisayanh PS, Warren SJ, Liu Z, Diaz LA, Lin MS. A subset of pemphigus foliaceus patients exhibits pathogenic autoantibodies against both desmoglein‐1 and desmoglein‐3. J Invest Dermatol 2002; 118:806–11. [DOI] [PubMed] [Google Scholar]
- 3. Aoki V, Millikan RC, Rivitti EA, Hans‐Filho G, Eaton DP, Warren SJ et al Environmental risk factors in endemic pemphigus foliaceus (fogo selvagem). J Investig Dermatol Symp Proc 2004; 9:34–40. [DOI] [PubMed] [Google Scholar]
- 4. Kallel Sellami M, Ben Ayed M, Mouquet H, Drouot L, Zitouni M, Mokni M et al Anti‐desmoglein 1 antibodies in Tunisian healthy subjects: arguments for the role of environmental factors in the occurrence of Tunisian pemphigus foliaceus. Clin Exp Immunol 2004; 137:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morini JP, Jomaa B, Gorgi Y, Saguem MH, Nouira R, Roujeau JC et al Pemphigus foliaceus in young women. An endemic focus in the Sousse area of Tunisia. Arch Dermatol 1993; 129:69–73. [DOI] [PubMed] [Google Scholar]
- 6. Ortega‐Loayza AG, Ramos W, Gutierrez EL, Jimenez G, Rojas I, Galarza C. Endemic pemphigus foliaceus in the Peruvian Amazon. Clin Exp Dermatol 2013; 38:594–600. [DOI] [PubMed] [Google Scholar]
- 7. Baum S, Sakka N, Artsi O, Trau H, Barzilai A. Diagnosis and classification of autoimmune blistering diseases. Autoimmun Rev 2014; 13:482–9. [DOI] [PubMed] [Google Scholar]
- 8. Qian Y, Culton DA, Jeong JS, Trupiano N, Valenzuela JG, Diaz LA. Non‐infectious environmental antigens as a trigger for the initiation of an autoimmune skin disease. Autoimmun Rev 2016; 15:923–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lombardi C, Borges PC, Chaul A, Sampaio SA, Rivitti EA, Friedman H et al Environmental risk factors in endemic pemphigus foliaceus (Fogo selvagem). J Invest Dermatol 1992; 98:847–50. [DOI] [PubMed] [Google Scholar]
- 10. Ruocco E, Ruocco V, Lo Schiavo A, Brunetti G, Wolf R. Viruses and pemphigus: an intriguing never‐ending story. Dermatology 2014; 229:310–5. [DOI] [PubMed] [Google Scholar]
- 11. Petzl‐Erler ML, Santamaria J. Are HLA class II genes controlling susceptibility and resistance to Brazilian pemphigus foliaceus (Fogo selvagem)? Tissue Antigens 1989; 33:408–14. [DOI] [PubMed] [Google Scholar]
- 12. Pavoni DP, Roxo VM, Marquart Filho A, Petzl‐Erler ML. Dissecting the associations of endemic pemphigus foliaceus (Fogo Selvagem) with HLA‐DRB1 alleles and genotypes. Genes Immun 2003; 4:110–6. [DOI] [PubMed] [Google Scholar]
- 13. Brochado MJ, Nascimento DF, Campos W, Deghaide NH, Donadi EA, Roselino AM. Differential HLA class I and class II associations in pemphigus foliaceus and pemphigus vulgaris patients from a prevalent Southeastern Brazilian region. J Autoimmun 2016; 72:19–24. [DOI] [PubMed] [Google Scholar]
- 14. Pereira NF, Hansen JA, Lin MT, Roxo VM, Braun K, Petzl‐Erler ML. Cytokine gene polymorphisms in endemic pemphigus foliaceus: a possible role for IL6 variants. Cytokine 2004; 28:233–41. [DOI] [PubMed] [Google Scholar]
- 15. Malheiros D, Petzl‐Erler ML. Individual and epistatic effects of genetic polymorphisms of B‐cell co‐stimulatory molecules on susceptibility to pemphigus foliaceus. Genes Immun 2009; 10:547–58. [DOI] [PubMed] [Google Scholar]
- 16. Dalla‐Costa R, Pincerati MR, Beltrame MH, Malheiros D, Petzl‐Erler ML. Polymorphisms in the 2q33 and 3q21 chromosome regions including T‐cell coreceptor and ligand genes may influence susceptibility to pemphigus foliaceus. Hum Immunol 2010; 71:809–17. [DOI] [PubMed] [Google Scholar]
- 17. Piovezan BZ, Petzl‐Erler ML. Both qualitative and quantitative genetic variation of MHC class II molecules may influence susceptibility to autoimmune diseases: the case of endemic pemphigus foliaceus. Hum Immunol 2013; 74:1134–40. [DOI] [PubMed] [Google Scholar]
- 18. Cipolla GA, Park JK, de Oliveira LA, Lobo‐Alves SC, de Almeida RC, Farias TD et al A 3′UTR polymorphism marks differential KLRG1 mRNA levels through disruption of a miR‐584‐5p binding site and associates with pemphigus foliaceus susceptibility. Biochim Biophys Acta 2016; 1859:1306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Salviano‐Silva A, Petzl‐Erler ML, Boldt ABW. CD59 polymorphisms are associated with gene expression and different sexual susceptibility to pemphigus foliaceus. Autoimmunity 2017; 50:377–85. [DOI] [PubMed] [Google Scholar]
- 20. Bumiller‐Bini V, Cipolla GA, de Almeida RC, Petzl‐Erler ML, Augusto DG, Boldt ABW. Sparking fire under the skin? Answers from the association of complement genes with pemphigus foliaceus. Front Immunol 2018; 9:695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wende H, Colonna M, Ziegler A, Volz A. Organization of the leukocyte receptor cluster (LRC) on human chromosome 19q13.4. Mamm Genome 1999; 10:154–60. [DOI] [PubMed] [Google Scholar]
- 22. Barrow AD, Trowsdale J. The extended human leukocyte receptor complex: diverse ways of modulating immune responses. Immunol Rev 2008; 224:98–123. [DOI] [PubMed] [Google Scholar]
- 23. Wende H, Volz A, Ziegler A. Extensive gene duplications and a large inversion characterize the human leukocyte receptor cluster. Immunogenetics 2000; 51:703–13. [DOI] [PubMed] [Google Scholar]
- 24. Augusto DG, Lobo‐Alves SC, Melo MF, Pereira NF, Petzl‐Erler ML. Activating KIR and HLA Bw4 ligands are associated to decreased susceptibility to pemphigus foliaceus, an autoimmune blistering skin disease. PLoS ONE 2012; 7:e39991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Camargo CM, Augusto DG, Petzl‐Erler ML. Differential gene expression levels might explain association of LAIR2 polymorphisms with pemphigus. Hum Genet 2016; 135:233–44. [DOI] [PubMed] [Google Scholar]
- 26. Augusto DG, O'Connor GM, Lobo‐Alves SC, Bass S, Martin MP, Carrington M et al Pemphigus is associated with KIR3DL2 expression levels and provides evidence that KIR3DL2 may bind HLA‐A3 and A11 in vivo. Eur J Immunol 2015; 45:2052–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Malheiros D, Panepucci RA, Roselino AM, Araújo AG, Zago MA, Petzl‐Erler ML. Genome‐wide gene expression profiling reveals unsuspected molecular alterations in pemphigus foliaceus. Immunology 2014; 143:381–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. New York: CSHL Press, 2001: 1448 p. [Google Scholar]
- 29. Anderson CA, Pettersson FH, Clarke GM, Cardon LR, Morris AP, Zondervan KT. Data quality control in genetic case‐control association studies. Nat Protoc 2010; 5:1564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second‐generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 2015; 4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hinrichs AS, Raney BJ, Speir ML, Rhead B, Casper J, Karolchik D et al UCSC data integrator and variant annotation integrator. Bioinformatics 2016; 32:1430–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McLaren W, Gil L, Hunt SE, Riat HS, Ritchie GR, Thormann A et al The Ensembl variant effect predictor. Genome Biol 2016; 17:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, Gliedt TP et al LocusZoom: regional visualization of genome‐wide association scan results. Bioinformatics 2010; 26:2336–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Arnold M, Raffler J, Pfeufer A, Suhre K, Kastenmüller G. SNiPA: an interactive, genetic variant‐centered annotation browser. Bioinformatics 2015; 31:1334–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Machiela MJ, Chanock SJ. LDlink: a web‐based application for exploring population‐specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics 2015; 31:3555–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res 2012; 40:D930–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boyle AP, Hong EL, Hariharan M, Cheng Y, Schaub MA, Kasowski M et al Annotation of functional variation in personal genomes using RegulomeDB. Genome Res 2012; 22:1790–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carithers LJ, Ardlie K, Barcus M, Branton PA, Britton A et al A Novel Approach to High‐Quality Postmortem Tissue Procurement: The GTEx Project. Biopreserv Biobank 2015; 13:311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kheradpour P, Kellis M. Systematic discovery and characterization of regulatory motifs in ENCODE TF binding experiments. Nucleic Acids Res 2014; 42:2976–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Labott AT, Lopez‐Pajares V. Epidermal differentiation gene regulatory networks controlled by MAF and MAFB. Cell Cycle 2016; 15:1405–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Klein RH, Andersen B. Dynamic networking for epidermal differentiation. Dev Cell 2015; 32:661–2. [DOI] [PubMed] [Google Scholar]
- 42. Köck J, Kreher S, Lehmann K, Riedel R, Bardua M, Lischke T et al Nuclear factor of activated T cells regulates the expression of interleukin‐4 in Th2 cells in an all‐or‐none fashion. J Biol Chem 2014; 289:26 752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baillat D, Hakimi MA, Näär AM, Shilatifard A, Cooch N, Shiekhattar R. Integrator, a multiprotein mediator of small nuclear RNA processing, associates with the C‐terminal repeat of RNA polymerase II. Cell 2005; 123:265–76. [DOI] [PubMed] [Google Scholar]
- 44. Hai TW, Liu F, Coukos WJ, Green MR. Transcription factor ATF cDNA clones: an extensive family of leucine zipper proteins able to selectively form DNA‐binding heterodimers. Genes Dev 1989; 3:2083–90. [DOI] [PubMed] [Google Scholar]
- 45. Guil S, Esteller M. Cis‐acting noncoding RNAs: friends and foes. Nat Struct Mol Biol 2012; 19:1068–75. [DOI] [PubMed] [Google Scholar]
- 46. Petryszak R, Keays M, Tang YA, Fonseca NA, Barrera E, Burdett T et al Expression Atlas update – an integrated database of gene and protein expression in humans, animals and plants. Nucleic Acids Res 2016; 44:D746–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Monteiro RC, Van De Winkel JG. IgA Fc receptors. Annu Rev Immunol 2003; 21:177–204. [DOI] [PubMed] [Google Scholar]
- 48. Aleyd E, Heineke MH, van Egmond M. The era of the immunoglobulin A Fc receptor FcαRI; its function and potential as target in disease. Immunol Rev 2015; 268:123–38. [DOI] [PubMed] [Google Scholar]
- 49. Lebbink RJ, van den Berg MC, de Ruiter T, Raynal N, van Roon JA, Lenting PJ et al The soluble leukocyte‐associated Ig‐like receptor (LAIR)‐2 antagonizes the collagen/LAIR‐1 inhibitory immune interaction. J Immunol 2008; 180:1662–9. [DOI] [PubMed] [Google Scholar]
- 50. Colombo BM, Canevali P, Magnani O, Rossi E, Puppo F, Zocchi MR et al Defective expression and function of the leukocyte associated Ig‐like receptor 1 in B lymphocytes from systemic lupus erythematosus patients. PLoS ONE 2012; 7:e31903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bonaccorsi I, Cantoni C, Carrega P, Oliveri D, Lui G, Conte R et al The immune inhibitory receptor LAIR‐1 is highly expressed by plasmacytoid dendritic cells and acts complementary with NKp44 to control IFNα production. PLoS ONE 2010; 5:e15080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang Y, Ding Y, Huang Y, Zhang C, Boquan J, Ran Z. Expression of leukocyte‐associated immunoglobulin‐like receptor‐1 (LAIR‐1) on osteoclasts and its potential role in rheumatoid arthritis. Clinics 2013; 68:475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang Y, Lv K, Zhang CM, Jin BQ, Zhuang R, Ding Y. The role of LAIR‐1 (CD305) in T cells and monocytes/macrophages in patients with rheumatoid arthritis. Cell Immunol 2014; 287:46–52. [DOI] [PubMed] [Google Scholar]
- 54. Perniola R. Twenty Years of AIRE. Frontiers in Immunology 2018; 9:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Anderson MS, Su MA. AIRE expands: new roles in immune tolerance and beyond. Nat Rev Immunol 2016; 16:247–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kuroki K, Tsuchiya N, Shiroishi M, Rasubala L, Yamashita Y, Matsuta K et al Extensive polymorphisms of LILRB1 (ILT2, LIR1) and their association with HLA‐DRB1 shared epitope negative rheumatoid arthritis. Hum Mol Genet 2005; 14:2469–80. [DOI] [PubMed] [Google Scholar]
- 57. Davidson CL, Li NL, Burshtyn DN. LILRB1 polymorphism and surface phenotypes of natural killer cells. Hum Immunol 2010; 71:942–9. [DOI] [PubMed] [Google Scholar]
- 58. Wagtmann N, Rojo S, Eichler E, Mohrenweiser H, Long EO. A new human gene complex encoding the killer cell inhibitory receptors and related monocyte/macrophage receptors. Curr Biol 1997; 7:615–8. [DOI] [PubMed] [Google Scholar]
- 59. Colonna M, Navarro F, Bellón T, Llano M, García P, Samaridis J et al A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J Exp Med 1997; 186:1809–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jones DC, Kosmoliaptsis V, Apps R, Lapaque N, Smith I, Kono A et al HLA class I allelic sequence and conformation regulate leukocyte Ig‐like receptor binding. J Immunol 2011; 186:2990–7. [DOI] [PubMed] [Google Scholar]
- 61. Vallabhapurapu S, Karin M. Regulation and function of NF‐κB transcription factors in the immune system. Annu Rev Immunol 2009; 27:693–733. [DOI] [PubMed] [Google Scholar]
- 62. Sun SC. The noncanonical NF‐κB pathway. Immunol Rev 2012; 246:125–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hayden MS, Ghosh S. Shared principles in NF‐κB signaling. Cell 2008; 132:344–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The expression quantitative trait (eQTL) effect of haplotype 3 SNPs on KIR (killer‐cell immunoglobulin like receptor) genes expression in whole blood.
Figure S2. The expression quantitative trait (eQTL) effect of the rs10423364 genotypes on leucocyte immunoglobulin like receptor B1 (LILRB1) gene expression in whole blood.
Table S1. List of 176 SNPs included in association analysis for endemic PF.
Table S2. Allele and genotype frequencies of SNPs associated with PF among patients and controls.
Table S3. Predicted regulatory effects of the SNPs rs465169, rs35336528 and rs1865097, and of the SNPs in strong LD with them (r 2 ≥ 0·8).
Table S4. Predicted regulatory effects of SNPs rs1325158, rs4806527 and rs2075731 from haplotype H1 associated with PF, and of SNPs in strong LD (r 2 ≥ 0·8) with them.
Table S5. Predicted regulatory effects of SNPs rs4806768 and rs61737870 from haplotype H2 associated with PF, and of SNPs in strong LD (r 2 ≥ 0·8) with them.
Table S6. Predicted regulatory effects of SNPs rs61739173 and rs11665986 from haplotype H3 associated with PF, and of SNPs in strong LD (r 2 ≥ 0·8) with them.
Table S7. Predicted regulatory effects of SNPs rs10423364, rs1061680 and rs61739173 from haplotype H4 associated with PF, and of SNPs in strong LD (r 2 ≥ 0·8) with them.


