Abstract
This review discusses recent advances towards understanding the sigma-1 receptor (S1R) as an endogenous neuro-protective mechanism in the retina, a favorable experimental model system. The exquisite architecture of the mammalian retina features layered and intricately wired neurons supported by non-neuronal cells. Ganglion neurons, photoreceptors, as well as the retinal pigment epithelium, are susceptible to degeneration that leads to major retinal diseases such as glaucoma, diabetic retinopathy, and age-related macular degeneration (AMD), and ultimately, blindness. The S1R protein is found essentially in every retinal cell type, with high abundance in the ganglion cell layer. Ultrastructural studies of photoreceptors, bipolar cells, and ganglion cells show a predominant localization of S1R in the nuclear envelope. A protective role of S1R for ganglion and photo-receptor cells is supported by in vitro and in vivo experiments. Most recently, studies suggest that S1R may also protect retinal neurons via its activities in Müller glia and microglia. The S1R functions in the retina may be attributed to a reduction of excitotoxicity, oxidative stress, ER stress response, or inflammation. S1R knockout mice are being used to delineate the S1R-specific effects. In summary, while significant progress has been made towards the objective of establishing a S1R-targeted paradigm for retinal neuro-protection, critical questions remain. In particular, context-dependent effects and potential side effects of interventions targeting S1R need to be studied in more diverse and more clinically relevant animal models.
Keywords: Sigma-1 receptor, Retinal diseases, Retinal ganglion neurons, Müller glia, Photoreceptors, Neuro-protection
19.1. Introduction
Earlier pharmacological profiling revealed two subtypes of sigma receptors (S1R and S2R) [1]. The S1R sequence has been cloned [2], while the identity of S2R remains unknown [3–5]. Despite numerous studies since its discovery [6, 7], S1R remains mysterious. Outstanding questions include the following: (1) Identity. No homolog of S1R is found in mammalian genomes. Curiously, the only protein that shares >30 % sequence identity with S1R is the yeast C-8,7 sterol isomerase [8]. But S1R is not found in yeast and it does not possess sterol isomerase activity. While an NMR structure of partial S1R was recently reported [9], an atomic structure of the whole protein has yet to be unveiled. (2) Function. In contrast to its unique identity, S1R is ubiquitously distributed, with high abundance in the central nervous system and liver [8]. Paradoxically, while S1R knockout mice do not exhibit overt phenotypes [10], S1R is linked to an array of pathological conditions such as cancer and neurological disorders (see review) [11]. These studies were conducted mostly using S1R ligands with only a handful employing S1R knockout mice. Hence, the S1R specificity of observed ligand functions awaits further investigations in knockout animals. (3) Endogenous ligands. Many synthetic ligands bind to S1R, including a few that have been intensively used for investigating S1R functions (see review) [12]. However, the identity of the true endogenous S1R ligand remains unclear. Several naturally occurring compounds show affinity for S1R, including steroids [13], trace amine [14], and lipids [15–17], but their S1R-specific roles are largely unknown.
Recently, there has been a surge of interest in S1R. In particular, important progress has been made to unravel its important role in the nervous system. A potential neuro-protective function of S1R is found in animal models of major neurode-generative diseases including Alzheimer’s disease [18], Parkinson’s disease [19], amyotrophic lateral sclerosis [20, 21], as well as retinal degenerative diseases [22–25]. The retina presents an excellent model system for studying S1R functions in the central nervous system. The main advantages include the following: (1) The retina is integral to, yet isolated from the brain, thus conveniently accessible for experimentation. (2) Animal models are available for major retinal degenerative diseases. (3) Despite being a thin sheet of tissue, the retina contains diverse cell types including neurons, epithelial cells, macroglia and microglia (see review) [26]. (4) Retinal cells are exquisitely organized into distinct layers [26], and hence advantageous for morphological and pathophysiological investigations (for example, see Fig. 19.1). (5) Since the eye is an immunologically privileged organ [27], immunogenic concerns caused by introducing experimental or therapeutic agents are relatively minor. In spite of a limited number of publications on S1R in the retina, progress has been achieved in identifying neuro-protective functions of S1R. While excellent reviews are available for studies of S1R in the nervous system in general [8, 11, 28], an overview is lacking for studies on S1R specifically in the retina. Here we discuss recent findings on the distribution, function, and molecular mechanisms of S1R in the mammalian retina.
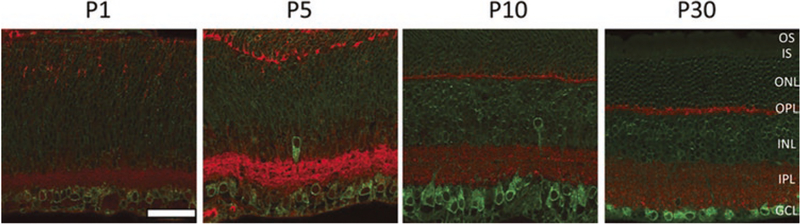
Fig. 19.1. Immunostaining of S1R on mouse retinal sections at different postnatal stages.
Green, S1R; red, synaptophysin. OS outer segment, IS inner segment, ONL outer nuclear layer, OPL outer plexiform layer, INL inner nuclear layer, IPL inner plexiform layer, GCL ganglion cell layer. Scale = 50 μm (Adapted from: Scientific Reports. 2015.2;5:10689)
19.2. General Molecular Functions of S1R
Mammalian S1R is a protein of 223 amino acids, with two transmembrane helices and a hydro-phobic C-terminal region that putatively form a ligand binding pocket(s) (see review) [12]. An N-terminal double-arginine sequence serves as an endoplasmic reticulum (ER) retention motif. Until the discovery of its ligand-operated chaperone function [29], molecular functions of S1R were not known. This study revealed that activated S1R regulates mitochondrial calcium homeostasis by stabilizing the IP3 type 3 receptor at ER/mitochondria contacts. Thus S1R is thought to support cell survival [29]. Follow-up studies suggest that S1R is a multitasking protein involved in a broad range of cellular activities. S1R has been reported to modulate the activity of various, e.g., Na+, K+, Ca2+, Cl−, ion channels, likely via direct interactions [30] (see review) [28]. S1R was also reported to interact with G-protein coupled receptors (see review) [12]. Recently, evidence showed that S1R is involved in autophagy [31, 32]. In accordance, S1R also participates in ER stress responses; e.g., S1R interacts with and stabilizes ER stress sensor IRE1 [29, 33, 34]. While interactions of S1R with several lipids were observed earlier (see review) [12], most recently the Su group reported that S1R transports myristic acid to support proper tau phosphorylation and axon extension [17]. They also found that S1R modulates transcriptional activities via interaction with a nuclear envelope protein [35], consistent with the presence of S1R in the nuclear envelope [36, 37].
Despite continuous discoveries about the molecular biology of S1R, it remains an open question as to whether there is a common thread connecting S1R functions. In other words, can all S1R actions be attributed to its chaperone activity? The S1R C-terminal half is believed to be responsible for its chaperone activity [29, 38]. If the C-terminus of S1R is confined in the ER lumen, how would S1R functionally interact with cytosolic proteins? What are the functions of the other S1R domains, for instance, the central loop region proposed to be cytosolic? It was reported that ligand binding to S1R alters its monomeric/oligomeric states [39–41]. How is this functionally related? In sum, many intriguing questions remain, which would inspire new investigations to help understand S1R functions in the retina as well as other systems.
19.3. Cellular and Sub-cellular Distribution of S1Rin the Retina
The neural retina is a sheet of light-sensitive tissue in the back of the eye. Its intricate structure contains three layers of neatly organized neurons: the outer nuclear layer (ONL), the inner nuclear layer (INL), and the ganglion cell layer (GCL). Sandwiched in between are two synaptic layers connecting neurons: the outer plexiform layer (OPL) and the inner plexiform layer (IPL) [26] (Fig. 19.1). The neural retina rests on a nourishing single layer of pigmented cells called retinal pigment epithelium (RPE). ONL is formed by photoreceptors. INL contains bipolar cells, horizontal cells, and amacrine cells. GCL is mainly composed of ganglion cells and displaced amacrine cells. Müller glia cells traverse the entire neural retina and are interconnected with retinal microglia cells [42]. Vision begins at photoreceptors, which convert light signals into chemical signals and then electrical impulses. Filtered through secondary neurons in INL, the electrical signals are transmitted to ganglion cells, and sent further into the brain through their axons (optic nerve) to be processed into images or other forms of vision.
While differences may exist among species, S1R is found in all cellular layers in the mouse retina, including GCL, INL, ONL, and RPE, as detected by both in-situ hybridization and immunohistochemistry [23, 37, 43, 44]. The specificity of S1R immunostaining is confirmed by the lack of S1R-positive staining in the retina of S1R knockout mouse [23]. S1R is abundant in GCL in mouse [23, 43], rat [45], monkey, pig, and human retinas [37]. In contrast, staining of S1R is less intense in ONL, unclear in the photoreceptor inner segment, and not detectable in the outer segment [23]. Consistently, using immuno- electron microscopy (EM) we did not observe S1R-positive staining on outer segment membrane discs, or in mitochondria or the ER which are concentrated in the photoreceptor inner segment [37] (Fig. 19.2). Rather, S1R is exclusively localized in photoreceptor nuclear membranes. Similarly in bipolar cells, EM data show that S1R is predominantly present in the nuclear envelope, in both outer and inner nuclear membranes [37]. In ganglion cells, S1R is found not only in nuclear membranes, but also in the ER and lipid droplets (Fig. 19.3). Interestingly, in accordance with S1R localization in nuclear membranes in retinal neurons [37] and Müller cells [36], Tsai et al. reported that S1R influences gene transcription by interacting with the nuclear envelope protein emerin to recruit chromatin-remodeling proteins [35].
Fig. 19.2. Electron microscopy images showing S1R distribution in the mouse photoreceptor subcellular compartments.
(a) Schematic of the compartments in the photoreceptor. (b) Ultrastructure of outer and inner segment. Asterisks label mitochondria. (c–e) Magnified images of the boxed areas in (b), showing the outer segment containing membrane discs, the connecting cilium (asterisk), and the inner segment (including ER), respectively. (f–h), Localization of S1R in the nuclear envelope. (f) nuclear region of several photoreceptor cells; (g) nuclear envelope of a single cell, (h) magnified box area in (g) showing S1R localization in the outer and inner membranes of the nuclear envelope (pointed to by arrows). (i) and (j), Photoreceptor synaptic terminal. The image in (j) is a magnified box area in (i) revealing the characteristic ribbon (asterisks) and vesicles. Scales: (b–e) and (g), 1 μm; (f), 3 μm; (h), 0.2 μm; (i), 0.5 μm; (j), 0.1 μm (Adapted from: Scientific Reports. 2015.2;5:10689)
Fig. 19.3. Subcellular localization of S1R in bipolar and ganglion cells of the mouse retina.
(a–c), Bipolar cells. (b) shows magnification of the boxed area in (a). Arrows point to S1R immunolabeling in the inner and outer membranes of the nuclear envelope. Arrowheads mark the plasma membrane. (c) shows S1R localization in the ER membrane (star) connected to the nuclear envelope (arrows). (d–f), Ganglion cells. (d) shows predomi nant S1R localization in the nuclear envelope (arrows) but not in the plasma membrane (arrow heads). (e) highlights the presence of S1R in the ER (boxed area). (f) shows the magnification of the boxed area in (e), revealing S1R localization in the ER cisternae (asterisks) that are adjacent to the plasma membrane (arrow heads). Scales: (a), (c–e), 2 μm; (f), 1 μm; (b), 0.25 μm (Adapted from: Scientific Reports. 2015.2;5:10689)
In spite of new findings, perplexing questions remain about the distribution of S1R in the retinal neurons. For example, S1R was identified as a molecular chaperone functioning at the ER/mitochondria junction [29]. What is the function of S1R in photoreceptor cells, where it is found neither in the ER or mitochondria? S1R has been reported to interact with multiple ion channels including NMDA receptors in the ganglion cell plasma membrane [46, 47]. However, EM data do not show S1R in the plasma membrane of reti nal neurons [37]. Is it possible some channels in the plasma membrane interact with S1R localized in the subsurface ER cisternae [20, 37]? Moreover, S1R expression in the embryonic (E16) mouse retina is barely detectable but continuously increases during development until a mature retina is formed [37]. Is there a possible link between the temporal S1R distribution and retinal development?
19.4. Functions of S1R in the Retina
High-affinity S1R-selective agonists, e.g., (+)-pentazocine, PRE084, SK10047, and antagonists, e.g., NE100, BD1047, BD1063, present convenient pharmacological tools for studying S1R functions in the retina [12]. Using S1R ligands to treat animals (or cells) and whole retina samples for analysis, early studies suggested a neuro-protective role of S1R in the retina [43, 48–52]. Taking advantage of the layered retinal structure that partitions different neurons, in recent studies investigators analyzed cell type- specific S1R functions in the retina (see details in the subsections below). Moreover, S1R knockout mice [10] and retinal disease models provide powerful genetic tools for delineating S1R- specific functions in a given disease or cell type. However, mechanistic studies using isolated retinal neurons, in particular, photoreceptor cells, are challenging, as these highly specialized neurons cannot maintain their physiology and viability in cell culture. While immortalized cell lines are often used to represent corresponding retinal neurons, they are very dissimilar to mature native neurons in morphology and pathophysiology. Moreover, they may be associated with identity complications, e.g., the RGC-5 cell line [53]. Likely because of available methods to culture primary ganglion cells and their high S1R abundance [37, 43, 44], a majority of S1R functional studies in the retina have focused on ganglion neurons and associated disease conditions. Studies have also been extended to other cell types, e.g., Müller glia and microglia. In the following subsections, published studies on each retinal cell type will be discussed.
19.4.1. Retinal Ganglion Cells (RGCs)
RGCs make the functional link between the retina and the brain. Approximately 20 subtypes of RGCs process complex visual information collected from bipolar cells and amacrine cells, and then send it to the brain as action potentials along RGC axons (see review) [26]. As such, RGCs play a critical role in vision, and their deteriora tion leads to vision loss or impairment. A good example is glaucoma, a prevalent retinal disease characterized by final-stage RGC loss and consequent visual field deficits (see review) [54]. Although no data is available with regard to S1R expression in each specific RGC subtype, it is conceivable that S1R is ubiquitously expressed, based on S1R-positive staining in essentially all GCL cells [23, 37, 43].
In vitro and in vivo studies from several research groups support a pro-survival role of S1R in RGCs. Using both primary mouse RGCs and a RGC-5 cell line, the Smith group showed that the S1R-specific agonist (+)-pentazocine protected against apoptosis induced by homocysteine or glutamate. The mechanism was attributed to the attenuation of excitotoxicity, which was mediated by the NMDA receptor [55, 56]. In a recent study, they observed that (+)-pentazocine also protected RGC-5 cells against oxidative stress; this effect was associated with down- regulation of ER stress proteins [57]. Using whole-cell patch clamp on RGC-5 cells, the Yorio group found that the S1R agonist SKF10047 promoted cell survival by inhibiting apoptosis-provoking Ca2+ influx mediated by the L-type Ca2+ channel [58, 59]. In purified rat primary RGCs, they were able to recapitulate the inhibitory effect of S1R activation on Ca2+ influx and a possible S1R/L-type channel interaction [60]. Their latest work showed that S1R protected RGCs in vitro against ischemic damage via ERK activation [61]. In an ex vivo study using patch clamp on rat retinal slices, the Yang group observed suppression of NMDA receptor-specific current responses in both ON and OFF types of RGCs following S1R activation [47]. Their data further suggested that this effect was mediated through a Ca2+-dependent PLC-PKC pathway. In sum, all the foregoing in vitro studies suggest a protective role of activated S1R in RGCs, via attenuation of oxidative stress, excitotoxicity, or Ca2+ toxicity involving ion channels. At present it is not clear whether these S1R actions are orchestrated in RGCs under cellular stresses.
In an in vivo study using a spontaneous diabetic retinopathy mouse model, the Smith group identified a prominent anti-oxidative effect of S1R activation [22]. Treating animals with (+)-pentazocine injection preserved the thickness of IPL and INL, cell number in GCL, as well as organization of Müller glia. Demonstrating a specific role of S1R in ganglion cell neuro-protection, the Guo group reported that cell loss in GCL was significantly faster in S1R knockout mice compared to wild type control after optic nerve crush, an acute glaucoma model [23] (Fig. 19.4). This observation was echoed by another study using S1R knockout mice from the Smith group [62]. While retinal morphology and electroretinogram (ERG) appeared normal in younger S1R knockout mice, decrease of ERG b-wave amplitudes and GCL nuclei number, as well as disrupted axon structure in the optic nerve head, occurred in S1R knockout mice compared to wild type at 12 months of age. Moreover, using S1R knockout mice, they confirmed a S1R-specific neuro- protective function of (+)-pentazocine in an induced diabetic mouse model [63]. Taken together, these studies support an important role of S1R in alleviating RGC stress and degeneration in RGC disease models.
Fig. 19.4. Comparison of the post-crush cell loss in the retinal ganglion cell layer between WT and Sigmar1−/− (S1R knockout) mice.
(a–d) Nissl-stained retinal whole-mounts from WT (a and b) and Sigmar1−/− (c and d) mice. Images were from representative fields (1000×) of the mid-peripheral inferior retinas of 12-month-old mice. For each mouse, while the right eye served as untreated control (a and c), the left eye was treated by optic nerve crush for 3 s (b and d). Retinal whole-mounts were prepared 7 days after surgery, and the side of the ganglion cell layer was stained. Healthy ganglion cells exhibited larger somas and nuclei with prominent nucleoli. Arrows point to apoptotic cells. (e) Quantification of cells remaining in the retinal ganglion cell layer 1 week after surgery. The number of remaining cells in the experimental eye is represented as a percentage of the untreated control. The data were pooled from three WT and Sigmar1−/− pairs of 6-month-old mice and two pairs of 12-month-old mice. There were 86.82 ± 7.90 % (mean±standard deviation [SD], n = 5) cells remaining in WT mice and 68.31 ± 3.36 % remaining in Sigmar1−/− mice. ** t-test, p = 0.0013 (Adapted from Mol Vis. 2011;17:1034–1043)
Given the complexity of the pathophysiology of retinal neuro-degeneration, it is a daunting challenge to delineate the molecular mechanisms of S1R-specific neuro-protection for RGCs. Since primary, mature RGCs do not divide and hence they cannot be expanded in cell culture, it is difficult to perform in vitro mechanistic studies using these cells. As RGC-5 which was long used as an RGC line recently proved false [53], it is imperative to establish an appropriate RGC line, for in vitro mechanistic research. Moreover, further investigation is needed to better correlate in vitro mechanisms to in vivo pathophysiology. To better understand the therapeutic potential of targeting S1R for interventions, in particular for treating chronic diseases such as glaucoma, more clinically relevant animal models, e.g., DBA/2J [54], may be utilized. To this end, local drug delivery methods integrating advanced bioengineering technologies would provide new insights and opportunities.
19.4.2. Müller Glia and Microglia
RGCs and Müller glia are closely situated, facilitating their functional interactions in RGC pathophysiology (see review) [42]. In a retinal transcriptome survey, Ha et al. did not find significant changes of ER stress genes in neural retinas isolated from S1R knockout mice compared to wild type control. Interestingly, however, marked expression changes of those genes were observed in Müller cells isolated from knockout versus wild type mice [24]. This finding implies an important role of Müller cells in previously observed S1R-mediated protection of RGCs.
Müller cells are a major glial cell type in the retina where they serve as anatomical conduits between neurons and their environment [42]. Müller cells are radially oriented, spanning the entire thickness of the retina from the inner limiting membrane to the outer limiting membrane. Studies suggest that Müller cells play essential roles in the retina (see review) [64]. In addition to supporting the structural integrity of the retina, they maintain retinal homeostasis by participating in essential processes such as glucose metabolism, antioxidant production, ion/substrate exchange, and vascular regulation. Müller cells, together with astrocytes and microglia, become reactive in retinal diseases [27].
In a recent report, the Smith group observed an increase of LPS-stimulated secretion of inflammatory proteins from Müller cells isolated from S1R knockout mice versus those from wild type control [65]. Furthermore, (+)-pentazocine treatment of Müller cells inhibited the secretion of inflammatory proteins and NFκB translocation to the nucleus. In a follow-up study, they found that Müller cells from S1R knockout mice compared to wild type cells manifested more severe oxidative stress, which could be explained by suppressed NRF2 signaling and impaired function of an L-cysteine/L-glutamate antiporter (system xc−) [66]. These studies uncovered an essential role of S1R in the suppression of oxidative stress and inflammation in retinal Müller glia. Reporting a different S1R action, Vogler et al. showed that PRE084 mitigated osmotic swelling of Müller cell somas induced by super- fusion of rat retinal slices with a hypo-osmotic solution [67]. This S1R effect was likely mediated through activation of a glutamatergic- purinergic signaling cascade known to prevent osmotic Müller cell swelling. Astrocytes are another type of retinal glia that are most abundant in the optic nerve head [64]. To our knowledge, there is no report investigating S1R function in this specific cell type in the retina.
Recently, S1R protein was also found in retinal microglia. Pretreatment of isolated microglia with (+)-pentazocine reduced LPS-stimulated morphological change, intracellular ROS pro duction, and secretion of inflammatory cytokines (TNF-α, IL-10, MCP-1). The (+)-pentazocine effects were blocked by S1R antagonist BD1063, suggesting a S1R-specific function [68]. These S1R-mediated responses likely involved suppression of the ERK/JNK MAPK pathway due to S1R activation.
Together, the foregoing reports have brought about a new perspective that S1R may protect RGCs through their functions in Müller glia and/or microglia. They also raise an interesting scenario in which the mechanisms of S1R-mediated retinal neuro-protection are multifactorial, likely involving both neuronal and non-neuronal cells and their interactions. An ensuing question is whether retinal glia or microglia cells can serve as effective therapeutic targets. These cells could play opposite roles. Whereas they are essential for maintaining retinal neuron homeostasis [64], when activated by stress conditions, they may transform into inflammatory cells causing harm to retinal neurons. On the other hand, these cells can be readily isolated from the retina, an advantage for in vitro experimental models. Nonetheless, more studies are warranted to understand their role in retinal neuro- degeneration and protection, in the context of specific S1R- associated regulations.
19.4.3. Bipolar Cells, Horizontal Cells, and Amacrine Cells
The nuclei of bipolar, horizontal, and amacrine cells are all in INL, which is situated in between the photoreceptor layer (ONL) and GCL. In mammalian retinas there are approximately a dozen types of bipolar cells, three types of horizontal cells, and 30 types of amacrine cells (see reviews) [26, 54]. Bipolar cells transfer visual signals either directly from photoreceptors to ganglion cells or indirectly through horizontal cells and amacrine cells. Whereas horizontal cells transmit (and modulate) the visual information from photoreceptors to bipolar cells, amacrine cells modulate the signals transmitted from bipolar cells to RGCs. Although S1R distribution in each subtype of the secondary neurons has not been completely delineated [45], immunostain ing shows S1R presence in majority of INL cells [23, 43, 45].
Because of a paucity of experimental evidence, the function of S1R in bipolar cells is not known. Vogler et al. reported that S1R activation protects against osmotic swelling of Müller cells, but not of bipolar cells [67]. On the other hand, one-year old S1R knockout mice showed reduced amplitudes of ERG b-wave, which measures the activity of the inner retinal neurons including bipolar cells [62]. Since S1R is found in bovine photoreceptor presynaptic terminals [37], it is tempting to speculate that S1R may modulate neurotransmission to postsynaptic bipolar cells under some circumstances. There is no data available about the function of S1R in horizontal and amacrine cells. Thus, more research is needed to explore S1R functions in these secondary neurons in visual signal transmission. Such information would provide important insight into possible side effects, e.g., disturbance of synaptic transmission, of S1R-targeted interventions.
19.4.4. Photoreceptor Cells
Photoreceptors are highly specialized neurons. Through a biochemical process of photo- transduction, they are capable of converting light signals into nerve impulses that eventually lead to vision (see review) [26]. There are two basic types of photoreceptors, rods and cones, each containing four morphologically and functionally distinct compartments. Rods are extremely light sensitive and responsible for night vision; cones respond to bright light and are responsible for day vision and color vision. Photoreceptors are highly susceptible to genetic defects, as well as insults from their environment. There are up to 100 photoreceptor gene loci that cause retinal diseases such as retinitis pigmentosa, a condition characterized by photoreceptor cell death (RetNet). While S1R is found in the nuclear envelope of photoreceptor cells [37], its function is not clear. Most recently, the Hara group demonstrated the importance of S1R for photoreceptors [25]. Using a 661W neuronal cell line, they found that high-affinity S1R ligand cutamesine (named SA4503) attenuated light-induced dis ruption of mitochondrial membrane potential and caspase-3/7 activation. Moreover, using a light- induced photoreceptor degeneration model of mice carrying a mutation in RPE65 (an RPE specific protein), cutamesine delivered by intravit-real injection partially rescued light-induced retinal dysfunction (reduced ERG) and ONL thinning. The cutamesine effect could be blocked by S1R antagonist BD1063, suggesting it was S1R specific. As photoreceptors and RPE cells are structurally and functionally dependent on each other [69], it remains unclear which cell type is the primary site of the observed S1R protective function. Of note, mechanisms of photo-receptor degeneration vary in different pathological contexts [70], and so may S1R function. Whether S1R activation is ubiquitously beneficial in the retina awaits more careful testing. Moreover, the predominance of S1R in the photoreceptor nuclear envelope raises an interesting question with regard to possible mechanisms of S1R-specific protection in photoreceptor cells. Therefore, different retinal degeneration models may be used in future experiments to comprehensively understand the role of S1R in photoreceptor pathophysiology.
19.4.5. Retinal Pigment Epithelium (RPE) Cells
RPE is a single layer of cells situated between the light-sensitive outer segments of photoreceptors and the choroid blood supply [26]. RPE possesses many functions essential to the visual process, the chief of which is to maintain photoreceptor homeostasis. Analyses of hereditary types of retinal degeneration reveal a strong dependence of RPE on photoreceptors and vice versa. Defects in RPE contribute to initiation and/or progression of AMD in humans (see review) [69]. Characterized by the loss of central vision, AMD is the leading cause of blindness in elderly populations, and no pharmacological treatment is available. Oxidative damage is considered as a major factor for disease onset and progression.
In situ hybridization indicated the presence of S1R mRNA in RPE [43]. However, its protein abundance and subcellular distribution in RPE cells remain unclear, partly because of the intense auto-fluorescence that masks specific S1R immunostaining. Nevertheless, in an earlier study using a human RPE cell line (ARPE-19) and adult human primary RPE cells, Bucolo et al. were able to reduce H2O2-induced DNA damage and cell loss by pre-treatment with PRE084, an effect abolished by S1R antagonists [71]. Most recently, using targeted siRNA screening in a human RPE1 cell line, MacVicar et al. identified S1R as a potential regulator of autophagosome homeostasis involving mitochondrial dynamics [31]. Autophagy is an important stress response pathway responsible for the removal and recycling of damaged or redundant cytosolic constituents. While autophagy is found to be an active process in the RPE in vivo [72], evidence from AMD donors indicates a decline of autophagic flux in the RPE [73]. Although not yet specifically investigated in RPE cells in vivo, S1R has been reported to influence autophagy in vitro [31, 32, 74]. A possible protective role of the S1R via autophagic regulations in RPE cells needs to be further determined experimentally. In light of an anti-oxidative function of S1R and its involvement in lipid metabolism, it appears reasonable that S1R may play a role in maintaining homeostasis of RPE cells, which are situated in a highly oxidative environment to process large amounts of lipids from phagocytosed photoreceptor membrane discs [69].
19.5. Concluding Remarks
The retina, which is composed of diverse cell types, represents a favorable model for studying the functions of S1R. In the past decade, considerable progress has been made in understanding the role of S1R in retinal degenerative diseases. While most efforts have been devoted to retinal ganglion neurons, reports on S1R in other retinal cell types are emerging. These studies generally support a protective role of S1R against stress-induced cell loss. To exploit the therapeutic potential of a S1R-targeted strategy for treating retinal diseases, more studies are required, particularly in the following areas: (1) Investigation using more diverse pre-clinical retinal disease models for a comprehensive understanding of S1R functions. (2) Delineation of S1R-specific and non-specific effects of S1R-binding drugs, using S1R knockout animals or cells. (3) Determination of the cellular and molecular mechanisms of S1R-mediated retinal neuro- protection. (4) Evaluation of combination therapies using S1R-targeting ligands and drugs targeting other pathways. A deeper understanding of S1R-specific functions and mechanisms in the retina would lead to new therapeutic opportunities, not only for retinal diseases but also other related disorders.
Acknowledgement
This work was supported by the National Eye Institute grant R01EY022678 and a Morgridge Institute for Research & the James Christenson Estate Macular Degeneration Research Award (to L-W Guo), and P30EY016665 and S10OD018221 (to the University of Wisconsin Vision Core). The project was also supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
We thank Drs. Miles L. Epstein and Arnold E. Ruoho for critical comments. We also thank Dr. Laura Hogan at the University of Wisconsin Institute for Clinical & Translational Research (ICTR) for editing and proof-reading.
References
- 1.Quirion R et al. (1992) A proposal for the classification of sigma binding sites. Trends Pharmacol Sci 13:85–86 [DOI] [PubMed] [Google Scholar]
- 2.Hanner M et al. (1996) Purification, molecular cloning, and expression of the mammalian sigma1- binding site. Proc Natl Acad Sci U S A 93:8072–8077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu J et al. (2011) Identification of the PGRMC1 protein complex as the putative sigma-2 receptor binding site. Nat Commun 2:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu UB MLC, Yang H, Guo L-W, Ruoho AE (2015) The Sigma-2 receptor and PGRMC1 are different binding sites derived from independent genes. EBioMedicine 2:1806–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hellewell SB, Bowen WD (1990) A sigma-like binding site in rat pheochromocytoma (PC12) cells: decreased affinity for (+)-benzomorphans and lower molecular weight suggest a different sigma receptor form from that of guinea pig brain. Brain Res 527:244–253 [DOI] [PubMed] [Google Scholar]
- 6.Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE (1976) The effects of morphine- and nalor phine- like drugs in the nondependent and morphine- dependent chronic spinal dog. J Pharmacol Exp Ther 197:517–532 [PubMed] [Google Scholar]
- 7.Su TP (1982) Evidence for sigma opioid receptor: binding of [3H]SKF-10047 to etorphine-inaccessible sites in guinea-pig brain. J Pharmacol Exp Ther 223:284–290 [PubMed] [Google Scholar]
- 8.Su TP, Hayashi T, Maurice T, Buch S, Ruoho AE (2010) The sigma-1 receptor chaperone as an inter- organelle signaling modulator. Trends Pharmacol Sci 31:557–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortega-Roldan JL, Ossa F, Amin NT, Schnell JR (2015) Solution NMR studies reveal the location of the second transmembrane domain of the human sigma-1 receptor. FEBS Lett 589:659–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langa F et al. (2003) Generation and phenotypic analysis of sigma receptor type I (sigma 1) knockout mice. Eur J Neurosci 18:2188–2196 [DOI] [PubMed] [Google Scholar]
- 11.Nguyen L et al. (2015) Role of sigma-1 receptors in neurodegenerative diseases. J Pharmacol Sci 127:17–29 [DOI] [PubMed] [Google Scholar]
- 12.Chu UB, Ruoho AE (2016) Biochemical pharmacology of the Sigma-1 receptor. Mol Pharmacol 89:142–153 [DOI] [PubMed] [Google Scholar]
- 13.Su TP, London ED, Jaffe JH (1988) Steroid binding at sigma receptors suggests a link between endocrine, nervous, and immune systems. Science 240:219–221 [DOI] [PubMed] [Google Scholar]
- 14.Fontanilla D et al. (2009) The hallucinogen N,N- dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science 323:934–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramachandran S et al. (2009) The sigma1 receptor interacts with N-alkyl amines and endogenous sphingolipids. Eur J Pharmacol 609:19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayashi T, Su T (2004) sigma-1 receptors at galactosylceramide- enriched lipid microdomains regulate oligodendrocyte differentiation. Proc Natl Acad Sci U S A 101:14949–14954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai SY, Pokrass MJ, Klauer NR, Nohara H, Su T (2015) sigma-1 receptor regulates Tau phosphorylation and axon extension by shaping p35 turnover via myristic acid. Proc Natl Acad Sci U S A 112:6742–6747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hedskog L et al. (2013) Modulation of the endoplasmic reticulum-mitochondria interface in Alzheimer’s disease and related models. Proc Natl Acad Sci U S A 110:7916–7921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Francardo V et al. (2014) Pharmacological stimulation of sigma-1 receptors has neurorestorative effects in experimental parkinsonism. Brain J Neurol 137:1998–2014 [DOI] [PubMed] [Google Scholar]
- 20.Mavlyutov TA et al. (2013) Lack of sigma-1 receptor exacerbates ALS progression in mice. Neuroscience 240:129–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mavlyutov TA, Guo LW, Epstein ML, Ruoho AE (2015) Role of the Sigma-1 receptor in Amyotrophic Lateral Sclerosis (ALS). J Pharmacol Sci 127:10–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith SB et al. (2008) In vivo protection against retinal neurodegeneration by sigma receptor 1 ligand (+)-pentazocine. Invest Ophthalmol Vis Sci 49:4154–4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mavlyutov TA, Nickells RW, Guo LW (2011) Accelerated retinal ganglion cell death in mice deficient in the Sigma-1 receptor. Mol Vis 17:1034–1043 [PMC free article] [PubMed] [Google Scholar]
- 24.Ha Y et al. (2014) Sigma receptor 1 modulates ER stress and Bcl2 in murine retina. Cell Tissue Res 356:15–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimazawa M, Sugitani S, Inoue Y, Tsuruma K, Hara H (2015) Effect of a sigma-1 receptor agonist, cutamesine dihydrochloride (SA4503), on photoreceptor cell death against light-induced damage. Exp Eye Res 132:64–72 [DOI] [PubMed] [Google Scholar]
- 26.Sung CH, Chuang JZ (2010) The cell biology of vision. J Cell Biol 190:953–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mac Nair CE, Nickells RW (2015) Neuroinflammation in glaucoma and optic nerve damage. Prog Mol Biol Transl Sci 134:343–363 [DOI] [PubMed] [Google Scholar]
- 28.Kourrich S, Su TP, Fujimoto M, Bonci A (2012) The sigma-1 receptor: roles in neuronal plasticity and disease. Trends Neurosci 35:762–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayashi T, Su T (2007) sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 131:596–610 [DOI] [PubMed] [Google Scholar]
- 30.Balasuriya D, Stewart AP, Edwardson JM (2013) The sigma-1 receptor interacts directly with GluN1 but not GluN2A in the GluN1/GluN2A NMDA receptor. J Neurosci 33:18219–18224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacVicar TD, Mannack LV, Lees RM, Lane JD (2015) Targeted siRNA Screens Identify ER-to- Mitochondrial Calcium Exchange in Autophagy and Mitophagy Responses in RPE1 Cells. Int J Mol Sci 16:13356–13380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vollrath JT et al. (2014) Loss of function of the ALS protein SigR1 leads to ER pathology associated with defective autophagy and lipid raft disturbances. Cell Death Dis 5:e1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Omi T et al. (2014) Fluvoxamine alleviates ER stress via induction of Sigma-1 receptor. Cell Death Dis 5:e1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mori T, Hayashi T, Hayashi E, Su T (2013) sigma-1 receptor chaperone at the ER-mitochondrion interface mediates the mitochondrion-ER-nucleus signaling for cellular survival. PLoS One 8:e76941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai SA et al. (2015) Sigma-1 receptor mediates cocaine-induced transcriptional regulation by recruiting chromatin-remodeling factors at the nuclear envelope. Proc Natl Acad Sci U S A 112(47): E6562–E6570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang G et al. (2006) Expression, subcellular localization, and regulation of sigma receptor in retinal muller cells. Invest Ophthalmol Vis Sci 47:5576–5582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mavlyutov TA, Epstein M, Guo LW (2015) Subcellular localization of the sigma-1 receptor in retinal neurons – an electron microscopy study. Sci Report 5:10689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu Z, Bowen WD (2008) Role of sigma-1 receptor C-terminal segment in inositol 1,4,5-trisphosphate receptor activation: constitutive enhancement of calcium signaling in MCF-7 tumor cells. J Biol Chem 283:28198–28215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mishra AK et al. (2015) The sigma-1 receptors are present in monomeric and oligomeric forms in living cells in the presence and absence of ligands. Biochem J 466:263–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gromek KA et al. (2014) The oligomeric states of the purified sigma-1 receptor are stabilized by ligands. J Biol Chem 289:20333–20344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chu UB, Ramachandran S, Hajipour AR, Ruoho AE (2013) Photoaffinity labeling of the sigma-1 receptor with N-[3-(4-nitrophenyl)propyl]-N-dodecylamine: evidence of receptor dimers. Biochemistry 52:859–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vecino E, Rodriguez FD, Ruzafa N, Pereiro X, Sharma SC (2015) Glia-neuron interactions in the mammalian retina. Prog Retin Eye Res 54(7):716–725 [DOI] [PubMed] [Google Scholar]
- 43.Ola MS et al. (2001) Expression pattern of sigma receptor 1 mRNA and protein in mammalian retina. Brain Res Mol Brain Res 95:86–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ola MS et al. (2002) Analysis of sigma receptor (sigmaR1) expression in retinal ganglion cells cultured under hyperglycemic conditions and in diabetic mice. Brain Res Mol Brain Res 107:97–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu LL, Wang L, Zhong YM, Yang XL (2010) Expression of sigma receptor 1 mRNA and protein in rat retina. Neuroscience 167:1151–1159 [DOI] [PubMed] [Google Scholar]
- 46.Zhang XJ et al. (2011) sigma receptor 1 is preferentially involved in modulation of N-methyl-D-aspartate receptor-mediated light-evoked excitatory postsynaptic currents in rat retinal ganglion cells. Neurosignals 19:110–116 [DOI] [PubMed] [Google Scholar]
- 47.Zhang XJ, Liu LL, Jiang SX, Zhong YM, Yang XL (2011) Activation of the zeta receptor 1 suppresses NMDA responses in rat retinal ganglion cells. Neuroscience 177:12–22 [DOI] [PubMed] [Google Scholar]
- 48.Bucolo C, Drago F (2004) Effects of neurosteroids on ischemia-reperfusion injury in the rat retina: role of sigma1 recognition sites. Eur J Pharmacol 498:111–114 [DOI] [PubMed] [Google Scholar]
- 49.Bucolo C, Drago F (2007) Neuroactive steroids protect retinal tissue through sigma1 receptors. Basic Clin Pharmacol Toxicol 100:214–216 [DOI] [PubMed] [Google Scholar]
- 50.Cantarella G et al. (2007) Protective effects of the sigma agonist Pre-084 in the rat retina. Br J Ophthalmol 91:1382–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bucolo C et al. (2006) A novel adamantane derivative attenuates retinal ischemia-reperfusion damage in the rat retina through sigma1 receptors. Eur J Pharmacol 536:200–203 [DOI] [PubMed] [Google Scholar]
- 52.Senda T, Mita S, Kaneda K, Kikuchi M, Akaike A (1998) Effect of SA4503, a novel sigma1 receptor agonist, against glutamate neurotoxicity in cultured rat retinal neurons. Eur J Pharmacol 342:105–111 [DOI] [PubMed] [Google Scholar]
- 53.Krishnamoorthy RR, Clark AF, Daudt D, Vishwanatha JK, Yorio T (2013) A forensic path to RGC-5 cell line identification: lessons learned. Invest Ophthalmol Vis Sci 54:5712–5719 [DOI] [PubMed] [Google Scholar]
- 54.Nickells RW, Howell GR, Soto I, John SW (2012) Under pressure: cellular and molecular responses during glaucoma, a common neurodegeneration with axonopathy. Annu Rev Neurosci 35:153–179 [DOI] [PubMed] [Google Scholar]
- 55.Martin PM, Ola MS, Agarwal N, Ganapathy V, Smith SB (2004) The sigma receptor ligand (+)-pentazocine prevents apoptotic retinal ganglion cell death induced in vitro by homocysteine and glutamate. Brain Res Mol Brain Res 123:66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dun Y, Thangaraju M, Prasad P, Ganapathy V, Smith SB (2007) Prevention of excitotoxicity in primary retinal ganglion cells by (+)-pentazocine, a sigma receptor-1 specific ligand. Invest Ophthalmol Vis Sci 48:4785–4794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ha Y et al. (2011) Sigma receptor 1 modulates endoplasmic reticulum stress in retinal neurons. Invest Ophthalmol Vis Sci 52:527–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tchedre KT, Yorio T (2008) Sigma-1 receptors protect RGC-5 cells from apoptosis by regulating intracellular calcium, Bax levels, and caspase-3 activation. Invest Ophthalmol Vis Sci 49:2577–2588 [DOI] [PubMed] [Google Scholar]
- 59.Tchedre KT et al. (2008) Sigma-1 receptor regulation of voltage-gated calcium channels involves a direct interaction. Invest Ophthalmol Vis Sci 49:4993–5002 [DOI] [PubMed] [Google Scholar]
- 60.Mueller BH 2nd et al. (2013) Sigma-1 receptor stimulation attenuates calcium influx through activated L-type Voltage Gated Calcium Channels in purified retinal ganglion cells. Exp Eye Res 107:21–31 [DOI] [PubMed] [Google Scholar]
- 61.Mueller BH 2nd et al. (2014) Sigma-1 receptor stimulation protects retinal ganglion cells from ischemia- like insult through the activation of extracellular-signal-regulated kinases 1/2. Exp Eye Res 128:156–169 [DOI] [PubMed] [Google Scholar]
- 62.Ha Y et al. (2011) Late-onset inner retinal dysfunction in mice lacking sigma receptor 1 (sigmaR1). Invest Ophthalmol Vis Sci 52:7749–7760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ha Y et al. (2012) Diabetes accelerates retinal ganglion cell dysfunction in mice lacking sigma receptor 1. Mol Vis 18:2860–2870 [PMC free article] [PubMed] [Google Scholar]
- 64.Chong RS, Martin KR (2015) Glial cell interactions and glaucoma. Curr Opin Ophthalmol 26:73–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shanmugam A et al. (2015) Sigma receptor 1 activation attenuates release of inflammatory cytokines MIP1gamma, MIP2, MIP3alpha, and IL12 (p40/p70) by retinal Muller glial cells. J Neurochem 132:546–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang J et al. (2015) Sigma 1 receptor regulates the oxidative stress response in primary retinal Muller glial cells via NRF2 signaling and system xc(−), the Na(+)-independent glutamate-cystine exchanger. Free Radic Biol Med 86:25–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vogler S et al. (2016) Sigma-1 receptor activation inhibits osmotic swelling of rat retinal glial (Muller) cells by transactivation of glutamatergic and purinergic receptors. Neurosci Lett 610:13–18 [DOI] [PubMed] [Google Scholar]
- 68.Zhao J et al. (2014) Sigma receptor ligand, (+)-pentazocine, suppresses inflammatory responses of retinal microglia. Invest Ophthalmol Vis Sci 55:3375–3384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ferrington DA, Sinha D, Kaarniranta K (2015) Defects in retinal pigment epithelial cell proteolysis and the pathology associated with age-related macular degeneration. Prog Retin Eye Res 51:69–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cuenca N et al. (2014) Cellular responses following retinal injuries and therapeutic approaches for neuro-degenerative diseases. Prog Retin Eye Res 43:17–75 [DOI] [PubMed] [Google Scholar]
- 71.Bucolo C, Drago F, Lin LR, Reddy VN (2006) Sigma receptor ligands protect human retinal cells against oxidative stress. Neuroreport 17:287–291 [DOI] [PubMed] [Google Scholar]
- 72.Kim JY et al. (2013) Noncanonical autophagy promotes the visual cycle. Cell 154:365–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaarniranta K et al. (2013) Autophagy and heterophagy dysregulation leads to retinal pigment epithelium dysfunction and development of age-related macular degeneration. Autophagy 9:973–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schrock JM et al. (2013) Sequential cytoprotective responses to Sigma1 ligand-induced endoplasmic reticulum stress. Mol Pharmacol 84:751–762 [DOI] [PMC free article] [PubMed] [Google Scholar]