Abstract
Purpose:
To systematically analyze the extent-of-disease in unifocal invasive lobular carcinoma (ILC) using ultrasound, with histopathology as the reference standard.
Methods:
In this single-institution retrospective study, 128 cases of ILC were identified over a 5-year period. After exclusions, the analyzed cohort consisted 66 cases. Ultrasound measurements of tumor extent along three axes were obtained. Tumor size was determined as the largest extent among the three axes and tumor volume by ellipsoidal approximation. Pathology review provided tumor size and volume. Correlation and regression analyses of tumor size and volume from ultrasound and pathology were performed. Tumor stage from ultrasound and pathology were used for concordance analyses.
Results:
The median (Ql, Q3) of tumor size from ultrasound and pathology were 12.5mm (9mm, 19mm) and 17mm (12mm, 25mm), respectively. The median (Ql, Q3) of tumor volume from ultrasound and pathology were 0.52cm3 (0.18cm3, 1.92cm3) and 1.04cm3 (0.45cm3, 2.49cm3), respectively. Ultrasound measurements were correlated with pathology reported tumor size (Spearman ρ=0.678, p<0.0001) and volume (Spearman ρ=0.699, p<0.0001). Ultrasound measured size and volume differed from pathology reported size and volume (p<0.0001, Wilcoxon signed ranks test). Concordancy between clinical tumor-size stage from ultrasound (cT) and pathology tumor-size stage (pT) varied with pT (p=0.0003, Fisher’s exact test), with the highest concordancy rate of 95.7% (95% CL: 85.2%−99.5%) observed for pT 1 tumors.
Conclusions:
Ultrasound underestimates tumor size and volume with the underestimation increasing for larger tumors. Hence, the concordancy rate in tumor-size stage between ultrasound and pathology is tumor-size dependent with highest concordancy rate observed for pT 1 tumors.
Keywords: Breast, Cancer, Ultrasound, Invasive Lobular Carcinoma
MICROABSTRACT
Pre-surgical ultrasound imaging can provide for tumor size measurements. In invasive lobular carcinoma (ILC), it is unclear if the concordancy in tumor-size stage (T-stage) between ultrasound and pathology is dependent on the tumor T-stage itself. From a cohort of 128 ILCs, 66 unifocal ILCs were analyzed. Concordancy between clinical tumor-size stage from ultrasound (cT) and pathology tumor-size stage (pT) varied with pT (p=0.0003, Fisher’s exact test), with the highest concordancy rate of 95.7% (95% CL: 85.2% - 99.5%) observed for pTl tumors. Caution is expressed for using ultrasound to stage ILCs larger than pT 1.
Introduction
Invasive lobular carcinoma (ILC) is the second most common histologic subtype of breast cancer 1. It accounts for approximately 10 % of all breast malignancies 2. ILC pose a diagnostic challenge, as they often are poorly circumscribed and fail to form discrete masses 3. This, perhaps is explained by the typical histopathological characteristics of ILC that include non-cohesive cells that infiltrate the stroma in a single-file pattern 4.
Ultrasound is widely available, inexpensive, and is valuable diagnostic tool as an adjunct to mammography 3. Some of the most common uses of ultrasound are to distinguish solid from cystic lesions, to evaluate palpable masses not visible in mammography, to evaluate young pregnant patients with palpable masses, and to characterize lymph nodes 5–7. The lack of radiation and real-time nature of the ultrasound makes it the method of choice for image-guided procedures 3,6. There is increasing evidence that ultrasound can detect occult cancers 6,8. Typically, ILC has a low mammographic sensitivity varying from 57–79 %, reflecting one of the most important causes of false negative rates 8. Also, for patients diagnosed with ILC, tumor size from mammography (r =0.27) is poorly correlated with histopathology 9. The role of ultrasound in the identification of ILC is well documented with sensitivity ranging from 81 to 83% 10. Meta-analysis of pooled data has shown that breast MRI has a sensitivity of 93.3% for detecting lobular carcinoma with additional lesions detected in the ipsilateral breast for 32% of patients and in the contralateral breast for 7% of patients 11.
Accurate tumor measurement may guide planning for surgery and systemic therapy 12,13. Analysis of the SEER-Medicare database with over 20,000 patients diagnosed with breast carcinoma, including approximately 2,000 ILCs, observed that patients with ILC are more likely to have an initial mastectomy (35% vs 30%), a reoperation (28% vs 21%), and a final mastectomy (44% vs 36%) compared to all breast cancer patients 14. Analyzing the data from the Netherlands Cancer Registry, for patients with early-stage (pathologically assessed T1 and T2) cancers, patients with ILCs were less likely to undergo breast conserving surgery (43% vs. 54%) than those with ductal cancers 15. Specific to lobular cancers, two large independent studies using data from the Netherlands Cancer Registry 16 and from the California Cancer Registry 17, have shown that the combination of adjuvant chemotherapy and adjuvant hormonal therapy did not improve 10-year survival rates over adjuvant hormonal therapy alone.
There have been prior reports on the correlation and concordance in ILC size between imaging and histopathology 9,18–23. However, analysis of tumor volume from ultrasound and its correlation and concordance with histopathology have not been adequately explored. The growing need for three-dimensional (3D) volume estimation to assess response to therapy prompted this investigation. Also, there is lack of clarity in literature as to whether the concordance in ILC size between ultrasound and histopathology is dependent on the tumor size itself. This is important in the context of neoadjuvant therapy. Among patients who undergo neoadjuvant chemotherapy, pathologic complete response rate is lower with ILCs than ductal cancers 24,25 and is dependent on receptor status 25,26. However, neoadjuvant chemotherapy, in particular for clinically-assessed T2 and T3 ILCs, can be of benefit in facilitating breast conserving surgery 24. Accurate measurement of tumor size in combination with receptor status 25,26 can help in identifying subjects that are likely to benefit from breast conservation following neoadjuvant chemotherapy. Hence, we conducted this systematic retrospective analysis in subjects diagnosed with unifocal ILC.
Patients and Methods
Human subjects
This study was performed in a large, urban, academic center in adherence to an institutional review board and health insurance portability and accountability act-compliant protocol. The institutional review board waived the informed consent for this retrospective study. Adult women with pathology-verified diagnosis of ILC over a 5-year period from January 1, 2007 through December 31, 2011 were eligible for inclusion in the study.
Search of the electronic medical records identified 127 subjects (130 breasts) with pathology-verified diagnosis of ILC over the study period. Electronic medical records of all 127 subjects were reviewed. The following subjects were excluded: loss to follow-up, including subjects who underwent surgery outside our institution (n=21), subjects who underwent neoadjuvant chemotherapy between diagnosis and surgical excision (n=5), subjects who had multifocal or multicentric disease (n=14), subjects who did not undergo an ultrasound exam (n=6), subjects who had a negative ultrasound exam (n=4), and subjects who had large lesions (larger than 65 mm in at least one dimension) that could not be reliably measured by ultrasound (n=2) were excluded. Subjects who underwent neoadjuvant chemotherapy between ultrasound imaging and surgical excision were excluded to avoid the confounding in tumor size/volume estimates due to therapy response. Due to the retrospective nature of the study, subjects with multifocal and multicentric disease were excluded, as the concordance in location between imaging and histopatholgy for the multiple foci could not be ascertained. Additional 9 cases were excluded because pathology reports did not include all three dimensions of the tumor, resulting in 66 cases for this study.
Bilateral mammography was performed in all patients. All ultrasound imaging was performed using a high-resolution ultrasound unit (IU-22, Philips Healthcare, Bothell, WA, USA) using a 17–5 MHz or a 12–5 MHz linear array transducer. Patients with dense breast tissue, or if clinically indicated, underwent bilateral breast MRI following appropriate standards of care. Pathologists with expertise in breast pathology processed the surgical specimens.
Data collection and preparation
The following demographic and clinical characteristics were obtained: age, mammograms and ultrasound results, type of biopsy, and preoperative histopathological diagnosis. Ultrasound measurements of the tumor extent (in cm) along the transverse (w), anteroposterior (d) and superior-inferior (h) directions were obtained from imaging reports, and the maximum of these three measurements was considered the ultrasound measured tumor size, i.e., S = MAX(w, d, h), where S is the tumor size. Ultrasound measured tumor volume (in cm3) was computed using the ellipsoidal approximation as: where V is the tumor volume. Review of pathology records following surgery provided the tumor extent along the three axes, from which the tumor size (S*) and tumor volume (V*) were obtained in a similar manner. The differences in tumor size and volume between ultrasound and pathology were computed respectively as, DS = S - S* and DV = V - V*. From the ultrasound measured size and from the pathology reported tumor size, the clinical tumor size stage (cT) and the pathology tumor size stage (pT), respectively, were determined as per a modified version of the American Joint Committee on Cancer (AJCC) guidelines. The modification pertains to the use of size alone for tumor size staging (T stage) and does not consider extension to the chest wall and/or to the skin.
Statistical Methods
All continuous variables were tested to determine if the normality assumption was satisfied. Depending upon the results, parametric or non-parametric tests were used for further analysis. Correlation analysis was performed. Statistical tests were conducted to determine if the tumor size, staging and volume differed between ultrasound and pathology. Simple binomial proportions were used to estimate the exact Clopper-Pearson confidence interval of the concordancy rates between pathology reported and ultrasound derived tumor stages. Effects associated with p<0.05 were considered statistically significant. All analyses were performed using statistical software (SAS® version 9.4. SAS Institute Inc., Cary, NC).
Results
The mean age (± standard deviation) of the analyzed cohort of 66 subjects was 62.4 ± 12.3 years. Diagnosis of ILC was ascertained by ultrasound-guided core-needle biopsy in 64/66 (97%) subjects, with the remainder by excisional biopsy. Among the 66 ILCs in the cohort, most (n=54) were of the classic type. The other variants were pleomorphic (n=8), mixed pleomorphic and classic (n=2), signet ring (n=1), and histiocytoid variant (n=1). The distribution of histologic grades were: grade 1 (n=42), grade 2 (n=11) and grade 3 (n=13). The receptor status distribution were: ER+/PR+/HER2− (n=54), ER+/PR+/HER2+ (n=1), ER+/PR−/HER2− (n=9), ER-/PR-/HER2+ (n=1), and triple-negative (n=1). Ultrasound measured tumor size and volume, pathology reported tumor size and volume, and the differences in tumor size and volume between ultrasound and pathology did not satisfy the normality assumption (p<0.0001, Shapiro-Wilks test).
Tumor size
The median, quartiles and range for tumor size measured by ultrasound, pathology, and their difference are summarized in Table 1. Ultrasound measured tumor size was correlated with pathology reported size (Spearman ρ = 0.678, p<0.0001). For these paired measurements, ultrasound measured size significantly differed from pathology reported size (p<0.0001, Wilcoxon signed ranks test). With pathology determined tumor size from surgery as the reference standard, the median underestimation by ultrasound was 3.5 mm. Robust regression analysis indicated that on average, ultrasound underestimated tumor size by 27.2% (95% confidence limits, CL: 17% - 37%).
Table 1.
Tumor size (in mm) measured by ultrasound, pathology and their difference.
| Median | Q1 | Q3 | Min | Max | |
|---|---|---|---|---|---|
| Ultrasound (S) | 12.5 | 9 | 19 | 4 | 61 |
| Pathology (S*) | 17 | 12 | 25 | 6 | 80 |
| Difference in size, DS = S − S* | −3.5 | −9 | 1 | −56 | 10 |
Tumor staging
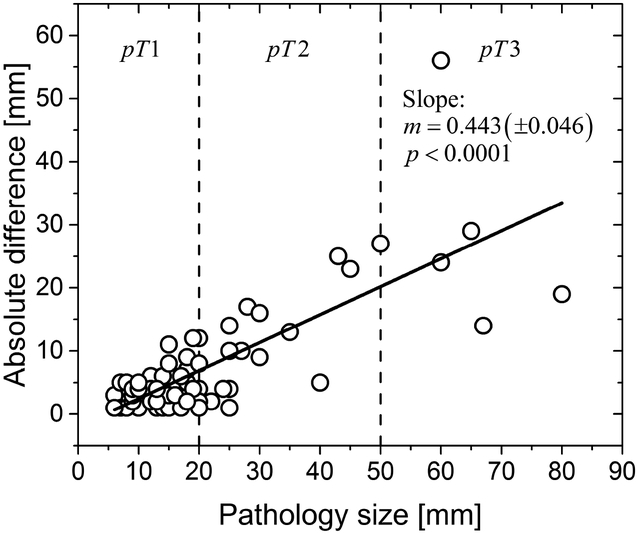
The data was analyzed by considering the pathology reported tumor stage (pT) that included the T1 subgroups (T1a, T1b and T1c) and by grouping all T1 subgroups. From Table 1, it can be observed that all ILCs were pT1 or greater, as the minima of the pathology reported size was 6 mm. Several prior studies reporting on the concordancy of imaging provided tumor size measurements with pathology had used fixed thresholds of 5 mm, 10 mm and 20 mm 9,18–23. The use of fixed thresholds is suitable, if the absolute difference in tumor size between ultrasound and pathology is independent of the pathology reported tumor size. Linear regression (Figure 1) of the absolute difference in tumor size with pathology reported size showed that the slope (m) was significantly different from zero (m = 0.443, p<0.0001). Also, the absolute difference in tumor size significantly varied with pathology reported tumor stage, pT (p<0.0001, Kruskal-Wallis test). Hence, concordance analyses were performed with ultrasound-derived clinical tumor size stage (cT). The clinical tumor stage (cT) differed from pathology reported tumor stage, pT, before and after consolidating the T1 subgroups (p<0.0001, Fisher’s exact test). For the cohort of 66 subjects, cT and pT were concordant in 37 subjects, resulting in overall concordancy rate of 56.1% (95% CL: 43.3% - 68.3%), before combining the T1 subgroups. After combining the T1 subgroups, cT and pT were concordant in 56/66 subjects, resulting in overall concordancy rate of 84.9% (95% CL: 73.9% - 92.5%). After combining the T1 subgroups, concordancy between cT and pT varied significantly with pT (p=0.0003, Fisher’s exact test). The pT dependent concordancy rates are summarized in Table 2. Among pT 1 tumors, 44/46 (95.7%) tumors were concordant and ultrasound overestimated the tumor stage as cT2 in the remaining 2 ILCs. Among pT2 ILCs, none were overestimated by ultrasound, with 5/15 pT2 reported as cT 1 by ultrasound. Among pT3 ILCs, none were overestimated by ultrasound, with 2/5 pT3 reported as cT2 and 1/5 pT3 reported as cT 1 by ultrasound.
Figure 1.
Linear regression of the absolute difference in tumor size between ultrasound and pathology with pathology reported size. The slope (m) of the fit was significantly different from zero (m = 0.443, standard error: ±0.046, p<0.0001), indicating that the differences increase with increasing pathology reported size. The vertical dashed lines correspond to the thresholds for tumor-size stage.
Table 2.
Concordancy between ultrasound-derived clinical tumor size stage (cT) and pathology tumor size stage (pT) for various pathology tumor size stages.
| Pathology tumor size stage (pT) | Number of cases concordant, nconc | Number of cases, ntot | Concordancy rate (%) | 95% confidence limits |
|---|---|---|---|---|
| T1 | 44 | 46 | 95.7% | 85.2% – 99.5% |
| T2 | 10 | 15 | 66.7% | 38.4% – 88.2% |
| T3 | 2 | 5 | 40% | 5.3% – 85.3% |
| All stages | 56 | 66 | 84.9% | 73.9% – 92.5% |
Tumor volume
The median, quartiles and range for tumor volume determined from the tumor extent in three dimensions measured by ultrasound, pathology, and their difference are summarized in Table 3. Ultrasound measured tumor volume was correlated with pathology reported volume (Spearman ρ = 0.699, p<0.0001). For these paired measurements, ultrasound measured volume significantly differed from pathology reported volume (p<0.0001, Wilcoxon signed ranks test). With pathology determined tumor volume from surgery as the reference standard, the median underestimation by ultrasound was 0.29 cm3. Regression analysis indicated that on average, the tumor volume measured by ultrasound was 2.61 times smaller than pathology. The difference in tumor volume between ultrasound and pathology varied significantly with pathology tumor size stage, pT (p<0.0001, Kruskal-Wallis test), with the difference in volume progressively increasing for larger tumors. The pT dependent tumor volume from ultrasound, pathology, and their difference are also included in Table 3.
Table 3.
Tumor volume (in cm3) measured by ultrasound, pathology and their difference for various pathology tumor size stages (pT).
| pT | n | Median | Q1 | Q3 | Min | Max | |
|---|---|---|---|---|---|---|---|
| Ultrasound (V) | All | 66 | 0.518 | 0.18 | 1.923 | 0.011 | 19.451 |
| T1 | 46 | 0.297 | 0.147 | 0.905 | 0.011 | 2.77 | |
| T2 | 15 | 3.508 | 0.792 | 4.147 | 0.467 | 12.147 | |
| T3 | 5 | 9.538 | 1.554 | 19.302 | 0.025 | 19.451 | |
| Pathology (V*) | All | 66 | 1.041 | 0.452 | 2.488 | 0.079 | 87.965 |
| T1 | 46 | 0.66 | 0.377 | 1.178 | 0.079 | 2.639 | |
| T2 | 15 | 4.712 | 2.356 | 4.712 | 2.111 | 20.617 | |
| T3 | 5 | 34.558 | 31.573 | 43.982 | 23.824 | 87.965 | |
| Difference in volume, DV = V − V* | All | 66 | −0.292 | −1.78 | 0.012 | −68.513 | 9.814 |
| T1 | 46 | −0.18 | −0.41 | 0.119 | −1.554 | 1.2 | |
| T2 | 15 | −2.545 | −8.681 | −1.780 | −17.092 | 9.814 | |
| T3 | 5 | −30.019 | −43.957 | −15.256 | −68.513 | −14.286 |
Discussion
This study retrospectively analyzed the correlation and concordance between tumor size assessed by ultrasound and histopathology. The study was designed to decrease variations that may cofound the ultrasound results, by including only unifocal lesions and excluding subjects who underwent neoadjuvant therapy. There is underestimation of tumor size and volume by ultrasound. However, this underestimation is dependent on the pathology reported tumor size. In general, the underestimation increased with increasing tumor size. This implies that using a fixed threshold for concordance analyses could result in varying concordance rates depending on the pathology reported tumor size distribution in an analyzed cohort. Since tumor size-stage, more often than measured tumor size, is largely used for clinical decisions, concordance in tumor size-stage between ultrasound and pathology was analyzed.
Receptor status 25,26 in conjunction with extent of disease 24 is important when considering neoadjuvant therapy for ILCs, especially when breast conservation is considered 24,27–29. Breast ultrasound is a widely available modality and can be used as a complement to further characterize lesions. Among histological subtypes, larger discrepancies in tumor size between ultrasound and pathology have been observed in lobular carcinomas 30. Our observation in this unifocal lobular carcinoma cohort was that ultrasound on average underestimated the tumor size by 27.2% and the median underestimation in tumor size was 3.5 mm. In comparison, an earlier study with 40 ILCs reported a median difference of 7.5 mm 30. However the size distribution in that study 30 was different with a median pathology reported size of 22 mm (range 7–140 mm) that was larger than that in this cohort (Table 1). As noted earlier, the underestimation increases with tumor size and hence is dependent on the size distribution in the study cohort. When tumor size-stage is used for concordance analysis, cT and pT were concordant in 56/66 (84.9%) subjects, after combining the T1 subgroups.
In a study of 111 cancers that included a small proportion of ILCs (11/111, 9.9%), it was observed that ultrasound underestimated the size for all tumors that were 30 mm or larger 31. Among the 12 cancers (both ductal and lobular) that were 30 mm or larger in that study, 7/12 (58.3%) were underestimated to be smaller than 30 mm. Our study differed in that it is specific to ILCs and that the underestimation increased with tumor size. Another study reported on size measurements in 95 cancers, comprising mostly pT 1 tumors (65/93, 69.9%) that included 15/95 (16.1%) ILCs. The study observed that the underestimation by ultrasound increased with tumor size. The results from this study, which is limited to unifocal ILCs, are consistent with this observation. In this study, none of the pT 1 tumors were underestimated by ultrasound, with 96% concordant with pathology and 4% that were overestimated by ultrasound. Among pT2 ILCs, 67% were concordant, none were overestimated by ultrasound, and the remaining 33% were underestimated as cTl by ultrasound. Among pT3 ILCs, 40% were concordant, none were overestimated by ultrasound, with underestimation in the remaining 60%, of which 40% were reported as cT2 and 20% as cT 1 by ultrasound. This suggests that ultrasound could be of value in staging pT 1 tumors, but caution should be exercised for pT2 and larger tumors.
While ellipsoidal approximation was used to determine the tumor volume from both pathology and ultrasound, the method of measuring tumor dimensions by ultrasound and histopathology is different and therefore, may partly account for the discrepancies observed. The dimensions measured by pathology is a composite of naked eye gross evaluation of surgical specimen, palpation of the tumor, and microscopic evaluation that may identify tumor cell infiltration into adjacent tissue or up to the surgical margin. On ultrasound imaging, the identified lesion may have inconspicuous borders, extensive acoustic shadowing or architectural distortion as the dominant image features 32,33, which present a challenge for accurate measurement of tumor dimensions. All these ultrasound features might be explained by the histological growth pattern that lobular carcinomas exhibit. The single file infiltration of atypical cells into the surrounding tissue without inducing a strong desmoplastic reaction results more often in irregular shape and irregular or indistinct margins in ILCs 34.
Tumor volume measurements along with breast dimensions are important for breast reconstruction surgical planning (fat grafting, breast implant or FLAP procedures), particularly when it is performed following lumpectomy. Ultrasound underestimated the measured tumor volume and the median underestimation was 0.29 cm3. When ultrasound measured tumor volume alone is available for surgical planning, the difference in tumor volume between ultrasound and pathology in Table 3 can provide an approximate guideline as to true extent of the disease.
Our study had limitations. It is a single-institution retrospective study, where most of the tumors (46/66, 70%) were pT 1. The sample size for tumors larger than pT 1 was relatively small and we observed higher discordancy rates in these tumors. Also, our study was limited to unifocal ILCs, while it is known that ILCs manifest more often as multifocal and multicentric disease than ductal cancers. The tumor volume was estimated based on ellipsoidal approximation; however, tumors are asymmetric and could have contributed to uncertainties in measurements. Regarding the use of MRI, it was not standard practice at our institution during the study period to perform MRI on all ILCs. Further, when MRI was performed it was done after the biopsy. Since the presence of clip artifacts and post-biopsy inflammation can confound the size measurements, it would not be a reliable/accurate comparison. Among the 66 subjects, only 33/66 subjects had an MRI exam, of which 4 had substantial clip artifacts and 2 had diffuse non mass-like enhancement, precluding size measurements. Hence, we did not include the MRI measured size and the scope of this study was to compare ultrasound and pathology measurements. A larger study, ideally with US and MRI measurements performed prior to biopsy, could be of clinical importance.
Conclusion
There is underestimation of tumor size and volume by ultrasound, when pathology is considered the reference standard. However, this underestimation is dependent on pathology reported tumor size and volume, with the underestimation increasing for larger tumors. Hence, the concordancy rate in tumor-size stage between ultrasound and pathology is tumor-size dependent with highest concordancy rate observed for pT 1 tumors. Caution should be exercised for tumor size evaluation in large invasive lobular carcinoma.
Clinical practice points.
Tumor size and volume are underestimated by ultrasound and the underestimation increases with tumor size. The concordancy rate in tumor-size stage between ultrasound and pathology is tumor-size dependent with highest concordancy rate observed for pT 1 tumors. Caution should be exercised for tumor size evaluation in large invasive lobular carcinoma.
Acknowledgements
This study was supported by National Cancer Institute (NCI) of the National Institutes of Health (NIH) grant R01 CA195512. The contents are solely the responsibility of the authors and do not reflect the official views of the NIH or the NCI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARTIONS OF INTEREST
Declarations of interest: None
Contributor Information
Gopal R. Vijayaraghavan, Radiology, University of Massachusetts Medical School, 55 Lake Avenue North, Worcester MA 01655. gopal.vijaraghavan@umassmemorial.org.
Srinivasan Vedantham, Medical Imaging, University of Arizona, 1501 N Campbell Avenue, Tucson AZ 85724. svedantham@radiology.arizona.edu.
Gabriela Santos-Nunez, Magnetic Resonance Imaging Fellow, University of Massachusetts Medical School., 55 Lake Avenue North, Worcester MA 01655. gabriela.santos-nunez@umassmemorial.org.
Rebecca Hultman, York Hospital Breast Care, 15 Hospital drive, York ME 03909, RHultman@yorkhospital.com.
References
- 1.Barroso-Sousa R, Metzger-Filho O. Differences between invasive lobular and invasive ductal carcinoma of the breast: results and therapeutic implications. Ther AdvMed Oncol. 2016;8(4):261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butler RS, Venta LA, Wiley EL, Ellis RL, Dempsey PJ, Rubin E. Sonographic evaluation of infiltrating lobular carcinoma. AJR Am JRoentgenol. 1999;172(2):325–330. [DOI] [PubMed] [Google Scholar]
- 3.Lopez JK, Bassett LW. Invasive lobular carcinoma of the breast: spectrum of mammographic, US, and MR imaging findings. Radiographics. 2009;29(1):165–176. [DOI] [PubMed] [Google Scholar]
- 4.Rakha EA, Ellis IO. Lobular breast carcinoma and its variants. Semin Diagn Pathol. 2010;27(1):49–61. [DOI] [PubMed] [Google Scholar]
- 5.Evans N, Lyons K. The use of ultrasound in the diagnosis of invasive lobular carcinoma of the breast less than 10 mm in size. Clin Radiol. 2000;55(4):261–263. [DOI] [PubMed] [Google Scholar]
- 6.Sehgal CM, Weinstein SP, Arger PH, Conant EF. A review of breast ultrasound. Journal of mammary gland biology and neoplasia. 2006; 11 (2): 113–123. [DOI] [PubMed] [Google Scholar]
- 7.Vijayaraghavan GR, Vedantham S, Kataoka M, DeBenedectis C, Quinlan RM. The Relevance of Ultrasound Imaging of Suspicious Axillary Lymph Nodes and Fine-needle Aspiration Biopsy in the Post-ACOSOG Z11 Era in Early Breast Cancer. Acad Radiol. 2017;24(3):308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oliveira TM, Elias J Jr., Melo AF, et al. Evolving concepts in breast lobular neoplasia and invasive lobular carcinoma, and their impact on imaging methods. Insights into imaging. 2014;5(2):183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mann RM, Veltman J, Barentsz JO, Wobbes T, Blickman JG, Boetes C. The value of MRI compared to mammography in the assessment of tumour extent in invasive lobular carcinoma of the breast. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2008;34(2):135–142. [DOI] [PubMed] [Google Scholar]
- 10.Molland JG, Donnellan M, Janu NC, Carmalt HL, Kennedy CW, Gillett DJ. Infiltrating lobular carcinoma--a comparison of diagnosis, management and outcome with infiltrating duct carcinoma. Breast. 2004;13(5):389–396. [DOI] [PubMed] [Google Scholar]
- 11.Mann RM, Hoogeveen YL, Blickman JG, Boetes C. MRI compared to conventional diagnostic work-up in the detection and evaluation of invasive lobular carcinoma of the breast: a review of existing literature. Breast cancer research and treatment. 2008;107(1): 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosen PP, Groshen S, Saigo PE, Kinne DW, Hellman S. Pathological prognostic factors in stage I (T1N0M0) and stage II (T1N1M0) breast carcinoma: a study of 644 patients with median follow-up of 18 years. J Clin Oncol. 1989;7(9):1239–1251. [DOI] [PubMed] [Google Scholar]
- 13.Singletary SE, Allred C, Ashley P, et al. Revision of the American Joint Committee on Cancer staging system for breast cancer. J Clin Oncol. 2002;20(17):3628–3636. [DOI] [PubMed] [Google Scholar]
- 14.Fortune-Greeley AK, Wheeler SB, Meyer AM, et al. Preoperative breast MRI and surgical outcomes in elderly women with invasive ductal and lobular carcinoma: a population-based study. Breast cancer research and treatment. 2014;143(1):203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Truin W, Roumen RM, Siesling S, et al. Patients with invasive lobular breast cancer are less likely to undergo breast-conserving surgery: a population based study in the Netherlands. Ann Surg Oncol. 2015;22(5):1471–1478. [DOI] [PubMed] [Google Scholar]
- 16.Truin W, Voogd AC, Vreugdenhil G, van der Heiden-van der Loo M, Siesling S, Roumen RM. Effect of adjuvant chemotherapy in postmenopausal patients with invasive ductal versus lobular breast cancer. Ann Oncol. 2012;23(11):2859–2865. [DOI] [PubMed] [Google Scholar]
- 17.Marmor S, Hui JYC, Huang JL, et al. Relative effectiveness of adjuvant chemotherapy for invasive lobular compared with invasive ductal carcinoma of the breast. Cancer. 2017;123(16):3015–3021. [DOI] [PubMed] [Google Scholar]
- 18.Gruber IV, Rueckert M, Kagan KO, et al. Measurement of tumour size with mammography, sonography and magnetic resonance imaging as compared to histological tumour size in primary breast cancer. BMC Cancer. 2013; 13:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onesti JK, Mangus BE, Helmer SD, Osland JS. Breast cancer tumor size: correlation between magnetic resonance imaging and pathology measurements. Am JSurg. 2008;196(6):844–848; discussion 849–850. [DOI] [PubMed] [Google Scholar]
- 20.McGhan LJ, Wasif N, Gray RJ, et al. Use of preoperative magnetic resonance imaging for invasive lobular cancer: good, better, but maybe not the best? Ann Surg Oncol. 2010;17 Suppl 3:255–262. [DOI] [PubMed] [Google Scholar]
- 21.Francis A, England DW, Rowlands DC, Wadley M, Walker C, Bradley SA. The diagnosis of invasive lobular breast carcinoma. Does MRI have a role? Breast. 2001;10(1):38–40. [DOI] [PubMed] [Google Scholar]
- 22.Boetes C, Veltman J, van Die L, Bult P, Wobbes T, Barentsz JO. The role of MRI in invasive lobular carcinoma. Breast cancer research and treatment. 2004;86(1):31–37. [DOI] [PubMed] [Google Scholar]
- 23.Berg WA, Gutierrez L, NessAiver MS, et al. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology. 2004;233(3):830–849. [DOI] [PubMed] [Google Scholar]
- 24.Truin W, Vugts G, Roumen RM, et al. Differences in Response and Surgical Management with Neoadjuvant Chemotherapy in Invasive Lobular Versus Ductal Breast Cancer. Ann Surg Oncol. 2016;23(1):51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lips EH, Mukhtar RA, Yau C, et al. Lobular histology and response to neoadjuvant chemotherapy in invasive breast cancer. Breast cancer research and treatment. 2012;136(1):35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petruolo OA, Pilewskie M, Patil S, et al. Standard Pathologic Features Can Be Used to Identify a Subset of Estrogen Receptor-Positive, HER2 Negative Patients Likely to Benefit from Neoadjuvant Chemotherapy. Ann Surg Oncol. 2017;24(9):2556–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dummin LJ, Cox M, Plant L. Prediction of breast tumor size by mammography and sonography--A breast screen experience. Breast. 2007;16(1):38–46. [DOI] [PubMed] [Google Scholar]
- 28.Shoma A, Moutamed A, Ameen M, Abdelwahab A. Ultrasound for accurate measurement of invasive breast cancer tumor size. Breast J. 2006;12(3):252–256. [DOI] [PubMed] [Google Scholar]
- 29.Haraldsdottir KH, Jonsson T, Halldorsdottir AB, Tranberg KG, Asgeirsson KS. Tumor Size of Invasive Breast Cancer on Magnetic Resonance Imaging and Conventional Imaging (Mammogram/Ultrasound): Comparison with Pathological Size and Clinical Implications. Scand JSurg. 2017;106(1):68–73. [DOI] [PubMed] [Google Scholar]
- 30.Pritt B, Ashikaga T, Oppenheimer RG, Weaver DL. Influence of breast cancer histology on the relationship between ultrasound and pathology tumor size measurements. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2004;17(8):905–910. [DOI] [PubMed] [Google Scholar]
- 31.Snelling JD, Abdullah N, Brown G, King DM, Moskovic E, Gui GP.Measurement of tumour size in case selection for breast cancer therapy by clinical assessment and ultrasound. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2004;30(1):5–9. [DOI] [PubMed] [Google Scholar]
- 32.Verma VP, Kaur N, Agarwal N, et al. Intra-operative measurement of tumour size in breast cancer and its comparison with other methods: a prospective study. Ecancermedicalscience. 2008;2:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung HN, Shin JH, Han BK, Ko EY, Cho EY. Are the imaging features of the pleomorphic variant of invasive lobular carcinoma different from classic ILC of the breast? Breast. 2013;22(3):324–329. [DOI] [PubMed] [Google Scholar]
- 34.Watermann DO, Tempfer C, Hefler LA, Parat C, Stickeler E. Ultrasound morphology of invasive lobular breast cancer is different compared with other types of breast cancer. Ultrasound in medicine & biology. 2005;31(2):167–174. [DOI] [PubMed] [Google Scholar]