Abstract
Objective:
To quantify differences in assessing preterm delivery when calculating gestational age from last menstrual period (LMP) versus ultrasonography biometry.
Methods:
The Zambian Preterm Birth Prevention Study is an ongoing prospective cohort study that commenced enrolment in August 2015 at Women and Newborn Hospital of University Teaching Hospital in Lusaka, Zambia. Women at less than 20 weeks of pregnancy who were enrolled between August 17, 2015, and August 31, 2017, and underwent ultrasonography examination were included in the present analysis. The primary outcome was the difference between ultrasonography- and LMP-based estimated gestational age. Associations between baseline predictors and outcomes were assessed using simple regression. The proportion of preterm deliveries using LMP- and ultrasonography-derived gestational dating was calculated using Kaplan–Meier analysis.
Results:
The analysis included 942 women. The discrepancy between estimating gestational age using ultrasonography and LMP increased with greater gestational age at presentation and among patients with no history of preterm delivery. In a Kaplan–Meier analysis of 692 deliveries, 140 (20.2%, 95% confidence interval [CI] 17.7–23.0) and 79 (11.4%, 95% CI 9.6–13.6) deliveries were classified as preterm by LMP and ultrasonography estimates, respectively.
Conclusion:
Taking ultrasonography as a standard, a bias was observed in LMP-based gestational age estimates; these increased with advancing gestation at presentation. This resulted in misclassification of term deliveries as preterm.
Keywords: Gestational age estimation, Last menstrual period, Gestational age, Preterm birth, Ultrasonography, Zambia
1. INTRODUCTION
Each year, 15 million babies (11% of births worldwide) are born preterm (less than 37 weeks of pregnancy) and 1 million die as a direct result of being born early [1]. Although preterm birth (PTB) reaches nearly every corner of the globe, the disease burden is not distributed uniformly. Reported rates of PTB vary widely among countries: in several European countries the proportion of births that occur before term is 5%, compared to 18% or higher reported in some Sub-Saharan African countries [1].
Accurate estimates of PTB rates are necessary for resource allocation and to design appropriate interventions for the prevention and treatment of prematurity and its sequelae. However, in some settings (especially those where early ultrasonography is scarce and women present late to prenatal care), data are inadequate and national estimates must be modeled [1,2]. Nearly all obstetric studies performed in Africa, such as those that describe the effects of HIV and antiretroviral therapy on adverse birth outcomes, use definitions of gestational age based only on last menstrual period (LMP), symphysis–fundal height, and/or neonatal assessment [3–6]. Basing gestational age estimates on LMP alone can misclassify births owing to imperfect maternal recall, irregular menses, misinterpretation of vaginal bleeding in early pregnancy as a menstrual cycle, and individual variation in menstrual cycle length [7]. Whereas studies in resource-rich settings have suggested that using LMP alone to determine gestational age overestimates PTB rates [8], data from sub-Saharan Africa are scarce.
The American College of Obstetricians and Gynecologists (ACOG) recommends that gestational age be assigned according to the best obstetric estimate [9]. This approach employs an “innocent until proven guilty” algorithm, where the LMP-derived gestational age is assumed to be correct unless it differs from ultrasonography-derived gestational age by a specified amount (in which case the ultrasonography estimate is used). Ultrasonography biometry works on the principle that gestational age can be estimated by measuring specific fetal structures (e.g., femur length, head circumference) and comparing them to known standards. As pregnancy progresses, the variability in these measurements increases and thus a gestational age estimate derived from these measurements becomes less accurate. For this reason, ultrasonography is superior to LMP as a means of estimating gestational age in early pregnancy if estimates differ by as little as +/− 5 days, whereas this tolerance is increased to ±21 days after 28 weeks of pregnancy. Thus, the ACOG approach acknowledges the potential for increasing imprecision in ultrasonography dating as gestation advances. However, it does not account for the potential for any systematic error in the LMP estimate of gestational age.
The aim of the present study was to seek to understand whether deriving gestational age estimates from LMP rather than ultrasonography would affect the assessment of PTB in an urban African cohort, and whether the discrepancy between LMP- and ultrasonography-based dates was greater with later presentation to care or associated with certain baseline maternal clinical and demographic characteristics. It was hypothesized that the proportion of deliveries categorized as preterm would be higher when gestational age was calculated from reported LMP as compared to an estimate based on ultrasonography biometry. It was further hypothesized that recall bias with later presentation to care might introduce systematic error into the LMP-derived estimate of gestational age.
2. MATERIALS AND METHODS
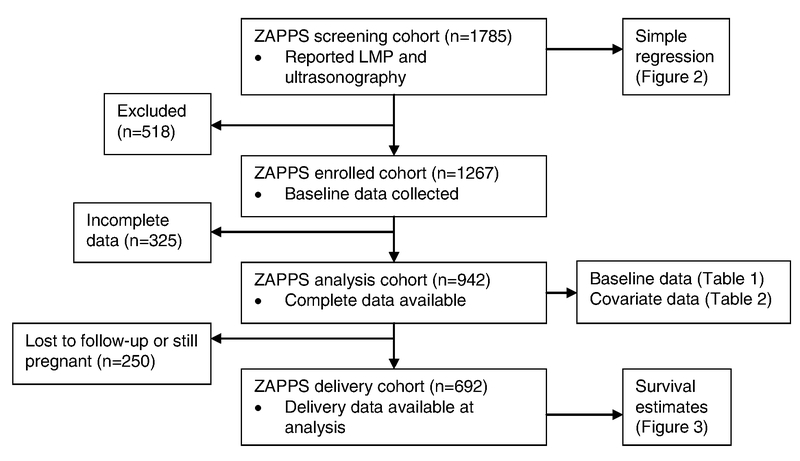
The present study included data from an ongoing prenatal cohort in Zambia. The Zambian Prematurity Prevention Study (ZAPPS) is a prospective cohort that enrolls participants prior to 20 weeks of pregnancy from five prenatal care clinics in the Lusaka urban district [10]. For the purposes of enrollment eligibility, gestational age was determined by best obstetric estimate [9]. The data presented in the present study were collected between August 17, 2015, and August 31, 2017. Within the ZAPPS cohort, data from three sub-groups were analyzed: all women screened for participation in the ZAPPS study made up the “screening cohort”; those who went on to be enrolled formed the “enrolled cohort”; and those who had delivered by the time of the analysis formed the “delivery cohort” (Figure 1). Pregnant women who met the following enrollment criteria were eligible for inclusion in the ZAPPS study: (1) aged 18 years or older; (2) viable intrauterine singleton or twin pregnancy; (3) presentation to prenatal care prior to 20 weeks of pregnancy among patients not infected with HIV or 24 weeks if infected; (4) residing within Lusaka with no plans to relocate during the study follow-up period; (5) willing to provide written, informed consent; (6) willing to allow participation of their neonate(s) in the study; (7) willing to be included in at-home follow-up for delivery outcomes if necessary [10].
Figure 1.
Cohort populations used for analyses. Abbreviations: LMP, last menstrual period; ZAPPS, Zambian Prematurity Prevention Study.
The University of Zambia biomedical research ethics committee and the University of North Carolina institutional review board granted approval to conduct this research before commencement of data collection. All participants provided individual written informed consent prior to enrollment. Ultrasonography data from patients who underwent screening but were not ultimately enrolled were de-identified.
Women interested in participating in ZAPPS were screened for eligibility by ultrasonography biometry. For this analysis, ultrasonography-based estimated gestational age was determined by the INTERGROWTH-21 protocol for standard ultrasonography measurements of either crown rump length (for less than 14 weeks of pregnancy) or head circumference and femur length (for greater than or equal to 14 weeks of pregnancy) [11,12]. The LMP-based estimated gestational age at presentation was derived from the first day of the participant’s LMP based on self-report. Using the ZAPPS screening cohort, the difference was calculated between the ultrasonography-based and LMP-based estimated gestational age at presentation for each participant as a continuous outcome variable (termed, “US–LMP discrepancy”; calculated by subtracting the LMP-derived gestational age from the ultrasonography-derived gestational age).
For the baseline analysis, only data from the ZAPPS enrolled cohort were reported as these were not collected from prospective participants who were screened but not ultimately enrolled (Figure 1). These analyses were further limited to women for whom all covariate data was available. Among these women, descriptive statistics were examined including medians and interquartile ranges (IQR) of each continuous variable and frequencies and percentages of each categorical variable. Crude associations between independent baseline covariates and US–LMP discrepancy were investigated (i.e., were any baseline characteristics correlated with discrepancies in the ultrasonography and LMP gestational age estimates). Key baseline participant characteristics and the US–LMP discrepancy in the participants included in the analysis were analyzed using simple regression to examine whether any particular characteristics were associated with a discrepancy in the estimates. Multiple regression of each key baseline characteristic and the US–LMP discrepancy was also performed, adjusting for gestational age at presentation and all other baseline covariates. A sensitivity analysis was then performed using inverse probability weighting to account for participants with missing covariate information.
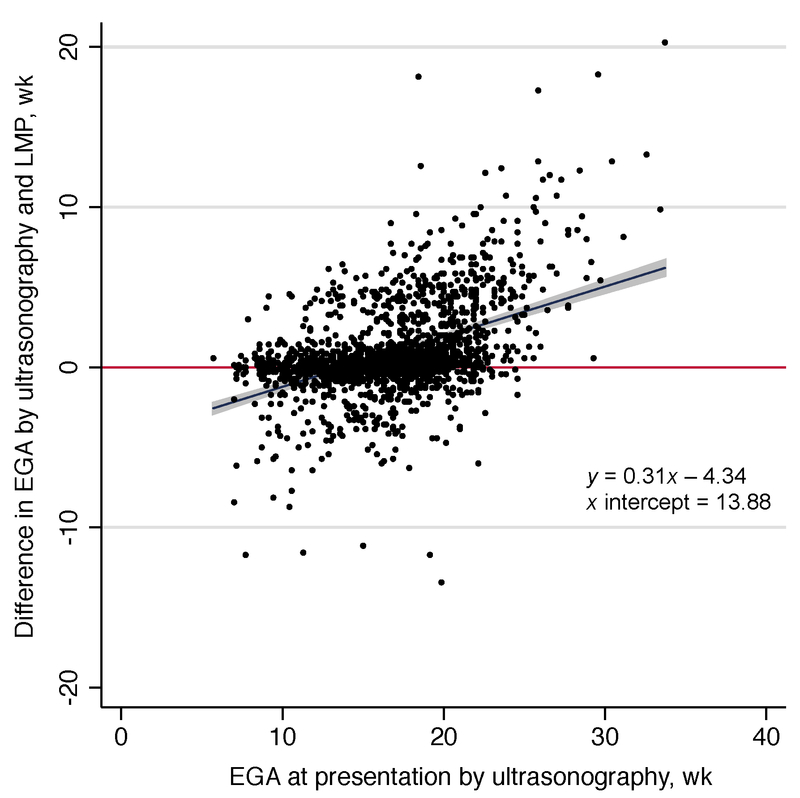
To investigate whether the discrepancy between LMP- and ultrasonography-based gestational age estimates would widen with advancing gestation at presentation, LMP and ultrasonography data were used in the screening cohort to fit a line of the US–LMP discrepancy by the ultrasonography-based gestational age estimate at presentation via simple regression (Figure 2). Using a Kaplan–Meier approach and data from ZAPPS participants who had delivered by the time of the analysis, the proportion of deliveries that occurred preterm using gestational age estimates based solely on LMP were calculated first, and then the proportion of deliveries that occurred preterm using gestational age estimates based solely on the ultrasonography-derived calculation. Finally, the proportion of deliveries classified as post-term by LMP-based estimates compared to ultrasonography estimates was calculated. All analyses described were performed using Stata release 14 (College Station, TX, USA). P<0.05 was considered statistically significant.
Figure 2.
Discrepancy between ultrasonography- and LMP-based EGA by ultrasonography-based gestational age at presentation (n=1785). Abbreviations: EGA, estimate gestational age; LMP, last menstrual period.
3. RESULTS
The ZAPPS screening cohort comprised 1785 women seeking prenatal care between August 2015 and August 2017 with both self-reported LMP and screening ultrasonography data (Figure 1). Of 1267 (70.9%) women in the enrolled cohort, 942 (74.3%) had complete data available for analysis. In this analysis, the median age was 27 years (IQR 22–31) (Table 1). The majority of the participants (590 [62.6%]) had been pregnant at least once before their current pregnancy and the median number of previous pregnancies was 1 (IQR 0–2). Of 590 participants who had been pregnant previously, 285 (48.3%) reported a prior PTB and 132 (22.4%) reported a previous spontaneous abortion. The median body mass index (BMI, calculated as weight in kilograms divided by the square of height in meters) of all patients included in the analysis was 24, and the median gestational age at presentation (by ultrasonography) was 15 weeks (IQR 13–18). Of those enrolled, 300 (31.8%) presented before 14 weeks of pregnancy.
Table 1.
Baseline characteristics (n=942).
| Characteristic | Valuea |
|---|---|
| Maternal age, y | 27 (22–31) |
| <20 | 83 (8.8) |
| 20–24 | 271 (28.8) |
| 25–30 | 278 (29.5) |
| ≥30 | 310 (32.9) |
| Maternal education | |
| No education | 13 (1.4) |
| Primary | 398 (42.3) |
| Secondary | 412 (43.7) |
| Post-secondary | 119 (12.6) |
| Marital status | |
| Never married | 131 (13.9) |
| Married/living together | 791 (84.0) |
| Divorced/separated/widowed | 19 (2.0) |
| BMI | 23.6 (21.2–27.1) |
| <18.5 | 47 (5.0) |
| 18.5–24 | 509 (54.0) |
| ≥25 | 386 (41.0) |
| Parity | 1 (0–2) |
| Prior spontaneous abortion | |
| 0 (nulliparous) | 352 (37.4) |
| 0 (parous) | 458 (48.6) |
| ≥1 | 132 (14.0) |
| Prior preterm birth(s) | |
| 0 (nulliparous) | 352 (37.4) |
| 0 (parous) | 305 (32.4) |
| ≥1 | 285 (30.3) |
| Vaginal bleeding at presentation | 75 (8.0) |
| HIV at enrollment | |
| Non-reactive | 747 (79.3) |
| Reactive | 195 (20.7) |
| Ultrasonography-estimated gestational age at presentation, wk | 15 (13–18) |
| <14 | 300 (31.9) |
| 14–24 | 642 (68.2) |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by the square of height in meters).
Values are given as median (interquartile range) or number (percentage).
In a regression of baseline characteristics against the US–LMP discrepancy (in days), absence of a previous spontaneous abortion (regression coefficient 3.40 days, 95% confidence interval [CI] 0.56–6.25), no reported history of PTB (regression coefficient 3.96 days, 95% CI 1.47–6.45), and having reached at least 14 weeks of gestation at presentation (regression coefficient 5.02 days, 95% CI 2.92–7.12) were associated with a greater US–LMP discrepancy (Table 2). In a multiple regression adjusting for all other baseline covariates, being at 14 weeks of pregnancy or later (regression coefficient 4.58 days, 95% CI 2.43–6.74) remained significantly associated with increased US–LMP discrepancy, while no reported history of PTB demonstrated a marginal, non-significant association (regression coefficient 2.92 days, 95% CI −0.03 to 5.88).
Table 2.
Regression of key baseline characteristics and mean discrepancy between gestation estimated by ultrasonography and by reported LMP in ZAPPS analysis cohort (n=942).
| Characteristic | No. | Mean US–LMP discrepancy, d | Unadjusted analysisa | Adjusted analysisa | ||
|---|---|---|---|---|---|---|
| Regression coefficient (95% CI) | P valueb | Regression coefficient (95% CI) | P valueb | |||
| Age, y (continuous) | 942 | 2.21 | 0.05 (−0.12 to 0.22) | 0.546 | 0.13 (−0.07 to 0.33) | 0.196 |
| Education | ||||||
| None | 13 | 5.77 | 5.72 (−3.16 to 14.59) | 0.206 | 3.84 (−5.03 to 12.71) | 0.396 |
| Primary | 398 | 2.49 | 2.43 (−0.74 to 5.60) | 0.132 | 1.65 (−1.68 to 4.99) | 0.331 |
| Secondary | 412 | 2.44 | 2.38 (−0.78 to 5.54) | 0.139 | 1.77 (−1.45 to 4.98) | 0.281 |
| Post-secondary | 119 | 0.06 | 1 (reference) | 1 (reference) | ||
| BMI | ||||||
| <18.5 | 47 | 4.11 | 2.07 (−2.57 to 6.70) | 0.381 | 2.60 (−2.00 to 7.20) | 0.267 |
| 18.5–24 | 509 | 2.04 | 1 (reference) | 1 (reference) | ||
| ≥25 | 386 | 2.20 | 0.16 (−1.89 to 2.21) | 0.880 | 0.28 (−1.89 to 2.45) | 0.799 |
| Parity | ||||||
| 0 | 352 | 1.88 | −0.52 (−2.57 to 1.53) | 0.617 | ||
| ≥1 | 590 | 2.40 | 1 (reference) | |||
| Prior spontaneous abortion | ||||||
| 0 | 810 | 2.68 | 3.40 (0.56–6.25) | 0.019 | 1.34 (−2.23 to 4.92) | 0.461 |
| ≥1 | 132 | −0.72 | 1 (reference) | 1 (reference) | ||
| Vaginal bleeding at enrollment | ||||||
| No | 867 | 2.25 | 1 (reference) | 1 (reference) | ||
| Yes | 75 | 1.71 | −0.54 (−4.20 to 3.11) | 0.771 | 0.26 (−3.42 to 3.94) | 0.891 |
| HIV | ||||||
| No | 747 | 1.90 | 1 (reference) | 1 (reference) | ||
| Yes | 195 | 3.40 | 1.90 (−0.94 to 3.95) | 0.226 | 0.92 (−1.59 to 3.44) | 0.471 |
| Prior preterm birth | ||||||
| Nulliparous | 352 | 1.88 | 1.53 (−0.88 to 3.94) | 0.214 | 1.63 (−1.52 to 4.78) | 0.310 |
| 0 | 305 | 4.31 | 3.96 (1.47–6.45) | 0.002 | 2.92 (−0.03 to 5.88) | 0.053 |
| ≥1 | 285 | 0.35 | 1 (reference) | 1 (reference) | ||
| Ultrasonography-estimated gestational age, wk | ||||||
| <14 | 300 | −1.22 | 1 (reference) | 1 (reference) | ||
| ≥14 | 642 | 3.81 | 5.02 (2.92–7.12) | <0.001 | 4.58 (2.43–6.74) | <0.001 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by the square of height in meters); LMP, last menstrual period; US–LMP, ultrasonography and last menstrual period; ZAPPS, Zambian Prematurity Prevention Study.
Model adjusted for all covariates listed in Table 2, except parity.
P values calculated by regression for unadjusted and adjusted estimates and were considered significant at <0.05.
In a sensitivity analysis accounting for missing covariate data using inverse probability weighting, the findings were similar (Table S1). While gestational age at presentation of 14 weeks or later was associated with increased US–LMP discrepancy (regression coefficient 5.01 days, 95% CI 2.86–7.17) in adjusted analysis, the association with no reported history of PTB was attenuated (regression coefficient 3.03 days, 95% CI −0.37 to 6.43).
Using the ZAPPS screening cohort, the plot of US–LMP discrepancy at presentation by the ultrasonography-based gestational age estimate in weeks demonstrated an associated regression line with a positive slope of 0.31 weeks that crossed zero at approximately 14 weeks of pregnancy (Figure 2). Women who presented prior to 14 weeks of pregnancy had an overall negative difference in gestational age based on ultrasonography compared with LMP (i.e., the LMP estimate was later than the ultrasonography estimate), whereas patients presenting at 14 weeks or later had a positive difference in gestational age estimated by ultrasonography compared with LMP (i.e., the ultrasonography estimate was later than the LMP estimate). With increasing gestation at presentation beyond 14 weeks, the difference between the LMP- and ultrasonography-estimated gestational age widened.
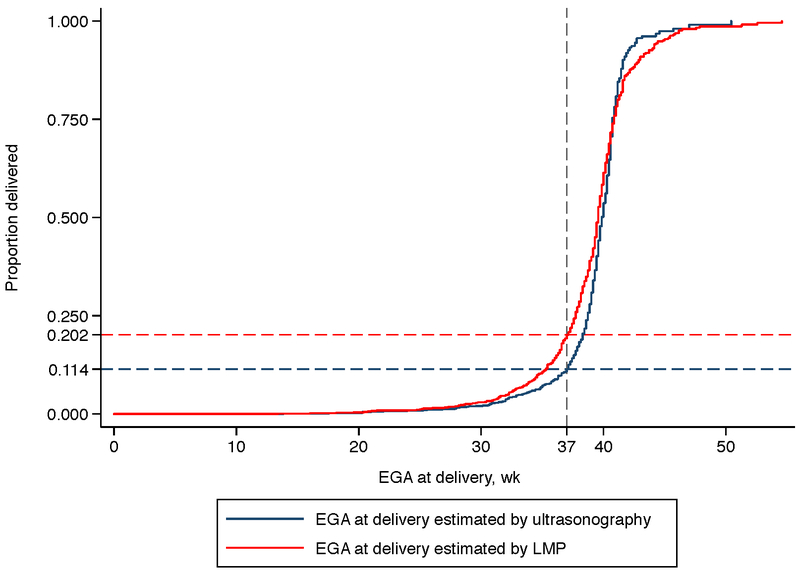
Based on Kaplan–Meier estimates derived from the ZAPPS delivery cohort (n=692), the proportion of deliveries that were preterm was 11.4% (95% CI 9.6–13.6%) using ultrasonography-based gestational age estimates and 20.2% (95% CI 17.7–23.0%) using LMP estimates (Figure 3). The proportion of post-term deliveries (i.e., occurring at 42 weeks or later) was similarly overestimated by LMP dating. Using gestational age based on ultrasonography, 7.4% (95% CI 5.2–10.2%) of deliveries were post-term compared with 13.2% (95% CI 10.6–16.1%) by LMP.
Figure 3.
Kaplan–Meier estimates of gestational age at delivery by ultrasonography and by LMP. Abbreviations: EGA, estimated gestational age; LMP, last menstrual period.
4. DISCUSSION
The present study had two primary findings. First, it confirmed the widely known limitation of LMP for pregnancy dating. Using ultrasonography as the gold standard, LMP-derived dates artificially doubled the proportion of PTB. Second, the study suggested that the error in gestational age measurement introduced by self-reported LMP may not be random. Among women who presented after 14 weeks of pregnancy, LMP-derived dates systematically underestimated current gestational age. This discrepancy appeared to be associated with obstetric history, specifically, whether or not a woman reported a previous PTB.
The finding of the present study that LMP-based gestational age overestimated PTB when compared to ultrasonography-based gestational age corroborates population data in the USA [8,13]. Until recently, the National Center for Health Statistics and the Centers for Disease Control and Prevention (CDC) in the USA-based national estimates of PTB on LMP dating, the sole determinant of gestational age at delivery on birth certificates in many states. In 2014, the CDC began to incorporate sonography into its estimates. In a report that explained the rationale for this shift, the CDC demonstrated that the PTB rate in 2013 was 11.39% based on LMP alone, compared to 9.62% based on the best obstetric estimate [13]. Another study using data from the USA reported a similar difference in PTB rates based on LMP alone compared to obstetric estimate in 2012 [8]. The authors also demonstrated that two objective indicators of clinical prematurity—NICU admission and low birth weight —correlated more closely with the obstetric estimates of gestational age at delivery than with LMP dating [8].
The present study demonstrated that the discrepancy between LMP and ultrasonography estimates of gestational age was related to the number of weeks of pregnancy at presentation. Women presenting prior to 14 weeks of pregnancy tended to report their LMP as less recent than ultrasonography would suggest it was, while women presenting after 14 weeks of pregnancy reported the opposite (Figure 2). This effect could at least partially explain the very high rates of PTB in some reports in which the median gestational age at presentation is considerably later than 14 weeks [14].
Other demographic and clinical covariates might predict discrepancy between LMP and ultrasonography dating if they were to interfere with menstrual regularity (e.g., BMI) or with recollection of LMP (e.g., educational attainment or prior adverse pregnancy outcome) [7,15,16]. In the present study cohort, the only factor marginally associated with US–LMP discrepancy was a history of PTB. It is conceivable that women who have had a prior PTB may track their menses more vigilantly or simply recall their LMP more accurately than women without this history. After controlling for gestational age at presentation to care, the findings of the present study did not support a strong association between US–LMP discrepancy and maternal age, educational attainment, BMI, parity, baseline hemoglobin, prior spontaneous abortion, vaginal bleeding at presentation, or HIV serostatus.
Limitations of the present study are as follows. First, the cohort was recruited primarily from the University Teaching Hospital, which cares for higher-risk patients referred from surrounding public-sector clinics [14]. This may limit the generalizability of the findings. While the bias demonstrated could exist in any setting where LMP is used as the sole determinant of gestational age, the magnitude of that bias may depend on population characteristics that affect menstrual regularity and recall. A second limitation, also related to generalizability, is that screening procedures excluded women whose pregnancies were advanced. The median gestational age at presentation in the Lusaka public sector is 23 weeks [17], and the present study cohort did not include many women presenting at this relatively late stage. Finally, the sample was not large enough to fully interrogate the relationships between all baseline characteristics and US–LMP discrepancy. Wide confidence intervals around some estimates (e.g., education, BMI, HIV, and even prior PTB) indicate imprecision due to the moderate sample size.
The present study has shown that estimates of PTB among pregnancies dated by LMP, a less accurate method of determining gestational age, may suffer from bias. It would appear that women tend to report their LMP as more recent with later presentation to care, which is a common occurrence in many low-income settings [17–19]. Observational research studies that rely on LMP alone would then report a falsely elevated risk of PTB among groups who present later. For instance, while HIV infection itself did not increase the discrepancy between LMP and ultrasonography gestational dating in our analysis, associations between HIV, its treatment, and PTB could be biased in studies that employ inaccurate gestational dating methods and do not account for the range of gestational ages at presentation to care [6,20].
One obvious solution to this problem would be widespread introduction of obstetric ultrasonography into under-resourced settings. In recent years remarkable progress has been made in ultrasonography transducer technology, with low-cost probes that can now transmit images to a smart phone or tablet. The benefit of prenatal ultrasonography goes well beyond gestation dating to the diagnosis of important—and sometimes life-threatening—pregnancy complications, including: ectopic pregnancy, abnormal placenta, multiple gestation, fetal malpresentation, fetal growth restriction, macrosomia, oligohydramnios, polyhydramnios, and intrauterine fetal death. The expansion of this essential technology to prenatal clinics worldwide is eagerly awaited. In the meantime, it is critical that investigations into adverse birth outcomes are based on accurate gestational age estimates and account for differing gestational ages at presentation to minimize this bias.
Supplementary Material
Regression of key baseline characteristics and mean discrepancy between estimated gestational age by ultrasound and by reported last menstrual period in complete ZAPPS enrolled cohort using inverse probability weighting (n=942).
Synopsis: Basing gestational age estimates on last menstrual period introduces a bias that worsens with late presentation to care and can misclassify term deliveries as preterm.
Acknowledgments
The study presented in this manuscript was partially funded by the Global Alliance to Prevent Prematurity and Stillbirth (Seattle Children’s Hospital/GAPPS 13008) and the Center for AIDS Research (CFAR P30–050410). Trainee support for Joan Price has been provided through the National Institutes of Health (T32 HD075731; K01 TW010857). The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health.
Footnotes
Conflicts of Interest:
The authors have no conflicts of interest.
REFERENCES
- 1.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. The Lancet. 2012;379(9832):2162–2172. [DOI] [PubMed] [Google Scholar]
- 2.Smid MC, Stringer EM, Stringer JS. A Worldwide Epidemic: The Problem and Challenges of Preterm Birth in Low- and Middle-Income Countries. Am J Perinatol. 2016;33(3):276–289. [DOI] [PubMed] [Google Scholar]
- 3.Zash R, Souda S, Chen JY, et al. Reassuring Birth Outcomes With Tenofovir/Emtricitabine/Efavirenz Used for Prevention of Mother-to-Child Transmission of HIV in Botswana. J Acquir Immune Defic Syndr. 2016;71(4):428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen JY, Ribaudo HJ, Souda S, et al. Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in Botswana. J Infect Dis. 2012;206(11):1695–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li N, Sando MM, Spiegelman D, et al. Antiretroviral Therapy in Relation to Birth Outcomes among HIV-infected Women: A Cohort Study. J Infect Dis. 2016;213(7):1057–1064. [DOI] [PubMed] [Google Scholar]
- 6.Uthman OA, Nachega JB, Anderson J, et al. Timing of initiation of antiretroviral therapy and adverse pregnancy outcomes: a systematic review and meta-analysis. Lancet HIV. 2017;4(1):e21–e30. [DOI] [PubMed] [Google Scholar]
- 7.Savitz DA, Terry JW Jr., Dole N, Thorp JM Jr., Siega-Riz AM, Herring AH. Comparison of pregnancy dating by last menstrual period, ultrasound scanning, and their combination. Am J Obstet Gynecol. 2002;187(6):1660–1666. [DOI] [PubMed] [Google Scholar]
- 8.Ambrose CS, Caspard H, Rizzo C, Stepka EC, Keenan G. Standard methods based on last menstrual period dates misclassify and overestimate US preterm births. J Perinatol. 2015;35(6):411–414. [DOI] [PubMed] [Google Scholar]
- 9.Committee opinion no 611: method for estimating due date. Obstet Gynecol. 2014;124(4):863–866. [DOI] [PubMed] [Google Scholar]
- 10.Castillo MC, Fuseini NM, Rittenhouse K et al. The Zambian Preterm Birth Prevention Study (ZAPPS): Cohort characteristics at enrollment. Gates Open Res 2018, 2:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papageorghiou AT, Kemp B, Stones W, et al. Ultrasound-based gestational-age estimation in late pregnancy. Ultrasound Obstet Gynecol. 2016;48(6):719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papageorghiou AT, Kennedy SH, Salomon LJ, et al. International standards for early fetal size and pregnancy dating based on ultrasound measurement of crown-rump length in the first trimester of pregnancy. Ultrasound Obstet Gynecol. 2014;44(6):641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin JA, Osterman MJK, Kirmeyer SE, Gregory ECW. Measuring Gestational Age in Vital Statistics Data: Transitioning to the Obstetric Estimate. Hyattsville, MD: National Center for Health Statistics; 2015. [PubMed] [Google Scholar]
- 14.Vwalika B, Stoner MC, Mwanahamuntu M, et al. Maternal and newborn outcomes at a tertiary care hospital in Lusaka, Zambia, 2008–2012. Int J Gynecol Obstet. 2017;136:180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin C, Hsia J, Berg CJ. Variation between last-menstrual-period and clinical estimates of gestational age in vital records. Am J Epidemiol. 2008;167(6):646–652. [DOI] [PubMed] [Google Scholar]
- 16.Morin I, Morin L, Zhang X, et al. Determinants and consequences of discrepancies in menstrual and ultrasonographic gestational age estimates. BJOG. 2005;112(2):145–152. [DOI] [PubMed] [Google Scholar]
- 17.Chi BH, Vwalika B, Killam WP, et al. Implementation of the Zambia electronic perinatal record system for comprehensive prenatal and delivery care. Int J Gynecol Obstet. 2011;113:131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Central Statistical Office Ministry of Health Zambia and ICF International. Zambia Demographic and Health Survey 2013–2014. In: ICF International, ed. Rockville, MD: 2014. [Google Scholar]
- 19.National Statistical Office (NSO) and ICF Macro. Malawi Demographic and Health Survey 2015–2016. Zomba, Malawi, and Rockville, Maryland, USA: 2017. [Google Scholar]
- 20.Stoner MCD, Cole SR, Price J, Winston J, Stringer JSA. Timing of Initiation of Antiretroviral Therapy and Risk of Preterm Birth in Studies of HIV-infected Pregnant Women: The Role of Selection Bias. Epidemiology. 2018;29(2):224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Regression of key baseline characteristics and mean discrepancy between estimated gestational age by ultrasound and by reported last menstrual period in complete ZAPPS enrolled cohort using inverse probability weighting (n=942).