Abstract
Background
Patients with chronic kidney disease (CKD) and stable ischemic heart disease (SIHD) are at markedly increased risk of cardiovascular events. Prior trials comparing a strategy of optimal medical therapy (OMT) with or without revascularization have largely excluded patients with advanced CKD. Whether a routine invasive approach when compared with a conservative strategy is beneficial in such patients is unknown.
Methods
ISCHEMIA-CKD is a National Heart, Lung, and Blood Institute-funded randomized trial designed to determine the comparative effectiveness of an initial invasive strategy (cardiac catheterization and optimal revascularization (percutaneous coronary intervention [PCI] or coronary artery bypass graft surgery [CABG], if suitable), plus OMT) versus a conservative strategy (OMT alone, with cardiac catheterization and revascularization [PCI or CABG, if suitable] reserved for failure of OMT) on long-term clinical outcomes, in 777 patients with advanced CKD (defined as those with estimated glomerular filtration rate [eGFR] <30 ml/min/1.73m2 or on dialysis) and moderate or severe ischemia on stress testing. Participants were randomized in a 1:1 fashion to the invasive or a conservative strategy. The primary endpoint is a composite of death or nonfatal myocardial infarction (MI). Major secondary endpoints are a composite of death, nonfatal MI, hospitalization for unstable angina, hospitalization for heart failure, or resuscitated cardiac arrest; angina control and disease-specific quality of life. Safety outcomes such as initiation of maintenance dialysis and a composite of initiation of maintenance dialysis or death will be reported. The trial is projected to have 80% power to detect a 22% to 24% reduction in the primary composite endpoint with the invasive strategy when compared with the conservative strategy.
Conclusions
ISCHEMIA-CKD will determine whether an initial invasive management strategy improves clinical outcomes when added to OMT in patients with advanced CKD and SIHD.
Keywords: catheterization, chronic kidney disease, optimal medical therapy, revascularization, stable ischemic heart disease
Background
Cardiovascular disease is the leading cause of death among patients with chronic kidney disease (CKD), 1, 2 a rate 15–30 times higher than age-adjusted cardiovascular mortality in the general population.3, 4 Patients with CKD are more likely to die from cardiovascular causes than to experience progression to end-stage renal disease requiring dialysis or transplantation.5, 6 Despite the strong link between CKD and cardiovascular disease, ~80% of contemporary stable ischemic heart disease (SIHD) trials have excluded patients with CKD, presumably because of this high antecedent risk. 7, 8 Most treatments aimed at reducing cardiovascular events in patients with CKD are extrapolated from prior trials enrolling largely patients without CKD. Patients with CKD were also under-represented in contemporary SIHD trials comparing revascularization with medical therapy, including Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial,9 the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial,10 and the Fractional Flow Reserve versus Angiography for Multivessel Evaluation 2 (FAME 2) trial.11 The above trials failed to show that an initial invasive strategy reduced the risk of death or myocardial infarction (MI) when compared with an initial conservative strategy of medical therapy alone.
Patients with CKD are at increased risk for procedural complications, including acute kidney injury (AKI), major bleeding, vessel dissection, MI and death. 12–14 Observational studies in CKD suggest lower long-term mortality rates in patients with SIHD who were revascularized when compared with patients who did not undergo revascularization,15 despite an increase in short-term risks. However, these “benefits” may be due to selection biases, compounded by the fact that the medical therapy in these studies was variable and not optimized. For this reason, there is an unmet need to better understand the optimal management of patients with SIHD and advanced CKD.16–18
To address these uncertainties, we are conducting the ISCHEMIA-CKD (NCT01985360) trial, an international, randomized comparative effectiveness trial that is designed to test the incremental value of an initial invasive strategy when added to optimal medical therapy (OMT) in patients with SIHD and advanced CKD.
Methods
Funding from NIH grants U01HL117904 and U01HL117905 were used to support the research and creation of this paper. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper, and its final contents.
Study Design and Population
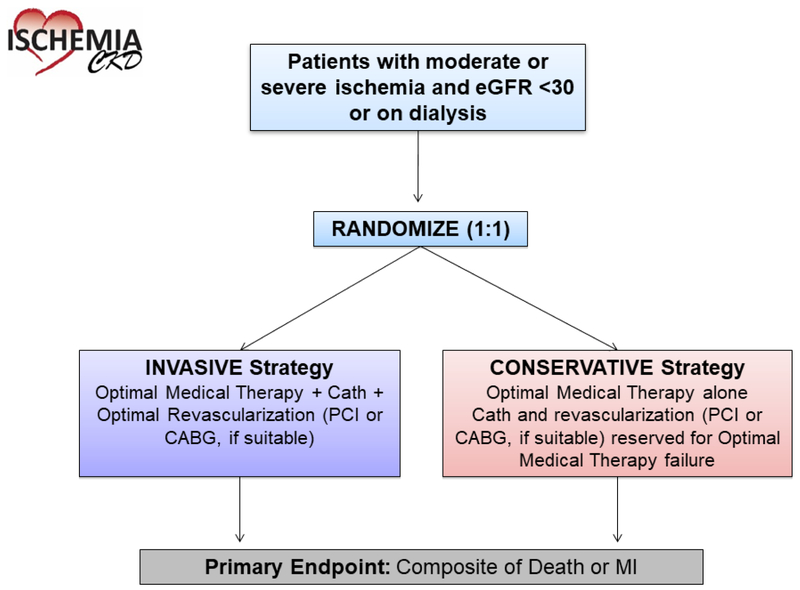
The primary aim of ISCHEMIA-CKD is to determine whether an initial invasive strategy of cardiac catheterization and revascularization (percutaneous coronary intervention [PCI] or coronary artery bypass graft surgery [CABG]), if suitable, in addition to OMT, will reduce the primary endpoint of all-cause death or non-fatal MI in 777 participants with moderate or severe ischemia and advanced CKD (defined as patients with estimated glomerular filtration rate [eGFR] <30 ml/min/1.73m2 or on dialysis), compared with a conservative strategy of OMT alone, with cardiac catheterization and revascularization (PCI or CABG, if suitable) reserved for failure of OMT (Figure 1). The secondary aims are to compare other clinical and economic outcomes as prespecified in the protocol and/or the Statistical Analysis Plan. Safety outcomes such as initiation of maintenance dialysis and a composite of initiation of maintenance dialysis or death will be reported. ISCHEMIA-CKD was designed to run in parallel with the ISCHEMIA Trial (NCT01471522), which randomized patients with eGFR ≥30 ml/min/1.73 m2 and moderate or severe ischemia in 1:1 fashion to invasive or conservative strategies.
Figure 1.
Study Design
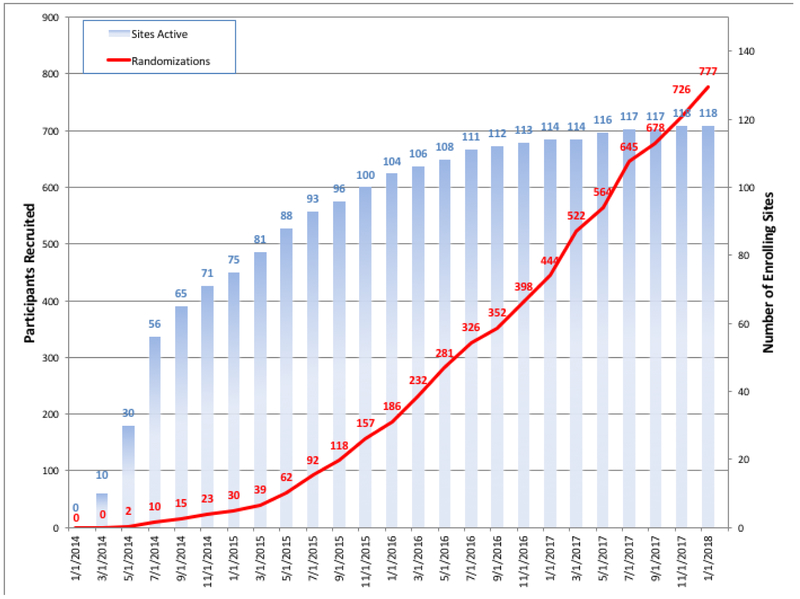
Patients with advanced CKD and moderate or severe ischemia on stress testing were screened for study inclusion, the details of which are shown in Table 1. The first patient was randomized on May 12, 2014 and the last patient on January 31, 2018 (Figure 2).
Table 1.
Inclusion and Exclusion Criteria
| Inclusion Criteria | • At least moderate ischemia on an exercise or pharmacologic stress test |
| • End-stage renal disease on dialysis or estimated glomerular filtration rate (eGFR) <30mL/min/1.73m2 | |
| • Willingness to comply with all aspects of the protocol, including adherence to the assigned strategy, medical therapy and follow-up visits | |
| • Willingness to give written informed consent | |
| • Age ≥ 21 years | |
| Exclusion Criteria | • Left ventricular ejection fraction < 35% |
| • History of unprotected left main stenosis ≥50% on prior coronary computed tomography angiography (CCTA) or prior cardiac catheterization (if available) | |
| • Finding of “no obstructive coronary artery disease ” (<50% stenosis in all major epicardial vessels) on prior CCTA or prior catheterization, performed within 12 months | |
| • Coronary anatomy unsuitable for either percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) | |
| • Unacceptable level of angina despite maximal medical therapy | |
| • Very dissatisfied with medical management of angina | |
| • History of noncompliance with medical therapy | |
| • Acute coronary syndrome within the previous 2 months | |
| • PCI within the previous 12 months | |
| • Stroke within the previous 6 months or spontaneous intracranial hemorrhage at any time | |
| • History of ventricular tachycardia requiring therapy for termination, or symptomatic sustained ventricular tachycardia not due to a transient reversible cause | |
| • NYHA class III-IV heart failure at entry or hospitalization for exacerbation of chronic heart failure within the previous 6 months | |
| • Non-ischemic dilated or hypertrophic cardiomyopathy | |
| • Severe valvular disease or valvular disease likely to require surgery or percutaneous valve replacement during the trial | |
| • Allergy to radiographic contrast that cannot be adequately pre-medicated, or any prior anaphylaxis to radiographic contrast | |
| • Planned major surgery necessitating interruption of dual antiplatelet therapy (note that patients may be eligible after planned surgery) | |
| • Life expectancy less than the duration of the trial due to non-cardiovascular comorbidity | |
| • Pregnancy | |
| • High likelihood of significant unprotected left main stenosis, in the judgment of the patient’s physician | |
| • Enrollment in a competing trial that involves a non-approved cardiac drug or device | |
| • Inability to comply with the protocol | |
| • Body weight or size exceeding the limit for cardiac catheterization at the site | |
| • Canadian Cardiovascular Society Class III angina of recent onset, OR angina of any class with a rapidly progressive or accelerating pattern | |
| • Canadian Cardiovascular Society Class IV angina, including unprovoked rest angina | |
| • High risk of bleeding which would contraindicate the use of dual antiplatelet therapy | |
| • Cardiac transplant recipient | |
| • Prior CABG, unless CABG was performed more than 12 months ago, and coronary anatomy has been demonstrated to be suitable for PCI or repeat CABG to accomplish complete revascularization of ischemic areas | |
Figure 2.
Participant Recruitment and Active Randomizing Sites
Patients who met the clinical and ischemia (site-interpreted) eligibility criteria (Table 2) and who gave informed written consent were randomized in a 1:1 fashion to the invasive or conservative strategies (Figure 1). Randomized allocation of treatment strategy was performed via a central interactive voice response system (IVRS) and interactive web response system (IXRS). Treatment allocation was open label. Participants were randomized over approximately 3.7 years, with follow-up projected for an average of approximately 2.8 years. The minimum follow-up period will be approximately 18 months following randomization of the final subject. The study is being performed in accordance with ethical principles of the Declaration of Helsinki, and the International Conference on Harmonization Good Clinical Practice guidelines. The final study protocol and informed consent were reviewed and approved by the corresponding health authorities and ethics boards/institutional review boards of all participating study sites. The trial was funded by the National Heart, Lung, and Blood Institute (NHLBI). The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper, and its final contents.
Table 2.
Ischemia Eligibility Criteria by Stress Test Modality
| Stress Test Modality | Diagnostic Criteria for Moderate or Severe Ischemia |
|---|---|
| Nuclear perfusion via SPECT or PET | ≥10% myocardium ischemic1 |
| Echocardiography | ≥3/16 segments with stress-induced severe hypokinesis or akinesis |
| Cardiac Magnetic Resonance | Perfusion: ≥12% myocardium ischemic, and/or Wall motion: ≥3/16 segments with stress-induced severe hypokinesis or akinesis |
| Exercise Test without Imaging (criteria 1–3 must all be met) | 1. Absence of resting ST-segment depression ≥1.0 mm or confounders that render exercise ECG non-interpretable (LBBB, LVH with repolarization, pacemaker, etc.) |
| 2. As compared to the baseline tracing, additional exercise-induced horizontal or downsloping ST-segment depression ≥1.5 mm in 2 leads or ≥2.0 mm in any lead; ST-segment elevation ≥1mm in a non-infarct territory. Both the J-point and the ST-segment at 80 msec need to meet criteria. When the HR is >130/min, the ST-segment at 60 msec. may be used if the segment at 80 msec. cannot be determined. | |
| 3. Either of the following: a. Workload at which ST-segment criteria are met is not to exceed completion of stage 2 of a standard Bruce protocol or 7 METs if a non-Bruce protocol is used or b. ST segment criteria are met at <75% of the maximum predicted HR |
Management Strategies and Study Assessments
Conservative Strategy
In participants randomized to the conservative strategy, management is focused on the provision of OMT. A fundamental principle of the conservative strategy was to restrict cardiac catheterization (and revascularization if suitable) only to participants who fail OMT, i.e., those who experience an acute coronary syndrome, acute ischemic heart failure or resuscitated cardiac arrest or who have angina refractory to medical therapy (Figure 3). If angina worsens, medical therapy is intensified first (eFigure 1). If symptoms are refractory to maximally tolerated medical therapy or become unstable, participants undergo cardiac catheterization. In such participants, revascularization (PCI or CABG) is performed using the principles of optimal revascularization as described below.
Figure 3.
Invasive Procedures and Protocol Adherence. 1 Heart-kidney team recommended for left main disease, complex multivessel disease or whenever in doubt. 2 FFR is recommended for stenosis <50% if PCI is considered and stress imaging shows ischemia in the corresponding territory; FFR is recommended for stenosis <80% if PCI is considered and stress imaging does not show ischemia in the corresponding territory. 3 Use of instantaneous wave-free ratio (iFR) instead of FFR (where available) was permitted, using a cutoff of ≤0.89 for physiologic significance. 4 Includes suspected hospitalization for unstable angina, ischemic heart failure, resuscitated sudden cardiac arrest or myocardial infarction.
Optimal Medical Therapy (OMT)
Optimal medical therapy consists of intensive, comprehensive secondary prevention with lifestyle and pharmacologic intervention applied equally to both treatment groups using individualized treatment regimens based on treat-to-target algorithms. The site study team works in collaboration with the participant’s principal care physician (internal medicine physician, nephrologist or cardiologist) to achieve OMT goals. Study coordinators have been trained to provide lifestyle counseling focused on smoking cessation, nutrition, physical activity, and medication adherence. Pharmacologic interventions include anti-atherothrombotic and anti-ischemic medications. Treatment algorithms were developed and recommended for management of low-density lipoprotein (LDL) cholesterol, blood pressure, and angina (eFigure 1). The minimum goals of OMT are those recommended by the American College of Cardiology (ACC), American Heart Association (AHA), the European Society of Cardiology (ESC) for secondary prevention and angina management, the National Kidney Foundation and the UK Renal Association.19–25 There are challenges to the implementation of OMT, as the evidence upon which the guidelines are based for patients with advanced CKD, and especially those on dialysis, is limited (most randomized trials excluded these high-risk patients). However, cardiovascular disease guideline recommendations are the existing standards and are used in ISCHEMIA-CKD with some modification, as outlined in Table 3. In addition, diagnosis, evaluation, prevention and treatment of CKD-related conditions, including anemia and mineral and bone disorders, are based on local practice and in accordance with national and international guidelines for kidney disease. Moreover, the choice of dialysis modality, the access site for dialysis, duration of dialysis and other strategies for adequate dialysis (such as dialysate temperature) are based on local practice. Dose adjustments are recommended for medications that are renally excreted or dialyzed.
Table 3.
Goals of Optimal Medical Therapy
| RISK FACTOR | GOALS |
|---|---|
| Behavioral | |
| Smoking | Smoking cessation |
| Physical activity | ≥30 minutes of moderate intensity ≥5 times/week |
| Saturated fat | <7% calories |
| Physiological | |
| Blood pressure | <130/80 mm Hg1
Significantly lower BP (e.g., <110 mm Hg systolic) should be avoided |
| LDL cholesterol | LDL-C <70 mg/dl (1.8 mmol/L) |
| Body Mass Index (kg/m22) | Initial BMI Weight Loss Goal |
| 25–27.5 BMI <25 | |
| >27.5 10% relative weight loss | |
| Diabetes | HbA1c <8% |
| Pharmacological agents2 | Indications |
| Aspirin | 75–162 mg daily |
| Statin | Pre-dialysis: Maximum tolerated dose of high-intensity statin (atorvastatin 40–80 mg or rosuvastatin 20–40 mg) Dialysis: Maximum tolerated dose of moderate- or high-intensity statin (atorvastatin 20–80 mg or rosuvastatin 10–40 mg) |
| ACEi/ARB | All participants (as tolerated) |
| Beta blocker | Use for history of MI or LVEF <40% |
| P2Y12 receptor antagonist | Use for participants with contraindication to aspirin; In combination with aspirin for participants who receive PCI (duration depends on BMS vs. DES); post-MI/ACS for 1 year |
| Ezetimibe | Use for participants unable to reach LDL-C goal on maximally tolerated statin dose |
This risk factor goal was changed in April 2018. Prior goal was <140/90 mm Hg in participants without proteinuria and for participants on dialysis, and a goal of <130/80 mm Hg in participants with proteinuria (>300mg/day).
Dose adjustments are required for medications that are renally excreted or dialyzed
BMI = body mass index; HbA1c = hemoglobin A1c; MI = myocardial infarction; ACEi/ARB = angiotensin converting enzyme inhibitor/angiotensin receptor blocker; eGFR = estimated glomerular filtration rate; LVEF = left ventricular ejection fraction; MI = myocardial infarction; PCI = percutaneous coronary intervention; BMS = bare metal stent; DES = drug-eluting stent; ACS = acute coronary syndrome; LDL-C = low-density lipoprotein cholesterol.
In patients on dialysis, there is no clear consensus as to which (pre-dialysis, post-dialysis or interdialytic) blood pressure should be used as the guide for therapy. Based on the 2017 ACC/AHA blood pressure guidelines 26 (even though specific recommendations for subjects on dialysis are lacking), we recommend an office blood pressure goal of <130/80 mm Hg for all participants in the ISCHEMIA-CKD trial. Significantly lower blood pressure (e.g., <110 mm Hg systolic) should be avoided. Controversy persists about the safety and efficacy of statins in patients with advanced CKD. Two large randomized controlled trials of statins in patients receiving hemodialysis (Die Deutsche Diabetes Dialyse Studie [the 4D study] and the Assessment of Survival and Cardiovascular Events [AURORA] trial) failed to show benefits.27, 28 However, in the Study of Heart and Renal Protection (SHARP),29 simvastatin with ezetimibe resulted in a significant 17% (95% confidence interval 6 to 26%) reduction in the risk of major adverse cardiac events when compared with placebo in patients with advanced CKD on or not on dialysis. While the effect of simvastatin + ezetimibe was significant in the non-dialysis CKD stratum and not significant in the dialysis stratum, there was no significant interaction observed between the two strata, prompting clinical practice guidelines to recommend lipid lowering therapy in advanced CKD, specifically for the prevention of atherosclerotic events.23, 24 However, neither SHARP nor the 4-D or AURORA trials enrolled a patient population similar to the ISCHEMIA-CKD population. Thus, for participants with advanced, non-dialysis-requiring CKD in ISCHEMIA-CKD, high-intensity statin therapy is recommended. If high-intensity statin therapy cannot be tolerated, moderate-intensity statin therapy is recommended. For subjects on dialysis, moderate- or high-intensity statin treatment is recommended as tolerated.
Optimizing Medical Therapy Goal Attainment
To improve medication adherence, the trial is able to provide certain medications at no cost to participants in some countries. Monthly reports on attainment of risk factor goals are provided to sites by the Clinical Coordinating Center. Sites where the attainment of OMT goals is below average are re-educated and site-specific corrective action plans are developed in conjunction with the study team. In addition, medication adherence is tracked using the Morisky-Green-Levine medication adherence survey. 30
Management of Angina
Medical management of angina in the conservative strategy is intensified according to the ISCHEMIA-CKD angina treatment algorithm (eFigure 1). The goal for participants randomized to the conservative strategy arm is to control angina such that participants report a satisfactory angina-related quality of life. If the level of angina is unacceptable to the participant despite maximally tolerated medical therapy, cardiac catheterization and revascularization (PCI or CABG if suitable) is recommended, consistent with standard of care.
Participants randomized to the invasive strategy who experience angina following revascularization may be treated medically, as per the ISCHEMIA-CKD angina treatment algorithm (eFigure 1). The goal for participants randomized to the invasive strategy is to control angina such that participants report a satisfactory angina-related quality of life. Unlike the approach to the participants randomized to the conservative strategy, participants randomized to the invasive strategy could undergo repeat cardiac catheterization and revascularization (PCI or CABG if suitable) without first maximizing medical therapy.
Invasive Strategy
Participants randomized to the invasive strategy underwent cardiac catheterization within 30 days of randomization, if feasible, with optimal revascularization (PCI or CABG) therapy based upon coronary anatomy and other clinical considerations soon thereafter as appropriate. In addition, all invasive participants receive OMT as outlined above.
Optimal Revascularization Therapy
Optimal revascularization therapy was performed based on findings from the diagnostic catheterization and relevant clinical information. The selection of PCI vs. CABG (or medical therapy in cases of non-obstructive CAD, diffuse small vessel disease, etc.) was left to the discretion of the treating team per local standards and expertise. The general principle of the trial was to select a revascularization modality that had the highest likelihood to safely and effectively relieve ischemia (using non-invasive imaging, invasive coronary angiography, or fractional flow reserve (FFR)/instantaneous wave-free ratio (iFR®)) in all viable myocardial territories of at least moderate size while minimizing the risk of acute kidney injury (AKI). Guidelines from professional societies and appropriateness criteria were recommended to be incorporated into the decision process. Use of a study Heart-Kidney Team (general cardiologist, nephrologist, interventional cardiologist, and cardiac surgeon) was recommended (Figure 3) to discuss complex cases after the diagnostic angiogram was performed to reach a consensus as to the best revascularization technique and approach.
Optimizing PCI
Patients with advanced non-dialysis-requiring CKD are at increased risk of AKI following PCI. However, there is controversy in the medical literature regarding the incidence (<1% to >30%), effective treatment (saline hydration, N-acetyl cysteine, or sodium bicarbonate) and prognosis of AKI (<0.5% to >5% requiring dialysis).31–34 The strategies to reduce the risk of AKI in participants enrolled in the ISCHEMIA-CKD trial are outlined in eTable 1. For participants randomized to the invasive strategy and not on dialysis, a customized hydration protocol based on the Prevention of Contrast Renal Injury with Different Hydration Strategies (POSEIDON) trial35 and contrast volume threshold (for hard stop) was provided to the site based on the participant’s eGFR and body weight, along with protocols for ultra-low volume36 and zero contrast PCI techniques.37 In addition, other recommendations to optimize PCI in this high-risk group included emphasis on complete functional revascularization (based on results of non-invasive stress test, FFR/iFR® or angiography), use of newer generation drug eluting stents (preferably the everolimus-eluting stents [Xience] and slow-release zotarolimus-eluting stents [Endeavor Resolute], which were provided free of charge for trial participants randomized to the invasive strategy), techniques to reduce the risk of bleeding (use of transradial angiography, renal dosing of antithrombotic medications), and liberal use of FFR/iFR® for intermediate lesions (FFR wires from St. Jude Medical and Phillips were provided free of charge for trial participants randomized to the invasive strategy). The use of bare metal stents, other DES, or bioresorbable scaffolds was discouraged.
Optimizing CABG
Patients with advanced non-dialysis-requiring CKD are at increased risk of AKI following CABG. Strategies to reduce the risk of AKI in those undergoing CABG are outlined in eTable 2. Other recommendations to optimize CABG included accurate assessment and evaluation of potential CABG participants; complete revascularization when feasible; optimal intraoperative management, including protection from myocardial, renal, and associated organ and system injury ; maximize opportunity for long-term graft patency by using multiple arterial grafts and especially a left internal mammary artery graft; and provision of optimal secondary prevention of atherosclerotic cardiovascular events following CABG.
Visit Schedule and Follow-up
Randomized participants are followed-up at 1.5, 3, 6, and 12 months following randomization during the first year and every 6 months thereafter until planned follow-up completion in June 2019 (eTable 3 for schedule of visits). During follow-up visits, participants are assessed for potential endpoint events, medication adherence (using the Morisky-Green-Levine medication adherence survey), lifestyle adherence (using Patient-Centered Assessment and Counseling for Exercise (PACE)), and health-related quality of life (QoL) (using brief symptoms/QoL assessment described below). At every follow-up visit the research team, in collaboration with the treating physician(s), evaluates the effectiveness of medical therapy and optimizes therapy as needed, according to guideline recommendations and study algorithms. All participants are followed regardless of whether they adhere to the randomized treatment strategy.
Study Endpoints
The primary endpoint is a composite of death or nonfatal MI. The major secondary endpoints are a composite of death, nonfatal MI, hospitalization for unstable angina, hospitalization for heart failure, or resuscitated cardiac arrest; angina control using the brief Seattle Angina Questionnaire (SAQ) Angina Frequency Scale and the QOL Scale. Safety outcomes such as initiation of maintenance dialysis and a composite of initiation of maintenance dialysis or death will be reported. Other endpoints are listed in Table 4 and outlined in the study protocol and/or the Statistical Analysis Plan.
Table 4.
Study Endpoints*
| Primary Endpoint |
Composite of death or nonfatal myocardial infarction. |
|---|---|
| Major Secondary Endpoints | 1. Composite of death, nonfatal myocardial infarction, resuscitated cardiac arrest, or hospitalization for unstable angina or heart failure |
| 2. Angina per SAQ Angina Frequency Scale | |
| 3. Disease-specific quality of life per SAQ Quality of Life Scale | |
| Other Secondary Endpoints | 1. Composite of death, nonfatal myocardial infarction, hospitalization for unstable angina, hospitalization for heart failure, resuscitated cardiac arrest, or stroke |
| 2. Composite of death, nonfatal myocardial infarction, or stroke | |
| 3. Composite endpoints incorporating cardiovascular death | |
| 4. Composite endpoints incorporating other definitions of MI as defined in the clinical event charter | |
| 5. Individual components of the primary and major secondary endpoints | |
| 6. Stroke | |
| 7. Health resource utilization, costs, and cost-effectiveness | |
| Safety Outcomes | 1. Composite of initiation of maintenance dialysis or death |
| 2. Initiation of maintenance dialysis | |
| 3. Other procedural complications | |
SAQ= Seattle Angina Questionnaire.
As outlined in the Study Protocol and/or the Statistical Analysis Plan
An independent clinical events adjudication committee (CEC) reviews and adjudicates all site-reported primary endpoint events and selected secondary endpoints in a blinded fashion based on study definitions (eTable 3). The following endpoints are adjudicated: death (including cause), MI, resuscitated cardiac arrest, hospitalization for unstable angina, hospitalization for heart failure, and stroke/transient ischemic attack. Because the trial is an open strategy trial, bias in the ascertainment of events is mitigated in several ways. These include carefully constructed data collection instruments (such as use of triggers/queries and algorithms to capture potential missed events [for example, cardiac biomarker elevation; hospitalization for other reasons, including chest pain, dyspnea, or pneumonia; and New York Heart Association class IV or Canadian Cardiovascular Society class IV on study visits]), screening of core lab data, site investigator and coordinator awareness regarding standard CEC processes and requests, and manual triggering of events found during review of source documents. Extensive cross variable checks, random and for-cause document reviews, and queries have been underway from trial inception to ensure complete ascertainment and site reporting of any potential endpoint event. Later in the trial, examination of site variation in anticipated vs. observed event reporting was initiated, with additional monitoring for site with low numbers of events. No concerns have been identified to date based on these efforts.
Statistical Considerations
All major treatment comparisons between the randomized groups will be performed according to the principle of “intention-to-treat;” that is, participants will be analyzed (and endpoints attributed) according to the randomized strategy assignment, irrespective of treatments actually received. Statistical comparisons will be performed using two-sided significance tests.
The statistical comparison of the two randomized groups with respect to the primary composite endpoint will be a “time-to-event” analysis, and will therefore be based on the time from randomization to the first occurrence of either death or nonfatal MI. To account for heterogeneity among trial participants, the overall comparison will be adjusted for a pre-specified set of prognostically important baseline covariates including age, sex, kidney function (dialysis status; eGFR among participants not on dialysis), ejection fraction, and the presence of diabetes. The level of significance for the assessment of the primary endpoint will be α=0.05. In addition to Cox regression analyses, event-free survival probabilities not assuming proportional hazards will be estimated as a function of follow-up time in each treatment group using the Kaplan-Meier method and presented with pointwise 95% confidence intervals. If the data provide evidence of an overall difference in outcome between management strategy groups, we will further examine whether the therapeutic effect is similar for all categories of participants, or whether it varies according to specific participant characteristics, as pre-specified in the Statistical Analysis Plan.
ISCHEMIA-CKD was originally designed to randomize approximately 1,000 participants. The sample size was estimated to provide 80–95% power to detect a 15% to 19% relative reduction in the primary composite endpoint assuming the 4-year cumulative rate of the primary composite endpoint is 50–70% in participants randomized to the conservative strategy. The projected event rate for the primary composite endpoint in the conservative strategy participants was based on multiple observational studies although none enrolled precisely the cohort under consideration. Slower than expected recruitment led study leadership to request approval from NHLBI to reduce the target sample size to 500–700 participants. This change was justified on the basis of an updated literature review supporting a range of possible treatment effects consistent with adequate power under the reduced sample size. This request was approved by NHLBI in late 2015. In an attempt to mitigate the loss of statistical power, follow-up was extended by 6 months. The revised target was ultimately exceeded due to higher than expected recruitment in 2016–2017. The final sample size of 777 participants is estimated to provide 80% power to detect a 22% to 24% relative reduction in the 4-year rate of the primary endpoint under assumptions consistent with the accumulating trial data.
Participants enrolled in the ISCHEMIA-CKD trial are a high-risk cohort. There is precedent from prior cardiovascular trials in high-risk cohorts to assume a relatively large effect size. Prior NHLBI-funded trials such as the Surgical Treatment for Ischemic Heart Failure (STICH) trial (heart failure with reduced ejection fraction) was powered for a 25% reduction, and the Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) trial 38 of patients with diabetes and multivessel coronary disease was powered for a 27% reduction in risk. Similarly, more recent trials in patients with ST-segment elevation myocardial infarction such as the Complete Versus Lesion-Only Primary PCI Trial (CvLPRIT) trial,39 Preventive Angioplasty in Acute Myocardial Infarction (PRAMI)40 and the DANAMI-3-PRIMULTI41 were powered for a 30% reduction in risk. The only randomized trial to date in the advanced CKD population, reported by Manske et al. in 1992,42 observed a 57% reduction in risk of cardiac death, MI or unstable angina with revascularization (PCI or CABG) when compared with medical therapy (aspirin and short acting nifedipine); however, the trial was prematurely terminated after only 26 participants were enrolled. Similarly, in a meta-analysis of 38 nonrandomized studies with 85,731 patients with CKD, a 27% lower mortality was observed with coronary revascularization when compared with no revascularization.15
Analysis of Quality of Life Outcomes
Patients’ QOL is assessed using the disease-specific brief Seattle Angina Questionnaire (SAQ)43 and the Rose Dyspnea Scale. The SAQ has been extensively validated in patients with SIHD.44, 45 The primary analyses will be conducted using continuous health status scores, which will also be categorized to facilitate clinical interpretation (e.g. any angina vs. none). Given that participants are expected to experience a nonlinear improvement in QOL following randomization, with rapid gains during the first 6 months, followed by continued gradual improvement through the end of follow-up, we will use piecewise linear growth curves with knots at 3 and 6 months to quantify changes through 3 months, between 3 and 6 months, and between 6 months and the end of follow-up. Quality of life analyses will therefore fit these curves utilizing hierarchical linear (for continuous outcomes) or generalized linear (for dichotomous and ordinal outcomes) models and will include participant-specific random effects for the growth-curve parameters, corresponding fixed-effect parameters representing the trajectory for an “average” participant, fixed effects for treatment and treatment-by-trajectory interaction terms, and adjustment for baseline QOL as a covariate. The key outcome of interest to be compared between treatment groups is the average QOL over the duration of follow-up, calculated as the area under the mean model-estimated growth curve for each treatment group.
Data and Safety Monitoring Committee
A Data and Safety Monitoring Board (DSMB) was appointed by the NHLBI to monitor participant safety and to independently review performance of the trial. The ISCHEMIA-CKD DSMB membership includes all individuals on the ISCHEMIA trial DSMB which was expanded to include a nephrologist. The DSMB reviews interim analyses of primary and secondary endpoints, additional safety events, and other information as requested by the Board.
Interim Monitoring
Interim treatment group comparisons are planned for the primary endpoint at least 3 times during the study and will be monitored with the use of two-sided symmetric O’Brien-Fleming type boundaries.46, 47 An α-spending function will be used to control the overall type-I error probability for the primary endpoint at 5%.
Study Organization
The Clinical Coordinating Center (CCC) is at the New York University School of Medicine and the Statistical and Data Coordinating Center (SDCC) at Duke Clinical Research Institute (eTable 5). The 777 randomized participants are from 118 sites in 30 countries. Country coordination is performed by cardiology and nephrology country leaders (eTable 6). As in the ISCHEMIA trial, each enrolling site was encouraged to have a lead non-invasive cardiologist principal investigator (PI) (typically the corresponding PI), a lead interventional cardiologist and a lead cardiovascular surgeon. In addition, each site was encouraged to have a lead nephrologist participate in the management of CKD issues in the participants and network with nephrology groups to help patient recruitment and management. The list of participating sites and personnel is provided in the supplementary appendix (eTable 6).
Discussion
Patients with SIHD and advanced CKD are underrepresented in contemporary clinical trials comparing optimal coronary revascularization and medical therapy versus optimal medical therapy alone, providing very limited data on management of these challenging patients. We considered including patients with advanced CKD in the ISCHEMIA trial, i.e., having no eGFR exclusion criterion. The COURAGE trial was designed without a creatinine cutoff for trial entry, yet only 16 patients with advanced CKD were enrolled in COURAGE, emphasizing the need for a dedicated trial to focus attention on this high-risk cohort and for successful enrollment. In addition, individuals with advanced CKD might have a high early risk-benefit ratio. It is unknown if these short-term increased risks could be offset by long-term clinical benefits. We were fortunate to secure a separate award from NHLBI to focus efforts on this understudied, high-risk population.
ISCHEMIA-CKD and ISCHEMIA: Similarities and Differences
The ISCHEMIA-CKD trial was designed using the ISCHEMIA trial infrastructure. This resulted in cost efficiencies because we utilized the same platforms as the ISCHEMIA trial (CCC, SDCC, InForm and IVRS, same sites and organization). For these reasons, the cost of conducting the CKD trial was substantially lower than for a true stand-alone trial. ISCHEMIA-CKD differs from ISCHEMIA in several ways: 1) The ISCHEMIA-CKD primary endpoint is a 2-component composite (death or MI) vs. the 5-component ISCHEMIA primary endpoint (cardiovascular death, MI, hospitalization for unstable angina, hospitalization for heart failure, or resuscitated cardiac arrest). The pre-specified change in the ISCHEMIA trial primary endpoint from a 2-component composite primary endpoint (cardiovascular death or MI) to a 5-component composite endpoint is described elsewhere.48, 49 An updated power analysis using aggregate event rate data in January 2018 indicated that the ISCHEMIA-CKD trial will have sufficient power for a range of possible treatment effects for the 2-component composite, and the incremental gain in power with the 5-component composite was marginal. A decision was therefore made to continue with the 2-component primary composite for ISCHEMIA-CKD. 2) Ischemia eligibility was determined by sites without core laboratory confirmation of ischemia severity (there are no imaging core labs for ISCHEMIA-CKD trial due to cost considerations and at the request of the NHLBI). 3) There was no requirement of coronary CT angiography prior to randomization to rule out left main disease or the absence of obstructive disease due to higher risk of AKI in those with non-dialysis advanced CKD. In subjects on dialysis, the use of coronary CT angiography was left to the discretion of the site but not mandated given that heavy calcification may preclude accurate assessment of coronary stenosis. Exclusion of potential left main disease was left to site investigators based on clinical judgment using the stress test and hemodynamic findings. 4) Randomized treatment strategies were optimized to reduce the risk of renal injury. 5) Although the majority of ISCHEMIA trial sites were invited for participation in ISCHEMIA-CKD, additional sites not participating in ISCHEMIA were chosen based on their potential to enroll patients with advanced CKD.
In summary, ISCHEMIA-CKD will provide much needed evidence as to whether an initial invasive management strategy improves long-term clinical outcomes when added to optimal medical therapy in patients with SIHD, moderate or severe ischemia on stress testing, and advanced CKD.
Supplementary Material
Acknowledgments
Funding Source:
The study was funded by the NHLBI (U01HL117904, U01HL117905).
Footnotes
Disclosures:
Dr. Sripal Bangalore: Ad hoc consultant/speaker: Abbott Vascular, Pfizer, Daiichi Sankyo, Merck, Medicines Company, Astra Zeneca. Research Grant: Abbott Vascular, NHLBI. Travel Grant: Boston Scientific, Medtronic
Dr. John Spertus: Ad hoc consultant: United Healthcare, Novartis, Bayer, Janssen, AstraZeneca Research Grant: Abbott Vascular; Intellectual Property: Copyright to Seattle Angina Questionnaire; Equity: Health Outcomes Sciences
Dr. Glenn M. Chertow: Member, Board of Directors: Satellite Healthcare, Ad hoc consultant: Akebia, AMAG, Amgen Ardelyx, Astra Zeneca, Durect, Gilead, Keryx, Outset, Physiowave, Puracath, Reata
Dr. Daniel Mark: Research grant support from Eli Lilly, Merck, Oxygen Biotherapeutics, and AstraZeneca.
The other authors have no relevant disclosures.
Disclaimer: The content of this manuscript is solely the responsibility of the authors and does not necessarily reflect the views of the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the United States Department of Health and Human Services.
References
- 1.Shlipak MG, Heidenreich PA, Noguchi H, Chertow GM, Browner WS, McClellan MB. Association of renal insufficiency with treatment and outcomes after myocardial infarction in elderly patients. Ann Intern Med 2002;137(7):555–62. [DOI] [PubMed] [Google Scholar]
- 2.Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, et al. Chronic kidney disease and mortality risk: a systematic review. Journal of the American Society of Nephrology : JASN 2006;17(7):2034–47. [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351(13):1296–305. [DOI] [PubMed] [Google Scholar]
- 4.Hachamovitch R, Berman DS, Kiat H, Cohen I, Cabico JA, Friedman J, et al. Exercise myocardial perfusion SPECT in patients without known coronary artery disease: incremental prognostic value and use in risk stratification. Circulation 1996;93(5):905–14. [DOI] [PubMed] [Google Scholar]
- 5.US Renal Data System. USRDS 2002 Annual Data Report: Atlas of End-Stage Renal Disease in the United States 2002. Bethesda, MD: National Institute of Health, National Institutes of Diabetes and Digestive and Kidney Diseases. [Google Scholar]
- 6.Foley RN, Murray AM, Li S, Herzog CA, McBean AM, Eggers PW, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 2005;16(2):489–95. [DOI] [PubMed] [Google Scholar]
- 7.Charytan D, Kuntz RE. The exclusion of patients with chronic kidney disease from clinical trials in coronary artery disease. Kidney international 2006;70(11):2021–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zannad F, Rossignol P. Cardiovascular Outcome Trials in Patients With Advanced Kidney Disease: Time for Action. Circulation 2017;135(19):1769–1771. [DOI] [PubMed] [Google Scholar]
- 9.Group BDS, Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. The New England journal of medicine 2009;360(24):2503–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sedlis SP, Jurkovitz CT, Hartigan PM, Goldfarb DS, Lorin JD, Dada M, et al. Optimal medical therapy with or without percutaneous coronary intervention for patients with stable coronary artery disease and chronic kidney disease. The American journal of cardiology 2009;104(12):1647–53. [DOI] [PubMed] [Google Scholar]
- 11.De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012;367(11):991–1001. [DOI] [PubMed] [Google Scholar]
- 12.McCullough PA. Contrast-induced acute kidney injury. Journal of the American College of Cardiology 2008;51(15):1419–28. [DOI] [PubMed] [Google Scholar]
- 13.Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. Journal of the American College of Cardiology 2004;44(7):1393–9. [DOI] [PubMed] [Google Scholar]
- 14.Tsai TT, Patel UD, Chang TI, Kennedy KF, Masoudi FA, Matheny ME, et al. Validated contemporary risk model of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the National Cardiovascular Data Registry Cath-PCI Registry. Journal of the American Heart Association 2014;3(6):e001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volodarskiy A, Kumar S, Amin S, Bangalore S. Optimal Treatment Strategies in Patients with Chronic Kidney Disease and Coronary Artery Disease. The American journal of medicine 2016;129(12):1288–1298. [DOI] [PubMed] [Google Scholar]
- 16.Reddan DN, Szczech LA, Tuttle RH, Shaw LK, Jones RH, Schwab SJ, et al. Chronic kidney disease, mortality, and treatment strategies among patients with clinically significant coronary artery disease. J Am Soc Nephrol 2003;14(9):2373–80. [DOI] [PubMed] [Google Scholar]
- 17.Szummer K, Lundman P, Jacobson SH, Schon S, Lindback J, Stenestrand U, et al. Influence of renal function on the effects of early revascularization in non-ST-elevation myocardial infarction: data from the Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART). Circulation 2009;120(10):851–8. [DOI] [PubMed] [Google Scholar]
- 18.Keeley EC, Kadakia R, Soman S, Borzak S, McCullough PA. Analysis of long-term survival after revascularization in patients with chronic kidney disease presenting with acute coronary syndromes. The American journal of cardiology 2003;92(5):509–14. [DOI] [PubMed] [Google Scholar]
- 19.Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R, et al. European guidelines on cardiovascular disease prevention in clinical practice: executive summary. Eur Heart J 2007;28(19):2375–414. [DOI] [PubMed] [Google Scholar]
- 20.Smith SC Jr., Allen J, Blair SN, Bonow RO, Brass LM, Fonarow GC, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute. Circulation 2006;113(19):2363–72. [DOI] [PubMed] [Google Scholar]
- 21.Gibbons RJ, Abrams J, Chatterjee K, Daley J, Deedwania PC, Douglas JS, et al. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina--summary article: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on the Management of Patients With Chronic Stable Angina). Journal of the American College of Cardiology 2003;41(1):159–68. [DOI] [PubMed] [Google Scholar]
- 22.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106(25):3143–421. [PubMed] [Google Scholar]
- 23.National Kidney Foundation. KDOQI Clinical Practice Guidelines for Cardiovascular Disease in Dialysis Patients. American journal of kidney diseases : the official journal of the National Kidney Foundation 2005;45(suppl 3):S1–S154. [PubMed] [Google Scholar]
- 24.Holt S, Goldsmith D. Renal Association Clinical Practice Guideline on cardiovascular disease in CKD. Nephron Clinical practice 2011;118 Suppl 1:c125–44. [DOI] [PubMed] [Google Scholar]
- 25.Stone NJ, Robinson J, Lichtenstein AH, Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2013. [Google Scholar]
- 26.Whelton PK, Carey RM, Aronow WS, Casey DE, Jr., Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology 2018;71(19):e127–e248. [DOI] [PubMed] [Google Scholar]
- 27.Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 2005;353(3):238–48. [DOI] [PubMed] [Google Scholar]
- 28.Fellstrom BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. The New England journal of medicine 2009;360(14):1395–407. [DOI] [PubMed] [Google Scholar]
- 29.Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011;377(9784):2181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care 1986;24(1):67–74. [DOI] [PubMed] [Google Scholar]
- 31.Josephson SA, Dillon WP, Smith WS. Incidence of contrast nephropathy from cerebral CT angiography and CT perfusion imaging. Neurology 2005;64(10):1805–6. [DOI] [PubMed] [Google Scholar]
- 32.Chertow GM, Normand SL, McNeil BJ. “Renalism”: inappropriately low rates of coronary angiography in elderly individuals with renal insufficiency. Journal of the American Society of Nephrology : JASN 2004;15(9):2462–8. [DOI] [PubMed] [Google Scholar]
- 33.James MT, Ghali WA, Knudtson ML, Ravani P, Tonelli M, Faris P, et al. Associations between acute kidney injury and cardiovascular and renal outcomes after coronary angiography. Circulation 2011;123(4):409–16. [DOI] [PubMed] [Google Scholar]
- 34.Rudnick MR, Goldfarb S, Wexler L, Ludbrook PA, Murphy MJ, Halpern EF, et al. Nephrotoxicity of ionic and nonionic contrast media in 1196 patients: a randomized trial. The Iohexol Cooperative Study. Kidney international 1995;47(1):254–61. [DOI] [PubMed] [Google Scholar]
- 35.Brar SS, Aharonian V, Mansukhani P, Moore N, Shen AY, Jorgensen M, et al. Haemodynamic-guided fluid administration for the prevention of contrast-induced acute kidney injury: the POSEIDON randomised controlled trial. Lancet 2014;383(9931):1814–23. [DOI] [PubMed] [Google Scholar]
- 36.Nayak KR, Mehta HS, Price MJ, Russo RJ, Stinis CT, Moses JW, et al. A novel technique for ultra-low contrast administration during angiography or intervention. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions 2010;75(7):1076–83. [DOI] [PubMed] [Google Scholar]
- 37.Ali ZA, Karimi Galougahi K, Nazif T, Maehara A, Hardy MA, Cohen DJ, et al. Imaging- and physiology-guided percutaneous coronary intervention without contrast administration in advanced renal failure: a feasibility, safety, and outcome study. Eur Heart J 2016;37(40):3090–3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, et al. Strategies for multivessel revascularization in patients with diabetes. The New England journal of medicine 2012;367(25):2375–84. [DOI] [PubMed] [Google Scholar]
- 39.Gershlick AH, Khan JN, Kelly DJ, Greenwood JP, Sasikaran T, Curzen N, et al. Randomized Trial of Complete Versus Lesion-Only Revascularization in Patients Undergoing Primary Percutaneous Coronary Intervention for STEMI and Multivessel DiseaseThe CvLPRIT Trial. Journal of the American College of Cardiology 2015;65(10):963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wald DS, Morris JK, Wald NJ, Chase AJ, Edwards RJ, Hughes LO, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med 2013;369(12):1115–23. [DOI] [PubMed] [Google Scholar]
- 41.Lonborg J, Engstrom T, Kelbaek H, Helqvist S, Klovgaard L, Holmvang L, et al. Fractional Flow Reserve-Guided Complete Revascularization Improves the Prognosis in Patients With ST-Segment-Elevation Myocardial Infarction and Severe Nonculprit Disease: A DANAMI 3-PRIMULTI Substudy (Primary PCI in Patients With ST-Elevation Myocardial Infarction and Multivessel Disease: Treatment of Culprit Lesion Only or Complete Revascularization). Circ Cardiovasc Interv 2017;10(4). [DOI] [PubMed] [Google Scholar]
- 42.Manske CL, Wang Y, Rector T, Wilson RF, White CW. Coronary revascularisation in insulin-dependent diabetic patients with chronic renal failure. Lancet 1992;340(8826):998–1002. [DOI] [PubMed] [Google Scholar]
- 43.Chan PS, Jones PG, Arnold SA, Spertus JA. Development and validation of a short version of the Seattle angina questionnaire. Circulation Cardiovascular quality and outcomes 2014;7(5):640–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arnold SV, Kosiborod M, Li Y, Jones PG, Yue P, Belardinelli L, et al. Comparison of the Seattle Angina Questionnaire With Daily Angina Diary in the TERISA Clinical Trial. Circ Cardiovasc Qual Outcomes 2014;7(6):844–50. [DOI] [PubMed] [Google Scholar]
- 45.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Fihn SD. Monitoring the quality of life in patients with coronary artery disease. The American journal of cardiology 1994;74(12):1240–4. [DOI] [PubMed] [Google Scholar]
- 46.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics 1979;35(3):549–56. [PubMed] [Google Scholar]
- 47.Lan K DD. Discrete sequential boundaries for clinical trials. Biometrika 1983;70:659–663. [Google Scholar]
- 48.Group ITR, Maron DJ, Hochman JS, O’Brien SM, Reynolds HR, Boden WE, et al. International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial: Rationale and design. Am Heart J 2018;201:124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bangalore S, Maron DJ, Reynolds HR, Stone GW, O’Brien SM, Alexander KP, et al. ISCHEMIA: Establishing the Primary End Point. Circulation Cardiovascular quality and outcomes 2018;11(5):e004791. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.