Abstract
Background:
The relationship between diuretic use or change in diuretic use and outcomes in chronic heart failure (HF) remains poorly defined. We evaluated the association between diuretic use and changes in health status, exercise capacity, and clinical events in a large randomized trial of subjects with HF.
Methods:
HF-ACTION randomized 2331 outpatients with HF and ejection fraction ≤ 35% to aerobic exercise training versus usual care. We grouped patients according to loop diuretic use from baseline through 6 months: continued-use, never-use, initiated, discontinued. The association between diuretic use and changes in health status, exercise capacity, and clinical outcomes (all-cause mortality/hospitalization, CV mortality and HF hospitalization) through 12 months were assessed using Cox proportional hazards models and generalized linear regression models, respectively.
Results:
A total of 2004 (86%) patients had complete data on diuretic use. There was no association between diuretic status and Kansas City Cardiomyopathy Questionnaire (KCCQ), 6-minute walk distance or peak VO2 in adjusted analyses (all P>0.05). A dose increase was associated with decrease in 6-minute walk distance (−4.25m, SE 1.12m, P<0.001) and change in KCCQ overall score (−0.56m, SE 0.24m, P=0.02). There were no between-group differences for all cause death or hospitalization comparing continuous use versus never-use (adjusted HR 0.91; 95%CI 0.72–1.15; P= 0.432).
Conclusions:
The initiation or discontinuation of diuretics over a 6-month time frame was not associated with a difference in mortality, hospitalizations, exercise or health status outcomes but a dose increase in HF patients was associated with worse exercise and health status outcomes.
INTRODUCTION
In order to manage volume overload and congestion, the use of loop diuretics is a mainstay of heart failure (HF) therapy. Despite widespread use, diuretic use has not been consistently shown to improve major clinical outcomes in large analyses (1). To the contrary, multiple studies have demonstrated potential harm associated with loop diuretic use (2–4) yet the relationship is likely confounded by the indication. Prior studies have focused principally on acute hospitalization for HF or the immediate post-hospitalization period. Analyses of chronic HF outpatients have focused primarily on patients with advanced HF in which high diuretic doses has been associated with poor clinical outcomes. This is believed to be secondary to acquired diuretic resistance in the later stages of HF (3). Furthermore, there have been no analyses evaluating the association of loop diuretic use with health related quality of life (HRQoL) and exercise capacity in chronic HF patients.
Our analysis utilized the HF-ACTION randomized clinical trial dataset of chronic HF with reduced ejection fraction (HFrEF) patients randomized to exercise training or standard of care to assess the relationship of diuretic use, initiation, discontinuation and dose escalation on clinical outcomes, HRQoL and exercise function.
METHODS
Overview
The design (5) and primary results (6,7) of the HF-ACTION study have been previously reported. HF-ACTION was a multicenter, randomized, placebo-controlled trial designed to evaluate the long-term efficacy and safety of a structured aerobic exercise intervention in medically stable outpatients with chronic HF and a reduced EF (6,7). A total of 2331 patients were enrolled from 82 centers in the North America and Europe between April 2003 and February 2007. Enrollment criteria included an EF ≤ 35%, New York Heart Association (NYHA) functional class II-IV symptoms and optimal medical therapy for at least 6 weeks duration, as well as the ability and willingness to exercise. Eligible participants were randomized 1:1 to aerobic exercise training versus usual care, with continued optimal background medical therapy. Supervised training involved aerobic exercise (walking, treadmill, or cycle ergometer) 3 times weekly for 36 sessions, followed by transition to a home-based exercise program for an additional 2 years. The exercise goal was 90 min per week for the first 3 months, followed by 120 min per week thereafter. Follow-up occurred over a median of 2.6 years.
Diuretic Status and Outcomes
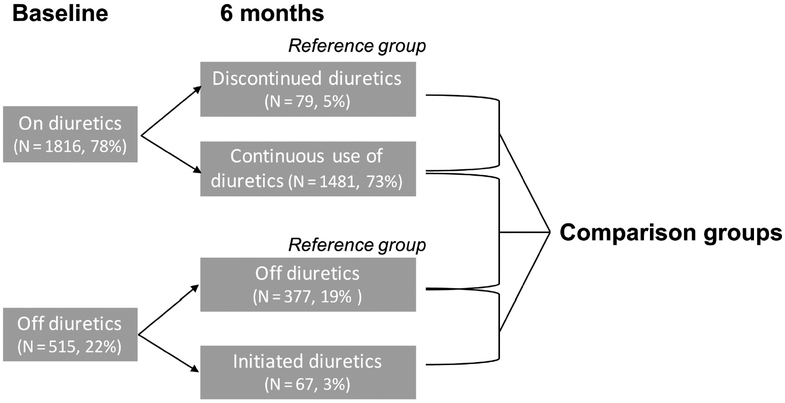
Patients were divided in 4 categories according to loop diuretic use at baseline and over the first 6 months of the trial: continuous-use, never-use, newly initiated and newly discontinued (Figure 1). Patient characteristics, medical history, health status, and physiological parameters at rest and during exercise testing were collected on standardized forms at baseline and repeated at 3 months, 6 months, 12 months, and 24 months. Data collection included current loop diuretic use, type of loop diuretic, and total daily dose. Diuretic use was assessed at baseline and 6 months. Loop diuretics were converted to furosemide equivalents by the following algorithm: 20 mg of torsemide to 40 mg of furosemide and 1 mg of bumetanide to 40 mg of furosemide.
Figure 1:
Comparison groups for statistical analysis.
Our endpoints were HRQoL, which was measured using the disease-specific Kansas City Cardiomyopathy Questionnaire (KCCQ) (8) and the general EuroQol-5 Dimensions (EQ-5D) (9) survey. The KCCQ (8) is a 23-item, self-administered disease-specific questionnaire that quantifies HRQoL in ambulatory HF patients. The KCCQ provides an overall summary score but also comprises seven domains (physical limitation, symptom stability, symptom burden, symptom frequency, self-efficacy, quality of life and social limitation). The KCCQ is scored from 0 to 100 with higher scores representing better HRQoL. Further we measured 6-minute walk test distance and peak VO2.
Additional endpoints included a composite of all-cause mortality or all-cause hospitalization and composite of CV mortality or HF hospitalization. Although blinding was not possible due to the nature of the exercise intervention, deaths and CV hospitalizations for each patient were adjudicated by an independent clinical events committee. Once a patient had a HF hospitalization that was confirmed by the clinical events committee, no future hospitalizations were adjudicated for that patient.
Statistical Analysis
All continuous data were reported as mean and standard deviation (SD) or median and (25th, 75th) percentiles, and categorical data as frequencies and percentages. Patients with known loop diuretic status at baseline and 6 months post-randomization were included in the analysis. Baseline clinical characteristics including demographics, medical history, laboratory values, medication use, HRQoL, and exercise parameters were compared based on baseline loop diuretic status (continuous-use vs newly discontinued, never-use vs newly initiated). Comparisons for continuous variables were based on the Wilcoxon rank-sum test, while categorical variables were assessed using x2 test or Fisher’s exact test, as appropriate. Cox proportional hazards regression models were used to evaluate the relationship between change in diuretic use and clinical endpoints (hospitalization and mortality). Diuretic use was defined as continuous use of diuretics at 0 and 6 months. Patients were only included in the analyses if they were alive and event-free at the time of their 6-month visit. The exercise capacity and HRQoL outcomes were assessed using generalized linear models, assuming a t-distribution and identity link. The exercise capacity outcomes of 6-minute walk test and cardiopulmonary exercise testing were analyzed using the change from 3 months to 12 months; the health status outcomes were analyzed using the change from 6 months to 12 months (time points we selected based on the availability of testing closest to the analyses landmark of 6 months). Models were adjusted for covariates (baseline characteristics) previously identified as being associated with clinical outcomes (10). For analyses using a smaller sample size, a limited set of adjustment variables (age, treatment arm, sex, BMI, BUN, LVEF, NYHA class, and loop diuretic dose) was selected given the established strong relationship with clinical outcomes. All modeling assumptions were assessed and none were significantly violated.
A second analysis was preformed to evaluate the association between diuretic dose changes with the change in exercise and HRQoL outcomes from baseline to 12 months, as well as the association between diuretic dose change and clinical outcomes. We correlated the change in diuretic dose with the change in outcomes described above from baseline to 12 months. Only patients who were on diuretics at baseline and/or 6 months were included in the analysis. The model calculated the dose change on a continuous scale (for 1 unit increase or decrease) and for the purposes of presentation the unit increase was multiplied by 20 to show an increase/decrease by 20mg of diuretic. A 20mg diuretic dose increase could mean an initiation of a diuretic or a diuretic dose increase in a patient who was already on diuretics at baseline. The association between dose change and changes in exercise capacity/HRQoL outcomes was analyzed using a generalized linear model, assuming a t-distribution and identity link. Clinical outcomes were assessed using Cox proportional hazards models. Modeling assumptions were again assessed and dose change was non-linearly related to the clinical outcomes and the change in peak VO2. Thus, dose-change was transformed using piece-wise linear splines with a single interior knot at the inflection point of 0mg.
P-values ≤0.05 from two-sided tests were considered statistically significant. Adjustments were not made for multiple comparisons due to the hypothesis generating nature of this secondary manuscript. All analyses were performed using SAS 9.4 (SAS Institute Inc. Cary, North Carolina, USA).
Funding and Manuscript Preparation
The HF-ACTION trial was funded by the National Heart, Lung, and Blood Institute (ClinicalTrials.gov Number: NCT00047437). Database management and statistical analysis was performed by the Duke Clinical Research Institute. The authors take full responsibility for the manuscript’s integrity and had complete control and authority over its preparation and the decision to publish.
RESULTS
Study Population
Of the 2331 patients enrolled in the HF-ACTION trial, the majority (78%) of patients were on loop diuretics at baseline. Six months after enrollment, 2004 patients (86% of initial trial population) had complete data on diuretic use. Baseline characteristics by inclusion and exclusion can be found in Supplemental Table 1. During the first 6 months after enrollment, 1481 (73%) remained continuously on diuretics and 377 (19%) remained off diuretics. Table 1 compares the baseline characteristics between patients on and off diuretics at 6 month follow up. Despite similar age and sex distribution, patients on loop diuretics tended to have a greater BMI, more diabetes and a greater proportion of NYHA class III/IV versus class II symptoms. All patients reported >90% use of beta-blockers and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers at baseline with a comparable use of both drugs amongst patients on and off diuretics.
Table 1:
Baseline Characteristics by Loop Diuretic Status
| General Characteristics | Continuous Use (N=1481) |
Never Use (N=377) |
P-value |
|---|---|---|---|
| Age, years | 59.8 (51.8–68.2) | 60.3 (52.0–69.6) | 0.320 |
| Female sex | 428/1481 (28.9%) | 99/377 (26.3%) | 0.310 |
| Race | <.001 | ||
| Black or African American | 496/1456 (34.1%) | 79/374 (21.1%) | |
| White | 874/1456 (60.0%) | 282/374 (75.4%) | |
| Diabetes | 523/1481 (35.3%) | 72/377 (19.1%) | <.001 |
| Previous MI | 631/1481 (42.6%) | 166/377 (44.0%) | 0.618 |
| Hypertension | 894/1470 (60.8%) | 207/375 (55.2%) | 0.048 |
| Blood Urea Nitrogen, mg/dL | 26.3 (29.6) | 19.7 (9.3) | <.001 |
| Creatinine | 1.2 (1.0–1.5) | 1.1 (0.9–1.3) | <.001 |
| NYHA Class | <.001 | ||
| II | 887/1481 (59.9%) | 290/377 (76.9%) | |
| III/IV | 594/1481 (40.1%) | 87/377 (23.1%) | |
| Angina Class | 0.086 | ||
| No Angina | 1223/1479 (82.7%) | 327/377 (86.7%) | |
| I | 134/1479 (9.1%) | 31/377 (8.2%) | |
| II–IV | 122/1479 (8.2%) | 19/377 (5.0%) | |
| Left Ventricular Ejection Fraction, % | 24.1 (19.9–29.8) | 26.8 (22.5–32.4) | <.001 |
| BMI, kg/m2 | 30.4 (26.2–35.5) | 28.2 (25.0–32.0) | <.001 |
| Severe Mitral Regurgitation | 175/1362 (12.8%) | 25/345 (7.2%) | 0.004 |
| Beta Blocker | 1401/1481 (94.6%) | 356/377 (94.4%) | 0.897 |
| Beta Blocker dose (mg/day Carvedilol equivalent) | 25.0 (13.0–50.0) | 38.0 (13.0–50.0) | 0.566 |
| Loop Diuretic dose (median in mg/day Furosemide equivalent) at 6 month | 40.0 (40.0–80.0) | 0.0 (0.0–0.0) | |
| Mean (SE) | 68.6 (60.1) | ||
| ACEI/ARB Use | 1410 (95.2%) | 361 (95.8%) | 0.652 |
| Peak VO2, mL/kg/min | 14.0 (11.2–17.2) | 16.4 (13.4–20.2) | <.001 |
| 6 Minute Walk Distance, meters | 366 (295–427) | 400 (338–459) | <.001 |
| Kansas City Cardiomyopathy Questionnaire, Overall Score | 66.4 (49.7–81.9) | 76.0 (60.4–88.5) | <.001 |
Diuretic initiation (N= 67, 3.3%) and discontinuation (N= 79, 5.1%) were infrequent, whereas an adjustment in diuretic dose was observed in about 32% of cases. The median (IQR) diuretic dose change between baseline and 6 months were −40mg (−80, −20) for patients who discontinued diuretics, +40mg (20, 40) for patients who initiated diuretics. The median (IQR) diuretic dose change was 0mg (0, 0) for those on continuous diuretic use from baseline to 6 months. Baseline characteristics of those not on diuretics at baseline, grouped by 6-month diuretic status (newly initiated vs. never-use), are presented in Supplement Table 2A. Compared to patients never on diuretics, patients who newly initiated diuretics had a lower peak VO2 (16.4mL/kg/min vs 14.6mL/kg/min; P= 0.005) and lower 6-minute walk distance (400m vs 377m; P= 0.033). Baseline characteristics of those on diuretics at baseline, by 6-month status (continuous-use vs newly discontinued), are presented in Supplement Table 2B. No significant difference in baseline characteristics were noted.
Diuretic Use, Health Status and Exercise Status
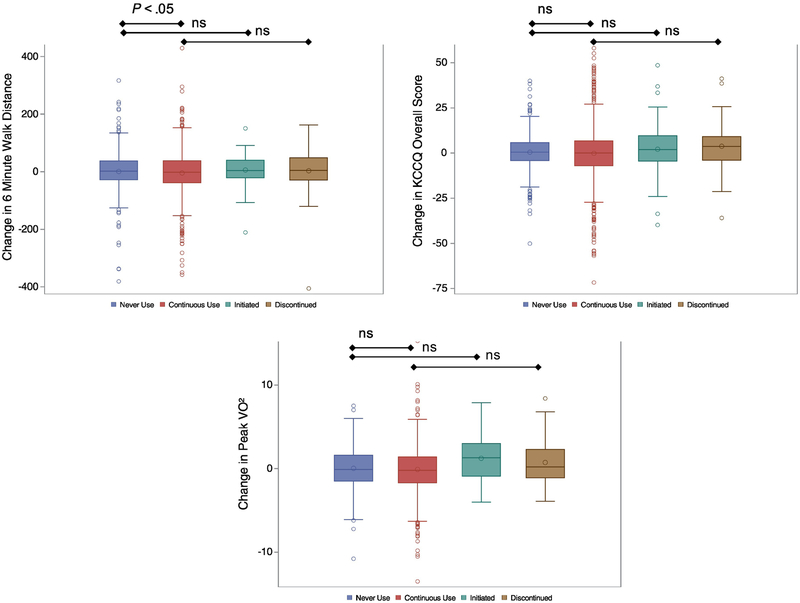
Between 3 and 12 months, patients in the “continuous diuretic use” group on average walked (unadjusted −10.30m, SE 4.34m, P=0.018) less during the 6-minute walk test than those who “never used diuretics” (Figure 2). The significant correlation persisted when the models were adjusted with the limited covariate list (−10.94m, SE 5.22m, P=0.036), but it did not hold when the full set of covariates was used (−8.05m, SE 5.15m, P=0.119) (Table 2). There were no significant differences in the peak VO2, KCCQ overall score, KCCQ symptom burden score, or KCCQ symptom frequency score between groups (Figure 2/Table 2). There were no significant differences in 6-minute walk distance, peak VO2, KCCQ overall score or either of the KCCQ domains between “initiation/never on” or “discontinuation/continuous use” over the 6-months period in the adjusted analysis (Table 2).
Figure 2:
Unadjusted changes in exercise (change in 6-min walk and peak VO2 from 3 to 12 months) and HRQoL (change in overall KCCQ from 6 to 12 months) in all four diuretic groups. Abbreviations: ns=non-significant
Table 2.
Association between diuretic loop use from baseline to 6 moths and exercise/HRQoL (* marks reference groups).
| Continuous Use vs Never Use* | ||||||
| Outcome |
Adjusted[1] (Partial Model) Mean Difference (SE) |
P-value |
Adjusted[2–6] (Full Model) Mean Difference (SE) |
P-value | ||
| Change in 6 Minute Walk Distance (m) from 3 to 12 months | −10.94 (5.22) | 0.036 | −8.05 (5.15) | 0.119 | ||
| Change in Peak VO2 (mL/kg/min) from 3 to 12 months | −0.35 (0.20) | 0.084 | −0.25 (0.20) | 0.224 | ||
| Change in KCCQ Overall Score from 6 to 12 months | 0.64 (0.89) | 0.470 | 0.51 (0.89) | 0.565 | ||
| Change in KCCQ Symptom Frequency Score from 6 to 12 months | 0.36 (1.06) | 0.730 | 0.37 (1.06) | 0.724 | ||
| Change in KCCQ Symptom Burden Score from 6 to 12 months | 1.62 (0.99) | 0.101 | 1.59 (0.99) | 0.111 | ||
| Discontinuation vs Continuous Use* | ||||||
|
Adjusted[1] Partial Model) Mean Difference (SE) |
||||||
| Change in 6 Minute Walk Distance (m) from 3 to 12 months | 10.84 (8.94) | 0.226 | ||||
| Change in Peak VO2 (mL/kg/min) from 3 to 12 months | 0.61 (0.36) | 0.092 | ||||
| Change in KCCQ Overall Score from 6 to 12 months | 1.41 (1.58) | 0.373 | ||||
| Change in KCCQ Symptom Frequency Score from 6 to 12 months | 1.17 (1.92) | 0.543 | ||||
| Continuous Use vs Never Use* | ||||||
| Outcome |
Adjusted[1] (Partial Model) Mean Difference (SE) |
P-value |
Adjusted[2–6] (Full Model) Mean Difference (SE) |
P-value | ||
| Change in KCCQ Symptom Burden Score from 6 to 12 months | −0.21 (1.76) | 0.905 | ||||
| Initiation vs Never Use* | ||||||
|
Adjusted[1] (Partial Model) Mean Difference (SE) |
P-value | |||||
| Change in 6 Minute Walk Distance (m) from 3 to 12 months | 3.78 (12.11) | 0.755 | ||||
| Change in Peak VO2 (mL/kg/min) from 3 to 12 months | 0.58 (0.46) | 0.215 | ||||
| Change in KCCQ Overall Score from 6 to 12 months | 0.66 (1.97) | 0.736 | ||||
| Change in KCCQ Symptom Frequency Score from 6 to 12 months | −0.58 (2.47) | 0.815 | ||||
| Change in KCCQ Symptom Burden Score from 6 to 12 months | 0.27 (2.46) | 0.913 | ||||
In a secondary analysis, we assessed the association between the change in diuretic dose with the change in exercise and HRQoL outcomes from baseline to 12 months, as well as the association between diuretic dose change and clinical outcomes. Loop diuretic dose change was linear in relation to exercise and HRQoL outcomes (Table 3). Unadjusted, loop diuretic dose change (for an 20mg dose increase) was significantly associated with a reduction in the 6-minute walk distance (−3.61m; SE 1.03m, P<0.001) and KCCQ overall score (−0.52m; SE 0.22mg, P=0.019). Following risk adjustment, dose increase continued to be significantly associated with change in 6-minute walk distance (−4.25m, SE 1.12m, P<0.001) and change in KCCQ overall score (−0.56m, SE 0.24m, P=0.02).
Table 3.
The association between loop diuretic dose change from baseline to 6 months and exercise and HRQoL outcomes at 12 months.
| Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|
| Outcome | Increase in Furosemide Dose Equivalent | Mean Difference (SE) | P-value | Mean Difference (SE) | P-value |
| 6 Minute Walk Distance (m)[1] | 20 mg | −3.61 (1.03) | 0.0005 | −4.25 (1.12) | 0.0002 |
| KCCQ Overall Score[2] | 20 mg | −0.52 (0.22) | 0.0189 | −0.56 (0.24) | 0.0193 |
| KCCQ Symptom Frequency Score[3] | 20 mg | −0.82 (0.26) | 0.0019 | −0.87 (0.28) | 0.0017 |
| KCCQ Symptom Burden Score[4] | 20 mg | −0.63 (0.26) | 0.0176 | −0.79 (0.26) | 0.0029 |
| Peak VO2 (mL/kg/min)[5] | 20 mg | 0.07 (0.08) | 0.3877 | 0.06 (0.09) | 0.5078 |
| Peak VO2 (mL/kg/min)[5] | −20 mg | −0.06 (0.06) | 0.3126 | −0.10 (0.06) | 0.1212 |
Adjusted for baseline 6-minute walk distance, number of HF hospitalizations in the previous 6 months, resting heart rate, L VEF, KCCQ clinical summary score, BUN, peak RER, smoking status, CPX duration, peak VO2
Adjusted for baseline KCCQ summary score, age, peripheral artery disease, biventricular pacemaker, atrial fibrillation/flutter, Beck depression score, BUN, BMI, Canadian Cardiovascular Society angina class, peak VO2
Adjusted for baseline KCCQ symptom frequency score, age, peripheral artery disease, biventricular pacemaker, atrial fibrillation/flutter, Beck depression score, BUN, BMI, Canadian Cardiovascular Society angina class, peak VO2
Adjusted for baseline KCCQ symptom burden score, age, peripheral artery disease, biventricular pacemaker, atrial fibrillation/flutter, Beck depression score, BUN, BMI, Canadian Cardiovascular Society angina class, peak VO2
Adjusted for baseline VO2 Consumption, age, sex, number of HF hospitalizations in the previous 6 months, ischemic etiology, insulin use, pacemaker, LVEF, BUN, BMI, peak RER, race, CPX duration
Piece-wise linear splines were created for dose change, with a single inflection point at 0mg. This results in 2 hazard ratios, one that quantifies relative risk associated with a decrease in dose at 6 months, and the second quantifies relative risk associated with an increase in dose at 6 months. The spline was only used for the Peak VO2 outcome.
Finally, we observed no modification of the treatment effect (exercise training) on outcomes (mortality, hospitalization, exercise and HRQoL) by loop diuretic group (continuous use, never use, initiation and discontinuation), i.e. there is no interaction (P>0.05) between treatment and loop diuretic group.
Diuretic Use and Clinical Outcomes
Results of the unadjusted analyses for clinical outcomes were similar to those seen for health and exercise status. A total of 232 patients (20.6%) receiving diuretics at baseline and 73 patients (19.8%) not prescribed diuretics at baseline were hospitalized or died by 12 months. In unadjusted analyses, patients who were “continuously on” diuretics for 6 months were more likely to experience the outcomes of all-cause death or hospitalizations (HR 1.30, 95% CI 1.09 – 1.54, P=0.004) and CV death or HF hospitalizations (HR 2.36, 95% CI 1.76 – 3.16, P<0.001) as compared with patients who were “never on” diuretics over a 6-month period (Table 4). When using a partial model for the adjusted analysis of the CV death or HF hospitalization outcome, those subjects continuously on diuretics for 6 months maintained a significant difference versus those who were “never on” diuretics (HR 1.63, 95% CI 1.17 – 2.26, P=0.004). However, no significance was found using the full model (HR 1.25, 95% CI 0.87 – 1.79, P=0.224). Similarly, all-cause death or hospitalization was no longer significant after adjustment.
Table 4.
Associa tion between diure tic use at 6 months and clinical outcomes. * Reference group
| Outcome | Raw Event Rate # Events/Total | Raw Event Rate # Events/Total |
Unadjusted Hazard Ratio (95% CI) |
P-value |
Adjusted[1] (Partial Model) Hazard Ratio (95% CI) |
P-value |
Adjusted[2][3] (For Full Model) Hazard Ratio (95% CI) |
P-value |
| Continuous Use | Never on Diuretics* | |||||||
| All Cause Death or Hosp | 633/1073 (58.99%) | 158/325 (48.62%) | 1.30 (1.09 – 1.54) | 0.004 | 1.01 (0.82 – 1.24) | 0.9462 | 0.91 (0.72 – 1.15) | 0.4324 |
| CV Death or HF Hosp | 395/1364 (28.96%) | 51/374 (13.64%) | 2.36 (1.76 – 3.16) | <.0001 | 1.63 (1.17 – 2.26) | 0.004 | 1.25 (0.87 – 1.79) | 0.2240 |
| Initiated Diuretic Use | Never on Diuretics* | |||||||
| All Cause Death or Hosp | 21/43 (48.8%) | 158/325 (48.6%) | 1.03 (0.65 – 1.62) | 0.898 | 0.85 (0.49 – 1.46) | 0.5540 | ||
| CV Death or HF Hosp | 12/57 (21.1%) | 51/374 (13.6%) | 1.56 (0.83 – 2.93) | 0.166 | 1.42 (0.69 – 2.93) | 0.3352 | ||
| Discontinued Use | Continuous Use* | |||||||
| All Cause Death or Hosp | 31/52 (59.62%) | 633/1073 (58.99%) | 1.01 (0.70 – 1.44) | 0.978 | 1.06 (0.73 – 1.55) | 0.7538 | ||
| CV Death or HF Hosp | 17/69 (24.64%) | 395/1364 (28.96%) | 0.81 (0.50 – 1.32) | 0.407 | 0.91 (0.55 – 1.50) | 0.7058 | ||
All models adjusted for age, treatment arm, sex, BMI, BUN, LVEF, NYHA class and baseline loop diuretic dose.
CV Death or HF Hosp adjusted for treatment arm, LVEF, MR grade, ventricular conduction on CPX test, KCCQ symptom stability score, BUN, race, sex, age, weber class and VE/VO2
All Cause Death or Hosp adjusted for treatment arm, Weber class, KCCQ symptom stability score, BUN, country. LVEF, sex, beta blocker dosage, MR grade, ventricular conduction on CPX test
Unadjusted, there were no significant differences in risk of the all-cause death or hospitalization (HR 1.03; 95% CI 0.65 – 1.62, P=0.898) and CV death or HF hospitalization (HR 1.56; 95% CI 0.83 – 2.93, P=0.166) between those who initiated diuretics between baseline and 6 months and those who were never on them (Table 4). The relationship between diuretic initiation and patients who remained off diuretics with the primary outcome remained unchanged after adjustment. Furthermore, there were also no significant differences in risk of unadjusted or adjusted outcomes between those who discontinued diuretics and those who were continuously on them (Table 4).
We found no association between diuretic dose increase (20mg furosemide equivalents) and all-cause mortality or hospitalization in an adjusted analysis (HR 1.06, 95% CI 0.98 – 1.14, P=0.179) and CV mortality and HF hospitalization (HR 1.06, 95% CI 0.98 – 1.14, P= 0.156).
DISCUSSION
In our analysis of a chronic HFrEF population from the HF-ACTION trial, we found that patients with chronic HF with continuous use of diuretics compared to patients off diuretics were at comparable risk for all-cause death, cardiovascular death or HF related hospitalizations. Further, we found no difference in exercise or HRQoL parameters. Finally, the initiation or discontinuation of diuretics over 6-months was not associated with a difference in mortality, hospitalizations, exercise or HRQoL outcomes but a dose increase in patients on diuretics was associated with worse exercise and HRQoL outcomes.
Diuretics are widely used in HF as the primary treatment and effectively reduce congestion, which is a key marker of decompensated HF and closely linked to a poor prognosis. DeVore et. al. have shown that the initiation of diuretic therapy during a hospitalization for acute HF led to improved 30 day outcomes in ASCEND-HF, and discontinuation of diuretics led to poor outcomes (1). Current guidelines emphasize that diuretics are a treatment for the clinical signs and symptoms of congestion, yet there is no evidence of a favorable effect on disease progression. In fact, in chronic HF, prescription of diuretics remains, to a large extent, subjective and evidence-free (11,12). On the contrary, the majority of evidence to date shows a negative relationship between use and dose of loop diuretics and prognosis in patients with chronic HF. In previous studies the use of loop diuretics and larger doses of diuretics was associated with higher all-cause mortality rates in carefully adjusted/propensity matched analyses (2–4,13). Domanski et al. found that, using data from the SOLVD trial, the use of loop diuretics was associated with increased adjusted all-cause mortality (HR 1.28, 95% CI 1.19–1.49), while the use of potassium-sparing diuretics was not (14). Our analysis could not confirm these findings, although for CV mortality and HF we did see a comparable trend towards higher mortality and rehospitalization that was no longer present after full model adjustment.
The proposed mechanisms for adverse effects of diuretics in patients with chronic HF include increase in neurohormones and renal impairment. It is well established that loop diuretics activate the renin-angiotensin system in HF as a response to diuretic treatment rather than as a result of the disease process itself (15). Neurohormonal activation is likely the result of renal sodium loss, intravascular hypovolemia and/or renal hypoperfusion with subsequent drop in blood pressure and baroreceptor activation (16,17). The use of loop diuretics was also associated with a slightly greater rate of decline in glomerular filtration rate, independent of diuretic dose (18). Importantly, a number of studies found that patients with chronic HF are commonly euvolemic or even hypovolemic (19,20), suggesting that there may be serious detrimental effects of indiscriminate chronic diuretic use. However, it is important to acknowledge that the use of loop diuretics in cohort studies and non-randomized use in clinical trials is strongly confounded by the severity of HF as it was also seen in our study cohort. For example sicker patients are more likely to be given loop diuretics (and in higher doses) than less sick patients, thus patients treated with diuretics will be at higher risk of death as a result of more severe HF.
To our knowledge, this is the first analysis to examine the association between diuretic use (never use, continuous, initiation or discontinuation) and exercise capacity (6-minute walk test and peak VO2) and health status in a large randomized chronic HF trial. In a small single center prospective randomized study of 28 patients, Gupta et al. showed that 3 months’ diuretic use did not result in significant changes in peak VO2, mean N-terminal pro-hormone brain natriuretic peptide (NT-proBNP) levels, or measures of HRQoL when compared with placebo (16). Our findings support the cited prospective randomized study suggesting that HRQoL and exercise status appear to be largely unaffected by diuretic use.
Nevertheless, amongst patients already on diuretics or newly on diuretics, enrolled in the HF-ACTION trial, a dose increase in loop diuretics was associated with worse exercise and HRQoL outcomes. This relationship was linear, i.e. a reduction in diuretic dose was associated with an improvement in exercise and HRQoL outcomes. The discrepancies in outcomes between the first analysis (patients who initiated diuretics had no significant change in exercise and HRQoL) vs. second analysis (dose increase was associated with worse exercise and HRQoL) are two-fold. First, in the cohort of patients who “initiated diuretics”, patients were not on diuretics at baseline, whereas patients included in the “dose change” analysis were either continuously (majority) on diuretics for at least 6 months or initiated in the same period. Second, dose increases between patients who initiated loop diuretics or increased diuretics differed significantly, potentially indicating higher degrees of congestion in patients who were already on diuretics. In other words, our data suggests that de novo start of diuretics at low doses does not convey an increased risk of poor clinical outcomes, whereas a dose increase in the entire population does.
Clinical implications
The present study may suggest that the routine use of continuous diuretics in chronic stable HF patients is not associated with any long-term improvement in peak VO2 or HRQoL. However, increases in diuretic dose, which could be a sign of progressive disease or diuretic resistance, were associated with worse exercise and HRQoL. The neurohormonal, hemodynamic and renal changes seen with chronic diuretic use could be related to the detrimental effects of diuretics on exercise and HRQoL.
Although post-hoc secondary analyses such as the present study are hypothesis generating, our data could suggest that the lowest achievable diuretic dose to provide effective decongestion may be favored over higher doses in chronic HF if exercise and HRQoL are taken into account. Further, it raises the question whether escalation of diuretics for mild congestive symptoms should be discouraged but perhaps the patient should be encouraged to adhere to a salt restricted diet, exercise and other proven HF directed medical therapy should be adjusted. Alternatively increases in diuretics should be accompanied by adjustment in guideline directed medical therapy to block increases in neurohormones seen with diuretic use.
Limitations
This was a post-hoc secondary analysis of a randomized controlled trial, with the analyses carried out on non-randomized treatment. It is possible that there were unmeasured confounders, most notably the severity of HF (other than LVEF and NYHA stage), which may account for the associations observed, despite careful statistical analysis adjusting for these biases. The patients included in HF-ACTION all had HFrEF, and therefore the findings of this particular analysis cannot be generalized to all patients with HF, which is particularly important given the rise of diuretic use to achieve adequate decongestion in patients with HF with preserved EF. Furthermore, we had limited information on the degree of congestion at baseline or follow up other than functional assessment, HRQoL, NYHA functional class and NT-proBNP, and the patient’s ability to exercise (inclusion criterion) and were therefore unable to fully assess the relationship between the severity of congestion and diuretic use/dose. Finally, our analysis was limited by a small number of patients in the groups who initiated or discontinued diuretics.
Conclusions
Continuous diuretic use in stable chronic HF patients is not associated with an increased risk for CV related death and HF rehospitalizations. Unless patients experience a diuretic dose escalation the harm associated with chronic diuretic use does not seem to extend to exercise capacity and health related quality of life. Our analysis supports a cautious use of diuretics and paired with the knowledge of adverse mechanisms of diuretics, diuretic use and dose escalation should be avoided when possible.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: M.F. is supported by an American Heart Association Grant, 17MCPRP33460225 and NIH T32 grant 5T32HL007101 and reports consulting fees from AxonTherapeutics, GE Healthcare. C.M.O. reports consulting fees from Novella and Amgen; ownership/partnership/principal in Biscardia, LLC; and research support from Otsuka, Roche Diagnostics, bG Medicine, Critical Diagnostics, Astellas, Gilead, GE Healthcare, and ResMed. R.J.M. receives research support from Amgen, AstraZeneca, BMS, GSK, Gilead, Novartis, Otsuka, and ResMed; and honoraria from Thoratec. All other authors declare no relevant financial disclosures.
References
- 1.DeVore AD, Hasselblad V, Mentz RJ et al. Loop diuretic dose adjustments after a hospitalization for heart failure: insights from ASCEND-HF. European journal of heart failure 2015; 17:340–6. [DOI] [PubMed] [Google Scholar]
- 2.Dini FL, Ghio S, Klersy C et al. Effects on survival of loop diuretic dosing in ambulatory patients with chronic heart failure using a propensity score analysis. Int J Clin Pract 2013;67:656–64. [DOI] [PubMed] [Google Scholar]
- 3.Eshaghian S, Horwich TB, Fonarow GC. Relation of loop diuretic dose to mortality in advanced heart failure. The American journal of cardiology 2006;97:1759–64. [DOI] [PubMed] [Google Scholar]
- 4.Abdel-Qadir HM, Tu JV, Yun L, Austin PC, Newton GE, Lee DS. Diuretic dose and long-term outcomes in elderly patients with heart failure after hospitalization. American heart journal 2010;160:264–271 e1. [DOI] [PubMed] [Google Scholar]
- 5.Whellan DJ, O’Connor CM, Lee KL et al. Heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION): design and rationale. Am Heart J 2007;153:201–11. [DOI] [PubMed] [Google Scholar]
- 6.Flynn KE, Pina IL, Whellan DJ et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA : the journal of the American Medical Association 2009;301:1451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Connor CM, Whellan DJ, Lee KL et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA : the journal of the American Medical Association 2009;301:1439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol 2000;35:1245–55. [DOI] [PubMed] [Google Scholar]
- 9.EuroQol G. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 10.O’Connor CM, Whellan DJ, Wojdyla D et al. Factors related to morbidity and mortality in patients with chronic heart failure with systolic dysfunction: the HF-ACTION predictive risk score model. Circ Heart Fail 2012;5:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevenson LW, Nohria A, Mielniczuk L. Torrent or torment from the tubules? Challenge of the cardiorenal connections. Journal of the American College of Cardiology 2005;45:2004–7. [DOI] [PubMed] [Google Scholar]
- 12.Yancy CW, Jessup M, Bozkurt B et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Journal of cardiac failure 2017. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed A, Young JB, Love TE, Levesque R, Pitt B. A propensity-matched study of the effects of chronic diuretic therapy on mortality and hospitalization in older adults with heart failure. International journal of cardiology 2008;125:246–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Domanski M, Norman J, Pitt B et al. Diuretic use, progressive heart failure, and death in patients in the Studies Of Left Ventricular Dysfunction (SOLVD). Journal of the American College of Cardiology 2003;42:705–8. [DOI] [PubMed] [Google Scholar]
- 15.Bayliss J, Norell M, Canepa-Anson R, Sutton G, Poole-Wilson P. Untreated heart failure: clinical and neuroendocrine effects of introducing diuretics. Br Heart J 1987;57:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta S, Waywell C, Gandhi N et al. The effects of adding torasemide to standard therapy on peak oxygen consumption, natriuretic peptides, and quality of life in patients with compensated left ventricular systolic dysfunction. European journal of heart failure 2010;12:746–52. [DOI] [PubMed] [Google Scholar]
- 17.von Lueder TG, Atar D, Krum H. Diuretic use in heart failure and outcomes. Clinical pharmacology and therapeutics 2013;94:490–8. [DOI] [PubMed] [Google Scholar]
- 18.Damman K, Kjekshus J, Wikstrand J et al. Loop diuretics, renal function and clinical outcome in patients with heart failure and reduced ejection fraction. European journal of heart failure 2016;18:328–36. [DOI] [PubMed] [Google Scholar]
- 19.Nijst P, Verbrugge FH, Bertrand PB et al. Plasma Volume Is Normal but Heterogeneously Distributed, and True Anemia Is Highly Prevalent in Patients With Stable Heart Failure. Journal of cardiac failure 2017;23:138–144. [DOI] [PubMed] [Google Scholar]
- 20.Bonfils PK, Damgaard M, Taskiran M, Goetze JP, Norsk P, Gadsboll N. Impact of diuretic treatment and sodium intake on plasma volume in patients with compensated systolic heart failure. European journal of heart failure 2010;12:995–1001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.