Abstract
Background:
Recent data support the implication of accelerated titanium dissolution products in peri-implantitis. It is unknown whether these dissolution products have an effect on the peri-implant microbiome, the target of existing peri-implantitis therapies.
Purpose:
This study assessed the relationship between the peri-implant microbiome, dissolved titanium levels, and peri-implantitis.
Materials and methods:
Clinical, microbiome, and titanium data were collected from a periodontal population having implants in function for 10 years. Clinical examinations were performed, and submucosal plaque samples were collected from the deepest site per implant. An aliquot of the sample was used for 16S rRNA gene sequencing, with the remainder analyzed for titanium quantity using mass spectrometry. Sequences were clustered into taxonomic units at 97% minimum sequence similarity using the QIIME pipeline approach.
Results:
Fifteen implants were assessed. According to established case definitions, six had a diagnosis of peri-implantitis; nine were healthy. The genera Streptococcus, Prevotella and Haemophilus characterized peri-implant health. Peri-implantitis was associated with a marked increase in Veillonella. Quantities of dissolved titanium were identified in 40% of sites. Titanium presence was associated with peri-implant disease status (p=0.02) and correlated to the 1st principal component of the microbiome (rho=0.552) and its alpha-diversity (rho=−0.496). Canonical correlation analyses found that titanium levels, but not health or disease status of the implant, were significantly associated with the microbiota composition (p=0.045).
Conclusion:
These findings suggest an association between titanium dissolution products and peri-implantitis and support a role for these products in modifying the peri-implant microbiome structure and diversity.
Keywords: dental implant, peri-implantitis, titanium, corrosion, bacteria, microbiome
Introduction
Biocompatibility of titanium has been well established, and implants are an efficacious tooth replacement modality. Nevertheless, peri-implantitis affects a significant number of patients with a reported prevalence range between 13% - 25%.1, 2 Required treatment may lead to increased patient morbidity, expense and possible loss of the implant.1, 3 The etiology of peri-implantitis has been traditionally considered akin to that of periodontitis based on the observation of substantial qualitative and quantitative changes in the peri-implant microbiota in disease versus health.4 However, distinct differences exist between the two disease entities both in immunological and microbiological terms. The immunological response is similar yet more rapid and extensive. 5 More recent evidence suggests that there are differences in the microbiota between periodontitis and peri-implantitis suggesting that implants create a unique microenvironement. 6 In addition, new information, has implicated titanium dissolution products in peri-implant disease progression.7
The biocompatibility of titanium is determined by a thin surface layer of titanium dioxide (TiO2), which can be weakened or disrupted leading to titanium corrosion.8 In a clinical study performing cytological analyses of peri-implant tissue samples, the concentration of titanium was higher in the peri-implantitis group compared with the group without peri-implantitis; no traces of titanium were observed in controls.9 In fact, epidemiological research on peri-implant diseases suggests titanium corrosion to be relevant for later peri-implant bone loss.10 Thus, despite the stability of TiO2, triggering factors, such as surface instrumentation with ultrasonic instruments, may exist that lead to accelerated titanium dissolution, which has been suggested as one of the causes for peri-implantitis. 7, 9, 11, 12
The mechanism by which titanium dissolution products are implicated in peri-implantitis is not completely understood. Titanium corrosion products may elicit a foreign-body-reaction causing bone resorption,8 and bone cells have been shown to be sensitive to the currents produced during corrosion events of metallic implants.13 Titanium particles may work as a secondary stimulus for the inflammatory process, demonstrated by an increase in secretion of interleukin-1β when human macrophages are exposed to titanium particles and bacterial components. 14 Importantly, recent results from our group identified a nearly 8-fold increased presence of titanium in peri-implant plaque providing cues for a pathogenic role of titanium as a modifying factor of the peri-implant microbiome.7 It is important to understand that dental implants are placed into an oral environment that is colonized by hundreds of bacterial species. Bacterial colonization has been shown to occur from tooth to newly placed implant within thirty minutes of implant placement and remains stable at the implant site for an extended period of time.15, 16 The role of titanium surface properties on bacterial adhesion is a critical reason why the biofilms formed on implants and teeth are largely dissimilar.17, 18 However, the interplay between titanium particles and established peri-implant microbiome has not been thoroughly assessed.
The aim of this study was to determine whether titanium dissolution products are involved in modifying the peri-implant microbiome, and to analyze the relationship between titanium dissolution products, peri-implant microbiome and peri-implant status.
Methods
Study population
The sample population for this study included a subset of participants of a cross-sectional study that provided peri-implant microbiome samples. The Institutional Review Board at the University of Washington approved the study protocol (No. 41380) and all patients provided written informed consent. STROBE guidelines were followed. Prior publication provides detailed recruitment and prevalence information for the cross-sectional study of 96 patients with 225 implants placed between 1998 and 2003, regarding the conditions at time of placement including: the periodontal status of the patient, brands of implants, type of restoration, patient factors, and the subsequent clinical findings at follow-up exams.1 In brief, participants who had taken antibiotics in the last three months were excluded from the microbial sampling. Implants were included if they had a diagnosis of either health or peri-implantitis. Implants diagnosed with mucositis were excluded. Further recruitment information can be found at Daubert et al.1
Definitions
For this study, peri-implant mucositis was defined as the presence of bleeding on probing with no evidence of radiographic bone loss beyond expected remodeling. Peri-implantitis was defined as the presence of bleeding on probing and/or suppuration, with >2 mm of detectable bone loss following initial remodeling, and a probing depth of 4 mm or greater.1 The presence of 2 mm of bone loss alone without mucositis symptoms did not contribute as a case of peri-implantitis. Due to non-standardized radiographs at the prosthetic insertion and follow-up examination, we considered a threshold of 2 mm from the expected marginal bone level following remodeling post-implant placement according to prevailing guidelines.19 These patients were given their implant diagnosis prior to the recent publication of the new classification system of peri-implant diseases and conditions.20 Since patients were included who had both prior radiographs and prior probing depths, it can be concluded that the diagnosis made is consistent with the newly accepted definition of peri-implantitis.
Sample collection
After isolation and supragingival plaque removal, the subgingival plaque sample was collected from the deepest probing site at each dental implant utilizing a sterile 1/2 mini Gracey curette. The curette was carefully inserted to the base of the pocket with the blade facing the gingival side, away from the implant surface, and plaque was removed in an upward motion facing the gingiva. We have previously validated this technique for the assessment of titanium concentration in peri-implant plaque.7 The plaque was transferred to a screw-cap tube with 500μl of sterile water. A 350 μl was set aside for titanium assessment and the remaining for DNA isolation. Both were frozen at −80° C for future processing.
DNA isolation
DNA isolation was done according to our previously published protocol.21 Specifically, plaque was isolated using Chelex-100™ (Bio-Rad, USA), a styrene divinylbenzene copolymer containing paired iminodiacetate ions, which act as chelating groups in binding polyvalent metal ions.22 A 150 μl aliquot of suspended plaque was placed into a tube containing 10 mg Chelex 100 followed by addition of 50 μl of 120 mM Tris HCl pH 8.0 followed by addition of 10 μl of 10 mg/mL proteinase K. Proteinase K was dissolved in 30 mM Tris HCl, pH 8.0. The mix was incubated at 55 oC for 30 min followed by vortexing and incubation in a boiling-water bath for 8 min. Upon removal from the boiling water bath, the tubes were centrifuged at 10,000–15,000×g for 3 min and the supernatant was transferred to a clean 1.5 ml microcentrifuge tube. Prokaryotic 16S rRNA genes were amplified using universal primers (27F and 1392R) using the GemTaq kit from MGQuest (USA) (Cat# EP012). The PCR program involved a pre-amplification step of 10 cycles with annealing temperature of 56oC followed by 20 amplification cycles with annealing temperature 58oC. In each cycle, elongation time was 1 min 10 s, at 72oC. PCR was finalized by extended elongation for 5 min. PCR products were purified with DNA Clean & Concentrator columns (Zymo Research, USA) and quantified using the NanoDrop (Agilent, USA).
DNA Sequencing
DNA sequencing was done according to our previous protocol.21 Each purified PCR product (1μg) was labeled with a Multiplex Identifier (MID) during the Roche Rapid Library preparation step. Samples were combined in equimolar concentrations and subjected to emPCR and DNA sequencing protocols as specified by the manufacturer’s recommendations for the Roche 454 Jr instrument.
Titanium Quantification
The aliquots of microbial samples collected from patients were transferred to the lab for inductively coupled mass spectrometry as previously reported.7 Specifically, the samples were placed into digestion vessels (50 mL polypropylene centrifuge tubes) with four 1 ml rinses of digestion solution (50:50 (V/V) concentrated nitric acid trace-metal grade (Fisher, trace-metal grade, USA: deionized (DI) water (Fisher, Barnstead Nanopure, USA) with a trace amount of hydrofluoric acid and 10 ppm terbium as recovery standard. Each sample was brought to 5 ml with the digestion solution. Open vessel microwave (Mars Xpress, CEM, Berkeley, CA) digestion was used (power 800W, 100%, ramp 15 min to 100°C, hold for 45 min). After the digestion, samples were brought to 25 ml with DI water. We quantified measurable Ti in the samples by ICP-MS with a detection limit of 0.5 ng/ml.
DNA Data Analysis
Samples were demultiplexed, quality-trimmed and filtered via BBDuk,23 sequences less than 100bp were discarded. A total of 439,465 reads with a mean 527bp reads per sample were clustered into Operational Taxonomic Units (OTUs) at 97% similarity (using pick_open_reference_otus.py command), aligned with Mothurs24 and assigned a taxonomy using the HOMD database v14.5.25 OTU tables were rarefied to a sequence depth of 15,200 reads per sample to calculate alpha diversity measures. For beta diversity, weighted and unweighted Unifrac distances were calculated and further analyzed by Principal Coordinates Analysis (PCoA).26 R packages “phyloseq”, “ggPlot2” and Ampvis were used to compare groups by diversity and composition.27–29
Statistical Analysis
Alpha diversity estimates were obtained using Shannon’s index (diversity) and the Chao1 index (richness). For beta diversity assessment species relative abundances were calculated per sample ( x / sum(x) ) and principal component and principal coordinate analysis with unifrac distances were used to visualize microbiome coordinates. The exploratory examination of associations between implant disease status, titanium, and alpha diversity metrics was performed using descriptive statistics and scatterplots. P-values for inter-group differences were derived from Generalized Estimating Equation models with Gaussian links and independence covariance matrices to account for multiple implants per person. For titanium concentrations all values were incremented by 0.001 and log-transformed. Alpha diversity scores and loadings on the first principal component were extracted to determine the pairwise correlations between microbiome diversity and composition, respectively, and titanium. Correlation analysis was performed using Spearman’s rho at alpha=0.05. Constrained ordination and permutation tests were utilized to assess the effect of titanium and disease status on microbiome beta diversity.
Results
We recruited peri-implantitis cases and healthy controls from a previous cross-sectional study to assess differences in the peri-implant microbiome and the quantity of titanium in the submucosal plaque. A total of 36 patients with 61 implants that were included in our earlier analysis returned for an additional visit for plaque sampling between July and November 2014. All patients had prior periodontal treatment and were enrolled in a regular maintenance program. After exclusion criteria were applied, DNA extraction, purification and sequencing were completed on 17 individual implant samples from 11 patients. 9 subjects with 15 implants had a diagnosis of periodontitis. Of these, 6 implants had a diagnosis of peri-implantitis and 9 implants were diagnosed as healthy. The two remaining implants were healthy implants in periodontally healthy patients and were not included in our analysis to avoid confounding in the interpretation of the effects of titanium on the peri-implant microbiome. The patient demographics and implant data are presented in Table 1.
Table 1.
Patient and implant characteristics. The first four columns show patient characteristics and the last 4 columns show implant-level characteristics including titanium quantity and DNA quantity.
| Patient Characteristics | Implant-Specific Characteristics | ||||||
|---|---|---|---|---|---|---|---|
| Patient Number | Patient Gender | Patient Age (yrs) | Periodontal Disease Status | Tooth Number | Implant Disease Status | Titanium (ug/ml) | DNA (ng/ul) |
| 1 | Male | 71 | Periodontitis | 2 | Healthy | 0 | 29.2 |
| 3 | Healthy | 0 | 17.7 | ||||
| 14 | Healthy | 0 | 37.5 | ||||
| 2 | Male | 61 | Periodontitis | 5 | Healthy | 10.8 | 42.3 |
| 12 | Healthy | 0 | 31.7 | ||||
| 7 | Periimplantitis | 31.4 | 71.5 | ||||
| 10 | Periimplantitis | 74.4 | 75.2 | ||||
| 3 | Male | 62 | Periodontitis | 3 | Periimplantitis | 0 | 65.3 |
| 4 | Female | 76 | Periodontitis | 19 | Healthy | 0 | 35.2 |
| 20 | Healthy | 0 | 66.1 | ||||
| 5 | Male | 67 | Periodontitis | 28 | Healthy | 7 | 5.3 |
| 6 | Female | 75 | Periodontitis | 19 | Periimplantitis | 0 | 49.7 |
| 7 | Male | 81 | Periodontitis | 6 | Healthy | 0 | 33 |
| 8 | Male | 68 | Periodontitis | 31 | Periimplantitis | 6 | 27.1 |
| 9 | Female | 77 | Periodontitis | 20 | Periimplantitis | 90.2 | 70.9 |
Titanium levels increase in peri-implantitis
A significant association was found between measurable Ti in the plaque surrounding a dental implant and the peri-implantitis disease status of the implant. The observed difference between groups in titanium levels was 31.7ug/ml (p=0.03).
Within sample diversity of the peri-implant microbiome is reduced with titanium presence in disease
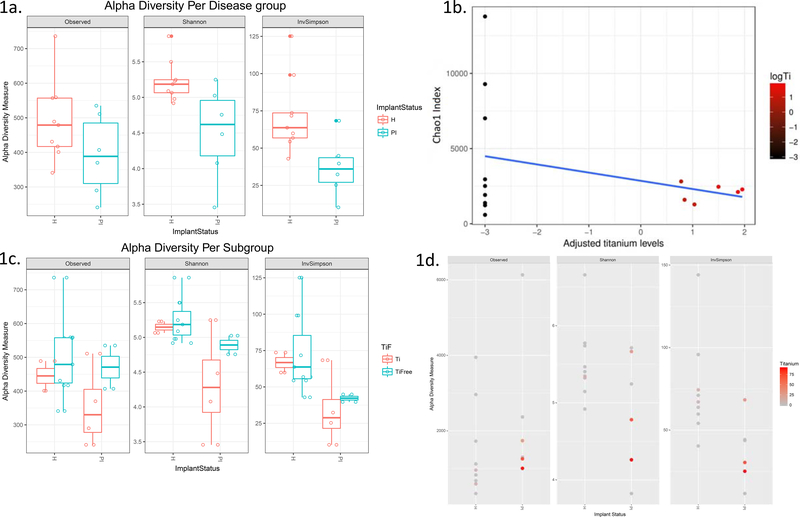
The assessment of the alpha diversity of the microbiome revealed that the peri-implant microbiome was less diverse in disease as compared to health (Fig. 1a; Shannon p=0.03, InvSimpson p=0.02). We then sought to determine whether a local environmental factor, i.e. titanium, in plaque affects within-sample diversity. We found an inverse correlation between Chao1, a species richness index, and log-transformed titanium levels in the samples (rho = −0.496). This correlation provides cues for reduced alpha diversity being linked to presence of titanium in the plaque. Next, we looked at subgroup analyses of alpha-diversity in titanium positive (Ti; Fig. 1c) versus titanium free (TiFree; Fig. 1c) samples and found a trend for less diversity in titanium positive versus titanium free samples in peri-implantitis, but not in health. This is illustrated with a color gradient for Titanium in Figure 1d.
Figure 1:
Alpha diversity of implant microbiome per disease status (1a) and titanium subgroups (1c). and adjusted titanium levels. 1c: Scatterplot of titanium levels (on log-scale) versus the Chao1 index (richness). Titanium levels are associated to decreased diversity as titanium levels increase (1d).
Titanium alters the microbial signature in the peri-implant sulci
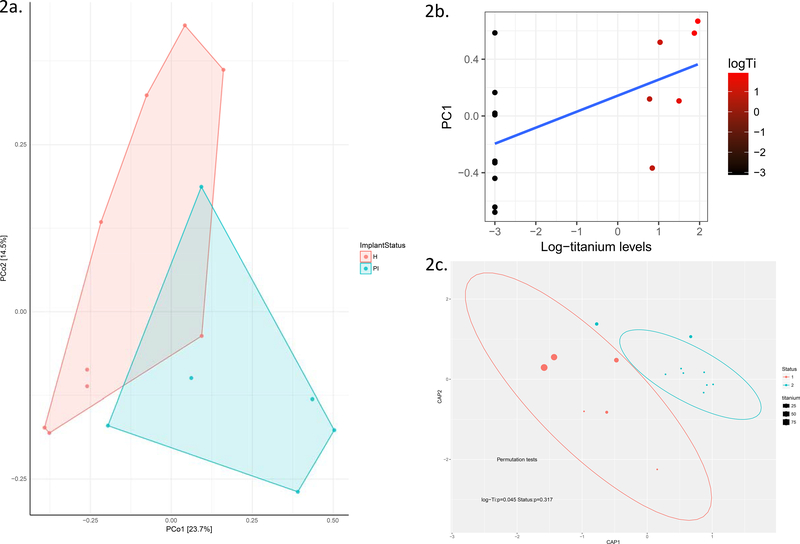
We calculated the principal coordinates of the microbiome in groups of health and disease utilizing principal coordinate analysis using a phylogenetically informed distance measure (unifraq) and found areas of overlap among healthy and diseased samples (Fig. 2a – unifraq ). In addition, we found a correlation between levels of titanium in the surrounding plaque and the first principal component of the microbial signature (rho=0.55; Fig. 2b). The first principal component accounts for the largest variability and converts the data set into a set of linearly uncorrelated variables. Next, we performed a canonical correspondence analysis (CCA), which is a constrained ordination method aimed at assessing the effect of titanium concentrations on the beta-diversity of the peri-implant microbiome. Levels of titanium were significantly associated to the microbiome ordinates (Permutation tests: p=0.045), while disease status was not (p=0.3; Fig. 2c) suggesting that Ti creates a unique niche in the oral cavity causing changes in microbiome structure.
Figure 2:
Principal coordinates of the microbiome in healthy implants (orange) and peri-implantitis (green). Note the overlap of healthy and diseased implant sites when plotted on the first two principal coordinates of the microbiome (2a). Scatterplot of titanium levels (log-transformed) versus the first principal component of the microbiome (2b). Canonical Analysis of principal coordinates showing titanium levels per site in embedded bubble plots in healthy implants (orange) and peri-implantitis (green) showing a clear separation between health and disease according to Ti presence in plaque (2c).
Titanium selectively enriches the peri-implant community for specific oral taxa
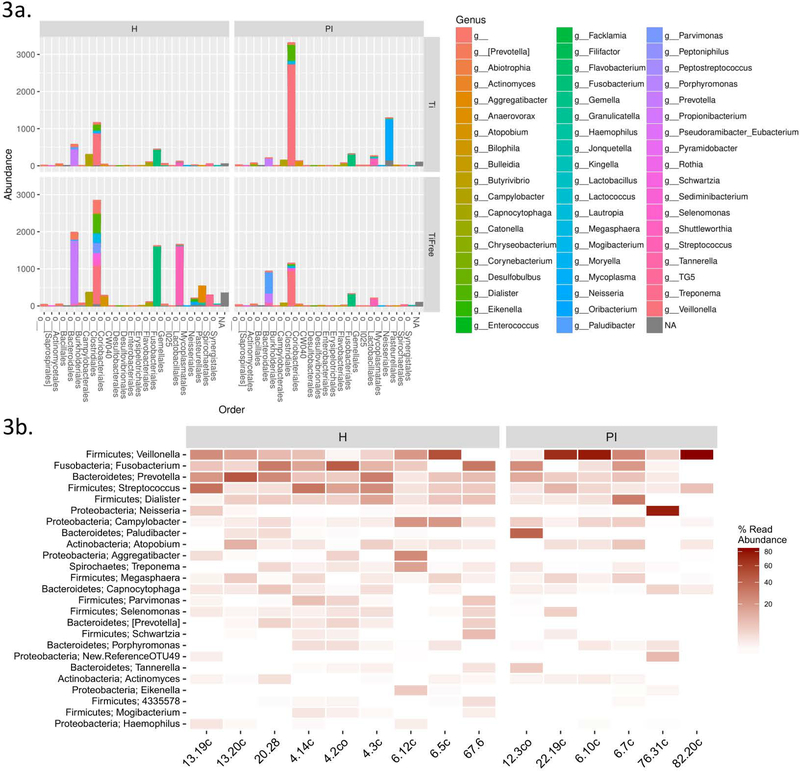
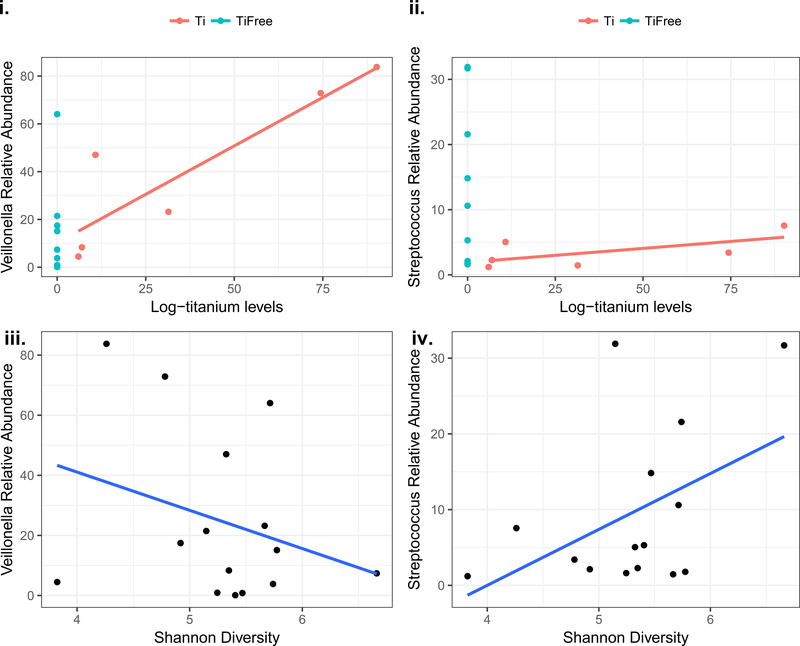
A total of 295 different taxa were identified across all samples. A genus-level taxonomic summary is shown in Figure 3a in subgroups of disease status and titanium presence. An enrichment in Veillonella and Neisseria is evident in the PI samples that include titanium (Fig. 3a). Investigation of the most abundant genera in health versus disease in Figure 3b suggests a preponderance of Veillonella in disease. Streptococcus, Prevotella and Haemophilus were more abundant in healthy samples. We then performed correlation analyses for Streptococcus (more abundant in health than disease) and Veillonella (more abundant in disease than health) to assess their associations to titanium levels, and alpha diversity. The relative abundance of Streptococcus was inversely associated to titanium levels (rho=−0.33) and was positively correlated to the microbiome diversity (rho=0.31), which is consistent with our findings of more diversity in health. On the contrary, Veillonella relative abundance was strongly correlated to titanium levels (rho=0.62) and inversely correlated to alpha diversity (rho=−0.28). The main genera associated with health (Streptococcus) and disease (Veillonella) plotted against alpha diversity and titanium levels are shown in Figure 4. These results are consistent to enrichment of Veillonella with increased titanium concentrations, and a less diverse microbiome in disease.
Figure 3:
Barplots of microbiome abundance per subgroups of titanium and disease status on a genus level (grouped by order) reveal the distinct profile of titanium vs. titanium free communities (3a). Heatmap of relative abundance of genera in groups of health and disease (3b).
Figure 4:
Scatterplots of main genera associated with health (Streptococcus) and disease (Veillonella) against alpha diversity, and log-transformed titanium levels.
Discussion
Using 16S rRNA gene sequencing and inductively coupled mass spectrometry, a significant relationship was found between the amount of titanium dissolution products and the microbial signature as well as the implant disease status. In detail, the most important clinical finding was that plaque samples from sites with peri-implantitis have higher titanium concentrations as compared to health, consistent with a previous report.7 When assessing the peri-implant microbiome in the presence of titanium our findings showed that disease was associated with a less diverse microbiota and higher titanium concentrations were correlated to this decrease in diversity. The former association has been previously established,17, 30 but the latter finding of an inverse association between titanium concentrations and microbiome diversity is novel. Lastly, titanium concentrations accounted for inter-group variability in the healthy and diseased microbiome in constrained ordination analysis. The titanium concentrations also enriched for the genera Veillonella, which may point to a role of titanium dissolution products as a modifier of the peri-implant microbiota via enrichment for selected oral taxa.
Strengths and limitations of the study
A major strength of the present study is the combination of the microbiome analysis with the quantitative measure of total titanium in plaque, which allowed us to assess the effect of titanium dissolution products as an environmental modifier of peri-implant microbiome structure and diversity. We have demonstrated that titanium dissolution products are a significant determinant of the microbiome around dental implants. This relationship between these products and peri-implant microbiome as well as peri-implantitis sheds light on the distinction between peri-implant disease and periodontitis; environmental factors unique to dental implant surfaces affect inflammatory disease processes. These findings have important ramifications in the design of implant-specific peri-implantitis treatment strategies.
Another strength of the study is the innovative approach to the study of the interplay between titanium particles and the peri-implant microbial community. A prior study from our lab21 found a significant effect of titanium electroconductivity and bacteria-mediated implant corrosion. We have also found a significant association between the amount of dissolved titanium in the peri-implant sulcus and the disease status of the implants.7 To our knowledge, no prior study thoroughly investigated the role of titanium dissolution products in shaping the peri-implant microbiome structure.
As with any cross-sectional study, the current findings provide evidence for a significant relationship, but no causality can be confirmed. The sample size is moderately small, and while other studies using 16S rRNA analysis have drawn conclusions with similar sample size, confirmatory studies in larger cohorts are necessary. As the disease severity of the included implants was moderate, it would also be beneficial to assess titanium dissolution products and microbiome on failed implants as well as implants with more severe peri-implantitis.
Interpretation of the study supported by existing evidence and possible mechanism
The presence of a titanium implant in the oral cavity creates a distinct microenvironment.
Extensive effort has gone into the characterization of the peri-implant biofilm. While recent advances in technology allows for a rich exploration of the microbial composition of both healthy versus diseased implants, and healthy versus diseased teeth, there remains a lack of consensus reporting both the complexity and the distinction of the microbiome at teeth and implant sites.17, 31–33 It is difficult to compare prior studies due to lack of consistency in case selection, disease definition, and reporting.17, 30, 34–36 A recent systematic review of the microbial profile associated with dental implants confirms that the lack of homogeneity of the reviewed studies did not allow for any direct comparison.37 The lack of a clear distinction of the biofilm associated with diseased implants suggests that unknown factors in addition to biofilm composition may account for the distinct pathology between peri-implantitis and periodontitis.33
It is difficult to assess peri-implantitis outside the context of periodontal disease, as periodontitis is recognized as a significant risk factor for peri-implantitis,1, 38- 41 and bacteria are considered to be a primary etiologic factor for both disease entities.17, 30, 31, 42- 44 To better illustrate, in a recently published 10 year retrospective study including 301 patients with 504 dental implants, 86% of these patients had periodontitis.32 Prior publications have associated gram negative bacteria with periodontal disease as well as peri-implantitis. 4, 45, 46 Recent metagenomics studies, however, have identified distinctive microbial signatures of the peri-implant and periodontal biofilms. 6, 47 Nevertheless, some of the bacteria found around implants with peri-implantitis have been similar species to those found around teeth that are diagnosed with chronic periodontitis.18, 43, 48 It is not clear if the bacteria occupy the peri-implant niche by proximity or whether they are associated with the etiology of peri-implant disease.
In addition, the oral microbiome has the potential to disrupt the biocompatible titanium dioxide surface layer.37 A specific example is Streptococcus mutans, which has been shown to increase the corrosion current49 and to induce titanium corrosion.50 We have shown that oral bacterial taxa are capable of affecting titanium electro-conductive properties and can lead to spontaneous generation of electrical potential and accelerated titanium dissolution from titanium implants.21 Further understanding of the interplay between the periodontal microbial community and titanium particles may provide insight into the prevention of peri-implant disease. Thus far indirect evidence supports that the pathogenesis of peri-implantitis may differ from periodontitis based on its faster rate of progression and defect configuration.18 The differences between periodontitis and peri-implantitis may lie in the material properties of titanium. Interestingly, it has been determined that while large titanium particles are biocompatible, fine titanium particles below 10 μm can be phagocytized by leukocytes and are cytotoxic both in vivo and in vitro.51
Next generation sequencing provides a method to examine the microbial signatures around dental implants and obtain information on the peri-implant microbiome that is not dependent on prior knowledge of bacterial species. Prior studies have examined the microbial biofilm around implants using 16s rRNA technology.17, 30, 31, 33, 34, 35, 36, 52 Each has used different parameters in patient selection and disease definition and in all cases the previous publications did not define the periodontal status of the implant patients, excluded patients with periodontitis, or used definitions of periodontitis and peri-implantitis that did not include attachment loss of 2mm or more. Most concur that the microbial community is distinct from the periodontal microbiome, Distinctions have been found in the core microbiome.53 There are also comparisons of peri-implant health and peri-implantitis microbiome. Sanz-Martin et al. compared peri-implantitis sites to healthy implants, finding distinctions between health and disease at all taxonomic levels.54
It is difficult, however, when assessing these studies to find any consensus on specific genera or species that characterize peri-implant disease. For example, one study found that health was characterized by Treponema, Prevotella, and S. mutans and disease was associated with Veillonella, Fusobacterium, and non-mutans Streptococcus,17 while others specified that Veillonella was lower in peri-implantitis compared to healthy implants.35, 52 Fürst et al. had identified Veillonella as the genera that increase in bacterial load from 30 minutes after placement to 1 week.15 Another group also found that Veillonella parvula was a dominent early subgingival colonizer.55 We found Veillonella associated with peri-implant disease. The lack of consensus provides additional evidence that there may be another factor shaping the peri-implant microbiome.
Based on our findings we present the possibility of titanium dissolution products as a modifier of the microbial signature and correlate of the disease status of the implant. In the present study, the microbiome alpha diversity of the peri-implant communities was reduced in disease versus health. These findings are corroborated by previous studies17, 30 and are of interest since they point to a reduction in the number of unique oral taxa with a small community of pathogens being associated with peri-implantitis. In fact, we identified a moderate correlation between streptococcus and alpha diversity, which is biologically plausible since streptococcus taxa are early colonizers that exploit adhesins for attachment to titanium substrates and support co-aggregation with other taxa. Notably, increased titanium levels were associate with reduced relative abundance of streptococcus as well as reduced alpha diversity measures. Therefore, the hypothesis that titanium dissolution products in the peri-implant sulcus may enrich for peri-implant pathogens warrants further investigation.
These findings highlight the importance of maintaining titanium surface biocompatibility during disease management. The fact that peri-implantitis samples differed in microbiome structure dependent upon the presence of titanium supports the hypothesis that different phenotypes of peri-implantitis exist, which may have distinct etiologies.
Acknowledgements
The authors would like to thank Sandra Bordin, Brian Leroux, Phillipe Hujoel and Peter Noble for their contributions to this project.
Funding
This project was supported by the University of Washington School of Dentistry Elam M. and Georgina E. Hack Memorial Research Fund, and National Institutes of Health NIDCR RO1DEO23810 to J.S.M.
Footnotes
Conflict of Interest
The authors have no conflict of interest related to this work.
Contributor Information
Diane Daubert, Department of Periodontics, Clinical and Periodontal Research Laboratory, University of Washington, Seattle, WA, USA.
Alexander Pozhitkov, Department of Restorative Dentistry, University of Washington, Seattle, WA, USA.
Jeff McLean, Department of Periodontics and Oral Health Sciences, Adjunct Associate Professor Department of Microbiology, University of Washington, Seattle, WA, USA.
Georgios Kotsakis, Department of Periodontics, Clinical and Periodontal Research Laboratory, University of Washington, Seattle, WA, USA.
References
- 1.Daubert DM, Weinstein BF, Bordin S, Leroux BG, Flemming TF. Prevalence and predictive factors for peri-implant disease and implant failure: a cross-sectional analysis. J Periodontol 2015; 86: 337–347. [DOI] [PubMed] [Google Scholar]
- 2.Konstantinidis IK, Kotsakis GA, Gerdes S, Walter MH. Cross-sectional study on the prevalence and risk indicators of peri-implant diseases. Eur J Oral Implantol 2015; 8: 75–88. [PubMed] [Google Scholar]
- 3.Derks J, Tomasi C. Peri-implant health and disease. A systematic review of current epidemiology. J Clin Periodontol 2015; 42 Suppl 16: S158–171. [DOI] [PubMed] [Google Scholar]
- 4.Charalampakis G, Leonhardt Å, Rabe P, Dahlén G. Clinical and microbiological characteristics of peri-implantitis cases: a retrospective multicentre study. Clin Oral Implants Res 2012; 23: 1045–1054. [DOI] [PubMed] [Google Scholar]
- 5.Belibasakis GN, Charalampakis G, Bostanci N, Stadlinger B. Peri-implant infections of oral biofilm etiology. Adv Exp Med Biol 2015; 830: 69–84. [DOI] [PubMed] [Google Scholar]
- 6.Robitaille N, Reed DN, Walters JD, Kumar PS. Periodontal and peri-implant diseases: identical or fraternal infections? Mol Oral Microbiol 2016; 31: 285–301. [DOI] [PubMed] [Google Scholar]
- 7.Safioti LM, Kotsakis GA, Pozhitkov AE, Chung WO, Daubert DM. Increased levels of dissolved titanium are associated with peri-implantitis - a cross-sectional study. J Periodontol 2017; 88: 436–442. [DOI] [PubMed] [Google Scholar]
- 8.Mouhyi J, Dohan Ehrenfest DM, Albrektsson T. The peri-implantitis: implant surfaces, microstructure, and physicochemical aspects. Clin Implant Dent Relat Res 2012; 14: 170–183. [DOI] [PubMed] [Google Scholar]
- 9.Olmedo DG, Nalli G, Verdú S, Paparella ML, Cabrini RL. Exfoliative cytology and titanium dental implants: a pilot study. J Periodontol 2013; 84: 78–83. [DOI] [PubMed] [Google Scholar]
- 10.Klinge B, Meyle J, Working G. Peri-implant tissue destruction. The Third EAO Consensus Conference 2012. Clin Oral Implants Res 2012; 23: 108–110. [DOI] [PubMed] [Google Scholar]
- 11.Olmedo DG, Paparella ML, Spielberg M, Brandizzi D, Guglielmotti MB, Cabrini RL. Oral mucosa tissue response to titanium cover screws. J Periodontol 2012; 83: 973–980. [DOI] [PubMed] [Google Scholar]
- 12.Rodrigues DC, Valderrama P, Wilson TG, Palmer K, Thomas A, Sridhar S, Adapalli A, Burbano M, Wadhwani C. Titanium Corrosion Mechanisms in the Oral Environment: A Retrieval Study. Materials 2013; 6: 5258–5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gittens RA, Olivares-Navarrete R, Tannenbaum R, Boyan BD, Schwartz Z. Electrical implications of corrosion for osseointegration of titanium implants. J Dent Res 2011; 90: 1389–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pettersson M, Kelk P, Belibasakis GN, Bylund D, Molin Thorén M, Johansson A. Titanium ions form particles that activate and execute interleukin-1β release from lipopolysaccharide-primed macrophages. J Periodontal Res 2017; 52: 21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fürst MM, Salvi GE, Lang NP, Persson GR. Bacterial colonization immediately after installation on oral titanium implants. Clin Oral Implants Res 2007; 18: 501–508. [DOI] [PubMed] [Google Scholar]
- 16.Heitz-Mayfield LJ, Lang NP. Comparative biology of chronic and aggressive periodontitis vs. peri-implantitis. Periodontol 2000 2010; 53: 167–181. [DOI] [PubMed] [Google Scholar]
- 17.Kumar PS, Mason MR, Brooker MR, O’Brien K. Pyrosequencing reveals unique microbial signatures associated with healthy and failing dental implants. J Clin Periodontol 2012; 39: 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lang NP, Berglundh T, Working Grp 4 Seventh European W. Periimplant diseases: where are we now? - Consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol 2011; 38: 178–181. [DOI] [PubMed] [Google Scholar]
- 19.Sanz M, Chapple IL, Periodontology Working Group VII European Consensus. Clinical research on peri-implant diseases: consensus report of Working Group 4. J Clin Periodontol 2012; 39 Suppl 12: 202–206. [DOI] [PubMed] [Google Scholar]
- 20.Berglundh T, Armitage G, Araujo MG, et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol 2018; 89 Suppl 1: S313–S318. [DOI] [PubMed] [Google Scholar]
- 21.Pozhitkov AE, Daubert DM, Brochwicz DA, et al. Interruption of electrical conductivity of titanium dental implants suggests a path towards elimination of corrosion. PLoS One 2015; 10: e0140393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bassetti M, Schär D, Wicki B, et al. Anti-infective therapy of peri-implantitis with adjunctive local drug delivery or photodynamic therapy: 12-month outcomes of a randomized controlled clinical trial. Clin Oral Implants Res 2014; 25: 279–287. [DOI] [PubMed] [Google Scholar]
- 23.Bushnell B BBMap (version 35.14) [Software]. Available at https://sourceforge.net/projects/bbmap/ 2015.
- 24.Schloss PD, Westcott SL, Ryabin T, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Applied and Environment Micro 2009; 75: 7537–7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dewhirst FE, Chen T, Izard J, et al. The Human oral microbiome. J Bacteriology 2010; 192: 5002–5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Applied and Environmental Microbiology 2005; 71: 8228–8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMurdie PJ, Holmes S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. Plos One 2013; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wickham H ggplot2. 2009.
- 29.Albertsen M, Karst SM, Ziegler AS, Kirkegaard RH, Nielsen PH. Back to Basics - The Influence of DNA Extraction and Primer Choice on Phylogenetic Analysis of Activated Sludge Communities. Plos One 2015; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dabdoub SM, Tsigarida AA, Kumar PS. Patient-specific analysis of periodontal and peri-implant microbiomes. J Dent Res 2013; 92: 168S–175S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koyanagi T, Sakamoto M, Takeuchi Y, Maruyama N, Ohkuma M, Izumi Y. Comprehensive microbiological findings in peri-implantitis and periodontitis. J Clin Periodontol 2013; 40: 218–226. [DOI] [PubMed] [Google Scholar]
- 32.Eick S, Ramseier CA, Rothenberger K, Bragger U, Buser D, Salvi GE. Microbiota at teeth and implants in partially edentulous patients. A 10-year retrospective study. Clin Oral Implants Res 2016; 27: 218–225. [DOI] [PubMed] [Google Scholar]
- 33.Schaumann S, Staufenbiel I, Scherer R, et al. Pyrosequencing of supra- and subgingival biofilms from inflamed peri-implant and periodontal sites. Bmc Oral Health 2014; 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koyanagi T, Sakamoto M, Takeuchi Y, Ohkuma M, Izumi Y. Analysis of microbiota associated with peri-implantitis using 16S rRNA gene clone library. J Oral Microbiol 2010; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.da Silva ESC, Feres M, Figueiredo LC, Shibli JA, Ramiro FS, Faveri M. Microbiological diversity of peri-implantitis biofilm by Sanger sequencing. Clinl Oral Implants Res 2014; 25: 1192–1199. [DOI] [PubMed] [Google Scholar]
- 36.Zheng H, Xu LX, Wang ZC, et al. Subgingival microbiome in patients with healthy and ailing dental implants. Scientific Reports 2015; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rakic M, Grusovin MG, Canullo L. The Microbiologic Proile Associated with Peri-Implantitis in Humans: A Systematic Review. Int J Oral & Maxillofac Implants 2016; 31: 359–368. [DOI] [PubMed] [Google Scholar]
- 38.Berglundh T, Lindhe J, Marinello C, Ericsson I, Liljenberg B. Soft tissue reaction to de novo plaque formation on implants and teeth. An experimental study in the dog. Clin Oral Implants Res 1992; 3: 1–8. [DOI] [PubMed] [Google Scholar]
- 39.Karoussis IK, Salvi GE, Heitz-Mayfield LJA, Bragger U, Hammerle CHF, Lang NP. Long-term implant prognosis in patients with and without a history of chronic periodontitis: a 10-year prospective cohort study of the ITI (R) Dental Implant System. Clinical Oral Implants Research 2003; 14: 329–339. [DOI] [PubMed] [Google Scholar]
- 40.Matarasso S, Rasperini G, Siciliano VI, Salvi GE, Lang NP, Aglietta M. A 10-year retrospective analysis of radiographic bone-level changes of implants supporting single-unit crowns in periodontally compromised vs. periodontally healthy patients. Clinical Oral Implants Research 2010; 21: 898–903. [DOI] [PubMed] [Google Scholar]
- 41.Ferreira SD, Silva GLM, Cortelli JR, Costa JE, Costa FO. Prevalence and risk variables for peri-implant disease in Brazilian subjects. J Clin Periodontol 2006; 33: 929–935. [DOI] [PubMed] [Google Scholar]
- 42.Mombelli A, Lang NP. The diagnosis and treatment of peri-implantitis. Perio 2000 1998; 17: 63–76. [DOI] [PubMed] [Google Scholar]
- 43.Salvi GE, Aglietta M, Eick S, Sculean A, Lang NP, Ramseier CA. Reversibility of experimental peri-implant mucositis compared with experimental gingivitis in humans. Clin Oral Implants Res 2012; 23: 182–190. [DOI] [PubMed] [Google Scholar]
- 44.Quirynen M, Vogels R, Peeters W, van Steenberghe D, Naert I, Haffajee A. Dynamics of initial subgingival colonization of ‘pristine’ peri-implant pockets. Clin Oral Implants Res 2006; 17: 25–37. [DOI] [PubMed] [Google Scholar]
- 45.Bower RC, Radny NR, Wall CD, Henry PJ. Clinical and microscopic findings in edentulous patients 3 years after incorporation of osseointegrated implant-supported bridgework. J Clin Periodontol 1989; 16: 580–587. [DOI] [PubMed] [Google Scholar]
- 46.Mombelli A, van Oosten MA, Schurch E, Land NP. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol Immunol 1987; 2: 145–151. [DOI] [PubMed] [Google Scholar]
- 47.Charalampakis G, Belibasakis GN. Microbiome of peri-implant infections: lessons from conventional, molecular and metagenomic analyses. Virulence 2015; 6: 183–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pontoriero R, Tonelli MP, Carnevale G, Mombelli A, Nyman SR, Lang NP. Experimentally induced peri-implant mucositis. A clinical study in humans. Clin Oral Implants Res 1994; 5: 254–259. [DOI] [PubMed] [Google Scholar]
- 49.Fukushima A, Mayanagi G, Nakajo K, Sasaki K, Takahashi N. Microbiologically induced corrosive properties of the titanium surface. J Dent Res 2014; 93: 525–529. [DOI] [PubMed] [Google Scholar]
- 50.Sridhar S, Wilson TG, Palmer KL, et al. In Vitro investigation of the effect of oral bacteria in the surface oxidation of dental implants. Clin Implant Dent Relat Res 2015. [DOI] [PubMed] [Google Scholar]
- 51.Kumazawa R, Watari F, Takashi N, Tanimura Y, Uo M, Totsuka Y. Effects of Ti ions and particles on neutrophil function and morphology. Biomaterials 2002; 23: 3757–3764. [DOI] [PubMed] [Google Scholar]
- 52.Tamura N, Ochi M, Miyakawa H, Nakazawa F. Analysis of bacterial flora associated with peri-implantitis using obligate anaerobic culture technique and 16S rDNA gene sequence. Int J Oral Maxillofac Implants 2013; 28: 1521–1529. [DOI] [PubMed] [Google Scholar]
- 53.Maruyama N, Maruyama F, Takeuchi Y, Aikawa C, Izumi Y, Nakagawa I. Intraindividual variation in core microbiota in peri-implantitis and periodontitis. Sci Rep 2014; 4: 6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanz-Martin I, Doolittle-Hall J, Teles RP, et al. Exploring the microbiome of healthy and diseased peri-implant sites using Illumina sequencing. J Clin Periodontol 2017; 44: 1274–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakou M, Mikx FH, Oosterwaal PJ, Kruijsen JC. Early microbial colonization of permucosal implants in edentulous patients. J Dent Res 1987; 66: 1654–1657. [DOI] [PubMed] [Google Scholar]