Abstract
Functionally important regions of sensory maps are overrepresented in the sensory pathways and cortex, but the underlying developmental mechanisms are not clear. In the spinal cord dorsal horn (DH), we recently showed that paw innervating Mrgprd+ non-peptidergic nociceptors display distinctive central arbor morphologies that well correlate with increased synapse transmission efficiency and heightened sensitivity of distal limb skin. Given that peripheral and central arbor formation of Mrgprd+ neurons co-occurs around the time of birth, we tested whether peripheral cues from different skin areas and/or postnatal reorganization mechanisms could instruct this somatotopic difference among central arbors. We found that, while terminal outgrowth/refinement occurs during early postnatal development in both the skin and the DH, postnatal refinement of central terminals precedes that of peripheral terminals. Further, we used single-cell ablation of Ret to genetically disrupt epidermal innervation of Mrgprd+ neurons and revealed that the somatotopic difference among their central arbors was unaffected by this manipulation. Finally, we saw that region-specific Mrgprd+ central terminal arbors are present from the earliest postnatal stages, before skin terminals are evident. In ummary, we find that region-specific organization of Mrgprd+ neuron central arbors is present shortly after initial central terminal formation, which likely develops independently of peripheral target innervation. Our data suggest that either cell-intrinsic and/or DH pre-patterning mechanisms are likely to establish this somatotopic difference.
Keywords: nociceptor, spinal cord dorsal horn, somatotopy, region-specific organization, RRID:MMRRC_036772-UNC, RRID:IMSR_JAX:031286, RRID:IMSR_JAX:009253, RRID:MGI:4459058, RRID:IMSR_JAX:028548, RRID:IMSR_HAR:3359, RRID:AB_10000240, RRID:AB_221569
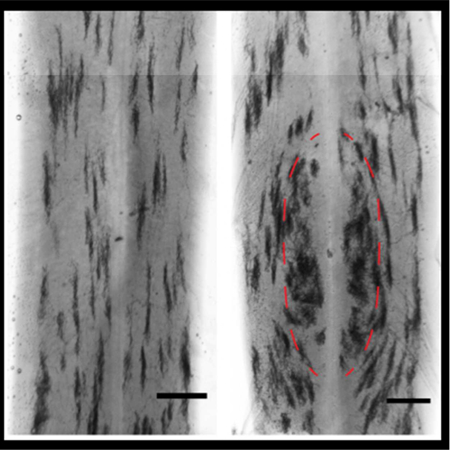
Somatotopic organization of a major class of mammalian nociceptors is evident at early postnatal stages. We performed sparse genetic tracing of Mrgprd+ nociceptor axonal arbors in P3 mouse spinal cords. The presence of region-specific morphologies at early stages of spinal cord innervation suggests that pre-patterning mechanisms could establish these differences.
1. Introduction
Animals use distal limb regions (paws/hands) for exploration and object manipulation. To facilitate these behavioral requirements, distal limb somatosensory circuits have several region-specific (somatotopic) organization mechanisms to increase the sensitivity of these skin regions. One such mechanism involves the ‘magnification’ of distal limb representations in the central nervous system, meaning distal limb regions are overrepresented in the spinal cord and cortex circuits (Fitzgerald & Swett, 1983; Millecchia et al., 1991; Penfield & Boldrey, 1937). While other sensory systems use analogous forms of regional magnification in various species (Ahnelt, 1998; Challis et al., 2015; Daniel & Whitteridge, 1961; Suga et al. 1987), the developmental mechanisms used by sensory systems to differentially allocate circuit space in this manner have not been clearly defined.
Similar to light touch, humans have increased sensitivity for pain in the distal limbs and fingertips (Mancini et al., 2014; Mancini et al., 2013). Recently, we used single-cell genetic tracing of Mrgprd+ mouse nociceptors to characterize the somatotopic organization of mammalian pain neurons (Olson et al., 2017). While we found no obvious mechanisms for increased sensitivity in paw peripheral circuits (these neurons have lower innervation density in the paw glabrous skin compared to the limb hairy skin and have similar terminal areas across skin regions), we identified a novel region-specific organization of the central terminal arbors of these neurons in the spinal cord dorsal horn (DH) (Figure 1A). Specifically, paw and trunk innervating nociceptors have distinct terminal morphologies (‘round’ vs. ‘long’) in the DH, such that paw nociceptors have a much wider mediolateral spread. Interestingly, this central terminal morphological distinction closely correlates with increased synaptic transmission efficiency in the spinal cord and decreased threshold for activating these afferents in the paw skin. This work suggests that ‘magnification’ of these nociceptors through region-specific central arbors could expand the representation of the paw in the spinal cord and downstream circuits. These results also indicate that Mrgprd+ neurons, which as a population show very similar molecular markers and anatomical features, differentially direct their central terminal arbor formation based on their somatotopic location during development. Our ability to trace and genetically manipulate single primary afferents from this population offers a unique ability to study the developmental mechanisms underlying region-specific organization of this sensory circuit.
Figure 1. Models of potential developmental mechanisms for region-specific Mrgprd+ central arbors.
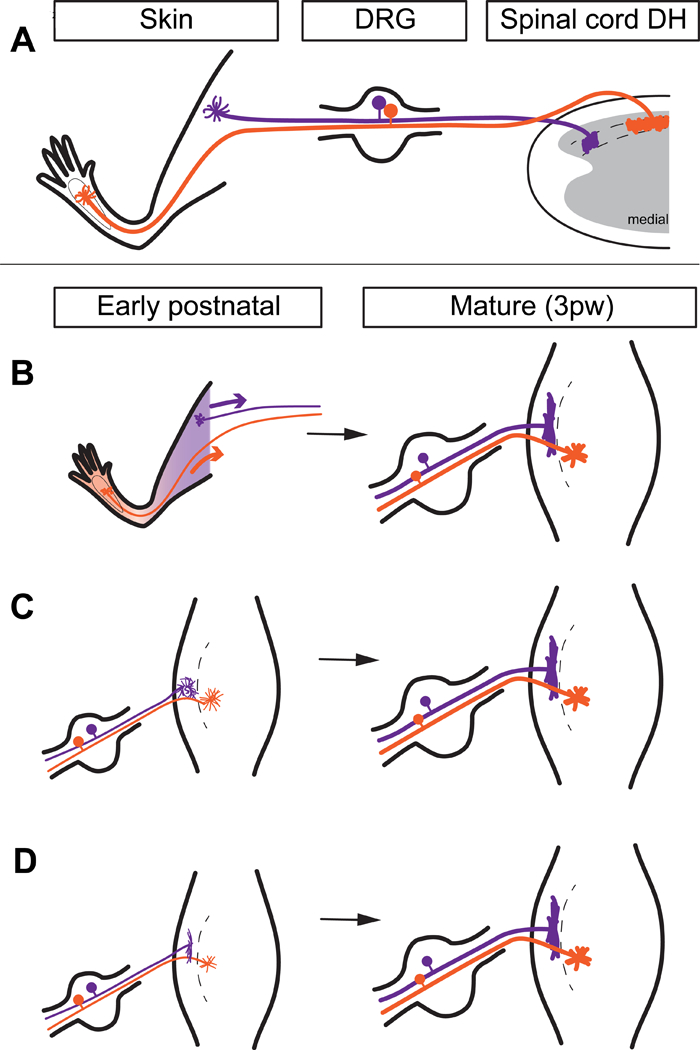
A, Mature somatotopic organization of Mrgprd+ afferents. Proximal hind limb afferents (purple) grow ‘long and thin’ central arbors in the lateral DH, while plantar paw afferents (orange) grow ‘round and wide’ central arbors in the medial DH. DH is drawn as a transverse section. B, Peripheral cues model. Upon innervation of the skin around birth, cues from different skin regions direct region-specific developmental of central terminal arbors. DH is drawn from a top-down view. C, Central reorganization model. Afferents grow immature, somatotopically homogenous arbors that are postnatally reorganized into region-specific arbor morphologies. D, Pre-patterned model. Afferents grow somatotopically distinct arbor morphologies during their initial innervation of the DH. These models are not mutually exclusive.
Previous research has characterized the development of somatosensory topographic maps and investigated potential mechanisms. Somatosensory afferents, including nociceptors, are dorsal root ganglion (DRG) or trigeminal ganglion neurons that have two axonal branches innervating the skin (or other tissues) and the spinal cord, respectively. Somatosensory circuits of the DH have a ‘flipped’ topographic map: in the lumbar enlargement (innervating the hindlimbs), the distal limbs (foot and toes) are represented in medial DH while the proximal limbs are represented in the lateral DH (Figure 1A) (Brown & Fuchs, 1975; Swett & Woolf, 1985). Nerve tracing experiments have shown that cutaneous sensory topographic maps formed early in development are similar to the mature pattern (Mendelson et al. 1992; Smith, 1983). Based on the rough coincidence of peripheral and central target innervation, and based on the proximal-to-distal progression of hind limb epidermal innervation, it was proposed that peripheral innervation could drive formation of a correct somatotopic map in the DH (Reynolds et al., 1991). However, subsequent studies provided evidence against this model (Mirnics & Koerber, 1995; Sharma et al., 1994; Wang & Scott, 2002). In general, the mechanisms directing the somatotopically-appropriate wiring of somatosensory afferents remain unclear.
Since the previous work could not resolve the single-cell structure of DRG neurons, the mechanisms for the disproportionate representation (magnification) of paw regions in somatosensory circuits have not been examined. Multiple potential models exist for the mechanisms that could instruct the magnification of paw regions among Mrgprd+ afferents. Peripheral cues from different skin regions (such as region-specific signaling molecules and/or activity-dependent processes) could play a major instructive role for the formation of region- specific central arbor morphologies in the DH (Figure 1B). Alternatively, Mrgprd+ neurons could form immature, somatotopically homogenous arbors that are postnatally reorganized into region-specific morphologies (Figure 1C). Finally, DRG afferents may form region-specific arbor morphologies during their initial terminal formation, suggesting mechanisms that ‘pre-pattern’ this somatotopic difference prior to DH innervation (Figure 1D). These models are not mutually exclusive of one another. For example, peripheral cues could even be involved in the prepatterned model, if such cues could act very quickly (Jackman & Fitzgerald, 2000).
Here, we used population-level tracing to characterize the postnatal development of Mrgprd+ nociceptor central and peripheral terminal arbors. In addition, we performed single-cell ablation of Ret to disrupt peripheral target innervation of these neurons and analyze the effect on their central arbor morphology in the DH. Lastly, we performed single-cell tracing of Mrgprd+ neurons in early postnatal animals, right after their initial innervation of the DH. These experiments show that region-specific arbors are present from the very early postnatal time we examined (supporting the ‘pre-patterned’ model), and that central terminal development slightly precedes, and occurs independently of, peripheral terminal development/refinement. Taken together, our results indicate that the region-specific single-cell organization of mammalian nociceptor central terminal arbors is likely to be dictated through pre-patterning mechanisms that are intrinsic to the DRG neurons themselves and/or by mechanisms within the spinal cord.
2. Materials and Methods
2.1. Mouse strains
Mice were raised in a barrier facility in Hill Pavilion, University of Pennsylvania. All procedures were conducted according to animal protocols approved by Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania and National Institutes of Health guidelines. MrgprdEGFPf (RRID:MMRRC_036772-UNC, MMRRC Cat# 036772-UNC), MrgprdCreERT2 (RRID:IMSR_JAX:031286, IMSR Cat# JAX:031286), RosaiAP (RRID:IMSR_JAX:009253, IMSR Cat# JAX:009253), and Retf(CFP) (RRID:MGI:4459058, MGI Cat# 4459058) mice have been previously described (Badea et al., 2009; Olson et al., 2017; Uesaka et al., 2008; Zylka et al., 2005). Retnull allele mice were generated by crossing a conditional Ret line (Retf/f) (RRID:IMSR_JAX:028548, IMSR Cat# JAX:028548) (Luo et al., 2007) with a germline Cre mouse line (Sox2Cre) (RRID:IMSR_HAR:3359, IMSR Cat# HAR:3359) (Hayashi et al., 2002).
2.2. Genetic labeling of Mrgprd+ nociceptors
To sparsely label Mrgprd+ nociceptors, we set up timed pregnancy matings of MrgprdCreERT2 mice with RosaiAP or Retf(CFP) mice. Population-level labeling was achieved through either prenatal or postnatal tamoxifen treatment. For prenatal treatment, pregnant females were given tamoxifen (Sigma, T5648) along with estradiol (Sigma, E8875, at a 1:1000 mass estradiol: mass tamoxifen ratio) and progesterone (Sigma, P3972, at a 1:2 mass progesterone: mass tamoxifen ratio) in sunflower seed oil via oral gavage at E16.5-E17.5, when Mrgprd is highly expressed in mouse non-peptidergic nociceptors (Chen et al., 2006).
2.3. Tissue preparation and histology
Procedures were conducted as previously described (Fleming et al., 2012; Niu et al., 2013). Briefly, mice were euthanized with CO2 and transcardially perfused with 4% PFA/PBS, and dissected tissue (skin, spinal cord, DRG) was post-fixed for 2 hr in 4% PFA/PBS at 4° C. Tissue used for section immunostaining was cryo-protected in 30% sucrose/PBS (4% overnight). Frozen glabrous skin and DRG/spinal cord sections (20–30 μm) were cut on a Leica CM1950 cryostat. Immunostaining was performed as described previously. DRGs for whole mount immunostaining were treated as described directly after post-fixation. The following antibodies were used: chicken anti-GFP (RRID:AB_10000240, Aves Labs Cat# GFP-1020, 1:1000), rabbit anti-GFP (RRID:AB_221569, Molecular Probes Cat# A-11122, 1:1000). The specificity of these commercial antibodies has been well documented in previous literature (Fleming et al., 2015; Niu et al., 2013). Tissue (skin or spinal cord with attached DRGs) for whole mount AP color reaction with BCIP/NBT substrate was treated as previously described. Following AP color reaction labeling, tissue was cleared in 1:2 (v:v) benzyl alcohol + benzyl benzoate (BABB) for imaging (Niu et al., 2013).
2.4. Image acquisition and data analysis
Images were acquired either on a Leica DM5000B microscope (brightfield with a Leica DFC 295 camera and fluorescent with a Leica 345 FX camera), on a Lecia SP5II confocal microscope (fluorescent), or on a Leica M205 C stereoscope with a Leica DFC 450 C camera (brightfield). Image quantification was performed in ImageJ. Graphs and statistical analyses were created in GraphPad Prism5.
For growth normalization of skin terminal densities (Figure 2), plantar paws (n=3 animals for each age) were imaged and fitted ellipses were drawn over the six mouse foot pads. Major axis lengths (proximodistal axis) were averaged across animals. Absolute skin terminal densities were multiplied by a normalization factor: Growth-normalized density (Age) = Absolute density (Age) X (Mean paw length (Age) / Mean paw length (3 pw)).
Figure 2. Postnatal central and peripheral terminal development of MrgprdEGFPf non-peptidergic nociceptors.
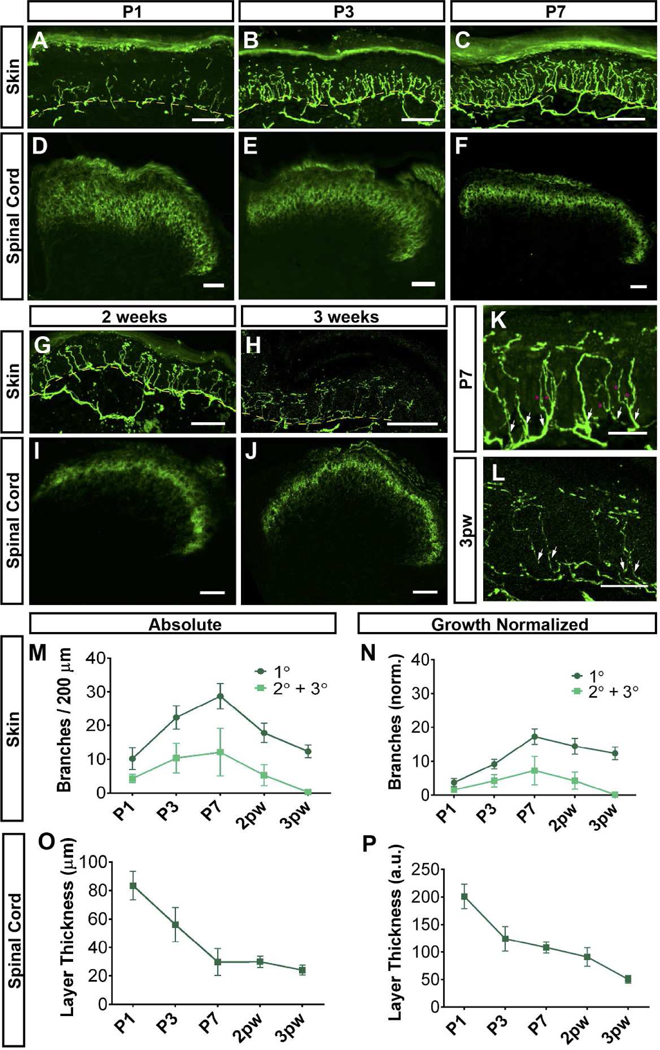
A-J, GFP immunostaining of glabrous skin (A-C, G, H) and DH (D-F,I, J) sections from MrgprdEGFPf mice at the indicated ages. K, L, Higher magnification views of peripheral terminals, indicating secondary/tertiary branches (pink asterisks) growing off primary branches (white arrows) in P7 but not 3pw skin. M, N, Quantification of densities of absolute and growth-normalized (N, see Methods) glabrous skin primary, secondary and tertiary branches during postnatal development. Skin terminals show overgrowth during the first week. Dotted yellow lines (A-C, G, H) indicate dermal/epidermal border. O, P, Quantification of absolute (O) and growth-normalized (P, see Methods) DH layer thickness at the indicated ages. DH terminals show a refinement during the first week, at which point they remain at their mature thickness. n = 3 animals per stage. Scale bars = 50 µm (A-J), 20 µm (K, L).
For growth normalization of DH layer thickness (Figure 2), the maximum mediolateral width of the MrgprdEGFPf innervation layer was measured (n=3 sections from separate animals for each age). Absolute layer thickness measurements were multiplied by a normalization factor: Growth-normalized thickness (Age) = Absolute thickness (Age) X (Mean DH width (Age) / Mean DH width (3 pw)).
For single-cell width measurements in sectioned DH tissue (Figure 3), serial DH sections were imaged, and individual arbors were identified by comparing adjacent sections. The DH of lumbar enlargement sections (L3-L5) were divided into thirds based on the maximum mediolateral width of the DH. Cells with most of their width lying in the medial third were classified as “Medial Lumbar”, cells were otherwise classified as “Lateral Lumbar”.
Figure 3. Genetic disruption of peripheral target innervation does not affect region-specific central arbor morphologies.
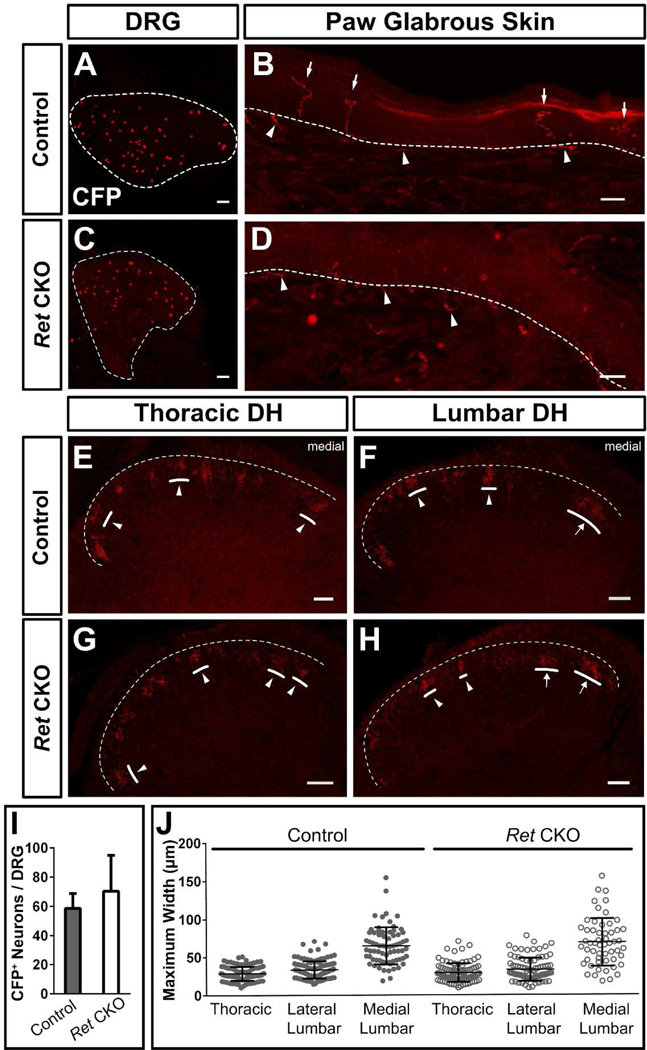
A, C, Whole mount CFP immunostaining of sparse labeled 2–3pw MrgprdCreERT2; Retf(CFP) / + (control, A) and MrgprdCreERT2; Retf(CFP) / null (mutant, C) DRGs (0.5 mg tamoxifen at E16.5-E17.5). B, D, CFP immunostaining of sectioned glabrous skin shows epidermal endings in control (B) but not mutant (D) mice, indicating a lack of peripheral terminals in Ret null nociceptors. White arrows, mature epidermal endings. White arrowheads, dermal axonal bundles. E-H, CFP immunostaining of serial DH sections from control (E&F) and mutant (G&H) mice shows sparse labeled terminals. I, Quantification of the number of CFP+ neurons / DRG. n = 14–22 DRGs from 3 animals per genotype. J, Maximal mediolateral width of sparse labeled neurons from control and mutant mice shows that the round-vs.-long distinction is still present in mutant mice. n = 239 (mutant), 287 (control) neurons from 3 mice per genotype. Scale bars = 100 µm (A&C), 20 µm (B&D), 50 µm (E-H).
3. Results
3.1. Population-level characterization of postnatal development of peripheral and central terminals of Mrgprd+ DRG neurons
To investigate whether region-specific arbor development may be driven by peripheral and/or central mechanisms, we used MrgprdEGFPf knock-in mice (Zylka et al., 2005) to characterize the postnatal (P1 – 3 postnatal weeks, pw) innervation of non-peptidergic nociceptors in the paw glabrous skin and the lumbar spinal cord enlargement DH. Mrgprd is first expressed at E16.5 in mice and specifically marks the non-peptidergic nociceptor population (Chen et al., 2006). EGFP expression in this knock-in mouse line faithfully indicates expression of Mrgprd. This genetic tool offers advantages over previous approaches since it specifically labels non-peptidergic fibers (unlike nerve filling) and avoids issues related to the dynamic expression of immunostaining markers (Jackman & Fitzgerald, 2000; Reynolds et al., 1991).
Peripherally, mature non-peptidergic nociceptor axons travel to the skin in the cutaneous nerves, grow a fiber plexus parallel to the skin surface in the dermis, and send perpendicular terminals out of the subepidermal plexus that penetrate the epidermis (Olson et al., 2017; Zylka et al., 2005). Most of the paw epidermis is not innervated by MrgprdEGFPf fibers at P1, except for a few rudimentary terminals (Figure 2A). From P1 to P7, there is a rapid phase of nerve terminal growth, as indicated by a great increase in density of both primary (leaving the subepidermal plexus) as well as secondary/tertiary (branches off primary terminals) Mrgprd+ fiber branches (Figure 2A-C, K, M, N). It is then followed by a refinement phase, as indicated by a decrease in density from P7 to 3pw. By 3pw, no secondary or tertiary branches are present (Figure 2G, H, L-N). This pattern is true when quantified as absolute terminal densities or as growth-normalized values (Figure 2M, N).
Centrally, non-peptidergic nociceptor axons travel through the dorsal roots, grow for 0–2 spinal segments rostrally or caudally in Lisseur’s tract at the dorsolateral margin of the spinal cord, and then dive ventrally to innervate layer II of the DH (Olson et al., 2017; Zylka et al., 2005). MrgprdEGFPf fibers have established a thick (in the dorsoventral extent) terminal layer by P1 in the DH (Figure 2D). This layer shows a 4-fold (absolute) decrease in layer thickness, reaching the mature layer thickness by P7 (Figure 2D-F, I, J). This decrease of layer thickness is also true when quantified by growth-normalized values (Figure 2O, P). In summary, while MrgprdEGFPf peripheral terminals are still undergoing initial outgrowth in the epidermis (the first postnatal week), their central terminals are in the process of refining to their mature thickness in the DH. These results indicate that, although both central and peripheral terminals show outgrowth and refinement during the postnatal period, central terminal development of Mrgprd+ neurons precedes peripheral development in this regard.
3.2. Non-peptidergic nociceptor central arbor formation is independent of peripheral terminal innervation
Next, to investigate whether non-peptidergic nociceptors utilize peripheral cues or processes to establish region-specific central arbor morphologies, we took advantage of a genetic manipulation to disrupt their peripheral target innervation. Previous work has shown that, upon DRG-specific deletion of the Glial cell line-derived neurotrophic factor receptor Ret, non-peptidergic nociceptors fail to innervate their final peripheral target, the skin epidermis, while their central terminals remain in DH layer II (Luo et al., 2007). However, these experiments did not trace single-cell morphology, so it remains unknown whether their central arbors are altered after this failure in peripheral target innervation.
We ablated Ret from individual non-peptidergic nociceptors and quantitatively measured their regional single-cell width (mediolateral) in the DH. We used a mouse line in which Cre- dependent inactivation of Ret also leads to expression of CFP (Retf(CFP)) (Uesaka et al., 2008). In MrgprdCreERT2 /+; Retf(CFP)/ null mice (Ret CKO), which carry the Retf(CFP) allele along with a Ret null allele, low-dose prenatal tamoxifen (0.5 mg at E16.5-E17.5) generated sparse Ret-null non-peptidergic nociceptors that are labeled with CFP (Figure 3C). In MrgprdCreERT2 /+; Ret f(CFP)/ + littermate controls (Control), this same treatment sparsely labeled Ret heterozygous non-peptidergic neurons with CFP (Figure 3A). As expected for Ret deletion, sparsely labeled CFP+ DRG cell bodies were smaller in MrgprdCreERT2 /+; Retf(CFP)/ null mutant mice (data not shown), but the number of CFP+ neurons was not decreased (average CFP+ neurons/DRG: control = 58.7 10.3, n = 14 DRGs from 3 animals, mutant = 70.4 ± 24.6 n = 22 DRGs from 3 animals) (Figure 3A, C, I). In addition, while sections of glabrous skin from control mice showed sparsely labeled terminals with mature epidermal endings, sections from mutant skin showed axon bundles in the dermis but no mature endings in the epidermis, consistent with previous work (Luo et al., 2007) (Figure 3B, D).
Lastly, serial sectioning of control and mutant thoracic (T6-T12) and lumbar (L3-L6) spinal cords showed that the round-vs.-long distinction in central terminal morphology was unaffected by Ret deletion (Figure 3E-H). We imaged through serial sections and measured the maximum mediolateral width of sparse-labeled neurons. The medial lumbar neurons were roughly twice as wide, on average, as either lateral lumbar or thoracic neurons (thoracic = 28.7 ± 5.2 μm, lateral lumbar = 33.5 ± 6.5 μm, medial lumbar = 64.7 ± 14.2 μm, n = 287 neurons from 3 mice) in control mice (Figure 3E&F, J). A similar difference was also seen in mutant spinal cords (thoracic = 29.0 ± 7.0 μm, lateral lumbar = 33.1 ± 8.7 μm, medial lumbar = 68.6 ± 18.1 μm, n = 239 neurons from 3 mice (Figure 3G&H, J), indicating that the round-vs.-long distinction is maintained in these mutant neurons. Additionally, no difference in the average width of round or long terminals was observed between control and Ret null non-peptidergic neurons (Figure 3J), suggesting that Mrgprd+ neurons grow normal central terminal arbor morphologies in the absence of Ret. In summary, this genetic disruption experiment suggests that Mrgprd+ afferent peripheral target (epidermal) innervation is dispensable for the formation of region-specific central arbors.
3.3. Sparse labeling reveals region-specific Mrgprd+ central arbors from the earliest stages of central innervation
Lastly, we asked whether the region-specific central terminal arbors of non-peptidergic DRG neurons might be established through postnatal reorganization (Figure 1C), or instead are present from the earliest stages of DH innervation (Figure 1D). We performed sparse genetic tracing of non-peptidergic neurons at early postnatal stages by crossing tamoxifen-dependent MrgprdCreERT2 mice with RosaiAP alkaline phosphatase reporter mice (0.25 mg of tamoxifen was given at E17.5) (Olson et al., 2017). Sparse terminals were seen after AP staining of skin and spinal cord tissue. Immature skin terminals could be seen at P3 but not P1 (Figure 4E&F), consistent with the few MrgprdEGFPf fibers in the epidermis at P1 (Figure 2). In addition, while their central arbors still appear immature at P1 and P3, region-specific arbor morphologies (the somatotopic organization of central arbors) are seen in both P1 and P3 spinal cords (Figure 4A-D). Like the mature DH organization of these afferents (Olson et al., 2017), medial lumbar enlargement (paw representation, outlined in Figure 4B, D) regions contain mediolaterally wide arbors while lateral lumbar enlargement and thoracic (proximal hindlimb, trunk) regions contain mediolaterally thin arbors (Figure 1A). While terminals at this stage still appear immature, this result indicates that these neurons form region-specific arbor morphologies during their initial innervation.
Figure 4. Region-specific Mrgprd+ DH arbors are evident during the earliest stages of innervation.
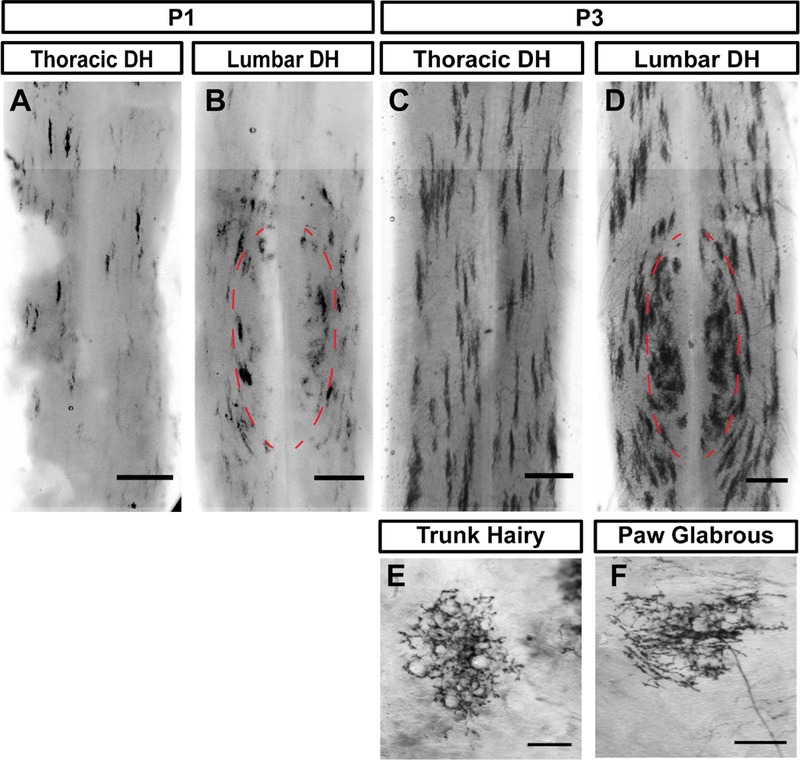
A-F, AP color reaction of spinal cord (A-D) and skin (E&F) tissue from P1 or P3 MrgprdCreERT2; RosaiAP mice (0.25 mg tamoxifen at E17.5). Sparse skin terminals were not seen at P1. While early postnatal nociceptors are still immature, the round vs. long distinction can be seen in B & D. Scale bars = 250 µm (A-D), 100 µm (E&F).
4. Discussion
In summary, we found that (1) postnatal development/refinement of central terminals precedes development/refinement in the periphery, (2) genetic disruption of peripheral target innervation had a negligible effect on region-specific central arbors, and (3) region-specific arbors are present from the earliest postnatal stages. While non-peptidergic central terminal arbors do show very clear postnatal layer thickness refinement (in the dorsoventral axis) (Figure 2), and while early postnatal arbors have a somewhat immature morphology (Figure 4), their region-specific structure is apparent from the earliest stages of DH innervation. Our results suggest that, while clear postnatal refinement does occur for both central and peripheral terminals, these neurons do not rely on postnatal refinement in order to establish their region-specific central arbor morphologies.
Trophic factor signaling plays important roles in neurite arbor morphogenesis. For example, Ret signaling is absolutely required in rapidly adapting Aβ low-threshold mechanoreceptors for the growth of central collaterals innervating the spinal cord DH (Fleming et al., 2015; Luo et al., 2009). Ret is also the main neurotrophic factor receptor expressed by Mrgprd+ DRG neurons (Luo et al., 2007). For these neurons, however, Ret deletion did not affect their DH arbor morphology at all, despite the obvious deficit of peripheral epidermis innervation. Given that (1) severely disrupting epidermal innervation had a negligible effect on central arbor morphologies and that (2) region-specific arbors are evident at P1, before distal limb Mrgprd+ nociceptors even innervate epidermis, we think that peripheral epidermal cues play a minor role in instructing formation of somatotopic central arbors of Mrgprd+ neurons. Past work has established that molecular cues from the dermis induce the development of hair follicles in hairy skin during embryogenesis, thereby playing critical roles in regional skin patterning (Millar, 2002; Sengel, 1990). Many molecular players in the dermis and epidermis have been identified, including the Wnt/β-catenin pathway, although the exact identity of the initial dermal signal remains to be determined (Millar, 2002). While our developmental characterization and Ret deletion experiments suggest that pre-patterning mechanisms are likely to control regional Mrgprd+ central arbor growth, our results do not rule out that Ret deleted neurons may receive dermally or epidermally-derived cues in the dermis. Future studies could examine whether mutant mice with severe skin patterning deficits, such as Lef1 mutants (van Genderen et al., 1994), might still display region-specific Mrgprd+ morphologies.
Earlier spinal nerve backfilling experiments indicated that DRG central projections form topographically appropriate innervation patterns from the earliest stages of innervation (Mendelson et al., 1992; Smith, 1983) and that spinal cord somatotopic map formation of DRG afferents does not rely on cues from the periphery (Sharma et al., 1994; Wang & Scott, 2002). Here we used single-cell tracing and genetic disruption to show that development of region-specific arbor morphologies, an important mechanism for magnifying paw representation in the central nervous system, occurs early and independent of peripheral target innervation. Our results suggest that DRG-intrinsic and/or DH-intrinsic pre-patterning mechanisms may instruct this difference. While much is known regarding the mechanisms differentiating lower motor neuron groups innervating various muscles (Dasen & Jessell, 2009; Dasen et al., 2005), very little is understood regarding mechanisms that might differentiate cutaneous somatosensory afferents innervating various regions. These could include transcription factor cascades and/or progressive neurogenesis, and future studies could use RNA sequencing at various developmental stages and/or neuronal birth dating techniques to investigate these mechanisms.
Acknowledgements
We would like to thank Drs. Steven Scherer, Michael Granato and Greg Bashaw for suggestions regarding the experimental design for this study. This work was supported by National Institutes of Health (NIH) (NS083702 and NS094224 to W.L and NS092297 to W.O.) and the Klingenstein-Simons Fellowship Award in the Neurosciences to W.L.
References
- Ahnelt PK (1998). The photoreceptor mosaic. Eye, 12 (Pt 3b, 531–540. doi:10.1038/eye.1998.142 [DOI] [PubMed] [Google Scholar]
- Badea TC, Hua ZL, Smallwood PM, Williams J, Rotolo T, Ye X, & Nathans J (2009). New mouse lines for the analysis of neuronal morphology using CreER(T)/loxP-directed sparse labeling. PLoS One, 4(11), e7859. doi:10.1371/journal.pone.0007859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PB, & Fuchs JL (1975). Somatotopic representation of hindlimb skin in cat dorsal horn. Journal of neurophysiology, 38(1), 1–9. [DOI] [PubMed] [Google Scholar]
- Challis RC, Tian H, Wang J, He J, Jiang J, Chen X, … Ma M. (2015). An olfactory cilia pattern in the mammalian nose ensures high sensitivity to odors. Current Biology, 25(19), 2503–2512. doi:10.1016/j.cub.2015.07.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CL, Broom DC, Liu Y, de Nooij JC, Li Z, Cen C, … Ma Q (2006). Runx1 determines nociceptive sensory neuron phenotype and is required for thermal and neuropathic pain. Neuron, 49(3), 365–377. doi:10.1016/j.neuron.2005.10.036 [DOI] [PubMed] [Google Scholar]
- Daniel PM, & Whitteridge D (1961). The representation of the visual field on the cerebral cortex in monkeys. The Journal of physiology, 159(2), 203–221. doi:10.1113/jphysiol.1961.sp006803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasen JS, & Jessell TM (2009). Hox networks and the origins of motor neuron diversity. Curr Top Dev Biol, 88, 169–200. doi:10.1016/S0070-2153(09)88006-X [DOI] [PubMed] [Google Scholar]
- Dasen JS, Tice BC, Brenner-Morton S, & Jessell TM (2005). A Hox regulatory network establishes motor neuron pool identity and target-muscle connectivity. Cell, 123(3), 477–491. doi:10.1016/j.cell.2005.09.009 [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, & Swett J (1983). The termination pattern of sciatic nerve afferents in the substantia gelatinosa of neonatal rats. Neuroscience Letters, 43, 149–154. doi:10.1016/0304-3940(83)90179-9 [DOI] [PubMed] [Google Scholar]
- Fleming MS, Ramos D, Han SB, Zhao J, Son YJ, & Luo W (2012). The majority of dorsal spinal cord gastrin releasing peptide is synthesized locally whereas neuromedin B is highly expressed in pain- and itch-sensing somatosensory neurons. Mol Pain, 8, 52. doi:10.1186/1744-8069-8-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming MS, Vysochan A, Paixao S, Niu J, Klein R, Savitt JM, & Luo W (2015). Cis and trans RET signaling control the survival and central projection growth of rapidly adapting mechanoreceptors. Elife, 4, e06828. doi:10.7554/eLife.06828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S, Lewis P, Pevny L, & McMahon AP (2002). Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Gene Expr Patterns, 2(1–2), 93–97. [DOI] [PubMed] [Google Scholar]
- Jackman A, & Fitzgerald M (2000). Development of peripheral hindlimb and central spinal cord innervation by subpopulations of dorsal root ganglion cells in the embryonic rat. J Comp Neurol, 418(3), 281–298. [PubMed] [Google Scholar]
- Joo W, Hippenmeyer S, & Luo L (2014). Neurodevelopment. Dendrite morphogenesis depends on relative levels of NT-3/TrkC signaling. Science, 346(6209), 626–629. doi:10.1126/science.1258996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Enomoto H, Rice FL, Milbrandt J, & Ginty DD (2009). Molecular identification of rapidly adapting mechanoreceptors and their developmental dependence on ret signaling. Neuron, 64(6), 841–856. doi:10.1016/j.neuron.2009.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Wickramasinghe SR, Savitt JM, Griffin JW, Dawson TM, & Ginty DD (2007). A hierarchical NGF signaling cascade controls Ret-dependent and Ret-independent events during development of nonpeptidergic DRG neurons. Neuron, 54(5), 739–754. doi:10.1016/j.neuron.2007.04.027 [DOI] [PubMed] [Google Scholar]
- Mancini F, Bauleo A, Cole J, Lui F, Porro CA, Haggard P, & Iannetti GD (2014). Whole-body mapping of spatial acuity for pain and touch. Annals of Neurology, 75(6), 917–924. doi:10.1002/ana.24179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini F, Sambo CF, Ramirez JD, Bennett DLH, Haggard P, & Iannetti GD (2013). A fovea for pain at the fingertips. Current biology : CB, 23(6), 496–500. doi:10.1016/j.cub.2013.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson B, Koerber HR, & Frank E (1992). Development of cutaneous and proprioceptive afferent projections in the chick spinal cord. Neuroscience Letters, 138(1), 72–76. doi:10.1016/0304-3940(92)90475-M [DOI] [PubMed] [Google Scholar]
- Millar SE (2002). Molecular mechanisms regulating hair follicle development. J Invest Dermatol, 118(2), 216–225. doi:10.1046/j.0022-202x.2001.01670.x [DOI] [PubMed] [Google Scholar]
- Millecchia RJ, Pubols LM, Sonty RV, Culberson JL, Gladfelter WE, & Brown PB (1991). Influence of map scale on primary afferent terminal field geometry in cat dorsal horn. J Neurophysiol, 66(3), 696–704. doi:10.1152/jn.1991.66.3.696 [DOI] [PubMed] [Google Scholar]
- Mirnics K, & Koerber HR (1995). Prenatal development of rat primary afferent fibers: II. Central projections. The Journal of comparative neurology, 355, 601–614. doi:10.1002/cne.903550409 [DOI] [PubMed] [Google Scholar]
- Niu J, Ding L, Li JJ, Kim H, Liu J, Li H, … Luo W (2013). Modality-based organization of ascending somatosensory axons in the direct dorsal column pathway. J Neurosci, 33(45), 17691–17709. doi:10.1523/JNEUROSCI.3429-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson W, Abdus-Saboor I, Cui L, Burdge J, Raabe T, Ma M, & Luo W (2017). Sparse genetic tracing reveals regionally specific functional organization of mammalian nociceptors. Elife, 6. doi:10.7554/eLife.29507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W, & Boldrey E (1937). Somatic motor and sensory representation in the cerebral cortex of man as studeid by electrical stimulation. Brain, 60(4), 389–443. doi:10.1093/brain/60.4.389 [Google Scholar]
- Reynolds ML, Fitzgerald M, & Benowitz LI (1991). GAP-43 expression in developing cutaneous and muscle nerves in the rat hindlimb. Neuroscience, 41, 201–211. doi:10.1016/0306-4522(91)90210-F [DOI] [PubMed] [Google Scholar]
- Sengel P (1990). Pattern formation in skin development. International Journal of Developmental Biology, 34(1), 33–50. [PubMed] [Google Scholar]
- Sharma K, Korade Z, & Frank E (1994). Development of specific muscle and cutaneous sensory projections in cultured segments of spinal cord. Development (Cambridge, England), 120, 1315–1323. [DOI] [PubMed] [Google Scholar]
- Smith CL (1983). The development and postnatal organization of primary afferent projections to the rat thoracic spinal cord. The Journal of comparative neurology, 220, 29–43. doi:10.1002/cne.902200105 [DOI] [PubMed] [Google Scholar]
- Suga N, Niwa H, Taniguchi I, & Margoliash D (1987). The personalized auditory cortex of the mustached bat: adaptation for echolocation. Journal of neurophysiology, 58(4), 643–654. [DOI] [PubMed] [Google Scholar]
- Swett JE, & Woolf CJ (1985). The somatotopic organization of primary afferent terminals in the superficial laminae of the dorsal horn of the rat spinal cord. The Journal of comparative neurology, 231(1), 66–77. doi:10.1002/cne.902310106 [DOI] [PubMed] [Google Scholar]
- Uesaka T, Nagashimada M, Yonemura S, & Enomoto H (2008). Diminished Ret expression compromises neuronal survival in the colon and causes intestinal aganglionosis in mice. J Clin Invest, 118(5), 1890–1898. doi:10.1172/JCI34425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Genderen C, Okamura RM, Farinas I, Quo RG, Parslow TG, Bruhn L, & Grosschedl R (1994). Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev, 8(22), 2691–2703. [DOI] [PubMed] [Google Scholar]
- Wang G, & Scott SA (2002). Development of “normal” dermatomes and somatotopic maps by “abnormal” populations of cutaneous neurons. Dev Biol, 251(2), 424–433. [DOI] [PubMed] [Google Scholar]
- Xu B, Zang K, Ruff NL, Zhang YA, McConnell SK, Stryker MP, & Reichardt LF (2000). Cortical degeneration in the absence of neurotrophin signaling: dendritic retraction and neuronal loss after removal of the receptor TrkB. Neuron, 26(1), 233–245. [DOI] [PubMed] [Google Scholar]
- Zylka MJ, Rice FL, & Anderson DJ (2005). Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron, 45(1), 17–25. doi:10.1016/j.neuron.2004.12.015 [DOI] [PubMed] [Google Scholar]


