Abstract
Inter-individual variability is always an issue when treating patients of different races, genders, ages, pharmacogenetics, and pharmacokinetic characteristics. However, the development of novel dosage forms is limited by the huge investments required for production line modifications and dosages diversity. Additive manufacturing (AM) or 3D printing can be a novel alternative solution for the development of controlled release dosages because it can produce personalized or unique dosage forms and more complex drug-release profiles. The primary objective of this manuscript is to review the 3D printing processes that have been used in the pharmaceutical area, including their general aspects, materials, and the operation of each AM technique. Advantages and shortcomings of the technologies are discussed with respect to practice and practical applications. Thus, this review will provide an overview and discussion on advanced pharmaceutical AM technologies, which can be used to produce unique controlled drug delivery systems and personalized dosages for the future of personalized medicine.
Keywords: pharmaceutical additive manufacturing, 3D printing, drug delivery system, personalized dosages, patient-centered drug development
INTRODUCTION
“Drug delivery system” refers to the method or process of administering a pharmaceutical compound to achieve a therapeutic effect in humans or animals (1). Inter-individual variability is always an issue when treating patients of different races, genders, ages, pharmacogenetics, and pharmacokinetic characteristics. Currently, the development of novel dosages is limited by demand, huge costs associated with production line changes, and the wide array of dosages required (2–4). In addition, developing new drugs is complex, expensive, and time-consuming, so optimizing the bioavailability or therapeutic effect of existing drugs has gained much interest (5–7). Additive manufacturing (AM) is a new alternative solution for the development of controlled release dosages because it can produce personalized or unique dosage forms and more complex drug-release profiles (8,9).
AM, popularly recognized as 3D printing, is the formalized term for rapid prototyping (RP) (10,11). The AM process uses additive technologies and combines materials layer by layer (12,13). AM equipment and materials were first developed in the early 1980s mainly in chemistry optics and robotics research (14,15), but were quickly applied in various engineering and biomedical fields (16,17). Compared with the traditional dosage manufacturing process, 3D printing can create complex products, personalized products, and products made for immediate consumption (18).
Based on the advantages offered by 3D printing technology, interest in this technique within the pharmaceutical industry has grown over the last few years; this is reflected in the increasing number of scientific reports and patents describing the pharmaceutical applications of 3D printing (17–20). After the Food and Drug Administration (FDA) approved the first 3D-printed drug product in August 2015, 3D printing received a boost as a pharmaceutical manufacturing technique (21).
Conventional pharmaceutical manufacturing has a complicated downstream process for mass production (22,23); however, AM can produce personalized dosages inexpensively without the need for such downstream processes (Fig. 1) (24). To achieve personalized dosages with conventional methods, only the dose can be adjusted. With the assistance of computational design, a researcher can adjust the dose, customize the drug-release profiles by complex structure design, and combine multiple active pharmaceutical ingredients (APIs) into a single dose (25–28). In addition, AM is more efficient and economical by reducing the downstream process (29). In particular, the FDA encourages drug manufacturers to produce solid oral dosages that meet the increasing demands of oral drug delivery with respect to the bioavailability of APIs and drug release characteristics, in a patient-centered drug development process (30). AM has the potential to meet and exceed these expectations.
Fig. 1.
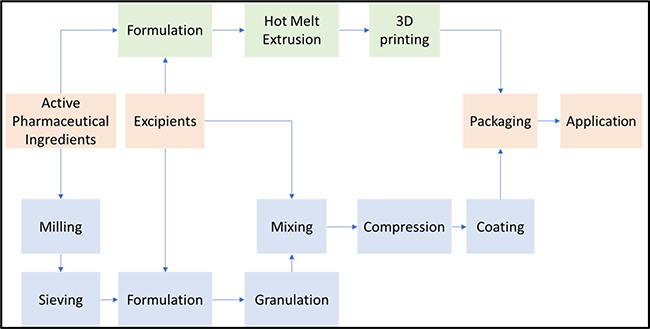
Representation of the conventional pharmaceutical tablets manufacturing process to 3D print process
PHARMACEUTICAL AM/3D PRINTING TECHNICAL ASPECTS
Pharmaceutical product development involves a number of steps from concept to physical production (31). Different pharmaceutical products will involve AM in different ways and degrees (19). With the development of AM/3D printing technologies, researchers and industry can now produce products with more complex structures and precise quality control (12,24,32,33). Pharmaceutical AM involves a series of operations that need to be performed sequentially. In this section, the different stages involved in the current AM process are discussed. Surely, there will be some variations depending on the technology that is being used and the design of the specific dosages. However, in general, currently available pharmaceutical AM can be classified into three main stages, namely modeling, building/printing, and finishing (34,35). These three major stages can be further subdivided into eight key steps (Fig. 2).
Fig. 2.

The schematic picture of the general pharmaceutical additive manufacturing processing
Modeling Conceptualization and Objective Design
Concepts and ideas will always be the first steps for any product development process and should include the type of the dosages, appearance, and function. The pharmaceutical concepts involve a large number of forms, from APIs, excipients, and dosage forms to the specific structure and manufacturing methods (36,37). Today, the modeling of 3D pharmaceutical products relies on computer-aided design (CAD).
“Slicing” the Objects
One of the most important steps in pharmaceutical AM is a deconstruction of the OBJ or STL file into a layer structure in a “G-code” file, which can be recognized by the specific 3D printing system (25). Researchers prefer to use the term “slicing” to describe such deconstruction steps. Obviously, the printing quality or resolution is dependent on the number of slicing steps (38). For the vertical Z-axis, the highest resolution reported to date is 16 μm, but 100 μm is typical of most AM instruments. For the horizontal resolution, compared to the 10–40 μm (600–2400 dpi) resolution of 2D printing, 3D printing can only reach as high as 30 μm in the X- and Y-axes (39,40).
Pre-formulation
To produce pharmaceutical products that take advantage of AM, three main properties of the raw materials should be considered: their chemical, physical, and mechanical properties. Different AM technologies offer the capacity to engineer complex structures from materials with specific physical properties, such as powders, pastes, liquids, or solids. In addition, chemical interactions, degradation, thermal stability, and photocurable properties should be considered during the pharmaceutical AM process. Improving the mechanical properties of the materials, such as the stiffness, hardness, and viscosity, will increase the success of producing AM pharmaceuticals (41).
Instrument Set-up
The production-ready material is loaded into the 3D printer. The instrument settings include general AM machine build parameters, such as the feeding rates, printing speeds, and build temperatures, as well as objective parameters, such as the resolution, layer thickness, and inner fill density. For some 3D printing that uses more than two nozzles, more parameters should be considered, such as the batch or dual extrusion settings.
Building/Printing
For most of the AM/3D printing process, the AM machine can finish the objective automatically without supervision. In some cases, the operator may need to continually monitor the process to ensure that there are no errors, such as material fail, software malfunction, or power issues during processing.
Removal/Cleaning
After the product is printed, it has to be removed, requiring minimal manual intervention, and the objects are ready for use. However, in most cases, after removing the 3D-printed parts from the build platform, they will require light cleanup by removing extra printed materials or supporting materials. In addition, the machine requires cleaning to avoid cross-contamination between different batches.
Post-Processing
Ideally, the printed objects should be ready to use, but in some cases, they will require further post-processing. Such post-processing can be classified into two major categories: (1) “subtraction,” which is removing extra materials and supporting structures added during the printing process, and involves polishing or sandpapering and (2) “addition,” where the product is physically combined with other parts for treatment or is coated for controlled release dosages. For some specific situations, there may also be chemical or thermal post-treatment of the printed products.
Application
After post-processing, the products should be ready for use. Compared to products obtained from conventional manufacturing methods, AM products may not behave as same as the standard material specifications, both the in vitro and in vivo shall be different with the conventional dosages due to the customized dose, structures, or fabrication paths. Recently, researchers developed some 4D products, in which the shape or geometry changes with time or drug-release from the matrix (42).
ADDITIVE MANUFACTURING TECHNOLOGIES
Currently, various standards have been applied to classify 3D printing technologies. However, it is difficult to come to a consensus on a universal standard of AM classification (43,44). For example, laser or resin solidification may be involved in liquid-based processes, whereas binder or laser may be involved in powder-based processes (25). There are three primary considerations for the AM printing process, which are: (1) hardware (i.e., the 3D printer); (2) software (used to communicate with the hardware and also software that converts the 3D objects into sliced layer files, which are recognized by the printers); and (3) materials used to print the objects. In this section, the AM technologies are classified according to the ways that the layers are built and the printing materials are used.
Extrusion-based System
Extrusion-based technologies were first used in the medical field for artificial implants, scaffolds, and bones, and then introduced into the pharmaceutical field in recent years (45). Because of advantages such as high drug-loading, adequate resolution, and excellent physical properties, they have been developed to overcome the limitations of the conventional pharmaceutical product development process (46). The extrusion-based system can be subdivided into several subsections based on material melting. The most common methods for extrusion use heat. However, there are other techniques that use, for example, premixed pastes or inks (47). The molten or liquefied material is extruded under pressure by a tractor-feed system (48).
Fused Deposition Modeling
Fused deposition modeling (FDM) is a typical material extrusion process and is trademarked by the company Stratasys®. In most FDM techniques, a molten thermoplastic polymer filament is extruded by two rollers through a high-temperature nozzle, where it is heated and is then deposited layer by layer (33). It is also known as fused filament fabrication (FFF) (49,50). It is the most widely used technique with many inexpensive, simple, desktop 3D printers. Several factors can influence the final model quality, such as the infill density, extrusion speed, layer heights, and nozzle temperature, but it has great potential and usefulness when these factors are controlled successfully (51–55).
Researchers have utilized drug-loaded polyvinyl alcohol (PVA) filaments, demonstrated the production of tablets and oral dispersible films, and investigated the impact of tablet infill density on the drug-release profiles (51,56). Both studies submerged commercially available PVA filaments into the drug solution for drug loading. However, the drug loading ratio by this method is low, and the drug concentrates on the surface of the filaments only (Fig. 3a). Similarly, Beck et al. used non-drug-loaded filaments and submerged the printed device into a polymeric nanocapsule suspension (Fig. 3b). In addition, erosion and shrinkage of the printed device during the drug loading process may be significant (57).
Fig. 3.
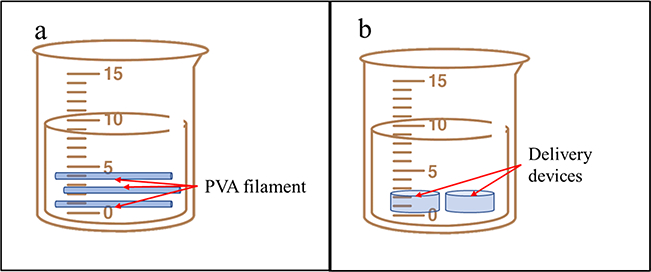
a Goyanes et al submerged the commercially available PVA filaments into drug solution for drug loading (65). b Beck et al. submerged the device into polymeric nanocapsules suspension for drug loading (72)
A better alternative to overcome low drug loading is to combine pharmaceutical hot-melt extrusion (HME) with AM for drug product developments. Researchers prefer to load the drug during the HME process, which improves the solubility of poorly water-soluble drugs by mixing APIs and excipients at the molecular level to form a homogenous amorphous solid dispersion. (Fig. 4) (41). Combining these two technologies potentially produces a continuous process, which is recommended by the FDA (58).
Fig. 4.
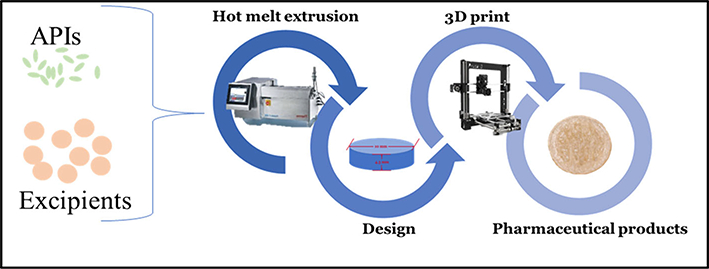
Zhang et al has combined pharmaceutical and FDM-based 3D printing as a potential continuous process for drug products development (41)
To address interpatient variability, modifying the drug loading is the most common way to personalize drug delivery. Recently, due to increased understanding of the diverse absorption, distribution, metabolism, and excretion (ADME) of particular drugs, researchers have been attempting to modify drug release profiles by complex drug product design. Goyanes et al. have studied the influence of oral dosage geometry on the drug release profiles (Fig. 5a). Changing the macro-geometry of the dosages led to surface area variation, which is the primary factor affecting drug release (52). Zhang et al. correlated the density gradient structure and drug release profile to produce zero-order release drug products, which provides an alternative means to engineer release profiles by controlling spatial distribution within a given polymer composition rather than creating a new host material (Fig. 5b) (26). Sadia et al. produced novel oral dosages with engineered channels within the immediate release tablet design, which increased the overall surface area to accelerate the drug release rate (Fig. 5c) (59). This indicates that the future trend of dosage form development will emerge to optimize drug release by controlling the media flow through these complex inner structures.
Fig. 5.
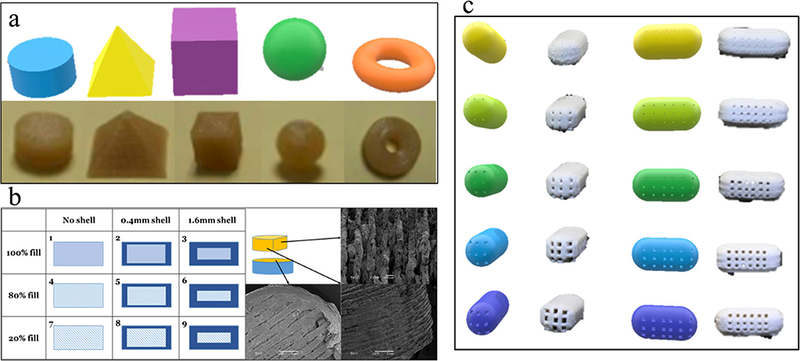
a Goyanes et al. have studied the geometry of the oral dosages influence on the drug release profiles (66). b Zhang et al. have investigated the density gradient structure and correlate to the drug release profile as well as produce the zero-order released drug products (26). c Sadia et al. have present novel oral dosages with engineered channels within the immediate release tablet design. (73)
The advantages of the FDM 3D printing process include the use of readily available filaments, which can produce pharmaceutical products with good structural properties and minimum post-processing. In a lab scale, it can be a more economical technique than conventional manufacturing methods, such as inject modeling. In addition, it can produce pharmaceutical products with properly controlled personalized dosages. The release profile of the incorporated drug can also be smoothly adjusted due to the limitless possibilities of printing a diverse range of geometries.
However, the quality (or resolution) of FDM 3D printed products is limited by the nozzle size and printing setups. The quality also is heavily dependent on the preformulated filaments or strings, which should have adequate mechanical properties. In addition, thermal-sensitive APIs cannot be used in these printing processes. In the next few years, FDM-based AM techniques will continue to dominate AM pharmaceutical development, unless there is a critical breakthrough in another technique.
Pressure-Assisted Microsyringes/Pressure-Assisted Bioprinting
Pressure-assisted microsyringes (PAM) printing was first introduced to create soft tissue scaffolds and for food printing in the early 2000s (60–62). As shown in Table I, the viscous semiliquid material is extruded from a syringe by pressure or force to build up the 3D-structured object. The process can be performed in a continuous flow at room temperature (33). The dispenser is usually a pressured-air piston (3–5 bars) (80). Biomaterials, such as solutions, pastes, and dispersions, have been widely used to print medical and pharmaceutical products with this technique (81–83).
Table I.
List of the Different Additive Manufacturing Techniques and Materials
| Techniques | Characteristics | Materials | References | |
|---|---|---|---|---|
| FDM | 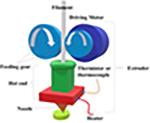 |
Widely used Simple Economical |
Polyvinyl alcohol (PVA), Hydroxypropyl cellulose (HPC), hydroxypropyl methylcellulose (HPMC), Hypromellos Acetate Succinate (HPMCAS), Soluplus ®, Eudragit |
(45)(46)(41)(26)( 47) |
| Low productivity, Low resolution, Heat process | ||||
| PAM | 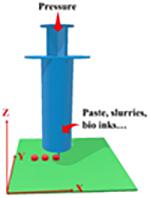 |
Simple, Bioprinting, Non-heat process, |
hydroxypropyl methylcellulose (HPMC) K4M, E15, K100M; polycaprolactone (PCL) and poly (lactic-co-glycolic) acid; Gelatin-based bioinks; Alginate- based bioinks |
(48) (48) (50)(51)(52) |
| Solidification issues, Material optimization | ||||
| MJS | 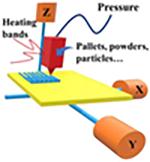 |
Applicability, | poly (D,L)-lactide | (53)(54) |
| Post- processing, heat process | ||||
| PED | 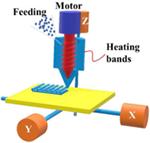 |
Applicability, | Polycaprolactone (PCL) |
(55) |
| Post- processing, heat process | ||||
| BJ-AM | 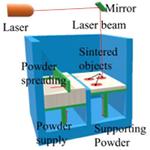 |
Applicability, High productivity, |
Aveicel PH301, Eudragit E-100 or RLPO |
(56)(57) |
| Post- processing, | ||||
| Powder bed fusion systems |
 |
Applicability, High productivity |
Polyethylene, polycaprolactone (PCL), poly-L-lactic acid (PLLA), polyetheretherketone (PEEK) |
(58)(59)(60) |
| Post- processing, heat process | ||||
| Resin based 3D printing |
 |
Bottom-up configuration, high resolution, |
Polypropylene, Acrylonitrile Butadiene Styrene (ABS), Polypropylene (PP) |
(61) (62) |
| low print speed, Costly, | ||||
 |
Top-down configuration, high resolution, |
|||
| low print speed, Costly, | ||||
| Droplet based 3D |
 |
simple, agility, precise control, |
Gelatin-based bioinks, Alginate- based bio inks, Pluronics, Collagen |
(63)(8)(64) |
| solvent needed, low resolution |
Li et al. produced gastro-floating tablets with hydroxypropyl methylcellulose-based dipyridamole paste using the PAM technique at 25 °C (Fig. 6b). Compared to the melt-based extrusion process, PAM technology allows printing at room temperature and avoids the potential thermal degradation of temperature-sensitive APIs (63,64). Khaled et al. also developed various sustained-release guaifenesin bilayer tablets (Fig. 6a), usinFg a double-syringe printer head with two different grades of hydroxypropyl methylcellulose. However, the tablet morphology showed low resolution and large thickness differences, indicating poor reproducibility (80).
Fig. 6.
a Khaled et al. also developed various sustained-release guaifenesin bilayer tablets using a double-syringe printer head (78). b Li et al., has produced the gastro-floating tablets with the hydroxypropyl methylcellulose-based dipyridamole paste using the PAM technique at 25 °C (47)
The significant drawbacks of this technique are the requirements of heat, solvents, or cross-linking agents for processing and solidification. The printing process or quality is dominated by the rheological properties of the slurries, which greatly depend on the type and amount of additives and solid material loading in the dispersion (84). In order to achieve pharmaceutical products with adequate appearance and homogeneity, the paste has to possess suitable viscosity and yield stress under the process shearing and pressure (85). In addition, due to the viscosity of the material, the printing speed is slower than that of other conventional methods. Although this technique has limitations, it is simple and versatile, and has been widely used for 3D printing of biomaterials (86).
Multiphase Jet Solidification
Multiphase jet solidification (MJS) was first developed by the Fraunhofer Institute for Applied Materials Research (IFAM) for manufacturing 3D structured parts using low melting point alloys or powder-binder mixtures by melting and extrusion through a computer-controlled nozzle (68,87). Unlike the filament-based FDM process, the loaded material can be a powder, pellet, or bar of the material-binder mixture (88). In addition, compared to that of precise extrusion deposition (PED, described below), a heated chamber or reservoir system is used to melt and liquefy the materials. Thus, the heated paste is extruded through a heated jet nozzle and used to build up the objects layer by layer. (89).
Unique in the MJS process is the “debinding” and sintering process of the printed parts, which are also known as “green parts” (90). Once the green part is obtained, solvent is applied to remove the binder and sinter the porous metal body to final density (95–98%) (Fig. 7). In general, a calculable linear shrinkage of the parts of 10–14% during the sintering process occurs, which depends on the content of the binder (91). Special care must be taken to avoid distortion by reducing the friction between the part and the support during the debinding process. To date, the wall thickness limit of the prototypes has been ~ 15 mm, so optimization will be necessary if thicker walls are required. Compared to play with the post process of the printed parts, the composition of the powder-binder system are future challenges to maximize wall thickness (92). However, the scope of MJS is limited in the pharmaceutical field due to the absence of a mixing process.
Fig. 7.
The debinding process of layerwise produced green part by submerging in a solvent then sintering to final density
Precise Extrusion Deposition
The PED technique was developed to manufacture scaffolds with controlled pore sizes and structural orientations, in order to provide the needed structural integrity, strength, and microenvironment for cell and tissue ingrowth and healing (9395). Compared to the other conventional FDM methods, the PED process does not use filaments and can form pellets according to the designed structures (96,97).
Polycaprolactone (PCL) is a widely used biodegradable polyester with a low melting point (98). It has been approved by the FDA in specific applications and is commonly used in implants, especially in long-term implants and controlled drug release applications (99). The pelleted PCL is fused and extruded by a pressure created by a turning precision screw as shown in Table I. Researchers have demonstrated the capability of the PED fabrication process in manufacturing PCL scaffolds with microstructure and pore sizes at a 250-μm scale (100). This process directly fabricates tissue scaffolds by converting designed architecture into layered deposition patterns without involving material preparation and direct casting, and thus opens opportunities for elaborate scaffold fabrication.
As mentioned above, PED has been approved more applicable in the pharmaceutical field than MJS and FDM techniques. In addition, from the engineering part of view, the extrusion-based AM shows great feasibility for continuous process which allows steady process parameters and better product quality. For example, current PED equipment uses a single screw for conveying the materials, which can also disperse the materials to build the objects with three-dimensional movement. A company called Arburg (Loßburg, Germany) is using a conventional single screw extruder to melt the powder or pellets and pulsed pressure to control the nozzle closure to disperse the molten materials (101). In the future, researchers could use both machines or combine these two technologies to exploit the advantages of both HME and PED. For example, a twin screw with kneading elements could be built into the extruder for good mixing and drug distribution. However, such technology has not been reported in the pharmaceutical field yet, but it has potential comprehensive applications.
The fact is that the development of continuous AM processes are driven by the purpose and the demand of the drug products. In general, the industrial scale requires the continuous process for the mass production of product because it can reduce processing time, saves costs for temporary storage, and enables full control of production. However, compared to conventional continuous processes, such as rotary press, the productivity of AM is low and the costs are higher. On the contrary, production costs may not be paramount for personalized or patient-centric dosage forms. In this situation, it may be better to maintain the AM in a batch process not requiring significant capital to install specialized equipment and special production lines.
Among the variety of the additive manufacturing techniques, the extrusion-based system is one technique that needs carefully selected materials with proper viscosity whether it melts or not. Based on the similar mechanism with melt extrusion process and automated drug-dispensing systems, the AM shared similar requirements on the rheology properties of the materials. Researchers are drawn on the experience of such techniques as well as based on their general experimental experience.
In addition, rheology was one of the most inconspicuous factors and easily ignored by the researchers, which has a dramatic effect on the printing process and quality of the final products. Even though researchers can successfully manufacture the drug products using a variety of materials, they are rarely focused on the rheological and viscoelasticity properties of the materials.
The AM has been introduced into the pharmaceutical area in the recent 20 years; it does not form a complete quality control and review system for 3D printing. So, like developing continuous process, bringing the production to competition and improving and perfecting the experimental parameters should be conducted for the pharmaceutical AM process.
Powder-based Systems
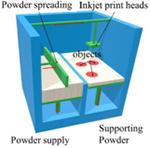
Binder Jetting Additive Manufacturing
The binder jetting additive manufacturing (BJ-AM) process, known sometimes as “binder jetting,” is a process that injects liquid binder through drop-on-demand (DOD) to selective areas of a spreading layer of powder material. Solidification of the saturated area has to occur once the binder is deposited, usually by heat application. Injet-solidification steps are repeated for each layer until the object is completed (102). The solidification mechanisms are similar to those in the wet granulation process, where the binder acts as a bridge between particles or dissolves and recrystallizes the particles (103). The quality and integrity of the objects produced using such techniques are primarily dominated by both the physicochemical properties of the liquid binder and the powder material (104,105).
BJ-AM allows microengineering of the release characteristics and development of highly controlled microstructures of the pharmaceutical products, so it is particularly useful in degradable tissue engineering matrices or controlled drug delivery development (71,106). Katstra et al. successfully produced controlled-release tablets using cellulose powder (Avicel PH-301) and Eudragit® E100 or Eudragit® RLPO as binder solutions (72). The first prescription 3D printed drug product (Spritam®; Aprecia) approved by US FDA was manufactured using the BJ-AM technology in 2015. Aprecia Pharmaceuticals’ named their 3D printing technology as ZipDose Technology (17). With this technique, drug particles are spread into thin layers, followed by selective jetting that bind the particles into thin porous layers. This process is repeated for a specific number of times according to the dose, thus building the product layer by layer. Spritam® is a rapidly disintegrating dosage form, where the levetiracetam dissolves in the mouth with a small amount of water in order to help patients who may have difficulty swallowing a tablet.
BJ-AM is similar to wet granulation. Compared to the other AM processes applied in the pharmaceutical field, BJ-AM is a potentially continuous process that shows high output, excellent reproducibility, and better quality. BJ-AM can be applied to a variety of raw materials in pharmaceutical products development.
Powder Bed Fusion Systems
Powder bed fusion differs from the traditional powder-based binder deposition systems mainly in the binding mechanism. Unlike inkjet deposition systems using binder solutions to bind particles together, powder bed fusion systems are based on different solidification mechanisms involving surface melting, congealing, and melting of binder powders with the accurate heating from a laser beam.
For pharmaceutical and medical applications, the most commonly used powder bed fusion system is selective laser sintering (SLS). SLS is a rapid prototyping process using a laser beam to solidify the powdered layer to manufacture complex 3D parts. There are two critical components of the SLS system, a beam deflection component, which allows the beam to scan each layer controlled by predefined CAD models, and a powder deposition component for distributing thin layers of powder before the layer is laser-sintered (107). Ligon et al. published a thorough review on polymers for 3D printing and customized additive manufacturing (108). Since SLS is a thermal process, the materials used in the process should meet specific requirements. The authors mentioned that, while many materials had been reported in the scientific literature or in patents, only a limited number of polymer powders for SLS were commercially available.
The application of SLS for producing medical and dental parts is well-established, but there are very few reports for drug delivery systems and pharmaceutical formulations (30). Fina et al. reported the first attempt at SLS printing for the preparation of drug-loaded oral dosage forms (tablets) using pharmaceutical grade excipients (44). The cylindrical printlets were designed with AutoCAD 2014, 10 mm in diameter and 3.6 mm in height. To achieve different dissolution properties, Kollicoat® IR was used to make immediate-release printlets, while Eutragit® L100–55 was used for modified-release printlets. Especially for the SLS process in this study, 3% Candurin® Gold Sheen was added to the excipient to enhance energy absorption from the laser. Interestingly, for all formulations in this study, the laser scanning speed, the chamber temperature, and the surface temperature were kept constant. Since the glass transition temperature (Tg) differs from polymer to polymer and changes in drug loading can influence the Tg, optimization of the process parameters for a specific formulation will be needed to produce high-quality drug formulations in the future. The appearances of the tablets that are printed by SLS are not ideal, but dissolution studies demonstrated the versatility of SLS to produce oral drug delivery systems with different pharmaceutical grade polymers and different drug loading for different release profiles, without drug degradation.
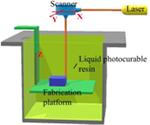
Resin-based 3D Systems
Resin-based 3D printing is widely used in biomedical applications (76). The advantages of this technique are its high resolution and mild treatment of the printing materials, which makes this technology suited for the fabrication of small objects, those requiring high precision, or those composed of materials that are prone to thermal or shear degradation (109). The objects are built based on photopolymerization, which is initiated by energy from laser light, to solidify the liquid resin layer by layer until a solid 3D object is produced. Controlling the energy and focal point of the laser can localize the solidification to predetermined positions. The energy transmitted by the laser is governed by the light source power, scan speed, and exposure time (33).
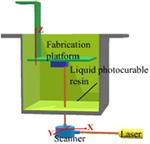
The older types of resin-based 3D printing used a bottom-up configuration where the laser beam scans the top of the resin surface. Today, more advanced printers can use a top-down arrangement, where the light is projected from the bottom of the resin bath through a laser-transparent window. A computer-controlled laser beam rastering on the free resin surface initiates photopolymerization and forms solidified resin following the slice pattern (110). After curing each layer, the support platform moves away from the surface one step. Thus, the newly built layer is recoated with fresh resin ready for curing the next layer. Because the depth of curing is set larger than the movement step of the platform, a good adherence between layers is formed by polymerization of unreacted groups on the solid surface with the illuminated resin of a new layer. The process is repeated until the complete 3D object is constructed. An additional step of washing excess resin and post-curing might be needed to improve the mechanical strength of the printed object.
One drawback of conventional stereolithography is that the printing speed is relatively slow due to the solidification happening one point at a time. Digital projection lithography (DLP) was developed to improve the printing speed. Instead of using a single laser beam, DLP uses a digital mirror device (DMD), with an array of up to millions of mirrors that can be rotated independently (111,112). The slice pattern is used to control the DMD, and the modulated light is projected as a two-dimension array of pixels onto the curable resin surface (113,114). The photopolymerization is initiated simultaneously at all illuminated pixels, and the illuminated regions are solidified with a single exposure. Meanwhile, the dark areas remain liquid. After the fabrication of one layer, the printing process continues by immersing the substrate in resin, and a new layer is solidified on top of the existing structure.
Recently, layerless lithography, called continuous liquid interface production (CLIP), was developed (115), which uses the top-down configuration and an oxygen-permeable illumination window. The photopolymerization inhibition mediated by oxygen creates a “dead zone” between the printing substrate and illumination window where the resin will not solidify (116). This prevents adherence to the window and allows the resin to freely flow into the dead zone, which enables a continuous photopolymerization process rather than layer-by-layer solidification. As a result, the production times are shorter and high printing resolution is obtained (116,117).
Very high lateral resolution two-photon stereolithography (TPS) is particularly compelling for printing in micro- and nanoscales, allowing miniaturized 3D objects to be printed precisely (118). TPS utilizes two photons simultaneously to initiate the polymerization reaction. The photons must combine to generate sufficient energy to initiate the photopolymerization reaction (119–121). The coincidence of two photons at a focal point restricts the excitation volume, and thus maintains a high spatial resolution (122). There is always a trade-off between printing resolution, building volume, and printing speed. With a spatial resolution as high as 100 nm, objects printed by TPS usually have a size limitation of 1 cm3 (123).
The resin print materials used in biomedical and pharmaceutical applications need to be both photopolymerizable and biologically safe. Schuster, Liska, and coworkers investigated the physical and chemical properties of resins and established a library of photopolymerizable resins that possessed low cytotoxicity and were suitable for long-term applications (124–126). Popular biodegradable macromers for stereolithography are based on poly (propylene fumarate), trimethylene carbonate co-ε-caprolactone, and poly(D, L-lactide)-fumarate (109). However, very few materials that can be used for resin-based printing have been approved by the FDA (18). Most cross-linked polymers are quite safe, but unpolymerized materials left in the sample after printing are toxic. Therefore, unreacted monomers or macromers residing in the matrices need to be thoroughly extracted from the objects after fabrication.
The high printing resolution and capability of utilizing clinical imaging data (MRI and CT scans) to build printing models make resin-based 3D printing particularly valuable for biomedical applications. This technique is widely used to fabricate patient-specific objects and models, such as functional body parts, implant devices, tissue engineering, and hydrogels containing cells (109). For pharmaceutical applications, this technique is especially powerful in the fabrication of highly precise drug deliveries and is suitable for thermally labile APIs. Several dosage forms have been successfully prepared by resin-based 3D printing, such as transdermal microneedles (116), porous scaffolds loaded with an antibiotic (127), and an oral modified-release dosage form (128). However, pharmaceutical applications might be limited because of low drug load, limited approved photosensitive polymers, and complications of the post-printing process.
Droplet-based Systems
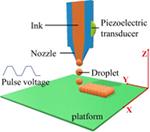
The dawn of droplet-based printing (DBP) was in 1951 when the first inkjet device was introduced (129). Over time, it has evolved to become a prominent technology in the printing industry in general and 3D printing in particular. DBP has many advantages over other 3D printing techniques, such as simplicity, agility, and precise control of the printing pattern. Because of its versatility, DBP has been applied to various areas, including biomedicine and pharmaceutics (130).
The general principle of DBP is that droplets of printing ink are formed at the tip of a printing nozzle or an array of printing nozzles before being deposited at predefined locations. The resolution of printing is controlled by droplet volumes that vary from several picoliters to nanoliters, which will result in droplet diameters of 10 to > 100 μm (131). Based on the droplet formation mechanisms, DBP can be classified as three printing types, namely inkjet, acoustic, and microvalve printing.
Inkjet Printing
Inkjet printing, including continuous, drop-on-demand and electrodynamic inkjet printing, manipulates the physical properties of ink solution to eject ink droplets onto a substrate with predetermined patterns. In continuous inkjet printing, the ink solution is pushed through a nozzle using pressure to form a stream of droplets at the tip of the nozzle (132). More efficient, drop-on-demand inkjet printing generates droplets in a more controlled manner. Each printer is equipped with one or more printheads that have ink chambers connected to one or more nozzles. Droplet demand is signaled as pulses from piezoelectric, electrostatic, or thermal actuators, which subsequently generate pressure pulses applied to the ink solution (133). When this pressure exceeds the surface tension of the ink solution at the nozzle orifice, an ink droplet is ejected out of the nozzle tip. Electrodynamic inkjet printing utilizes electric fields to pull ink solution through the nozzle orifice as droplets (134). The ink solution is fed into a metallic nozzle, and, under pressure at the back of the nozzle, the ink solution forms a meniscus at the nozzle tip. A high voltage applied to the nozzle and substrate generates an electric charge within the ink solution surrounded by the meniscus that pulls the ink toward the substrate. When the electrical force overcomes the surface tension, a droplet will leave the nozzle and deposit on the substrate (135).
Acoustic Printing
In acoustic printing, ink droplets are formed and ejected under the effect of an acoustic field. The printhead is equipped with an array of microchannels that can hold ink inside due to surface tension. Droplet demand is signaled to an acoustic generator, and the generated waves circulate and form an acoustic focal point near the channel outlets. When the force generated by the acoustic radiation overcomes the surface tension of the ink solution at the channel outlet, droplets are ejected from the microfluidic channels (136). Unlike inkjet printing, acoustic printing is very gentle on the ink. Thus, it is suitable for printing sensitive materials, such as cells and medicines. However, movement of the printing nozzle and/or substrate during printing disturbs the precision of the acoustic focal points. As a result, the droplet ejection is poorly controlled, resulting in low printing resolution. Moreover, the acoustic fields may be insufficiently strong to cause droplet ejection (130).
Review of pharmaceutical additive manufacturing
Microvalve Printing
In microvalve printing, droplet ejection is electromechanically controlled by a microvalve using a magnetic field. In the printhead, ink is filled in the fluid chamber under pressure applied from the back, and the gating of the nozzle orifice is controlled by a microvalve typically comprised of a solenoid-driven plunger (137). Controlling signals are transferred to the microvalve as voltage pulses applied to the coil. Subsequently, the generated magnetic field lifts the plunger to open the orifice, and a droplet is ejected due to the pressurized ink chamber (138). The microvalve can be opened and closed at a very high frequency, thousands of times per second. The printing operation can switch between continuous inkjet mode and drop-on-demand mode by adjusting the pressure applied and frequency of the microvalve gating.
Droplet-based 3D printing has been extensively applied in both biomedical and pharmaceutical fields. For biomedicine, the “ink” formulations contain various biologics, such as cells, protein, and DNA dispersed in a hydrogel delivery medium. Printable inks inherently possess low viscosity, biodegradability, biocompatibility, good adhesion, and high mechanical strength. These requirements limit the range of materials that can be used for drop-based 3D printing (130). Favorite materials used for biomedical printing include alginate, collagen type I, methacrylate gelatin, fibrin, and polyethylene glycol. Biomedical applications of drop-based 3D printing consist of generating tissue such as bone, heart, cartilage, liver, lung, and skin tissue (139); high throughput screening (140); and creating tumor tissue models to mimic cancer microenvironments (141). The applications of pharmaceutical ink formulations are more versatile than those of biomedical formulations. The composition of a pharmaceutical ink formulation consists of an API, carrier fluid, and excipients, as well as surface tension and viscosity modulators. The pharmaceutical applications of droplet-based 3D printing were intensively reviewed by Daly et al. (78). The primary dosage form for individualized treatments is inkjet printing of the formulation on a porous substrate such as paper (8) or fibrous gelatin matrix (79). The print sheets are then cut into predetermined dimensions and loaded into a hard capsule. Inkjet printing in conjunction with UV photoinitiation was used to fabricate tablets in which poly(ethylene glycol) diacrylate established crosslinks after the ink had been deposited on the printing substrate (142). Recently, controlled-release tablets with precise, complex structures were prepared using melting inkjet 3D printing, a solvent-free method (143). The results demonstrated that drug release could be modulated by the surface area, printing structure, and wetting property of the tablets. DBP is a potential approach to individualized treatment. However, it might be limited to very low drug dosages since the drug load and size of the dosage forms are very small.
CHALLENGES AND FUTURE PERSPECTIVES OF PHARMACEUTICAL AM
As an emerging technology in the pharmaceutical industry, there are many challenges to 3D printing. Some review articles have already raised points worthy of consideration. Liaw et al. reviewed the FDA regulations and challenges of 3D printing (144). Preis et al. systematically reviewed the current status of the 3D-printed drug for children and the challenges encountered (145). Our current review focuses on the challenges of the materials, equipment, and processes of additive manufacturing for personalized drug delivery systems.
Most additive manufacturing platforms were initially developed for applications in industries other than pharmaceutical, while the materials utilized were not pharmaceutical grade. To develop personalized drug delivery systems, pharmaceutical scientists have two important considerations: (1) find appropriate materials for the different types of 3D printing and (2) maintain the desired properties after the printing process. Polylactic acid (PLA) and acrylonitrile butadiene styrene (ABS) are the most used materials for FDM systems (54), but commercial filaments of pharmaceutical grade polymers are not available. SLS is a one-step technology that does not require high-quality intermediate filaments. However, commonly used materials for SLS are powdered plastics, ceramics, and metal alloys (107,146), most of which are not suitable for drug delivery systems. The challenges of using pharmaceutical grade materials for SLS processes are potential low absorption of laser energy and thermal degradation from high energy input. An alternative method to increase laser energy absorption is adding pharmaceutical excipients that have reasonable absorbance at the wavelength of the laser source or lowering the laser scanning speed to increase the energy transmission time. Regarding thermal degradation, a “design space” of process parameters is needed for all new materials utilized in the 3D printing process and all changes in processing conditions (147).
To ensure the quality of drug products, pharmaceutical material selection and process optimization remain a big challenge. At the beginning of the twenty-first century, the FDA first introduced the Quality by Design (QbD) concepts (148), namely a new guideline for developing and manufacturing the drug products. These concepts can achieve ensured quality with high efficiency, flexibility, and more important, minimum regulatory oversight (149). Currently, most of the pharmaceutical AM stays in the lab scale and researchers were struggling on the prophase studies, such as developing the suitable materials, standard operation procedure (SOP), as well as experimental on the processing window for a variety of materials and machines. In the lab scale, the design of experiment (DoE) has been applied which can guide through the optimization of the pharmaceutical AM process (150,151). In the event pharmaceutical AM has been industrialized or commercialized, the QbD should be applied to provide guidance on pharmaceutical development to the facility, the design of products, and processes that maximize the product’s efficacy and safety profile while enhancing product manufacturability.
Considerable effort has been made regarding regulation of 3D-printed medical devices. In 2017, the FDA released its final guidance on Technical Considerations for Additive Manufactured Medical Devices for industry and FDA staff (152). This guidance emphasized design and manufacturing process considerations (e.g., patient-matched device design, software workflow, material controls, and process validation) and device testing considerations (e.g., device testing, mechanical testing, and material characterization). Some of the critical attributes mentioned above can also apply to the pharmaceutical drug products manufactured by 3D printing techniques. However, a specific guidance for 3D-printed drug products is very much needed. Reexamining the first FDA-approved 3D-printed tablet, Spritam®, shows that it contains excipients that are present in conventional tablets and its manufacturing conditions are relatively mild compared with those of FDM or SLS. Considering novel excipients and more complicated printing processes, the regulatory agency needs a unique pathway for drug products manufactured by printing techniques. Can traditional in vitro testing methods be used for 3D-printed drug products? Furthermore, should 3D-printed dosage forms be required to meet more/extra/special requirements than conventional products? In the future, if a 3D-printed drug product is developed as a substitute for a current product, it will still make sense to evaluate and approve it following traditional guidelines. However, if it is designed with unique or personalized properties, a new pathway of approval and guidelines should likely be developed.
CONCLUSION
This review was organized systematically to present AM with respect to the development of pharmaceuticals. AM/3D printing is an emerging technology in dosage form development, but it has tremendous potential in the development of pharmaceutical products. This potential to develop personalized medicine is a revolutionary change in compounding the dosage forms with tailored doses, especially for drugs with narrow therapeutic ranges. This would have a profound effect on society and industry. However, appropriate regulations and policies must be developed for future research in this arena. 3D printing has provided new prospects for various industries in production paradigms and extending manufacturing to local and decentralized production. Such additive manufacturing has exceptional applications that need to be exploited in the manufacturing sector. Nevertheless, the use of different 3D printing technologies has recently increased in various industries in the recent past; it requires advancements (reducing production time, printer cost, and printer materials) to replace the conventional production methods. Wohler’s report in 2014 estimated that global revenue for AM will rise fourfold from US$5 billion in 2016 to US$21 billion in 2021. This suggests a considerable market potential for AM in the near future.
ACKNOWLEDGEMENTS
The authors thank the Pii Center for Pharmaceutical Technology for contributions in this project.
FUNDING INFORMATION
This work was partially supported by Grant Number P20GM104932 from the National Institute of General Medical Sciences (NIGMS), a component of NIH.
REFERENCES
- 1.Misra A, Ganesh S, Shahiwala A, Shah SP. Drug delivery to the central nervous system: a review. J Pharm Pharm Sci. 2003;6:252–73. [PubMed] [Google Scholar]
- 2.Langer R New methods of drug delivery Science (80-. ). JSTOR; 1990;1527–33. [DOI] [PubMed] [Google Scholar]
- 3.Lepourcelet M, Chen Y-NP, France DS, Wang H, Crews P, Petersen F, et al. Small-molecule antagonists of the oncogenic Tcf/β-catenin protein complex Cancer cell. Elsevier; 2004;5:91–102. [DOI] [PubMed] [Google Scholar]
- 4.Uhrich KE, Cannizzaro SM, Langer RS, Shakesheff KM. Polymeric systems for controlled drug release Chem Rev ACS Publications; 1999;99:3181–98. [DOI] [PubMed] [Google Scholar]
- 5.Crowley MM, Zhang F, Repka MA, Thumma S, Upadhye SB, Kumar Battu S, et al. Pharmaceutical applications of hot-melt extrusion: part I Drug Dev Ind Pharm Taylor & Francis; 2007;33:909–26. [DOI] [PubMed] [Google Scholar]
- 6.Blagden N, De Matas M, Gavan PT, York P. Crystal engineering of active pharmaceutical ingredients to improve solubility and dissolution rates Adv Drug Deliv Rev. Elsevier; 2007;59:617–30. [DOI] [PubMed] [Google Scholar]
- 7.Vasconcelos T, Sarmento B, Costa P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs Drug Discov Today Elsevier; 2007;12:1068–75. [DOI] [PubMed] [Google Scholar]
- 8.Sandler N, Määttänen A, Ihalainen P, Kronberg L, Meierjohann A, Viitala T, et al. Inkjet printing of drug substances and use of porous substrates-towards individualized dosing J Pharm Sci. Wiley Online Library; 2011;100:3386–95. [DOI] [PubMed] [Google Scholar]
- 9.Preis M, Breitkreutz J, Sandler N. Perspective: concepts of printing technologies for oral film formulations Int J Pharm. Elsevier; 2015;494:578–84. [DOI] [PubMed] [Google Scholar]
- 10.Maffezzoli A Rapid prototyping: an overview. Lecce: Univ. Lecce; 2000. p. 1–21. [Google Scholar]
- 11.American Society for Testing and Materials. Committee F42 on additive manufacturing technologies—scope [Internet]. ASTM. 2009. [cited 2017 Sep 24]. Available from: https://www.astm.org/COMMITTEE/F42.htm
- 12.Gross BC, Erkal JL, Lockwood SY, Chen C, Spence DM. Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences ACS Publications; 2014. [DOI] [PubMed] [Google Scholar]
- 13.Melchels FPW, Domingos MAN, Klein TJ, Malda J, Bartolo PJ, Hutmacher DW. Additive manufacturing of tissues and organs Prog Polym Sci. Elsevier; 2012;37:1079–104. [Google Scholar]
- 14.Kodama H Automatic method for fabricating a threedimensional plastic model with photo-hardening polymer. Rev Sci Instrum. AIP 1981;52:1770–3. [Google Scholar]
- 15.Kodama H A scheme for three-dimensional display by automatic fabrication of three-dimensional model. J IEICE. 1981;64:1981–4. [Google Scholar]
- 16.Bandyopadhyay A, Vahabzadeh S, Shivaram A, Bose S. Three-dimensional printing of biomaterials and soft materials MRS Bull Cambridge University Press; 2015;40:1162–9. [Google Scholar]
- 17.Norman J, Madurawe RD, Moore CMV, Khan MA, Khairuzzaman A. A new chapter in pharmaceutical manufacturing: 3D-printed drug products Adv Drug Deliv Rev. Elsevier; 2017;108:39–50. [DOI] [PubMed] [Google Scholar]
- 18.Goole J, Amighi K. 3D printing in pharmaceutics: a new tool for designing customized drug delivery systems Int J Pharm. Elsevier; 2016;499:376–94. [DOI] [PubMed] [Google Scholar]
- 19.Kalaskar DM, Serra T. 3d printing in medicine. 1st Editio. Kalaskar DM, editor. London: Woodhead Publishing; 2017. p. 16–32. [Google Scholar]
- 20.Desimone JM, Moshkin A, Ermoshkin N, Samulski ET, inventors. Sibley KD, assignee. Continuous liquid interphase printing. Patent WO/2014/126837 21 Aug. 2014. Available from: https://patentscope.wipo.int/search/en/detail.jsf? docId=WO2014126837&recNum=1&maxRec=&office=&prev-Filter=&sortOption=&queryString=&tab=PCT+Biblio
- 21.Aprecia Pharmaceuticals First FDA-approved medicine manufactured using 3D printing technology now available. 2016;6–8.
- 22.Carl SM, Lindley DJ, Knipp GT, Morris KR, Oliver E, Becker GW, et al. Biotechnology-derived drug product development Pharm. Manuf. Handb. Prod. Process Hoboken: John Wiley & Sons, Inc.; 2007. [Google Scholar]
- 23.Conway BR. Solid dosage forms Pharm. Manuf. Handb Hoboken: John Wiley & Sons, Inc.; 2008. p. 233–65. [Google Scholar]
- 24.Ventola CL. Medical applications for 3D printing: current and projected uses. Pharm Ther. MediMedia, USA. 2014;39:704. [PMC free article] [PubMed] [Google Scholar]
- 25.Do A-V, Smith R, Acri TM, Geary SM, Salem AK. 3D printing technologies for 3D scaffold engineering Funct 3D Tissue Eng Scaffolds. Elsevier; 2018;203–34. Available from: linkinghub.elsevier.com/retrieve/pii/B9780081009796000094 [Google Scholar]
- 26.Zhang J, Yang W, Vo AQ, Feng X, Ye X, Kim DW, et al. Hydroxypropyl methylcellulose-based controlled release dosage by melt extrusion and 3D printing: structure and drug release correlation. Carbohydr Polym. 2017;177:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goyanes A, Wang J, Buanz A, Martinez-Pacheco R, Telford R, Gaisford S, et al. 3D printing of medicines: engineering novel oral devices with unique design and drug release characteristics. Mol Pharm. American Chemical Society 2015;12:4077–84. [DOI] [PubMed] [Google Scholar]
- 28.Okwuosa TC, Pereira BC, Arafat B, Cieszynska M, Isreb A, Alhnan MA. Fabricating a shell-core delayed release tablet using dual FDM 3D printing for patient-centred therapy Pharm Res Springer US; 2017;34:427–37. [DOI] [PubMed] [Google Scholar]
- 29.Straub J Initial work on the characterization of additive manufacturing (3D printing) using software image analysis. Machines. Multidisciplinary Digital Publishing Institute; 2015;3:55–71. [Google Scholar]
- 30.Alhnan MA, Okwuosa TC, Sadia M, Wan KW, Ahmed W, Arafat B. Emergence of 3D printed dosage forms: opportunities and challenges. Pharm Res. 2016;33:1817–32. [DOI] [PubMed] [Google Scholar]
- 31.Gibson I, Rosen D, Stucker B. Introduction and Basic Principles Addit. Manuf. Technol New York: Springer; 2015. p. 1–18. [Google Scholar]
- 32.Cooke MN, Fisher JP, Dean D, Rimnac C, Mikos AG. Use of stereolithography to manufacture critical-sized 3D biodegradable scaffolds for bone ingrowth J Biomed Mater Res B Appl Biomater. Wiley Online Library; 2003;64:65–9. [DOI] [PubMed] [Google Scholar]
- 33.Chia HN, Wu BM. Recent advances in 3D printing of biomaterials. J Biol Eng. BioMed Central 2015;9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gibson I, Rosen D, Stucker B. Development of additive manufacturing technology Addit. Manuf. Technol New York: Springer; 2015. p. 19–42 [Google Scholar]
- 35.Gibson I, Rosen D, Stucker B. Generalized additive manufacturing process chain Addit. Manuf. Technol. New York: Springer; 2015. p. 43–61. [Google Scholar]
- 36.Chen B, Zhu L, Zhang F, Qiu Y. Process development and scale-up: twin-screw extrusion Dev. Solid Oral Dos. Forms Pharm. Theory Pract Second Ed. 2016. p. 821–68. [Google Scholar]
- 37.Qiu Y, He X, Zhu L, Chen B. Product and process development of solid oral dosage forms. Dev. Solid Oral Dos. Forms. 2017. p. 555–91. [Google Scholar]
- 38.Yaman U, Butt N, Sacks E, Hoffmann C. Slice coherence in a query-based architecture for 3D heterogeneous printing. Comput Des. 2016;75–76:27–38. [Google Scholar]
- 39.Low ZX, Chua YT, Ray BM, Mattia D, Metcalfe IS, Patterson DA. Perspective on 3D printing of separation membranes and comparison to related unconventional fabrication techniques J Memb Sci Elsevier; 2017;523:596–613. [Google Scholar]
- 40.Krivec M, Roshanghias A, Abram A, Binder A. Exploiting the combination of 3D polymer printing and inkjet Ag-nanoparticle printing for advanced packaging Microelectron Eng Elsevier; 2017;176:1–5. [Google Scholar]
- 41.Zhang J, Feng X, Patil H, Tiwari RV, Repka MA. Coupling 3D printing with hot-melt extrusion to produce controlled-release tablets. Int J Pharm. 2017;519:186–97. [DOI] [PubMed] [Google Scholar]
- 42.Miao S, Castro N, Nowicki M, Xia L, Cui H, Zhou X, et al. 4D printing of polymeric materials for tissue and organ regeneration Mater Today. Elsevier; 2017;20:577–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Birtchnell T, Urry J. 3D, SF and the future. Futures. Pergamon 2013;50:25–34. [Google Scholar]
- 44.Fina F, Goyanes A, Gaisford S, Basit AW. Selective laser sintering (SLS) 3D printing of medicines Int J Pharm. Elsevier; 2017;529:285–93. [DOI] [PubMed] [Google Scholar]
- 45.Peng W, Datta P, Ayan B, Ozbolat V, Sosnoski D, Ozbolat IT. 3D bioprinting for drug discovery and development in pharmaceutics Acta Biomater. Elsevier; 2017;57:26–46. [DOI] [PubMed] [Google Scholar]
- 46.Dimitrov D, Schreve K, De Beer N. Advances in three dimensional printing—state of the art and future perspectives Rapid Prototyp J. Emerald Group Publishing Limited; 2006;12:136–47. [Google Scholar]
- 47.Kozin ED, Black NL, Cheng JT, Cotler MJ, McKenna MJ, Lee DJ, et al. Design, fabrication, and in vitro testing of novel three-dimensionally printed tympanic membrane grafts. Hear Res. 2016;340:191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vaezi M, Yang S. Freeform fabrication of nanobiomaterials using 3D printing. Rapid Prototyp Biomater. 2014;16–74. [Google Scholar]
- 49.Reprap. Fused filament fabrication [Internet]. 2014. [cited 2017 Nov 8]. p. 1 Available from: http://reprap.org/wiki/Fused_filament_fabrication
- 50.Korpela J, Kokkari A, Korhonen H, Malin M, Närhi T, Seppälä J. Biodegradable and bioactive porous scaffold structures prepared using fused deposition modeling J Biomed Mater Res B Appl Biomater. Wiley online Library; 2013;101:610–9. [DOI] [PubMed] [Google Scholar]
- 51.Goyanes A, Buanz ABM, Basit AW, Gaisford S. Fused-filament 3D printing (3DP) for fabrication of tablets Int J Pharm Elsevier; 2014;476:88–92. [DOI] [PubMed] [Google Scholar]
- 52.Goyanes A, Robles Martinez P, Buanz A, Basit AW, Gaisford S. Effect of geometry on drug release from 3D printed tablets Int J Pharm Elsevier; 2015;494:657–63. [DOI] [PubMed] [Google Scholar]
- 53.Goyanes A, Buanz ABM, Hatton GB, Gaisford S, Basit AW. 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets Eur J Pharm Biopharm. Elsevier; 2015;89:157– 62. [DOI] [PubMed] [Google Scholar]
- 54.Skowyra J, Pietrzak K, Alhnan MA. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing. Eur J Pharm Sci 2015;68:11–7. Available from: http://www.sciencedirect.com/science/article/pii/S0928098714004370?via%3Dihub [DOI] [PubMed] [Google Scholar]
- 55.Pietrzak K, Isreb A, Alhnan MA. A flexible-dose dispenser for immediate and extended release 3D printed tablets Eur J Pharm Biopharm. Elsevier; 2015;96:380–7. [DOI] [PubMed] [Google Scholar]
- 56.Jamróz W, Kurek M, Łyszczarz E, Szafraniec J, Knapik-Kowalczuk J, Syrek K, et al. 3D printed orodispersible films with aripiprazole. Int J Pharm. 2017;533:413–20. [DOI] [PubMed] [Google Scholar]
- 57.Beck RCR, Chaves PS, Goyanes A, Vukosavljevic B, Buanz A, Windbergs M, et al. 3D printed tablets loaded with polymeric nanocapsules: an innovative approach to produce customized drug delivery systems. Int J Pharm. 2017;528:268–79. [DOI] [PubMed] [Google Scholar]
- 58.OConnor T, Lee S. Emerging technology for modernizing pharmaceutical production: continuous manufacturing Dev. Solid Oral Dos. Forms Pharm. Theory Pract. Second ed. 2016. p. 1031–46. [Google Scholar]
- 59.Sadia M, Arafat B, Ahmed W, Forbes RE, Alhnan MA. Channelled tablets: an innovative approach to accelerating drug release from 3D printed tablets. J Control Release. 2017;269:355–63. [DOI] [PubMed] [Google Scholar]
- 60.Chimate C, Koc B. Pressure assisted multi-syringe single nozzle deposition system for manufacturing of heterogeneous tissue scaffolds Int J Adv Manuf Technol, Springer; London: 2014;75:317–30. [Google Scholar]
- 61.Vozzi G, Flaim C, Ahluwalia A, Bhatia S. Fabrication of PLGA scaffolds using soft lithography and microsyringe deposition. Biomaterials. 2003;24:2533–40. [DOI] [PubMed] [Google Scholar]
- 62.Liu Z, Zhang M, Bhandari B, Wang Y. 3D printing: Printing precision and application in food sector Trends Food Sci Technol. Elsevier; 2017. p. 83–94. [Google Scholar]
- 63.Li Q, Guan X, Cui M, Zhu Z, Chen K, Wen H, et al. Preparation and investigation of novel gastro-floating tablets with 3D extrusion-based printing Int J Pharm. Elsevier; 2018;535:325–32. [DOI] [PubMed] [Google Scholar]
- 64.Li J, Chen M, Fan X, Zhou H. Recent advances in bioprinting techniques: approaches, applications and future prospects. J Transl Med. BioMed Central. 2016;14:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kolesky DB, Truby RL, Gladman AS, Busbee TA, Homan KA, Lewis JA. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater. 2014;26:3124–30. [DOI] [PubMed] [Google Scholar]
- 66.Colosi C, Shin SR, Manoharan V, Massa S, Costantini M, Barbetta A, et al. Microfluidic bioprinting of heterogeneous 3D tissue constructs using low-viscosity bioink. Adv Mater. 2016;28:677–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gu BK, Choi DJ, Park SJ, Kim MS, Kang CM, Kim C-H. 3-dimensional bioprinting for tissue engineering applications. Biomater Res. BioMed Central. 2016;20:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Greulich M, Greul M, Pintat T. Fast, functional prototypes via multiphase jet solidification Rapid Prototyp J MCB UP Ltd; 1995;1:20–5. Available from: http://www.emeraldinsight.com/doi/10.1108/13552549510146649 [Google Scholar]
- 69.Koch KU. Time-compression technologies ‘98 conference 1998. [Google Scholar]
- 70.Colosi C, Shin SR, Manoharan V, Massa S, Costantini M, Barbetta A, et al. Supporting information: microfluidic bioprinting of heterogeneous 3D tissue constructs using low-viscosity bioink. Adv Mater. 2016;28:677–684a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu BM, Borland SW, Giordano RA, Cima LG, Sachs EM, Cima MJ. Solid free-form fabrication of drug delivery devices. J Control Release. 1996;40:77–87. [Google Scholar]
- 72.Katstra WE, Palazzolo RD, Rowe CW, Giritlioglu B, Teung P, Cima MJ. Oral dosage forms fabricated by three dimensional printing (TM). J Control Release. 2000;66:1–9. [DOI] [PubMed] [Google Scholar]
- 73.Tan KH, Chua CK, Leong KF, Cheah CM, Gui WS, Tan WS, et al. Selective laser sintering of biocompatible polymers for applications in tissue engineering. Biomed Mater Eng. IOS Press. 2005;15:113–24. [PubMed] [Google Scholar]
- 74.Chen C-H, Lee M-Y, Shyu VB-H, Chen Y-C, Chen C-T, Chen J-P. Surface modification of polycaprolactone scaffolds fabricated via selective laser sintering for cartilage tissue engineering Mater Sci Eng C. Elsevier; 2014;40:389–97. [DOI] [PubMed] [Google Scholar]
- 75.Schmidt M, Pohle D, Rechtenwald T. Selective laser sintering of PEEK CIRP Ann Technol. Elsevier; 2007;56:205–8. [Google Scholar]
- 76.Arcaute K, Mann B, Wicker R. Stereolithography of spatially controlled multi-material bioactive poly (ethylene glycol) scaffolds Acta Biomater Elsevier; 2010;6:1047–54. [DOI] [PubMed] [Google Scholar]
- 77.Billiet T, Vandenhaute M, Schelfhout J, Van Vlierberghe S, Dubruel P. A review of trends and limitations in hydrogelrapid prototyping for tissue engineering Biomaterials. Elsevier; 2012;33:6020–41. [DOI] [PubMed] [Google Scholar]
- 78.Daly R, Harrington TS, Martin GD, Hutchings IM. Inkjet printing for pharmaceutics—a review of research and manufacturing Int J Pharm. Elsevier; 2015;494:554–67. [DOI] [PubMed] [Google Scholar]
- 79.Palo M, Kogermann K, Laidmäe I, Meos A, Preis M, Heinämäki J, et al. Development of oromucosal dosage forms by combining electrospinning and inkjet printing Mol Pharm. ACS Publications; 2017;14:808–20. [DOI] [PubMed] [Google Scholar]
- 80.Khaled SA, Burley JC, Alexander MR, Roberts CJ. Desktop 3D printing of controlled release pharmaceutical bilayer tablets Int J Pharm Elsevier; 2014;461:105–11. [DOI] [PubMed] [Google Scholar]
- 81.Knowlton S, Anand S, Shah T, Tasoglu S. Bioprinting for neural tissue engineering. Trends Neurosci. 2018;41:31–46. [DOI] [PubMed] [Google Scholar]
- 82.Jessop ZM, Al-Sabah A, Gardiner MD, Combellack E, Hawkins K, Whitaker IS. 3D bioprinting for reconstructive surgery: principles, applications and challenges J Plast Reconstr Aesthet Surg. Churchill Livingstone; 2017;70:1155–70. [DOI] [PubMed] [Google Scholar]
- 83.Ji S, Guvendiren M. Recent advances in bioink design for 3D bioprinting of tissues and organs Front Bioeng Biotechnol. Frontiers Media SA; 2017;5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aho J, Boetker JP, Baldursdottir S, Rantanen J. Rheology as a tool for evaluation of melt processability of innovative dosage forms. Int J Pharm. Elsevier 2015;494:623–42. [DOI] [PubMed] [Google Scholar]
- 85.Lewis JA, Gratson GM. Direct writing in three dimensions. Mater Today. Elsevier. 2004;7:32–9. [Google Scholar]
- 86.Tartarisco G, Gallone G, Carpi F, Vozzi G. Polyurethane unimorph bender microfabricated with pressure assisted microsyringe (PAM) for biomedical applications. Mater Sci Eng CElsevier. 2009;29:1835–41. [Google Scholar]
- 87.Smith-Moritz G “Multiphase jet solidification (MJS)”, Rapid Prototyping Report, CAD/CAM Publishing Inc. 1994;4;6. [Google Scholar]
- 88.Kennicott PR. An application reference model for layered manufacturing. Albuquerque: Sandia National Labs.; 1994. [Google Scholar]
- 89.Geiger M, Steger W, Greul M, Sindel M. Multiphase jet solidification Eur. Action Rapid Prototyp. Aarhus; 1994; EARP-Newsl. [Google Scholar]
- 90.Green body - Wikipedia [Internet]. [cited 2017 Dec 31]. Available from: https://en.wikipedia.org/wiki/Green_body
- 91.Greul M, Pintat T, Greulich M. Rapid prototyping of functional metallic parts Comput Ind. Elsevier; 1995;28:23–8. [Google Scholar]
- 92.Landers R, Mülhaupt R. Desktop manufacturing of complex objects, prototypes and biomedical scaffolds by means of computer-assisted design combined with computer-guided 3D plotting of polymers and reactive oligomers Macromol Mater Eng. Wiley subscription services, Inc., A Wiley Company; 2000;282:17–21. [Google Scholar]
- 93.Sun W, Lal P. Recent development on computer aided tissue engineering—a review Comput Methods Prog Biomed. Elsevier; 2002;67:85–103. [DOI] [PubMed] [Google Scholar]
- 94.Hollister SJ, Maddox RD, Taboas JM. Optimal design and fabrication of scaffolds to mimic tissue properties and satisfy biological constraints Biomaterials. Elsevier; 2002;23:4095–103. [DOI] [PubMed] [Google Scholar]
- 95.Hutmacher DW. Scaffolds in tissue engineering bone and cartilage Biomaterials. Elsevier; 2000;21:2529–43. [DOI] [PubMed] [Google Scholar]
- 96.Wang F, Shor L, Darling A, Khalil S, Sun W, Güçeri S, et al. Precision extruding deposition and characterization of cellular poly-e-caprolactone tissue scaffolds Rapid Prototyp J Emerald Group Publishing Limited; 2004;10:42–9. [Google Scholar]
- 97.Bellini A Fused deposition of ceramics: a comprehensive experimental, analytical and computational study of material behavior, fabrication process and equipment design. Doctrate thesis 2002. https://doi.org/10.16953/DEUSBED.74839 [Google Scholar]
- 98.Shor L, Güçeri S, Chang R, Gordon J, Kang Q, Hartsock L, et al. Precision extruding deposition (PED) fabrication of polycaprolactone (PCL) scaffolds for bone tissue engineering Biofabrication. IOP Publishing; 2009;1:15003. [DOI] [PubMed] [Google Scholar]
- 99.Ulery BD, Nair LS, Laurencin CT. Biomedical applications of biodegradable polymers. J Polym Sci B Polym Phys. 2011;49:832–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Darling A, Shor L, Sun W, Guceri S. Super-Sparger microcarrier beads and precision extrusion deposited poly-epsilon-Caprolactone structures for biological applications. Patent WO/2006/091921 31 August 2006. Available from: https://patentscope.wipo.int/search/en/detail.jsf? docId=WO2006091921
- 101.Neff M, Kessling O. Layered functional parts on an industrial scale. Kunst Int. 2014;104:40–3. [Google Scholar]
- 102.Sachs E, Cima M, Cornie J. Three-dimensional printing: rapid tooling and prototypes directly from a CAD model CIRP Ann Manuf Technol. Elsevier; 1990;39:201–4. [Google Scholar]
- 103.Aulton ME, Taylor K. Aulton’s pharmaceutics: the design and manufacture of medicines. Aulton’s pharm Des. Manuf. Med. Churchill Livingstone/Elsevier; 2013. [Google Scholar]
- 104.de Leon AC, Chen Q, Palaganas NB, Palaganas JO, Manapat J, Advincula RC. High performance polymer nanocomposites for additive manufacturing applications React Funct Polym. Elsevier; 2016;103:141–55. [Google Scholar]
- 105.Goh GL, Ma J, Chua KLF, Shweta A, Yeong WY, Zhang YP. Inkjet-printed patch antenna emitter for wireless communication application. Virtual Phys Prototyp. 2016;11:289–94. [Google Scholar]
- 106.Cima LG, Vacanti JP, Vacanti C, Ingber D, Mooney D, Langer R. Tissue engineering by cell transplantation using degradable polymer substrates J Biomech Eng American Society of Mechanical Engineers; 1991;113:143–51. [DOI] [PubMed] [Google Scholar]
- 107.Kruth JP, Wang X, Laoui T, Froyen L. Lasers and materials in selective laser sintering Assem Autom MCB UP Ltd; 2003;23:357–71. [Google Scholar]
- 108.Ligon SC, Liska R, Stampfl J, Gurr M, Mülhaupt R. Polymers for 3D printing and customized additive manufacturing. Chem Rev American Chemical Society. 2017;117:10212–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Melchels FPW, Feijen J, Grijpma DW. A review on stereolithography and its applications in biomedical engineering Biomaterials. Elsevier; 2010;31:6121–30. [DOI] [PubMed] [Google Scholar]
- 110.Jacobs PF. Rapid prototyping & manufacturing: fundamentals of stereolithography. Society of Manufacturing Engineers, in cooperation with the computer and Automated Systems Association of SME; McGraw-Hill, Inc. New York, NY, 1993. Available from: https://dl.acm.org/citation.cfm?id=541722 [Google Scholar]
- 111.Zheng X, Deotte J, Alonso MP, Farquar GR, Weisgraber TH, Gemberling S, et al. Design and optimization of a light-emitting diode projection micro-stereolithography three-dimensional manufacturing system. Rev Sci Instrum, AIP. 2012;83:125001. [DOI] [PubMed] [Google Scholar]
- 112.Sun C, Fang N, Wu DM, Zhang X. Projection microstereolithography using digital micro-mirror dynamic mask Sensors Actuators A Phys Elsevier; 2005;121:113–20. [Google Scholar]
- 113.Grogan SP, Chung PH, Soman P, Chen P, Lotz MK, Chen S, et al. Digital micromirror device projection printing system for meniscus tissue engineering Acta Biomater Elsevier; 2013;9:7218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee MP, Cooper GJT, Hinkley T, Gibson GM, Padgett MJ, Cronin L. Development of a 3D printer using scanning projection stereolithography Sci Rep Nature Publishing Group; 2015;5:9875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tumbleston JR, Shirvanyants D, Ermoshkin N, Janusziewicz R, Johnson AR, Kelly D, et al. Continuous liquid interface production of 3D objects Science (80-. ). American association for the Advancement of Science; 2015;347:1349–52. [DOI] [PubMed] [Google Scholar]
- 116.Johnson AR, Caudill CL, Tumbleston JR, Bloomquist CJ, Moga KA, Ermoshkin A, et al. Single-step fabrication of computationally designed microneedles by continuous liquid interface production. PLoS One Public Library of Science. 2016;11:e0162518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Janusziewicz R, Tumbleston JR, Quintanilla AL, Mecham SJ, DeSimone JM. Layerless fabrication with continuous liquid interface production. Proc Natl Acad Sci National Acad Sciences; 2016;201605271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gomez LPC, Spangenberg A, Ton X, Fuchs Y, Bokeloh F, Malval J, et al. Rapid prototyping of chemical microsensors based on molecularly imprinted polymers synthesized by two-photon stereolithography Adv Mater, Wiley Online Library; 2016;28:5931–7. [DOI] [PubMed] [Google Scholar]
- 119.Cumpston BH, Ananthavel SP, Barlow S, Dyer DL, Ehrlich JE, Erskine LL, et al. Two-photon polymerization initiators for three-dimensional optical data storage and microfabrication Nature Nature Publishing Group; 1999;398:51–4. [Google Scholar]
- 120.Xing J-F, Zheng M-L, Duan X-M. Two-photon polymerization microfabrication of hydrogels: an advanced 3D printing technology for tissue engineering and drug delivery. Chem Soc Rev Royal Society of Chemistry. 2015;44:5031–9. [DOI] [PubMed] [Google Scholar]
- 121.Claeyssens F, Hasan EA, Gaidukeviciute A, Achilleos DS, Ranella A, Reinhardt C, et al. Three-dimensional biodegradable structures fabricated by two-photon polymerization Langmuir. ACS Publications; 2009;25:3219–23. [DOI] [PubMed] [Google Scholar]
- 122.Weiβ T, Hildebrand G, Schade R, Liefeith K. Two-photon polymerization for microfabrication of three-dimensional scaffolds for tissue engineering application Eng Life Sci Wiley Online Library; 2009;9:384–90. [Google Scholar]
- 123.Truby RL, Lewis JA. Printing soft matter in three dimensions. Nature Nature Research. 2016;540:371–8. [DOI] [PubMed] [Google Scholar]
- 124.Liska R, Schuster M, Inführ R, Turecek C, Fritscher C, Seidl B, et al. Photopolymers for rapid prototyping J Coat Technol Res Springer; 2007;4:505–10. [Google Scholar]
- 125.Schuster M, Turecek C, Kaiser B, Stampfl J, Liska R, Varga F. Evaluation of biocompatible photopolymers I: photoreactivity and mechanical properties of reactive diluents J Macromol Sci A Taylor & Francis; 2007;44:547–57. [Google Scholar]
- 126.Schuster M, Turecek C, Mateos A, Stampfl J, Liska R, Varga F. Evaluation of biocompatible photopolymers II: further reactive diluents Monatshefte für Chemie/Chemical Mon. Springer, 2007;138:261–8. [Google Scholar]
- 127.Channasanon S, Udomkusonsri P, Chantaweroad S, Tesavibul P, Tanodekaew S. Gentamicin released from porous scaffolds fabricated by stereolithography. J Healthc Eng Hindawi. 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang J, Goyanes A, Gaisford S, Basit AW. Stereolithographic (SLA) 3D printing of oral modified-release dosage forms Int J Pharm. Elsevier; 2016;503:207–12. [DOI] [PubMed] [Google Scholar]
- 129.Le HP. Progress and trends in ink-jet printing technology. J Imaging Sci Technol Society for Imaging Science Technology. 1998;42:49–62. [Google Scholar]
- 130.Gudapati H, Dey M, Ozbolat I. A comprehensive review on droplet-based bioprinting: past, present and future Biomaterials. Elsevier; 2016;102:20–42. [DOI] [PubMed] [Google Scholar]
- 131.Tasoglu S, Demirci U. Bioprinting for stem cell research Trends Biotechnol. Elsevier; 2013;31:10–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Derby B Bioprinting: inkjet printing proteins and hybrid cell-containing materials and structures. J Mater Chem Royal Society of Chemistry. 2008;18:5717–21. [Google Scholar]
- 133.Derby B Inkjet printing of functional and structural materials: fluid property requirements, feature stability, and resolution. Annu Rev Mater Res. Annual Reviews. 2010;40:395–414. [Google Scholar]
- 134.Sutanto E, Shigeta K, Kim YK, Graf PG, Hoelzle DJ, Barton KL, et al. A multimaterial electrohydrodynamic jet (E-jet) printing system J Micromech Microeng IOP Publishing; 2012;22:45008. [Google Scholar]
- 135.Hayati I, Bailey AI, Tadros TF. Mechanism of stable jet formation in electrohydrodynamic atomization Nature Springer; 1986;319:41–3. [Google Scholar]
- 136.Demirci U Acoustic picoliter droplets for emerging applications in semiconductor industry and biotechnology. J Microelectromech Syst IEEE. 2006;15:957–66. [Google Scholar]
- 137.Moon S, Hasan SK, Song YS, Xu F, Keles HO, Manzur F, et al. Layer by layer three-dimensional tissue epitaxy by cell-laden hydrogel droplets Tissue Eng Part C Methods. Mary Ann Liebert, Inc. 140 Huguenot Street, 3rd Floor New Rochelle, NY 10801 USA: 2009;16:157–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Faulkner-Jones A, Greenhough S, King JA, Gardner J, Courtney A, Shu W. Development of a valve-based cell printer for the formation of human embryonic stem cell spheroid aggregates Biofabrication. IOP Publishing; 2013;5:15013. [DOI] [PubMed] [Google Scholar]
- 139.Gao G, Cui X. Three-dimensional bioprinting in tissue engineering and regenerative medicine Biotechnol Lett Springer; 2016;38:203–11. [DOI] [PubMed] [Google Scholar]
- 140.Xu F, Wu J, Wang S, Durmus NG, Gurkan UA, Demirci U. Microengineering methods for cell-based microarrays and high-throughput drug-screening applications. Biofabrication. IOP Publishing; 2011;3:34101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xu F, Celli J, Rizvi I, Moon S, Hasan T, Demirci U. A three-dimensional in vitro ovarian cancer coculture model using a high-throughput cell patterning platform Biotechnol J Wiley Online Library; 2011;6:204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Clark EA, Alexander MR, Irvine DJ, Roberts CJ, Wallace MJ, Sharpe S, et al. 3D printing of tablets using inkjet with UV photoinitiation Int J Pharm Elsevier; 2017;529:523–30. [DOI] [PubMed] [Google Scholar]
- 143.Kyobula M, Adedeji A, Alexander MR, Saleh E, Wildman R, Ashcroft I, et al. 3D inkjet printing of tablets exploiting bespoke complex geometries for controlled and tuneable drug release J Control Release Elsevier; 2017;261:207–15. [DOI] [PubMed] [Google Scholar]
- 144.Liaw C-Y, Guvendiren M. Current and emerging applications of 3D printing in medicine. Biofabrication. 2017;9:24102. [DOI] [PubMed] [Google Scholar]
- 145.Preis M, Öblom H. 3D-printed drugs for children—are we ready yet? AAPS PharmSciTech Springer; US: 2017;18:303–8. [DOI] [PubMed] [Google Scholar]
- 146.Shirazi SFS, Gharehkhani S, Mehrali M, Yarmand H, Metselaar HSC, Adib Kadri N, et al. A review on powder-based additive manufacturing for tissue engineering: selective laser sintering and inkjet 3D printing. Sci Technol Adv Mater. 2015;16:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bai J, Zhang B, Song J, Bi G, Wang P, Wei J. The effect of processing conditions on the mechanical properties of polyethylene produced by selective laser sintering. Polym Test. 2016;52:89–93. [Google Scholar]
- 148.Millennium N, Based R, Seite A, Practice GM, Approach R, Management MSR, et al. Pharmaceutical cGMPs for the 21 st Century : A Risk-Based Approach; 2003;21–3. [Google Scholar]
- 149.Tomba E, Facco P, Bezzo F, Barolo M. Latent variable modeling to assist the implementation of quality-by-design paradigms in pharmaceutical development and manufacturing: a review Int J Pharm. Elsevier; 2013;457:283–97. [DOI] [PubMed] [Google Scholar]
- 150.Guerra AJ, Ciurana J. 3D-printed bioabsordable polycaprolactone stent: the effect of process parameters on its physical features Mater Des Elsevier; 2018;137:430–7. [Google Scholar]
- 151.Liravi F, Toyserkani E. A hybrid additive manufacturing method for the fabrication of silicone bio-structures: 3D printing optimization and surface characterization Mater Des Elsevier; 2018;138:46–61. [Google Scholar]
- 152.Food and Drug Administration. Technical Considerations for Additive Manufactured Devices; draft guidance for industry and Food and Drug Administration staff. FDA; 2016 [Google Scholar]


