Abstract
Background.
Research indicates that DM1 are at increased risk of cancer and early death. Family data may provide insights given DM1 phenotypic heterogeneity, the broad range of non-muscular manifestations, and the usual delays in the diagnosis of DM1.
Method.
We collected family history data from 397 genetically- and/or clinically-confirmed DM1 patients (respondents) enrolled in the US or UK myotonic dystrophy registries. We calculated standardized mortality ratios for DM1 first-degree relatives (parents, siblings, and offspring) by their reported DM1 status (affected, unaffected, or unknown). For cancer-related analyses, we used mixed effects logistic regression models to evaluate factors associated with cancer development in DM1 families, including familial clustering.
Results.
467 deaths and 337 cancers were reported among 1,737 first-degree DM1 relatives. Mortality risk among relatives reported as DM1-unaffected was comparable to that of the general population (Standardized Mortality Ratio, SMR=0.82, p=0.06), while significantly higher mortality risks were noted in DM1-affected relatives (SMR=2.47, p<0.0001), and in those whose DM1-status was unknown (SMR=1.60, p<0.0001). In cancer risk analyses, risk was higher among families in which the DM1 respondent had cancer (OR=1.95, p=0.0001). Unknown-DM1 status in the siblings (OR=2.59, p=0.004) was associated with higher cancer risk.
Conclusion.
There is an increased risk of death, and probably cancer, in relatives with DM1 and those whose DM1 status is unkown. This suggests a need to perform a careful history and physical, supplemented by genetic testing, to identify family members at-risk for DM1 and who might benefit from disease specific, clinical care and surveillance.
Keywords: Myotonic dystrophy type I, mortality, cancer, tumor, genetic testing
INTRODUCTION
Myotonic dystrophy type 1 (DM1; OMIM 160900) is an autosomal dominant multisystem disorder characterized by progressive muscle weakness and myotonia [1]. The disease is caused by unstable CTG nucleotide repeat expansions in the 3’ untranslated region of the dystrophia myotonica-protein kinase (DMPK) gene on chromosome 19q13 [2–4] and characterized by genetic anticipation, in which offspring present at earlier ages and with more severe phenotypes than their parents [5].
Common, non-muscular clinical manifestations of DM1 include cataracts, cardiac conduction defects, respiratory insufficiency, sleep disturbances, central nervous system involvement, endocrine and gastrointestinal abnormalities [1, 6]. Life expectancy in DM1 patients is significantly reduced; median age-at-death is early-to-late 50s, with respiratory and cardiac complications, followed by malignancy comprising the main causes [7, 8]. Diagnostic delays and misdiagnosis are common occurrences in DM1, particularly in patients with mild or atypical clinical presentations; a recent study reported average diagnostic delays of 7 years [9].
Recent studies have provided evidence that DM1 patients are at high risk of certain cancers. The evidence is strongest for cancers of the endometrium, cutaneous melanoma, and thyroid [10–14], followed by cancers of the ovary, brain [10, 13], testis [11, 12], and possibly basal cell carcinoma of the skin [15]. Despite reports of the high relative risks associated with these cancers, their absolute risks are relatively modest [8, 16]. It has been suggested that cancer incidence in DM1 patients is obscured by the high competing death rates from non-cancer causes [8]. The risk factors and molecular mechanisms of DM1-carcinogenesis are largely unexplored, but are hypothesized to result from a genetic predisposition to cancer that is driven by specific aspects of DM1 patho-physiology [17].
Family studies in extended DM1 pedigrees are limited despite their potential to better characterize the full disease phenotype. Such studies may be of particular importance for severe non-muscular phenotypes that may appear prior to diagnosis. For example, cancer incidence studies in DM1 patients showed a high frequency of cancers prior to disease diagnosis [10, 16]. Similarly, a nationwide study from Denmark showed that the first year after DM1 diagnosis carried the highest risk of a new cardiac diagnosis suggesting that pre-existing prevalent conditions may be detected during the DM1 workup [18]. Also, the high risk of sudden death noted in patients with DM1[19], has recently been shown to be associated with a family history of sudden death [20].
In the current study, we evaluated risk of all-cause mortality and assessed determinants of cancer development in first-degree relatives of respondents in a large DM1 cohort.
METHODS
Data Collection and Study Participants:
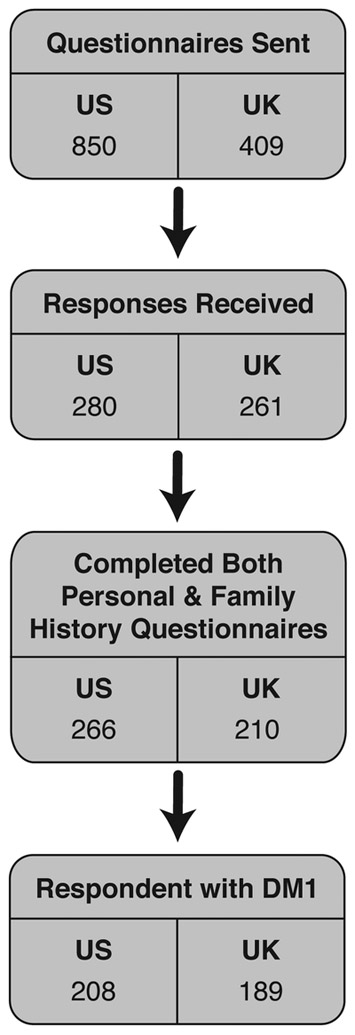
This study is a collaboration between the Clinical Genetics Branch, US National Cancer Institute, the University of Rochester, and Newcastle University. Detailed study design and patient characteristics have been reported [21, 22]. Briefly, we delivered study questionnaires to genetically- and/or clinically-confirmed DM patients from the US National Registry of Myotonic Dystrophy and Facioscapulohumeral Muscular Dystrophy Patients and Family Members [23] (US DM Registry; N=850) and the UK DM registry (N=409) [24]; a follow-up mail survey was sent to non-responders from both registries. The questionnaire collected personal history of benign and malignant tumors, selected lifestyle factors, and selected family history information. A total of 541 DM subjects responded to the questionnaire (US=280 and UK=261). We excluded patients with DM2, and those who did not complete either the family history or personal cancer history questionnaire, to ensure high quality data. The analysis included 397 DM1 patients (genetically confirmed=193; 48.6%) reporting on 1,737 first-degree relatives. Figure 1 represents a flow chart illustrating the patient selection process.
Figure 1:
Flow Chart of Patient Study Inclusion.
The family history questionnaire collected the following information from study participants regarding their first-degree relatives (parents, siblings, and offspring): year of birth, sex, DM1 status (affected, unaffected, unknown), history of cancer/tumor diagnosis (yes, no), cancer site and age at diagnosis, vital status, and year of death, if applicable. Tumor reports were reviewed and only malignant tumors were included in this analysis.
The study was approved by the Ethics Committees of the University of Rochester, Newcastle University, and the National Institutes of Health Office of Human Subjects Research. All patients provided informed consent prior to their participation.
Statistical Analysis:
Characteristics of the US and UK DM1 patients included in this study were compared using Fisher’s Exact and Wilcoxon Rank Sum tests.
Survival estimates were compared with population life-tables published by the US Centers for Disease Control available at (https://www.cdc.gov/nchs/data/nvsr/nvsr66/nvsr66_03.pdf), and the UK Office for National Statistics available at (https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesunitedkingdomreferencetables).
All analyses were conducted using R statistical software. Standardized mortality ratios (SMR), with corresponding 95% confidence intervals (CI), were calculated using the package ‘ems,’ version 1.0.0 [25]. Survival analyses were conducted using the ‘survival’ package, version 2.38 [26]. Generalized linear mixed models were fit using the ‘lme4’ package, version 1.1–15 [27]. Expected numbers of deaths among first-degree relatives were obtained using the published age-, and country-specific life tables cited above.
All estimates were stratified by reported DM1 status (affected, unaffected, or unknown). In parental dyads with one parent reported as DM1-affected and the other as DM1-unknown (n=16), we designated the second parent as DM1-unaffected, due to the population rarity of DM1 mutations. In parents both reported as DM1-unknown (n=65), or DM1-unaffected (n=43), both parents were assigned DM1-unknown status. DM1 status of siblings and offspring was used as reported.
We used multivariable mixed effects logistic regression for cancer risk models, clustering on family groups. The analysis focused on first reported cancer modeled as a dichotomous response (yes/no). Final models included country of data collection, reported DM1 status, sex of the relative, relationship status to the respondents, and cancer history in the respondent. Analyses were conducted for all family members combined, and were also stratified by relationship to the respondent. A p-value of <0.05 was considered statistically signicant for this study.
RESULTS
The study included 208 US, and 189 UK patients reporting on 693 parents, 656 siblings, 388 offspring). US and UK DM1 patients were comparable for age at questionnaire completion, DM1 paternal transmission, ages at DM1 onset and diagnosis, and sex (Table 1). More US DM1 patients with a history of cancer responded to the questionnaire versus the UK (US: N=62, 30%; UK: N=11; 6%; p-Fisher’s exact <0.0001). US participants had more children (p=0.004, Wilcoxon rank sum) and siblings (p<0.0001, Wilcoxon) than did UK respondents (Table 1). Among family members, 640 were reported to be DM1-affected (219 parents, 262 siblings, and 159 offspring), 651 unaffected (214 parents, 300 siblings, and 137 offspring), and 446 with DM1-unknown status (260 parents, 94 siblings, and 92 offspring).
Table 1:
Description of characteristics of the US and UK Myotonic Dystrophy Type 1 (DM1) study participants.
| US Cohort | UK Cohort | |
|---|---|---|
| Number of Participants with DM1 | 208 | 189 |
| Age at Interview;(years; Median (IQR))* |
53 (18.0) | 47 (19.5) |
| DM Inheritance: | ||
| Maternal | 47 (23%) | 35 (19%) |
| Paternal | 71 (34%) | 83 (44%) |
| Unknown | 90 (43%) | 71 (38%) |
| Age at Onset (years; Median (IQR))* |
25.5 (23.0) | 27.0 (20.5) |
| Age at Diagnosis (years; Median (IQR))* |
33.0 (23) | 33.0 (18) |
| Sex (% Female) | 115 (55%) | 98 (52%) |
| Personal History of Cancer (n/% Yes) |
62 (30%) | 11 (6%) |
| Personal History of benign Tumor(n/% Yes) |
34 (16%) | 17 (9%) |
| Number of Children: | ||
| 0 | 99 (48%) | 106 (56%) |
| 1 | 34 (16%) | 28 (15%) |
| 2 | 42 (20%) | 37 (20%) |
| ≥3 | 32 (15%) | 18 (10%) |
| Number of Siblings: | ||
| 0 | 18 (9%) | 41 (22%) |
| 1 | 62 (30%) | 73 (39%) |
| 2 | 58 (28%) | 52 (28%) |
| 3 | 35 (17%) | 9 (5%) |
| ≥4 | 35 (17%) | 7 (4%) |
IQR: inter-quartile range; defined as the difference between the 75th and 25th percentiles of the observed quantity.
Survival probability in first-degree relatives of DM1 patients
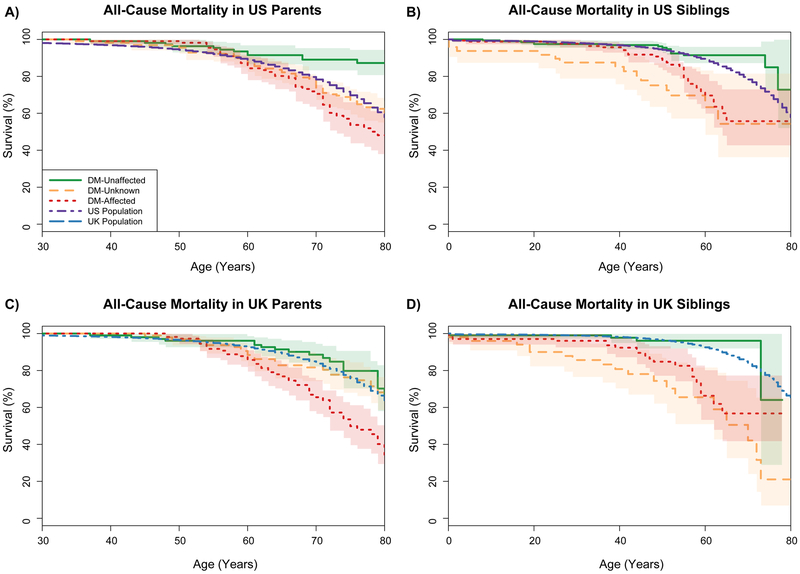
Four-hundred sixty-seven deaths were reported in patients’ first-degree relatives (296 in parents, 150 in siblings, and 21 in offspring). Figure 2 summarizes survival estimates in DM1 relatives by their reported DM1-status compared with corresponding general population estimates. There was no statistical significant difference in overall survival of DM1-unaffected relatives versus the general population (standardized mortality ratio, SMR=0.82, 95%CI=0.76–1.01, p=0.06). Relatives reported as DM1-affected or DM1-unknown were at increased mortality risk relative to the general population (SMR=2.47, 95%CI=2.29–2.67, p<0.0001 in DM1-affected relatives, and SMR=1.60, 95%CI=1.48–1.74, p<0.0001 in relatives with DM1-unknown status). For DM1-affected relatives, mortality risk increased in subsequent generations (parents: SMR=2.1, 95%CI=1.90–2.31, siblings: SMR=3.11, 95%CI=2.69–3.59, and offspring: SMR=5.53, 95%CI=4.53–6.75 in offspring). Detailed risk estimates by relationship and country of data collection are summarized in Table 2.
Figure 2:
Overall Survival in Parents (A, C) and Siblings (B, D) of DM1 respondents, by country (US: A, B; UK: C, D) and reported DM1 status, with overall population survival. Among siblings: green solid curves display survival among family members reported as DM-unaffected, orange dashed for family members reported as DM-unknown, and red dotted for family members reported as DM-affected.
Among parents: red curves correspond to parents reported as DM-affected, green curves to parents reported as DM-affected, and orange curves to parents from pairs in which both parents were reported as DM-unknown or DM-unaffected.
General population survival: in the US is depicted as a dashed purple line, and the UK is depicted as a dashed blue line.
Table 2:
Standardized Mortality Ratios (SMR) for DM1-affected Relatives by their Reported DM1-status
| DM1-Unaffected | DM1-Affected | DM1-Unknown | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| O | E | SMR (95% CI) | O | E | SMR (95% CI) | O | E | SMR (95% CI) | ||
| Parents | US | 29 | 38.27 | 0.76 (0.56, 1.02) | 55 | 32.32 | 1.70 (1.47, 1.97) | 85 | 64.47 | 1.32 (1.17, 1.48) |
| UK | 25 | 23.02 | 1.09 (0.81, 1.46) | 57 | 21.05 | 2.71 (2.38, 3.08) | 45 | 31.59 | 1.42 (1.20, 1.69) | |
| Total | 54 | 61.29 | 0.88 (0.71, 1.09) | 112 | 53.36 | 2.10 (1.90, 2.31) | 130 | 96.06 | 1.35 (1.23, 1.49) | |
| Siblings | US | 16 | 21.03 | 0.76 (0.46, 1.26) | 32 | 12.14 | 2.64 (2.16, 3.22) | 15 | 3.88 | 3.87 (3.06, 4.90) |
| UK | 4 | 4.64 | 0.86 (0.32, 2.34) | 20 | 4.58 | 4.37 (3.58, 5.34) | 18 | 2.95 | 6.11 (5.18, 7.21) | |
| Total | 20 | 25.67 | 0.78 (0.50, 1.22) | 52 | 16.72 | 3.11 (2.69, 3.59) | 33 | 6.82 | 4.84 (4.21, 5.56) | |
| Offspring | US | 0 | 2.27 | - | 6 | 2.22 | 2.70 (1.67, 4.36) | 1 | 1.01 | 0.99 (0.14, 6.90) |
| UK | 0 | 0.59 | - | 11 | 0.85 | 12.93 (10.98, 15.22) | 3 | 0.38 | 7.87 (5.27, 11.76) | |
| Total | 0 | 2.86 | - | 17 | 3.07 | 5.53 (4.53, 6.75) | 4 | 1.39 | 2.87 (1.62, 5.08) | |
| Total | 74 | 89.82 | 0.82 (0.67, 1.01) | 181 | 73.16 | 2.47 (2.29, 2.67) | 167 | 104.27 | 1.60 (1.48, 1.74) | |
Abbreviations: O (observed), E (expected), SMR (standardized mortality ratio), CI (confidence interval)
Cancer risk and determinants in first-degree relatives of DM1 patients
Cancer was reported in 337 relatives (233 parents, 81 siblings, 23 offspring). Relatives affected with DM1 and those reported as having unknown DM1-status developed cancers more frequently than those reported as DM1-unaffected (21.8% vs. 15.4%, p<0.001). In analyses adjusted for country of data collection, cancer risks in DM1-affected relatives or those with unknown status were modestly but not significantly elevated (OR=1.28 and 1.26, respectively). Risk was significantly higher in parents (OR=8.4, 95%CI=5.25–13.46, p<0.0001), and siblings (OR=2.39, 95%CI=1.47–3.91, p=0.0004) than offspring, and in families whose DM respondent had cancer (OR=1.95, 95%CI=1.39–2.73, p=0.0001) (Table 3). In analyses stratified by relationship, excess cancer risks in the DM1-unknown family members were noted in siblings (OR=2.59, 95% CI=1.37–4.88, p=0.004) and probably offspring (OR=2.68, 95%CI=0.81–8.86, p=0.1), but not in parents (OR=1.15, 95%CI=0.77–1.7, p=0.5). The cancer risks were elevated among parents of DM1 respondent with cancer (OR=2.65, 95%CI=1.76–4.01, p<0.0001), and possibly siblings (OR=1.43, 95%CI=0.80–2.54, p=0.2) but not among offspring (OR=0.77, 95%CI=0.26–2.28, p=0.6) (Table 3).
Table 3:
Results of multivariable logistic regression models for probability of cancer in all relatives combined (a) and stratified by their relationship to the reporting respondent (b-d)
| Covariate |
a) All 1st-Degree Relatives |
b) Parents | c) Siblings | d) Offspring | |||||
|---|---|---|---|---|---|---|---|---|---|
|
Odds Ratio |
95% CI |
Odds Ratio |
95% CI |
Odds Ratio |
95% CI |
Odds Ratio |
95% CI | ||
| US vs. UK | 1.42 | 1.06-1.91 | 1.09 | 0.77, 1.54 | 1.61 | 0.90, 2.90 |
2.51 | 0.83, 7.55 | |
|
Proband Cancer (Yes vs. No) |
1.95 | 1.39, 2.73 | 2.65 | 1.76, 4.01 | 1.43 | 0.80, 2.54 |
0.77 | 0.26, 2.28 | |
|
DM Status (vs. Unaffected) |
Unknown | 1.26 | (0.90, 1.75) | 1.15 | 0.77, 1.72 | 2.59 | 1.37, 4.88 |
2.68 | 0.81, 8.86 |
| Affected | 1.28 | (0.94, 1.75) | 1.11 | 0.72, 1.69 | 1.23 | 0.72, 2.12 |
1.71 | 0.53, 5.57 | |
| Male vs. female | 0.96 | (0.74, 1.24) | 1.44 | 1.03, 2.01 | 0.66 | 0.41, 1.08 |
0.36 | 0.13, 0.98 | |
DISCUSSION
In this study, we evaluated mortality risk and cancer determinants in first-degree relatives of DM1 patients. As expected, relatives who were themselves reported to have DM1 demonstrated higher mortality risk than the general population. For relatives with unknown-DM1 status (most likely comprised of a mixture of unaffected and affected individuals with an anticipated milder or atypical phenotype), mortality risks were comparable to or higher than those observed in DM1-affected individuals. Similarly, cancers were more frequently reported for DM1-affected and DM1-unknown relatives than for DM1-unaffected individuals, and risk was higher among families in which the DM1 respondent had cancer.
In agreement with previous literature [28, 29], our study showed a higher mortality risk in DM1-affected individuals. The higher mortality risks in subsequent generations (SMR=2.1 in parents, 3.1 in siblings, and 5.5 in offspring) likely reflect the known genetic anticipation phenomenon in patients with DM1.
Data from DM1 relatives whose DM1 status was unknown to the family’s respondent raise important questions related to the possible contribution of a non-muscular DM1-related phenotype in patient clinical outcome. The survival curve for parents of unknown DM1 status (when the DM1 status of one or both parents could not be determined) followed the expected Mendelian distribution of an equal mix of affected and unaffected (Supplemental Figure 1). This might be anticipated; however, these individuals were described as unknown-DM1 status by their children, who may not have recognized a less severe or delayed onset of manifestations of DM1 in their parents. In untested siblings and offspring those who were reported as unknown (possibly with a milder or atypical phenotype) had higher mortality risk than expected based on age- and country-specific survival data. Similarly, risk of cancer was higher in siblings and offspring reported to have a DM1-unknown status than DM1-affected or unaffected. These findings highlight the importance of considering DM1 genetic testing among relatives to facilitate early diagnosis and proper clinical management for serious disease phenotypes that may appear before, or in the absence of, the classic clinical DM1 phenotype.
Results from the multivariable cancer risk models showed an association between cancer status in DM1 respondent and that of their first-degree relatives. This suggests familial aggregation of cancer in DM1 families, which one would expect if the cancer phenotype is caused by the DM1 genotype. Familial cancer clustering is one of the hallmarks of cancer predisposition syndromes [30]. However, the association in our study was seen only in parents and was not in siblings or offspring. A previous large population-based study of cancer risk in family members of DM1 patients suggested that cancer risk in DM families (disease subtype was not available) was driven by individuals’ DM status [31].
The strengths of the current study include its large sample size and broad representation of DM1 patients through analyzing extended family data. We implemented several measures to ensure that we control for possible differences between patients enrolled from different countries, including: using of the same questionnaire, harmonizing of variables that we added from individual registry databases, when needed, standardizing mortality rates to country-specific rates, and adjusting for country of data collection in our multivariable models. The study is limited by the self-reported nature of the cancer and DM1 diagnostic data available. To ensure better data quality and to minimize misclassification, we focused on first-degree relatives, excluded family members of patients who did not report on their personal history of cancer, and evaluated all cancers combined rather than organ-specific cancers. Our results showing no significant mortality differences between relatives reported as DM1-unaffected are consistent with valid, reliable death reporting for family members, but under-reporting of family history of cancer is possible. A previous study evaluating the validity of population-based reporting of cancer family history concluded that reporting was not highly accurate, but higher validity for first-degree versus second-degree relatives was noted. [32] Patients included in this study may not be representative of the general DM1 patient population because of the voluntary enrollment nature of the registries. Similarly, the low response rate among US and moderate response rate among UK registry members may reduce the representativeness of our data, as seen by the oversampling of respondents with past cancer/tumor history in the US cohort. However, the focus of our analysis was on family members from at least 2 generations, which may minimize concerns regarding DM1 non-representativeness, because of its intergenerational phenotypic variation.
CONCLUSIONS
Our study showed that family members reported as DM1-affected, or DM1-unknown experienced increased mortality relative to the general population. The high risk of cancer and mortality in relatives with DM1-unknown status underscores the importance of a careful history and physical supplemented by genetic testing among unevaluated, first-degree relatives, to identify those individuals who might warrant DM1-related surveillance. Although such family members may not be readily recognized as classically affected due to their atypical syndromic presentation, their identification permits implementation of appropriate medical surveillance required to prevent and manage other DM1-related disease manifestations.
Supplementary Material
ACKNOWLEGEMENT
We thank the patients for investing the time and energy required to complete our study questionnaires, without which this analysis would not have been possible.
FUNDING SOURCE
This work was supported by the NCI Intramural Research Program, Division of Cancer Epidemiology and Genetics. The US Registry received support for this study through NCI contract # HHSN261201200318P and general support through the National Institute of Neurological Disorders and Stroke (NIH Senator Paul D. Wellstone Muscular Dystrophy Cooperative Research Center grant #U54-NS048843), the Saunders Family Foundation, and the Abrams Family Fund. The UK Registry received support for this study through NCI contract # HHSN261201400556P and general support from the Myotonic Dystrophy UK (MDUK) and Myotonic Dystrophy Support Group (MDSG).
Footnotes
CONFLICTS OF INTEREST
None
REFERENCE
- [1].Thornton CA. Myotonic dystrophy. Neurol Clin. 2014. 32: 705–719, viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fu YH, Pizzuti A, Fenwick RG Jr., et al. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science. 1992. 255: 1256–1258. [DOI] [PubMed] [Google Scholar]
- [3].Brook JD, Mccurrach ME, Harley HG, et al. Molecular-Basis of Myotonic-Dystrophy - Expansion of a Trinucleotide (Ctg) Repeat at the 3’ End of a Transcript Encoding a Protein-Kinase Family Member. Cell. 1992. 68: 799–808. [DOI] [PubMed] [Google Scholar]
- [4].Mahadevan M, Tsilfidis C, Sabourin L, et al. Myotonic dystrophy mutation: an unstable CTG repeat in the 3’ untranslated region of the gene. Science. 1992. 255: 1253–1255. [DOI] [PubMed] [Google Scholar]
- [5].Redman JB, Fenwick RG, Jr., Fu YH, Pizzuti A, Caskey CT. Relationship between parental trinucleotide GCT repeat length and severity of myotonic dystrophy in offspring. JAMA. 1993. 269: 1960–1965. [PubMed] [Google Scholar]
- [6].Meola G, Sansone V. Cerebral involvement in myotonic dystrophies. Muscle Nerve. 2007. 36: 294–306. [DOI] [PubMed] [Google Scholar]
- [7].de Die-Smulders CE, Howeler CJ, Thijs C, et al. Age and causes of death in adult-onset myotonic dystrophy. Brain. 1998. 121 (Pt 8): 1557–1563. [DOI] [PubMed] [Google Scholar]
- [8].Gadalla SM, Pfeiffer RM, Kristinsson SY, et al. Quantifying cancer absolute risk and cancer mortality in the presence of competing events after a myotonic dystrophy diagnosis. PLoS One. 2013. 8: e79851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hilbert JE, Ashizawa T, Day JW, et al. Diagnostic odyssey of patients with myotonic dystrophy. J Neurol. 2013. 260: 2497–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gadalla SM, Lund M, Pfeiffer RM, et al. Cancer risk among patients with myotonic muscular dystrophy. JAMA. 2011. 306: 2480–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Win AK, Perattur PG, Pulido JS, Pulido CM, Lindor NM. Increased cancer risks in myotonic dystrophy. Mayo Clin Proc. 2012. 87: 130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Abbott D, Johnson NE, Cannon-Albright LA. A Population-based survey of risk for cancer in individuals diagnosed with myotonic dystrophy. Muscle Nerve. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fernandez-Torron R, Garcia-Puga M, Emparanza JI, et al. Cancer risk in DM1 is sex-related and linked to miRNA-200/141 downregulation. Neurology. 2016. 87: 1250–1257. [DOI] [PubMed] [Google Scholar]
- [14].Mohamed S, Pruna L, Kaminsky P. Augmentation du risque de tumeurs dans la dystrophie myotonique de type 1. Presse Med. 2013. 42: e281–e284. [DOI] [PubMed] [Google Scholar]
- [15].Wang Y, Pfeiffer RM, Alsaggaf R, et al. Risk of skin cancer among patients with myotonic dystrophy type 1 based on primary care physician data from the U.K. Clinical Practice Research Datalink. Int J Cancer. 2018. 142: 1174–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gadalla SM, Pfeiffer RM, Kristinsson SY, Bjorkholm M, Landgren O, Greene MH. Brain tumors in patients with myotonic dystrophy: a population-based study. Eur J Neurol. 2016. 23: 542–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mueller CM, Hilbert JE, Martens W, Thornton CA, Moxley RT, 3rd, Greene MH. Hypothesis: neoplasms in myotonic dystrophy. Cancer Causes Control. 2009. 20: 2009–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lund M, Diaz LJ, Ranthe MF, et al. Cardiac involvement in myotonic dystrophy: a nationwide cohort study. Eur Heart J. 2014. 35: 2158–2164. [DOI] [PubMed] [Google Scholar]
- [19].Groh WJ, Groh MR, Saha C, et al. Electrocardiographic abnormalities and sudden death in myotonic dystrophy type 1. N Engl J Med. 2008. 358: 2688–2697. [DOI] [PubMed] [Google Scholar]
- [20].Wahbi K, Babuty D, Probst V, et al. Incidence and predictors of sudden death, major conduction defects and sustained ventricular tachyarrhythmias in 1388 patients with myotonic dystrophy type 1. Eur Heart J. 2017. 38: 751–758. [DOI] [PubMed] [Google Scholar]
- [21].Gadalla SM, Hilbert JE, Martens WB, Givens S, Moxley RT, 3rd, Greene MH. Pigmentation phenotype, photosensitivity and skin neoplasms in patients with myotonic dystrophy. Eur J Neurol. 2017. 24: 713–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Alsaggaf R, Wang Y, Bettolo C, et al. Benign and Malignant Tumors in the UK Myotonic Dystrophy Patient Registry. Muscle Nerve. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hilbert JE, Kissel JT, Luebbe EA, et al. If you build a rare disease registry, will they enroll and will they use it? Methods and data from the National Registry of Myotonic Dystrophy (DM) and Facioscapulohumeral Muscular Dystrophy (FSHD). ContempClinTrials. 2012. 33: 302–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wood L, Cordts I, Atalaia A, et al. The UK Myotonic Dystrophy Patient Registry: facilitating and accelerating clinical research. J Neurol. 2017. 264: 979–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Borges L, Brasil P. Epimed Solutions Collection for Data Editing, Analysis, and Benchmarking of Health Units. R package version 1.0.0. . 2017. [Google Scholar]
- [26].Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model : Springer, New York, 2000. [Google Scholar]
- [27].Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software. 2015. 67. [Google Scholar]
- [28].Mladenovic J, Pekmezovic T, Todorovic S, et al. Survival and mortality of myotonic dystrophy type 1 (Steinert’s disease) in the population of Belgrade. EurJNeurol. 2006. 13: 451–454. [DOI] [PubMed] [Google Scholar]
- [29].Mathieu J, Allard P, Potvin L, Prevost C, Begin P. A 10-year study of mortality in a cohort of patients with myotonic dystrophy. Neurology. 1999. 52: 1658–1662. [DOI] [PubMed] [Google Scholar]
- [30].Lindor NM, McMaster ML, Lindor CJ, Greene MH, National Cancer Institute DoCPCO, Prevention Trials Research G. Concise handbook of familial cancer susceptibility syndromes - second edition. J Natl Cancer Inst Monogr. 2008: 1–93. [DOI] [PubMed] [Google Scholar]
- [31].Lund M, Diaz LJ, Gortz S, et al. Risk of cancer in relatives of patients with myotonic dystrophy: a population-based cohort study. Eur J Neurol. 2014. 21: 1192–1197. [DOI] [PubMed] [Google Scholar]
- [32].Mai PL, Garceau AO, Graubard BI, et al. Confirmation of family cancer history reported in a population-based survey. J Natl Cancer Inst. 2011. 103: 788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.