Abstract
Background:
Infants born with critical congenital heart disease (cCHD) who require surgical intervention in the newborn period are often hospitalized in a Cardiac Intensive Care Unit (CICU). Cardiac surgery and the CICU environment are traumatic to infants and their families. Infants are exposed to overwhelming stress which can result in increased pain, physiologic instability, behavioral disorganization, disrupted attachment, and altered brain development. Individualized Family-centered Developmental Care (IFDC) is a model that can address the unique needs and developmental challenges of infants with cCHD.
Purpose:
The purpose of this manuscript is to: 1.) Clearly describe the uniqueness of the cCHD infant, including the medical, neurological, and parental challenges. 2.) Propose methods to apply IFDC in order to support recovery of infants with cCHD in the CICU.
Conclusions:
The experiences in the CICU shape the developing brain and alter recovery and healing, thus adversely impacting development. IFDC is a promising model of care that nurses can integrate into the CICU to promote neuroprotection and development. Nurses can effectively integrate IFDC into the CICU by understanding the unique characteristics of infants with cCHD and applying IFDC interventions that include both maturity and recovery perspectives.
Clinical Implications:
The incorporation of IFDC interventions is essential for the infant with cCHD and should be standard of care. Applying IFDC with a recovery perspective in all aspects of caregiving will provide opportunities for individualization of care and parent engagement, allowing infants in the CICU to recover from surgery while supporting both short and long-term neurodevelopment.
Introduction
Infants born with critical congenital heart disease (cCHD) are the most fragile subset of patients with CHD because they require open-heart surgery in the newborn period in order to survive. These infants are at three to four times the risk for developing learning disabilities, behavioral problems, mental health problems, and other developmental deficits or delays compared to children with structurally normal hearts1–6. Much focus has been given by researchers and clinicians on describing and mitigating these challenges in this at-risk population7. While the causes are clearly multifaceted, little attention has been given to the early care needs during the fragile newborn period. Infants born with cCHD are often hospitalized in a mixed pediatric intensive care unit or a dedicated cardiac intensive care unit (CICU). For the purposes of this manuscript, we will use CICU throughout. The developing infant brain needs closeness with its mother and family, including voice and affect regulation with family members to support security and trust, which are greatly reduced in the CICU.8 However, necessary care in the CICU exposes infants to overwhelming stress through a myriad of noxious stimuli, including painful procedures, invasive lines and tubes, and intense sensory stimulation. Bonding and attachment between parent and infant are disrupted. Mobility is limited. Neurotoxic medications are required to reduce pain and agitation. The combination of these negative experiences disrupt the infant’s synaptogenesis, brain maturation, and neurodevelopment9. The CICU environment creates a misalignment between what the developing brain needs and what the environment provides, impacting both short and long-term neurodevelopment.
Current research advocates for the integration of Individualized Family-centered Developmental Care (IFDC) into CICU care.9,10 IFDC is a model of care that minimizes the mismatch between infant neurobiological needs and the CICU environment, thus diminishing the frequency and/or severity of adverse effects on the high-risk infant. Core components of IFDC include parent engagement, cue-based care, and the provision of a supportive environment. Many positive outcomes of IFDC have been demonstrated.11–13 IFDC originally evolved from the Neonatal Individualized Developmental Care and Assessment Program (NIDCAP), which demonstrated positive outcomes for premature infants including enhanced brain structure and function, along with improved behavioral outcomes into school age.14 In addition, medical benefits have been reported such as decreased length of ICU and hospital stays, earlier oral feeding, and increased weight gain,12–17 along with parental engagement, parent attachment to their infant, and parental confidence in caregiving.18–22
IFDC has been integrated into Neonatal Intensive Care Units (NICUs) but only recently surfaced for CICUs.23–29 Currently, the use of IFDC in CICUs is variable; many centers report difficulty in implementation due to the perceived acuity of patients and lack of staff education.24 One contributing explanation may be that there is sparse literature describing the challenges of infants with cCHD during the neonatal period. Furthermore, most literature on IFDC is focused on the premature infant in the NICU and arises from a maturation perspective, where developmental supports are focused on enhancing development and promoting growth outside of the womb for the infant born early. Most infants with cCHD, however, are born near-term or full-term and struggle with recovery after cardiac surgery, along with challenges in growth and development. Although there are some similarities, we believe that the infant with cCHD in the CICU is fundamentally different from the premature infant in the NICU, requiring an approach to care that includes both an understanding of neurodevelopmental maturation and cardiac-specific recovery requirements. We propose that these unique characteristics create barriers and challenges for staff to fully integrate IFDC into CICUs. We believe that a synthesis of the literature regarding the behavioral and developmental profile of infants with cCHD will provide guidance to nurses and health care professionals in the CICU to understand the behavioral profile of the cCHD infant and to successfully apply IFDC into practice. The purpose of this manuscript is to: 1.) Clearly describe the uniqueness of the cCHD infant, including the medical, neurological, and parental challenges. 2.) Propose methods to apply IFDC in order to support recovery of infants with cCHD in the CICU.
Unique Considerations for the Infant with cCHD
Infants with cCHD show some similarities to other hospitalized newborns; however they also have their own unique behavioral profile with specific medical, neurological, and parental challenges (See Figure)25,30,31.
Figure.
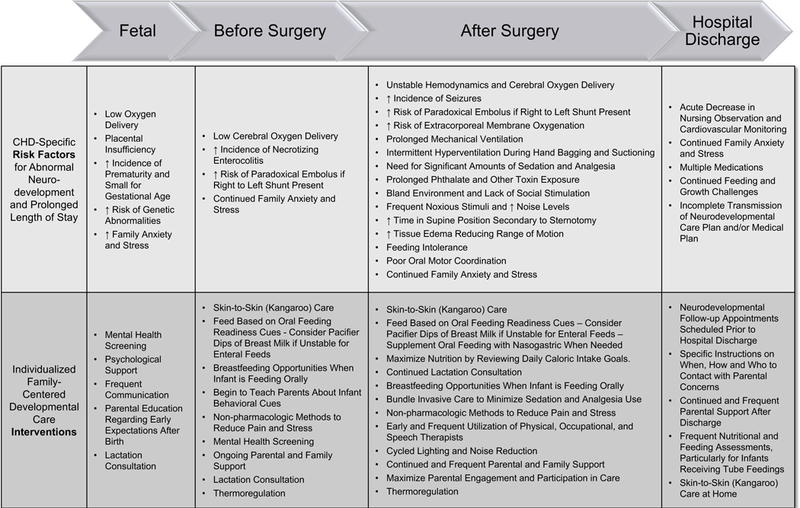
Medical Challenges
Infants with cCHD require frequent monitoring of physiological parameters including evaluation of heart rate and rhythm, blood pressure, central filling pressures, perfusion, volume status, urine output, and temperature regulation.32 Many are physiologically fragile, with minimal reserve to adapt to stressors in the environment. Routine nursing is a source of stress, impacting both sleep and pain. Sleep, which is essential for synapse formation and memory facilitation, becomes frequently disrupted.33,34 Painful experiences during early development shape the overall pain system and determine the final architecture of the adult brain.35 Pain must be monitored and managed, particularly after surgery, often requiring pharmacologic intervention, which places infants at increased risk for adverse effects of excessive sedation, respiratory depression, withdrawal, and diminished responsivity to caregivers and parents.36 Furthermore, infants are at risk for a myriad of consequences of open-heart surgery, including hemodynamic instability, persistent hypoxemia, infection, arrhythmias, cardiac arrest, seizures, cardiovascular collapse requiring extracorporeal membrane oxygenation, and a prolonged length of stay.
Neurological Challenges
Critical CHD is frequently associated with “congenital brain disease”, given the known prenatal and postnatal insults to brain development37,38. Up to 50% of infants with cCHD are at risk for neurodevelopmental impairments39. Brain injury may occur during intrauterine life and before cardiac surgery.22,40 Studies have shown that infants with cCHD present with higher rates of microcephaly, brain immaturity, and white matter injury at birth.37 Prenatal programming of the infant may also occur as a result of maternal stress during pregnancy, which has been shown in other populations to increase the risk for neurodevelopmental sequelae.41 Later, acquired neurologic injury may arise from the adverse effects of anesthetics, cardiopulmonary bypass, sedation, analgesia, hypoxia, seizures, or stroke.1,42
Delays in neurodevelopment can appear early in infancy and present as atypical autonomic, state, and motor organization, as well as feeding dysfunction.43 Infants with cCHD are easily overwhelmed by social and sensory stimulation, difficult to console, and have poor visual orienting.44–46 Motor challenges can occur related to neurologic injury, including hypo/hypertonia, asymmetry of movement, and general gross motor delays.5,30,43,47,48 Infants also often demonstrate weak sucking and few feeding readiness cues. Feeding difficulties, coupled with symptoms of congestive heart failure, frequently lead to tube feeding, delayed oral feeding, and poor weight gain and growth49–51. The etiology of feeding dysfunction is multifactorial and often related to the hemodynamic status of the infant, and in some, injury to the recurrent laryngeal nerve, phrenic nerve, and thoracic duct as a consequence of surgery near the aortic arch or left pulmonary artery. Additional factors influencing feeding dysfunction may include fatigue, increased respiratory rate, sedation levels, neurologic status, vocal cord dysfunction, and dysphasia. Neurologic insult may also contribute to poor feeding organization in this population52.
Parental Challenges
Parents of infants born with cCHD experience high stress from the time of infant diagnosis through the subsequent hospitalization(s) for cardiac surgery and beyond. Infants may be immediately separated from their parents after birth, causing an alteration in the normal attachment process.53 Parents are often unable to feed, hold, care, and at times even touch their critically ill infant, creating a sense of altered parental role.54,55 Mothers, in particular, experience heightened stress, anxiety, and depression.56–61 Parental stress influences the parent’s quality of life, mental health, and overall family functioning.62 Parental stress impacts the child’s overall development and research is emerging that maternal mental health has more significant influence on child behavioral outcomes than the physiologic impact of the cCHD.63
Applying IFDC to Support Recovery in the CICU
The challenge for nurses and other medical professionals in the CICU is to apply IFDC. We propose the application of IFDC in the CICU by including considerations for the infant’s postoperative recovery needs (Table 1). Unlike the premature infant who spends weeks and months in the NICU working on feeding and growth (maturation), most infants with cCHD may spend only days to weeks in the CICU, yet undergo an overwhelmingly stressful open-heart surgery, requiring invasive medical and surgical care and constant attention to their physiology in order to survive (recovery). It is important to note that approximately 20 percent of infants with cCHD have hospital lengths of stay of 2 months or greater; therefore, individualizing care based on their changing developmental needs is paramount64,65. Regardless of the length of stay, all fragile infants benefit from the IFDC approach to care. Optimal outcomes will occur when the healthcare team focuses both on physiology and infant behavior, in order to adjust caregiving practices and the physical and emotional environment to support the developmental needs of the infant. IFDC requires a shift in the critical care paradigm to include the evaluation of the infant’s behavior and building a relationship between caregiver, infant and family.
Table 1:
IFDC with a Recovery Perspective
| IFDC Core Components | Benefits of integrating IFDC into care: | Vulnerabilities when not integrating IFDC into care: | Examples of IFDC Interventions: |
|---|---|---|---|
| Parent Engagement | - Caregiver attunement to infant’s
experience - Considerations for emotional development: trust vs. mistrust, enhances development of trusting relationships with parents - Parents actively participate in infant care practices - Parents feel confident and competent in providing direct care (e.g. diaper change, feeding, wound care) |
- Isolation, parental separation for extended periods - Parent anxiety and stress - Missed opportunities for holding, skin-to-skin contact |
- Parent presence and participation in daily
medical rounds - Parent holding of infant, skin-to-skin contact preferred - Parent provides as much infant care as possible based on the clinical stability of the infant (mouth care, diaper change, feeding, turning, holding, comforting, touching) - Parent provides non-pharmacologic comfort measures (pacifier, facilitated tucking, holding) during routine nursing care - Mother encouraged to breastfeed preoperatively and as soon as infant is able to eat by mouth postoperatively. |
| Cue-Based Care (e.g. Infant state and attention) | - Individualized plan of care tailored to the
unique needs of the infant - Caregivers respond to infant behavioral cues - Infant organization is supported |
- Bedside caregivers and/or parents unaware of
when infant is most comfortable and available for social
interaction - Infant stressed during feeds - Infant requires medication to recover from a disorganized state |
- Incorporation of infant behavior into
routine assessment - Parents are taught to read and respond to infant behavioral cues - Feeds are provided to infants based on feeding readiness cues instead of a time-based schedule - Non-pharmacologic comfort measures are provided when infant shows stress cues |
| Supportive Environment (e.g. Thermoregulation, Positioning/Motor) | -Promotion of normothermia - Circadian rhythm is supported - Neutral tucked body alignment and developmental positioning supported |
- Often cared for on warming beds, not able to
wear clothes - At risk for hypothermia - Fixed extended postures for long periods without opportunities for neutral flexion. -Fixed postures with head to one side (particularly for infants with an endotracheal tube or extracorporeal membrane oxygenation via neck cannulation) |
- Provision of cycled lighting to facilitate
circadian rhythm - Low noise levels in the unit - Skin-to-skin contact offered as the preferred method of holding when infants require an artificial heat source (warming bed or incubator) - Positioning devices provided to maintain midline, neutral postures - Parents encouraged to provide containment and swaddling for the infant |
To the developing brain, all experience matters, both positive and negative; thus, each interaction shapes neural connections and long-term brain maturation. IFDC provides the essential framework for nurses to support the vulnerable developing brain while maintaining the medical stability of the recovering infant after cardiac surgery. In order to provide this care, nurses must add to the medical and task-oriented model of caregiving to include a relationship-based model of care where the infant is recognized as an individual who is communicating and interacting with the environment. All care must be regarded as “engaging with” the infant rather than “doing to” or “doing for.”66 An understanding of infant communication should occur to properly individualize caregiving to meet the expectations of the infant’s susceptible brain. Caregivers and parents should be engaged in continuous assessment of the infant, responding to the infant’s behavioral cues and providing a supportive environment.67
Nurses in the CICU are already acutely attuned to assessing the physiological status of the infant, but behavioral assessment should be included into care also. In current practice, most nurses begin caregiving with a physical assessment of the patient, then creation of a plan of care, implementation of the plan, and re-evaluation of the patient. Behavioral assessment should be integrated as the first step into IFDC implementation in order to guide the plan of care. Reading infant behavior is key to promote individualization of care and inform nursing interventions at the point of care.
Behavioral Assessment
Infant behavior is composed of four subsystems of functioning: autonomic, motor, state, and self-regulation. These systems exist simultaneously and mutually influence each other, occurring in continuous interaction with the environment.68 The cCHD infant is physiologically vulnerable and this instability is seen in all systems of functioning, such as poor coloring, low motor tone, prolonged metabolism of sedative medications and withdrawal, limited alertness, and poor self-regulation. Reading infant behavior is similar to learning any language, requiring education and practice. Even very fragile infants display reliably observable behaviors in the form of autonomic and visceral responses, movement patterns, level of alertness of state, and self-regulation.68,69 Medical providers can assess each subsystem while performing the standard head to toe body system assessment (Table 2). Infant behaviors can be categorized as organized when the infant is well regulated and disorganized when the infant has difficulty adapting to internal or external stimuli.70,71 Parent engagement is essential because they are the infant’s stable, familiar, predictable, and primary caregivers. Therefore, parents must also be educated on how to read and respond to infant behaviors and to discern when the infant is organized versus disorganized.
Table 2:
Behavioral Assessment
| Assessment Definition | Organized | Disorganized | |
|---|---|---|---|
| Autonomic | respiration patterns, color fluctuations, visceral system, heart rate variability | smooth respiration, good and stable color, and stable digestion | respiratory pauses and changes, color changes, tachypnea, cyanotic, grey, flushed, gagging, gasping, spitting up, hiccupping, bowel movement strains straining, gas, tremors, startling, coughing, sneezing, yawning, and sighing |
| Motor | postures, tone of trunk, extremities and face,
movement patterns, coordinated suck |
smooth well-modulated posture and
well-modulated tone, synchronous smooth movements with efficient
strategies such as: hand-on-face protection, hand clasping, foot clasping, finger folding, hand-to-mouth maneuvers, grasping, suck-searching and sucking, hand holding, tucking |
flaccidity, tuning out, low tone in face/gape
face, hypertonicity, hyperextensions, legs and arms (stretching out, stiffening, trying to brace), trunk (arching), tongue thrusting, finger splaying, facial grimacing, protective maneuvers such as high guard arm position and fisting, and frantic and diffuse activity |
| State | range, robustness, transitions | clear, robust sleep states, rhythmic, robust
crying, good self-quieting, robust, focused, shiny-eyed alertness with
intent and/or animated facial expression, frowning, cooing, smiling, cheek softening, mouth pursing to “ooh” face |
state related signals of
disorganization strained fussing or crying, glassy eyed, strained alertness, irritability, panicked or worried, staring or averting eyes, eye floating, diffuse sleep or awake states with whimpering sounds, facial twitches and discharge smiling, rapid state oscillations |
| Self-regulation | Behaviors the infant uses to maintain the integrity and balance of other subsystems and to move smoothly between states | Any of the organization signals above |
Any of the disorganization signals above |
Supporting Organization by Applying IFDC Interventions in the CICU
Autonomic.
Autonomic assessment in an infant with cCHD may be challenging as skin color is often atypical at baseline and sedative medications as well as excessive stimulation can cause abnormal movements. Nurses must consider other sources of autonomic instability, including pain, temperature instability, hemodynamic instability, atypical seizures, or withdrawal from analgesic or sedative mediations. The provision of calming techniques during and after caregiving, such as offering a pacifier, swaddling, or gentle touch has been shown to increase physiologic stability72. Another alternative is offering two-person care to fragile infants, where the nurse provides a medical intervention and a parent, therapist, or second nurse supports the infant. Parents should be given a key role in the CICU and encouraged to provide these forms of non-pharmacologic comfort measures. It is important to consider thermoregulation and the need for clothing and blankets when the infant is held or in the open environment. Partial swaddling during caregiving is helpful. For example, during a diaper change, the upper extremities and chest can be maintained swaddled in a blanket. A particularly vulnerable time for temperature instability is when the infant is transitioning out of an artificial heat source such as a warmer bed or incubator.
Motor.
Supporting the motor system may be challenging for post-surgical infants who must remain supine given medical risks, including sternotomy and delayed sternal closure, transthoracic intracardiac catheters, and chest tubes. These medically-necessary limitations present a challenge to creating a position supportive of the infant’s motor system. Postoperative infants often have low tone and need assistance to round their hips and shoulders forward and bringing hands and knees to midline. Small blanket rolls or positioning devices under both sides of the shoulders and hips are necessary, especially for the child who is sedated and paralyzed. Positioning devices should prevent hyperextension of the neck. If hyperextension of the neck is required to maintain an open airway for long periods of time, reintubation should be considered. Positioning devices should support flexion of the hips and knees towards midline, minimizing outward extension (“frog legs”), as often occurs in the immediate postoperative period. Supportive positions, such as prone in a well tucked position on the parent’s chest, is ideal and should be a goal for the infant with cCHD before and after surgery. In addition to holding, parents should be encouraged to support flexion and containment with their hands, also known as facilitated tucking.
State and Self-Regulation.
Post-surgical analgesia and sedation, along with a compromised autonomic system, creates challenges for infants to attain a quiet alert state. Caregiving interventions to support state regulation include remaining in-tune with the infant’s cues, providing breaks as needed by bundling care with pacing, and maintaining contact with the infant so that stimulation is not continually coming and going. If the infant is overwhelmed or stressed, nurses can modify caregiving to provide support for the infant. Environmental interventions such as minimizing sound, regulating light, and promoting sleep provide additional stress relief to the infant and support self-regulation. Televisions should not be used as a state regulator for infants, rather, supportive holding, swaddling, and a soft voice to promote quiet alertness. Sound machines, lullabies, soft music, and musical crib toys should be used sparingly as these often are overwhelming to the recovering infant and also add to the overall noise level in the room. Preferably, lower the sound level in the CICU rather than cover up the noise with more sound. Parents can read or sing to their infants or provide recordings of their voices if they are unable to be present at the bedside. Nurses can provide cycled lighting to promote infant circadian rhythm development, allowing low, indirect light during the day when awake and darkness at night. Care in the CICU will inevitably require bright light at times; however, infants’ eyes should be protected from the light with a dark cloth or a hand, even during periods of sedation. Excessive noise and light also disrupts sleep, which is critical in the newborn period. Sleep disturbances impact cognition, attentional, and psychosocial development.73
Supporting Family-Centered Care
The CICU team is uniquely positioned to enhance parental role and support attachment and bonding between infants and parents. Even when the infant is critically ill, parents should be viewed as the infant’s primary caregiver and participate in care and decision-making. Parents can and should be integrated into care, regardless of the infant’s medical fragility. For the most fragile infant, parent engagement might include assisting the nurse in routine care such as mouth care, diaper changing, positioning, touching, or providing facilitated tucking. Evidence is overwhelming in infant literature that holding and touch stabilizes the most fragile infants74–77. When holding is not possible, alternative types of nurturing touch can be employed, such as supporting the parent to cradle the infant in the crib. As infants recover, parental engagement can be expanded to include all aspects of care, including feeding, comforting, and holding the infant to support parent’s active participation in the care of their infant. The preferred method of holding for parents is skin-to-skin contact, also known as kangaroo care. Skin-to-skin contact protects infants from nosocomial infection, supports growth, and promotes temperature stability for infants requiring an artificial heat source in the pre or postoperative period78–80.
Conclusions and Implications
Infants with cCHD have an increased risk for abnormal neurodevelopment. The experiences in the CICU shape the developing brain and alter recovery and healing, thus adversely impacting development. IFDC is a promising model of care that nurses can integrate into the CICU to promote neuroprotection and development. Nurses can effectively integrate IFDC into the CICU by understanding the unique characteristics of infants with cCHD and applying IFDC interventions that include both maturity and recovery perspectives.
We have presented some examples of clinical interventions that can be incorporated into the care of infants with cCHD in the CICU using IFDC; however, many research implications also exist. Specific IFDC interventions should be studied to understand their impact on infants with cCHD, such as cycled lighting, holding and positive touch, or the use of non-pharmacologic comfort interventions. Future research should examine both short and long-term outcomes of IFCD on infant neurodevelopment. Instrument development for the measurement of developmental care in the CICU which accounts for the infant’s medical fragility would be beneficial to enable researchers to examine both individual-level and unit-level variables of IFDC. Finally, studies should examine the impact of parent engagement and participation in care on infant developmental outcomes as well as family outcomes such as parent stress, parent-infant attachment, or family functioning.
Acknowledgments
Funding:
This work was supported by the National Institute of Nursing Research (NINR T32NR007100).
Footnotes
Conflicts of Interests: The authors have no conflicts of interest to disclose.
References
- 1.Clouchoux C, Du Plessis A, Bouyssi-Kobar M, et al. Delayed cortical development in fetuses with complex congenital heart disease. Cereb Cortex. 2012;23(12):2932–2943. [DOI] [PubMed] [Google Scholar]
- 2.Mussatto KA, Hoffmann RG, Hoffman GM, et al. Risk and prevalence of developmental delay in young children with congenital heart disease. Pediatrics. 2014;133(3):e570–e577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newburger JW, Sleeper LA, Bellinger DC, et al. Early developmental outcome in children with hypoplastic left heart syndrome and related anomalies. Circulation. 2012;125(17):2081–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wernovsky G Current insights regarding neurological and developmental abnormalities in children and young adults with complex congenital cardiac disease. Cardiology in the Young. 2006;16(S1):92–104. [DOI] [PubMed] [Google Scholar]
- 5.Snookes SH, Gunn JK, Eldridge BJ, et al. A systematic review of motor and cognitive outcomes after early surgery for congenital heart disease. Pediatrics. 2010;125(4):e818–e827. [DOI] [PubMed] [Google Scholar]
- 6.Marino B S PHL, J.W. N, al. e. Neurodevelopmental outcomes in children with congenital heart disease: Evaluation and management: A scientific statement from the American Heart Association. Circulation. 2012;126:1143–1172. [DOI] [PubMed] [Google Scholar]
- 7.Wernovsky G, Licht DJ. Neurodevelopmental Outcomes in Children With Congenital Heart Disease-What Can We Impact? Pediatr Crit Care Med. 2016;17(8 Suppl 1): S232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofer MA. Early social relationships: A psychobiologist’s view. Child Development. 1987;58:633–647. [PubMed] [Google Scholar]
- 9.Anand KJS, Scalzo FM. Can adverse neonatal experiences alter brain development and subsequent behavior? Biology of the Neonate. 2000;77:69–82. [DOI] [PubMed] [Google Scholar]
- 10.Daniels JM, Harrison TM. A Case Study of the Environmental Experience of a Hospitalized Newborn Infant With Complex Congenital Heart Disease. The Journal of cardiovascular nursing. 2015. [DOI] [PubMed] [Google Scholar]
- 11.Als H, Lawhon g, Duffy FH, McAnulty GB, Gibes-Grossman R, Blickman JG. Individualized developmental care for the very low birthweight preterm infant: Medical and neurofunctional effects. Journal of the American Medical Association. 1994;272:853–858. [PubMed] [Google Scholar]
- 12.Als H, Gilkerson L, Duffy FH, et al. A three-center randomized controlled trial of individualized developmental care for very low birth weight preterm infants: Medical, neurodevelopmental, parenting and caregiving effects. Journal of Developmental and Behavioral Pediatrics. 2003;24(6):399–408. [DOI] [PubMed] [Google Scholar]
- 13.Als H, Duffy F, McAnulty GB, et al. Early experience alters brain function and structure. Pediatrics. 2004;113(4):846–857. [DOI] [PubMed] [Google Scholar]
- 14.McAnulty G, Duffy FH, Kosta S, et al. School age effects of the Newborn Individualized Developmental Care and Assessment Program for preterm medically low-risk preterm infants: Preliminary findings. Journal of Clinical Neonatology. 2012;1(4):184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Westrup B, Kleberg A, von Eichwald K, Stjernqvist K, Lagercrantz H. A randomized controlled trial to evaluate the effects of the Newborn Individualized Developmental Care and Assessment Program in a Swedish setting. Pediatrics. 2000;105(1):66–72. [DOI] [PubMed] [Google Scholar]
- 16.Kleberg A, Westrup B, Stjernqvist K, Lagercrantz H. Indications of improved cognitive development at one year of age among infants born very prematurely who received care based on the Newborn Individualized Developmental Care and Assessment Program (NIDCAP). Early Hum Dev. 2002;68:83–91. [DOI] [PubMed] [Google Scholar]
- 17.Als H, Lawhon G, Gibes R, Duffy FH, McAnulty GB, Blickman JG. Individualized behavioral and developmental care for the VLBW preterm infant at high risk for bronchopulmonary dysplasia and intraventricular hemorrhage: Study II NICU outcome. Paper presented at: New England Perinatal Association Annual Meeting1988; Woodstock, VT. [Google Scholar]
- 18.Als H, Duffy FH, McAnulty G, et al. NIDCAP improves brain function and structure in preterm infants with severe intrauterine growth restriction. J Perinatol. 2012;32:797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sannino P, Giannì ML, De Bon G, et al. Support to mothers of premature babies using NIDCAP method: a non-randomized controlled trial. Early Hum Dev. 2016;95:15–20. [DOI] [PubMed] [Google Scholar]
- 20.Kleberg A, Hellström-Westas L, Widström A-M. Mothers’ perception of Newborn Individualized Developmental Care and Assessment Program (NIDCAP) as compared to conventional care. Early Hum Devel. 2007;83(6):403–411. [DOI] [PubMed] [Google Scholar]
- 21.Nelson AM, Bedford PJ. Mothering a preterm infant receiving NIDCAP care in a level III newborn intensive care unit. Journal of Pediatric Nursing. 2016;31(4):e271–e282. [DOI] [PubMed] [Google Scholar]
- 22.Ohlsson A, Jacobs SE. NIDCAP: A Systematic Review and Meta-analyses of Randomized Controlled Trials. Pediatrics. 2013. [DOI] [PubMed] [Google Scholar]
- 23.Peterson JK. Supporting Optimal Neurodevelopmental Outcomes in Infants and Children With Congenital Heart Disease. Crit Care Nurse. 2018;38(3):68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sood E, Berends WM, Butcher JL, et al. Developmental Care in North American Pediatric Cardiac Intensive Care Units: Survey of Current Practices. Advances in Neonatal Care. 2016;16(3):211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torowicz D, Lisanti AJ, Rim J-S, Medoff-Cooper B. A developmental care framework for a cardiac intensive care unit: a paradigm shift. Advances in Neonatal Care. 2012;12(5S):S28–S32. [DOI] [PubMed] [Google Scholar]
- 26.Butler SC, Huyler K, Kaza A, Rachwal C. Filling a significant gap in the cardiac ICU: implementation of individualised developmental care. Cardiol Young. 2017;27(9):1797–1806. [DOI] [PubMed] [Google Scholar]
- 27.Lisanti AJ, Cribben J, Connock EM, Lessen R, Medoff-Cooper B. Developmental Care Rounds: An Interdisciplinary Approach to Support Developmentally Appropriate Care of Infants Born with Complex Congenital Heart Disease. Clinics in perinatology. 2016;43(1):147–156. [DOI] [PubMed] [Google Scholar]
- 28.Medoff-Cooper B, Irving SY, Hanlon AL, et al. The Association among Feeding Mode, Growth, and Developmental Outcomes in Infants with Complex Congenital Heart Disease at 6 and 12 Months of Age. J of Peds. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peterson JK, Evangelista LS. Developmentally Supportive Care in Congenital Heart Disease: A Concept Analysis. J Pediatr Nurs. 2017;36:241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ricci MF, Andersen JC, Joffe AR, et al. Chronic Neuromotor Disability After Complex Cardiac Surgery in Early Life. Pediatrics. 2015;136(4):e922–933. [DOI] [PubMed] [Google Scholar]
- 31.Sables-Baus S, Kaufman J, Cook P, da Cruz EM. Oral feeding outcomes in neonates with congenital cardiac disease undergoing cardiac surgery. Cardiol Young. 2012;22(1):42–48. [DOI] [PubMed] [Google Scholar]
- 32.Beke DM. Norwood Procedure for Palliation of Hypoplastic Left Heart Syndrome: Right Ventricle to Pulmonary Artery Conduit vs Modified Blalock-Taussig Shunt. Crit Care Nurse. 2016;36(6):42–51. [DOI] [PubMed] [Google Scholar]
- 33.Peng N-H, Chen C-H, Bachman J, et al. To explore the relationships between physiological stress signals and stress behaviors in preterm infants during periods of exposure to environmental stress in the hospital. Biological research for nursing. 2010:1099800410392020. [DOI] [PubMed] [Google Scholar]
- 34.Smith GC, Gutovich J, Smyser C, et al. Neonatal intensive care unit stress is associated with brain development in preterm infants. Ann Neurol. 2011;70(4):541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anand KJS, Carr DB. The neuroanatomy, neurophysiology, and neurochemistry of pain, stress, and analgesia in newborns and children. Pediatric Clinics of North America. 1989;36:795–822. [DOI] [PubMed] [Google Scholar]
- 36.Naguib AN, Dewhirst E, Winch PD, Simsic J, Galantowicz M, Tobias JD. Pain management after surgery for single-ventricle palliation using the hybrid approach. Pediatric cardiology. 2012;33(7):1104–1108. [DOI] [PubMed] [Google Scholar]
- 37.Wernovsky G, Licht DJ. Neurodevelopmental Outcomes in Children With Congenital Heart Disease—What Can We Impact? Pediatric Critical Care Medicine. 2016;17(8_suppl):S232–S242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Licht DJ, Shera DM, Clancy RR, et al. Brain maturation is delayed in infants with complex congenital heart defects. The Journal of Thoracic and Cardiovascular Surgery. 2009;137(3):529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mebius MJ, Kooi EM, Bilardo CM, Bos AF. Brain Injury and Neurodevelopmental Outcome in Congenital Heart Disease: A Systematic Review. Pediatrics. 2017:e20164055. [DOI] [PubMed] [Google Scholar]
- 40.Khalil A, Bennet S, Thilaganathan B, Paladini D, Griffiths P, Carvalho JS. Prevalence of prenatal brain abnormalities in fetuses with congenital heart disease: a systematic review. Ultrasound in Obstetrics & Gynecology. 2016;48(3):296–307. [DOI] [PubMed] [Google Scholar]
- 41.Kim DR, Bale TL, Epperson CN. Prenatal programming of mental illness: current understanding of relationship and mechanisms. Current psychiatry reports. 2015;17(2):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naguib AN, Winch PD, Tobias JD, et al. Neurodevelopmental outcome after cardiac surgery utilizing cardiopulmonary bypass in children. Saudi J Anaesth. 2015;9(1):12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Limperopoulos C, Majnemer A, Shevell MI, Rosenblatt B, Rohlicek C, Tchervenkov C. Neurodevelopmental status of newborns and infants with congenital heart defects before and after open heart surgery. Journal of Pediatrics. 2000;137(5):638–645. [DOI] [PubMed] [Google Scholar]
- 44.Owen M, Shevell M, Donofrio M, et al. Brain volume and neurobehavior in newborns with complex congenital heart defects. Journal of Pediatrics. 2014;164(5):1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Massaro AN, Glass P, Brown J, et al. Neurobehavioral abnormalities in newborns with congenital heart disease requiring open-heart surgery. J of Peds. 2011;158(4):678–681. e672. [DOI] [PubMed] [Google Scholar]
- 46.Butler S, Sadhwani A, Stopp C, et al. Neurodevelopmental Assessment of Infants with Congenital Heart Disease in the Early Postoperative Period. Second Annual Cardiac Neurodevelopmental Symposium; 2016; Cincinnati, Ohio. . [DOI] [PubMed] [Google Scholar]
- 47.Marino BS, Lipkin PH, Newburger JW, et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation. 2012;126(9):1143–1172. [DOI] [PubMed] [Google Scholar]
- 48.Miller G, Eggli KD, Contant C, Baylen BG, Myers JL. Postoperative neurologic complications after open heart surgery on young infants. Archives of Pediatrics and Adolescent Medicine. 1995;149(7):764–768. [DOI] [PubMed] [Google Scholar]
- 49.Davis D, Davis S, Cotman K, et al. Feeding difficulties and growth delay in children with hypoplastic left heart syndrome versus d-transposition of the great arteries. Pediatric cardiology. 2008;29(2):328–333. [DOI] [PubMed] [Google Scholar]
- 50.Imms C Feeding the infant with congenital heart disease: an occupational performance challenge. American Journal of Occupational Therapy. 2001;55(3):277–284. [DOI] [PubMed] [Google Scholar]
- 51.Costello CL, Gellatly M, Daniel J, Justo RN, Weir K. Growth restriction in infants and young children with congenital heart disease. Congenital heart disease. 2015;10(5):447–456. [DOI] [PubMed] [Google Scholar]
- 52.Medoff-Cooper B Feeding Bheaviors as an Index of Developmental Outcomes. International Congress of Infant Studies; 2002; Toronto, Canada. [Google Scholar]
- 53.Boztepe H, Ay A, Kerimoğlu Yıldız G, Çınar S. Does the visibility of a congenital anomaly affect maternal–infant attachment levels? Journal for Specialists in Pediatric Nursing. 2016;21(4):200–211. [DOI] [PubMed] [Google Scholar]
- 54.Lisanti AJ, Golfenshtein N, Medoff-Cooper B. The Pediatric Cardiac Intensive Care Unit Parental Stress Model: Refinement Using Directed Content Analysis. Advances in Nursing Science. 2017;40(4):319–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Franck LS, Mcquillan A, Wray J, Grocott MP, Goldman A. Parent stress levels during children’s hospital recovery after congenital heart surgery. Pediatric cardiology. 2010;31(7):961–968. [DOI] [PubMed] [Google Scholar]
- 56.Vrijmoet-Wiersma CJ, Ottenkamp J, van Roozendaal M, Grootenhuis MA, Koopman HM. A multicentric study of disease-related stress, and perceived vulnerability, in parents of children with congenital cardiac disease. Cardiol Young. 2009;19(6):608–614. [DOI] [PubMed] [Google Scholar]
- 57.Lawoko S, Soares JJ. Psychosocial morbidity among parents of children with congenital heart disease: a prospective longitudinal study. Heart & Lung: The Journal of Acute and Critical Care. 2006;35(5):301–314. [DOI] [PubMed] [Google Scholar]
- 58.Bevilacqua F, Palatta S, Mirante N, et al. Birth of a child with congenital heart disease: emotional reactions of mothers and fathers according to time of diagnosis. The Journal of Maternal-Fetal & Neonatal Medicine. 2013;26(12):1249–1253. [DOI] [PubMed] [Google Scholar]
- 59.Pinto NM, Weng C, Sheng X, et al. Modifiers of stress related to timing of diagnosis in parents of children with complex congenital heart disease. The Journal of Maternal-Fetal & Neonatal Medicine. 2016;29(20):3340–3346. [DOI] [PubMed] [Google Scholar]
- 60.Franich-Ray C, Bright MA, Anderson V, et al. Trauma reactions in mothers and fathers after their infant’s cardiac surgery. Journal of Pediatric Psychology. 2013;38(5):494–505. [DOI] [PubMed] [Google Scholar]
- 61.Utens E, Versluis-Den Bieman H, Verhulst F, Witsenburg M, Bogers A, Hess J. Psychological distress and styles of coping in parents of children awaiting elective cardiac surgery. . Cardiol Young. 2000;10(3):239–244. [DOI] [PubMed] [Google Scholar]
- 62.Ernst MM, Marino BS, Cassedy A, et al. Biopsychosocial predictors of quality of life outcomes in pediatric congenital heart disease. Pediatric cardiology. 2018;39(1):79–88. [DOI] [PubMed] [Google Scholar]
- 63.McCusker CG, Doherty NN, Molloy B, et al. Determinants of neuropsychological and behavioural outcomes in early childhood survivors of congenital heart disease. Archives of Disease in Childhood. 2007;92(2):137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tabbutt S, Ghanayem N, Ravishankar C, et al. Risk factors for hospital morbidity and mortality after the Norwood procedure: A report from the Pediatric Heart Network Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg. 2012;144(4):882–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown KL, Ridout DA, Goldman AP, Hoskote A, Penny DJ. Risk factors for long intensive care unit stay after cardiopulmonary bypass in children. Crit Care Med. 2003;31(1):28–33. [DOI] [PubMed] [Google Scholar]
- 66.Als H, Gilkerson L. The role of relationship-based developmentally supportive newborn intensive care in strengthening outcome of preterm infants. Semin Perinatol. 1997;21(3):178–189. [DOI] [PubMed] [Google Scholar]
- 67.Als H, Butler S. Neurobehavioral development of the preterm infant In: Martin AFMW R, ed. Fanaroff and Martin’s Neonatal-Perinatal Medicine: Diseases of the Fetus and Infant (9th ed.). St. Louis: Elsevier Mosby; 2011:1057–1074. [Google Scholar]
- 68.Als H A synactive model of neonatal behavioral organization: Framework for the assessment and support of the neurobehavioral development of the premature infant and his parents in the environment of the neonatal intensive care unit. Physical & Occupational Therapy in Pediatrics. 1986;6:3–53. [Google Scholar]
- 69.Als H Reading the premature infant In: Goldson E, ed. Developmental Interventions in the Neonatal Intensive Care Nursery. New York: Oxford University Press; 1999:18–85. [Google Scholar]
- 70.Als H, Lester BM, Brazelton TB. Dynamics of the behavioral organization of the premature infant: A theoretical perspective In: Field TM, Sostek AM, Goldberg S, Shuman HH, eds. Infants Born at Risk. New York: Spectrum Publications; 1979:173–193. [Google Scholar]
- 71.Als H Manual for the Naturalistic Observation of the Newborn (Preterm and Fullterm) Revision. Boston: The Children’s Hospital; 1984. [Google Scholar]
- 72.Committee On F, Newborn, Section On A, Pain M. Prevention and Management of Procedural Pain in the Neonate: An Update. Pediatrics. 2016;137(2):e20154271. [DOI] [PubMed] [Google Scholar]
- 73.Kurth S, Olini N, Huber R, LeBourgeois M. Sleep and early cortical development. Current sleep medicine reports. 2015;1(1):64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith JR. Comforting touch in the very preterm hospitalized infant: an integrative review. Advances in neonatal care : official journal of the National Association of Neonatal Nurses. 2012;12(6):349–365. [DOI] [PubMed] [Google Scholar]
- 75.Dominguez Rosales R, Albar Marin MJ, Tena Garcia B, et al. [Effectiveness of the application of therapeutic touch on weight, complications, and length of hospital stay in preterm newborns attended in a neonatal unit]. Enferm Clin. 2009;19(1):11–15. [DOI] [PubMed] [Google Scholar]
- 76.Harrison LL, Williams AK, Berbaum ML, Stem JT, Leeper J. Physiologic and behavioral effects of gentle human touch on preterm infants. Research in nursing & health. 2000;23(6):435–446. [DOI] [PubMed] [Google Scholar]
- 77.Reynolds LC, Duncan MM, Smith GC, et al. Parental presence and holding in the neonatal intensive care unit and associations with early neurobehavior. J Perinatol. 2013;33(8):636–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Conde-Agudelo A, Diaz-Rossello JL. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst Rev. 2016(8):CD002771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McCall EM, Alderdice F, Halliday HL, Jenkins JG, Vohra S. Interventions to prevent hypothermia at birth in preterm and/or low birthweight infants. Cochrane Database Syst Rev. 2010(3):CD004210. [DOI] [PubMed] [Google Scholar]
- 80.Ludington-Hoe SM. Thirty years of Kangaroo Care science and practice. Neonatal network : NN. 2011;30(5):357–362. [DOI] [PubMed] [Google Scholar]


