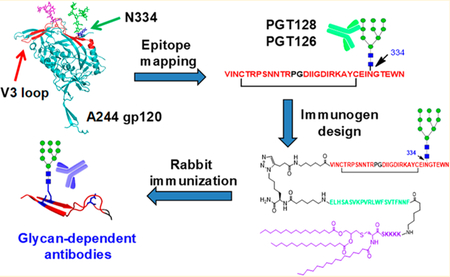
Abstract
The N332 high-mannose glycan on the HIV-1 gp120 V3-loop is the target of many bNAbs. About 17% HIV isolates carry the N332 to N334 mutation, but the antibody recognition of the N334 N-glycan and its immunogenicity are not well characterized. Here we report the chemoenzymatic synthesis, antigenicity, and immunogenicity of the V3 N334 glycopeptides from HIV-1 A244 gp120, a key component in the partially successful Thai clinical trials. We found that synthetic V3 glycopeptide carrying a N334 high-mannose glycan could be recognized by bNAb PGT128 and PGT126 but not by 10-1074. Rabbit immunization with the synthetic three-component A244 glycopeptide immunogen elicited substantial glycan-dependent antibodies with broad reactivity to various HIV-1 gp120/gp140 carrying N332 or N334 glycosylation sites. These results indicated that the N334 site is vulnerable and the A244 V3 glycopeptide represents a valuable immunogen for further HIV-1 vaccine studies.
Graphical Abstract
INTRODUCTION
Recently, broadly neutralizing antibodies (bNAbs) that target the high-mannose patch on HIV-1 have been discovered and show outstanding neutralization breadth and potency.1,2 For example, antibody PGT128 could neutralize over 70% of globally circulating viruses,1 while PGT121 has been shown to protect against high-dose vaginal simian human immunodeficiency virus (SHIV) challenge with passive immunization in macaques3 and to suppress virus replication in chronically infected macaques.4 Also, 10-1074 was able to suppress viral load when used together with other bNAbs in animal models.5–8 Previous studies have shown that bNAbs that target the V3-glycan bind the intrinsic high-mannose patch centered on the N332 high-mannose N-glycan.9–12 Indeed, we and others have demonstrated that the synthetic JR-FL V3 glycopeptide carrying a N332 high-mannose glycan could mimic the conserved epitope of several bNAbs and elicit glycan-dependent antibody responses by animal immunizations,13–16 suggesting the N332 high-mannose glycan can be recognized by the immune system as non-self component and synthetic V3 glycopeptide carrying a N332 high-mannose glycan could mimic the epitope of V3-glycan class of bNAbs.17 The V3 domain of HIV-1 typically contains three potential N-glycosylation sites with the N295 and N332 sites at the base and the N301 site within the loop.18 The highly conserved N-glycan at N332 is the center of the intrinsic high-mannose patch. However, there are about 17% isolates carrying the N332 to N334 mutation.10 The switch of the N-glycosylation from N332 to the N334 site was usually achieved by a double mutation, first at the 334 residue to introduce an Asn and then at the 336 residue to introduce a Ser or Thr residue, which generates a new NXS/T consensus sequence for N-glycosylation at the N334 site.10 In fact, many HIV isolates that switch the N332 glycosylation to the N334 N-glycosylation are highly resistant to some of the high-mannose patch targeting bNAbs.10 Therefore, it is interesting to examine how those bNAbs recognize the specific N-glycans in the V3 region with a shift of the N-glycosylation site from N332 to N334. In this study, we focused on the A244 strain V3 with a N334 glycosylation site. Interestingly, the corresponding gp120 envelope glycoprotein from the A244 strain was previously chosen as an important component of the vaccine used in the Thai clinical trials that showed a 31.5% reduction in the risk of acquiring infection.19,20 We sought to systematically study the antigenicity and immunogenicity of the HIV-1 A244 V3 glycopeptides with high-mannose or complex-type N-glycan attached at the N334, N301, and N295 sites. We efficiently synthesized the V3 glycopeptides derived from 244 strain using a chemoenzymatic method. Antibody binding studies indicated that the synthetic V3 glycopeptide with a high-mannose glycan at the N334, N301, or N295 was recognized by PGT128 and PGT126 but not by the N322 glycan-specific antibody 101074. Interestingly, 10-1074 was still unable to bind to our A244 V3 glycopeptide even after shifting the glycosylation site from N334 to N332. Preliminary rabbit immunization with a synthetic three-component immunogen containing the A244 glycopeptide elicited glycan-dependent antibodies that were cross-reactive to different HIV-1 gp120/gp140 glycoproteins and different synthetic high-mannose V3 glycopeptides with promiscuity of glycosylation sites.
RESULTS
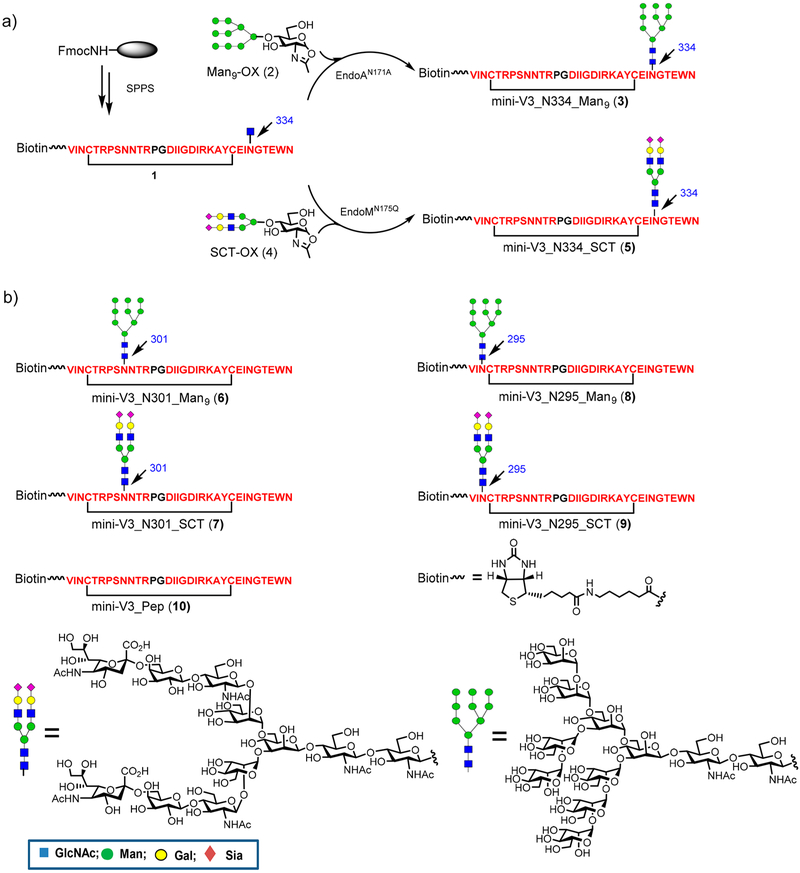
Chemoenzymatic Synthesis of HIV-1 A244 V3 Glycopeptides.
Similar to our previous studies on HIV-1 JR-FL glycopeptides,13,21 we selected the HIV-1 A244 mini-V3 domain (33-mer) corresponding to the residues 292–339, while the 305–320 tip residues were replaced by a dipeptide Pro-Gly (PG) insert as the basic peptide sequence.1 The chemoenzymatic synthesis of the target A244 glycopeptides used a strategy similar to our previously reported synthesis of JR-FL V3 glycopeptides13 Briefly, cyclic precursor peptides (1) carrying an N-acetylglucosamine (GlcNAc) moiety at the N334 site were synthesized by solid phase peptide synthesis (SPPS) (Figure 1). To achieve site-specific immobilization on streptavidin surface for binding analysis, a biotin tag was placed at the N-terminus. A Man9GlcNAc glycan was then transferred from the corresponding glycan oxazoline (2) using glycosynthase EndoA-N171A22 to afford the desired glycopeptide (3) with a natural glycosidic bond in excellent yield (Figure 1). Similarly, a sialylated complex-type glycan was transferred from the sialoglycan oxazoline (4) using EndoM-N175Q23 as the catalyst to afford glycopeptide (5). Glycopeptides (6, 7, 8, and 9) carrying a high-mannose and a complex type glycan at N301 and N295 sites, respectively, together with the aglycone V3 peptide (10) were also synthesized using the same strategy.
Figure 1.
Chemoenzymatic synthesis of HIV-1 A244 V3 glycopeptides: (a) synthesis of A244 V3 glycopeptides carrying a high-mannose or a complex-type N-glycan at the N334 site; (b) structures of additional synthetic A244 V3 peptides and glycopeptides carrying a high-mannose or a complex-type N-glycan at the N301 and N295 sites.
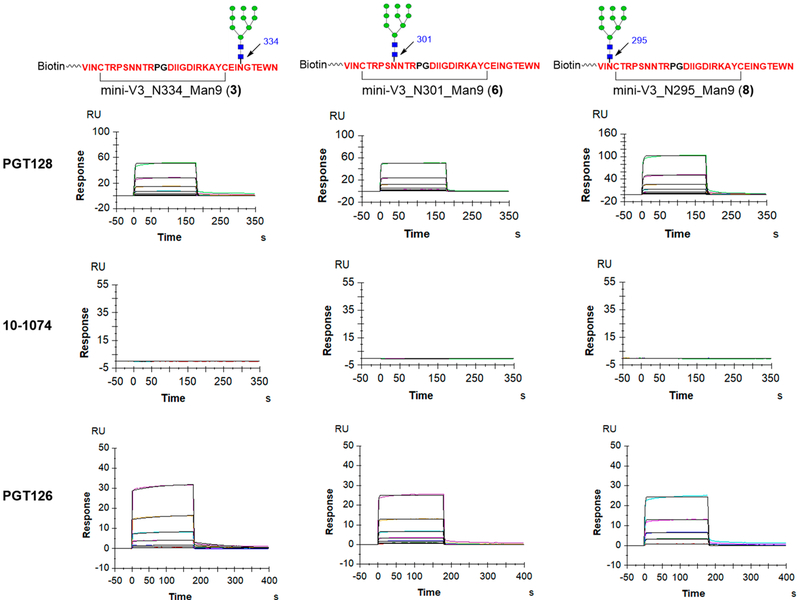
Binding of A244 V3 Glycopeptides to bNAbs.
The synthetic biotin tagged V3 glycopeptides were captured on NeutrAvidin immobilized CM5 chips with an identical response unit for binding evaluation of bNAbs PGT128, PGT126, PGT121, and 10-1074. We found that bNAb PGT128 could bind to all the V3 glycopeptides (3, 6, and 8) carrying a high-mannose glycan at the N334, N301, or N295 site with moderate affinity (Figure 2), but no binding was observed to V3 glycopeptides bearing sialylated complex-type glycan (Supporting Information, Figure S1.) Thus, shifting the high-mannose N-glycan from N332 to the N334 site did not abolish the binding of antibody PGT128. Similarly, we found that PGT126 could bind all the V3 glycopeptides carrying a high-mannose glycan but not the glycopeptides carrying the complex-type N-glycan. These results verified that the recognition of PGT128 and PGT126 to high-mannose glycan is promiscuous in the context of the V3 peptide, consistent with a previous mutational study.1 Interestingly, 10-1074 did not bind to any A244 V3 glycopeptides (Figure 2), while in a previous study we found that 10-1074 exhibited strong binding to JR-FL V3 glycopeptides carrying a high-mannose glycan at the N332 site.13,21 Switching the high-mannose glycan from N332 to N334 completely abolished binding of 10-1074. Similarly, PGT121 was unable to bind to the A244 glycopeptides bearing a high-mannose N-glycan but could bind to the glycopeptide (7) with a sialylated complex-type N-glycan placed at the N301 site (Figure S1). The binding specificity of PGT121 was consistent with our previous binding results with the JR-FL V3 glycopeptide.13 Lastly, all the bNAbs we tested demonstrated no binding to the aglycone V3 peptide (10) under our SPR conditions (Figure S1), confirming that the peptide alone is not sufficient to achieve high affinity for these antibodies.
Figure 2.
SPR sensorgrams of binding to A244 V3 high-mannose glycopeptides (3, 6, and 8) by bNAb PGT128, 10-1074, and PGT126.
Previous structural studies of PGT124, a homologous antibody of 10-1074, indicated that both the N332 high-mannose glycan and the GDIR motif at the base of V3 loop were critical for the binding to envelope glycoprotein gp120.9,24 As the A244 V3 peptide sequence includes the GDIR motif, the major difference between HIV-1 JR-FL and A224 V3 domains is that the conserved N-glycosylation at N332 site was moved to N334. We also synthesized a mutated A244 V3 glycopeptide (11) with the N334 shifted back to N332 and performed its binding to PGT128, PGT126, and 10-1074. This mutated A244 V3 glycopeptide (11) contains a similar N332 high-mannose glycan and the GDIR motif as the JR-FL V3 glycopeptide (12) (Supporting Information, Figure S2). PGT128 and PGT126 were able to bind the glycopeptide (11) with similar affinity as the other A244 glycopeptides which carried a high-mannose N-glycan at N334. Interestingly, the N332 glycan-specific 10-1074 antibody did not show any affinity to glycopeptide (11) (Supporting Information, Figure S2). These results further verified the promiscuous recognition of PGT128 and PGT126 to high-mannose glycan. However, the fact that 10-1074 did not show binding to glycopeptide (11) suggested that other amino acid residues in the V3 loop might be also critical for high-affinity recognition by 10-1074.
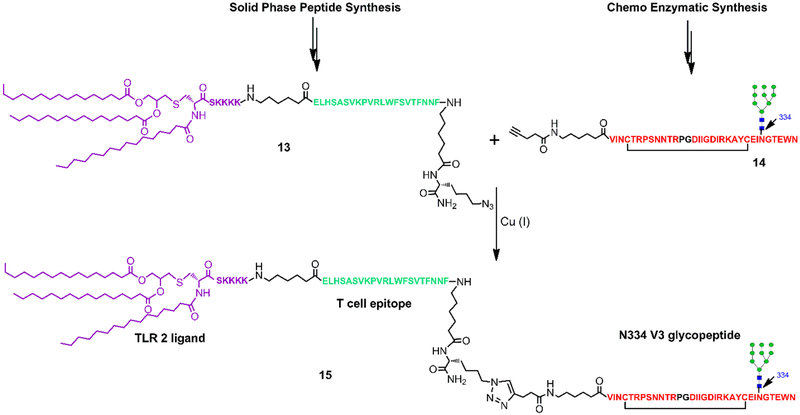
Synthesis of A244 V3 Glycopeptide Immunogen.
To evaluate the immunogenicity of the HIV-1 A244 V3 glycopeptide, we synthesized a self-adjuvanting three-component HIV immunogen which included the A244 V3 glycopeptide with a high-mannose glycan at the N334 site, the P30 T-helper epitope FNNFTVSFWLRVPKVSASHLE,25 and the Toll-like receptor 2 (TLR2) ligand lipopeptide Pam3CSK414,26 to activate the immune cells (Figure 3). Briefly, the lipopeptide (13), which contains the Pam3CSK4 component, the T-helper peptide epitope, and an azide moiety, was prepared following the previously described procedure.14 The A244 V3 glycopeptide (14) with an alkyne moiety placed at the N-terminus was synthesized by the chemoenzymatic method that involved the EndoM-N175A catalyzed transfer of a high-mannose N-glycan to the corresponding GlcNAc-peptide acceptor.14 Finally, the desired glycopeptide immunogen (15) was afforded in excellent yield by copper(I)-catalyzed alkyne–azide [3 + 2] cycloaddition reaction (click chemistry) between the lipopeptide (13) and the glycopeptide (14) (Figure 3). The identity and homogeneity of (15) were confirmed by analytical HPLC and LC–MS analysis.
Figure 3.
Synthesis of three-component HIV immunogen containing the A244 V3 N334 high-mannose glycopeptide.
Rabbit Immunization.
The three-component HIV-1 V3 glycopeptide immunogen (15) thus synthesized was formulated into liposomes following the previously reported procedures.14,27 The liposomes were then administered to rabbits (n = 3) at low-dosing of 50 μg of synthetic immunogen per immunization, delivered by subcutaneous and intramuscular injections. As the Pam3CSK4 could serve as an adjuvant, no external adjuvants were added. After priming, a total of three boosters were given at intervals of 21 days. Bleeding was done 7 days after the last injection, and the obtained antisera from the three immunized rabbits were combined and used for binding analysis.
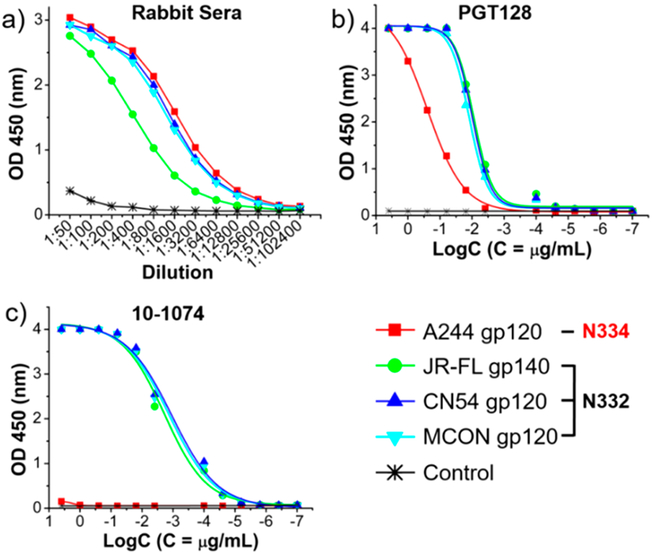
ELISA Binding to gp120/gp140.
We first evaluated the recognition of the antisera to an array of HIV-1 gp120 or gp140. The antisera showed significant binding to all the gp120/gp140s tested in our ELISA assays, including A244 gp120, JR-FL gp140, CN54 gp120, and MCON gp120, although the antisera binding to the JR-FL gp140 was relatively weak (Figure 4). This promiscuous binding to gp120/gp140 was similar to antibody PGT128 but different from 10-1074. PGT128 recognized all the tested gp120/gp140, but 10-1074 could only bind to the gp120/gp140 with a N332 glycosylation site (JR-FL, CN54, and MCON) but not a N334 site (A244). The broad recognition to gp120/gp140 by the antisera suggested that the N334 high-mannose N-glycan as part of the antigenic structure was also immunogenic and could direct specific immune responses. The antisera induced by previous synthesized V3 glycopeptide (JR-FL strain) immunogen carrying a N332 high-mannose glycan also showed broad recognition to the gp120/gp140.14 However, the recognition was significantly weaker than that by the A244 V3 glycopeptide immunogen (15) (Table S1). The end point titer to the JR-FL gp140 of the antisera induced by the JR-FL and A244 glycopeptide immunogen was the same. However, for the binding to the A244 gp120, CN54 gp120, and MCON gp120, the end point titer of the antisera elicited by the A244 V3 glycopeptide immunogen (15) was respectively 4-, 2-, and 4-fold greater than that by the JR-FL V3 glycopeptide immunogen (Table S1). Thus, the synthetic A244 glycopeptide immunogen (15) induced stronger antibody response than the JR-FL glycopeptide, suggesting the V3 glycopeptide bearing a N334 high-mannose glycan is more immunogenic than the V3 glycopeptide bearing a N332 high-mannose glycan.
Figure 4.
Comparison of the binding of the antisera and selected bNAbs to an array of HIV-1 gp120/gp140 envelope glycoproteins: (a) antisera from the A244 glycopeptide immunogen; (b) antibody PGT128; (c) antibody 10-1074. The HIV-1 A244 gp120 carries an N-glycan at the N334 site, while HIV-1 JR-FL gp140, CN54 gp120, and MCON gp120 have an N-glycan at the N332 site.
ELISA Binding to Synthetic A244 Glycopeptide.
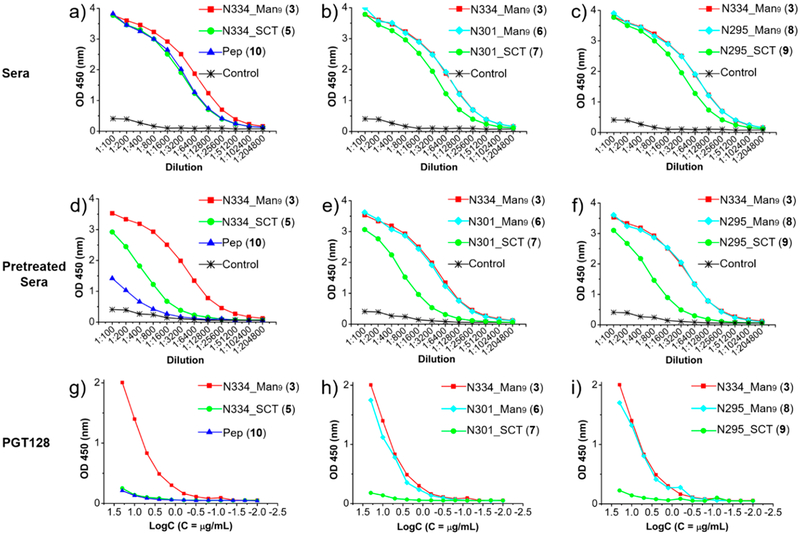
To further evaluate the antibody responses induced by the A244 glycopeptide immunogen, we measured the binding of rabbit antisera to the A244 V3 glycopeptides using ELISA. As shown in Figure 5a, the antisera exhibited strong binding to the N334 glycopeptide, while the affinity of the antisera to the N334 complex-type glycopeptide (5) and the aglycone V3 peptide (10) was weaker than that of the high-mannose glycopeptide (3). The antisera also exhibited strong recognition to the A244 glycopeptides (6 and 8) with a high-mannose N-glycan at the N301 and N295 site, respectively. Interestingly, the affinity of the antisera binding to the three high-mannose glycopeptides (3, 6, and 8) was about the same regardless of the conserved site of the high-mannose N-glycan along the V3 domain (Figure 5b,c), whereas the binding of the antisera to the complex type A244 glycopeptides (5, 7, and 9) was weaker than those of the corresponding high-mannose V3 glycopeptides (3, 6, and 8). These results suggest that antibodies raised by the three-component A244 glycopeptide immunogen bearing a high-mannose N-glycan at the N334 site may be glycan-dependent with binding specificity.
Figure 5.
ELISA analysis of the binding of the antisera and antibody PGT128 to synthetic A244 V3 glycopeptides: (a–c) ELISA binding of the rabbit antisera to A244 V3 glycopeptides; (d–f) ELISA binding of the pretreated (V3 peptide-depressed) rabbit sera to A244 V3 glycopeptides; (g–i) ELISA binding of PGT128 to A244 V3 glycopeptides.
Previous studies using animal immunizations have shown that gp120 V3 peptides are immunogenic and can elicit antibody responses that target the peptide epitopes.28,29 These peptide-specific antibodies may interfere the binding of glycopeptide-specific antibodies to glycopeptides. To remove these peptide binding antibodies from the rabbit sera, we pretreated the sera with the aglycone peptide (l0)-bound beads and performed ELISA binding using these pretreated sera, as demonstrated in our recent study.14 As shown in Figure 5d, the pretreated sera exhibited very weak binding to the aglycone peptide (10), indicating that most of the peptide specific antibodies were efficiently removed. Interestingly, the pretreated sera showed significantly decreased binding to the N334 complex-type glycopeptide (5) when compared to the nontreated sera (Figure 5a and Figure 5d). However, the pretreated sera still showed high-affinity binding to the N334 high-mannose glycopeptide (3) (Figure 5d), although the titer was slightly lower than that of the nontreated sera (Figure 5a). These results suggest that substantial glycopeptide binding antibodies are still left in the sera. We next performed ELISA analysis to the N301 and N295 V3 glycopeptides with the pretreated sera. Consistent with the N334 V3 glycopeptides, the binding of the pretreated sera to the complex-type V3 glycopeptides (7 and 9) was much weaker than that of the corresponding high-mannose V3 glycopeptides (6 and 8) (Figure 5e,f). More interestingly, the binding profiles to the N301 and N295 high-mannose glycopeptides (6 and 8) by the pretreated sera were identical with the N334 high-mannose glycopeptide (3) (Figure 5e,f). In comparison, antibody PGT128 also recognized all the V3 high-mannose glycopeptides in ELISA binding with similar binding profiles (Figure 5g–i), showing glycosylation site-promiscuity. Taken together, our results indicate that the synthetic V3 N334 high-mannose glycopeptide immunogen could induce substantial glycopeptide specific antibodies within a short-term immunization, and these antibodies show promiscuous recognition to HIV-1 gp120/gp140 envelope glycoproteins and synthetic V3 glycopeptides with apparent specificity to the high-mannose N-glycan. The broad recognition by the antisera raised by the A244 V3 glycopeptide immunogen was similar to PGT128 but differed from 10-1074, where PGT128 also recognized all the gp120/gp140 but 10-1074 recognized only the gp120/gp140 carrying a N332 glycan.
Preliminary Mapping of the Epitope of the Glycopeptide-Dependent Antibodies in the Antisera.
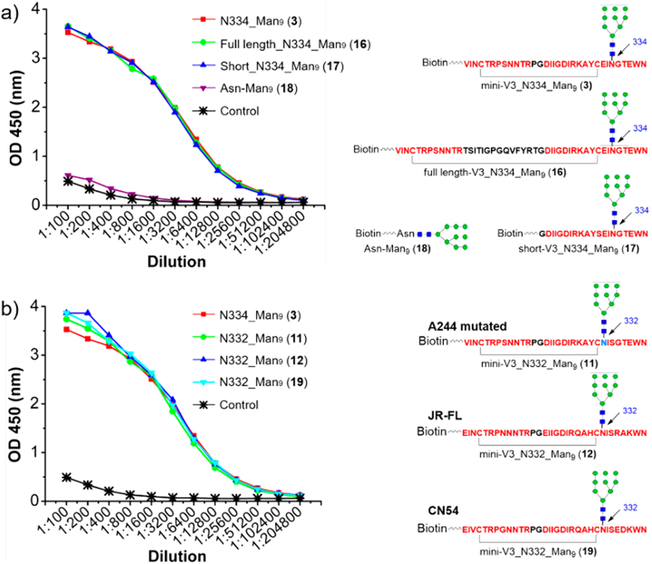
The high-mannose glycopeptide-specific nature of the antibodies raised by the A244 glycopeptide immunogen prompted us to map the epitopes of the antibodies. In order to compare the binding with the mini-V3 (33-mer) glycopeptide (3), we also synthesized a full-length (43-mer) A244 V3 glycopeptide (16), a short (20-mer) A244 V3 glycopeptide (17), and a biotinylated high-mannose type N-glycan (18) (Figure 6a). Surprisingly, the pretreated sera exhibited equal binding to the full-length, mini, and short V3 high-mannose glycopeptides (Figure 6a), indicating the recognition was mainly dependent by the high-mannose glycan. Some of the bNAbs including PGT128 have been shown to recognize free N-glycans,1 particularly in glycan arrays,30,31 but the binding affinity was much lower than that of the V3 glycopeptides.13 To verify whether the elicited antibodies targeted the free N-glycan, we performed the ELISA binding to the free high-mannose glycan (18). The pretreated sera showed only very weak recognition to the high-mannose glycan (18) (Figure 6a) and also to the aglycone peptide (10) (Figure 5d). In addition, we also examined the binding of the pretreated antisera with several V1V2 glycopeptides carrying a high-mannose N-glycan but having a peptide sequence that is unrelated to the V3 peptide.Similar to the free high-mannose type N-glycan binding study, no apparent binding of the rabbit antisera to the V1V2 glycopeptides was observed (data not shown). Taken together, these peptide binding results suggest that the antibodies elicited by the synthetic A244 glycopeptide immunogen specifically target the epitopes formed by the high-mannose glycan and the peptide epitope. Weak binding of the pretreated sera to the free high-mannose glycan was desired as this could avoid eliciting substantial anti-glycan self-reactive antibodies, which is one of the challenges in immunogen design for targeting the high-mannose patch.10 Next, we performed ELISA analysis to the N332 V3 glycopeptides with the pretreated sera. The sera antibodies bind to the A244 mutated N332 V3 glycopeptide (11), JR-FL V3 N332 glycopeptide (12), and CN54 V3 N332 glycopeptide (19), showing similar binding profiles as the A244 N334 glycopeptide (3) (Figure 6b). We also analyzed the cross binding of the A244 glycopeptides with the pretreated antisera elicited by the JR-FL N332 V3 glycopeptide immunogen that was previously described.14 We found that the pretreated sera showed similar binding profiles to the JR-FL V3 N332 glycopeptide (12) and A244 N334 glycopeptide (3), while the binding to the A244 N334 complex-type glycopeptide (5) was decreased (Figure S3). These results suggest that the A244 V3 glycopeptide has the potential to elicit high-mannose glycopeptide specific antibody responses with promiscuity of glycosylation sites, which is similar to PGT128 and PGT126, and the synthetic V3 glycopeptide with a N334 high-mannose glycan may be a potential antigen for HIV vaccine design.
Figure 6.
ELISA analysis of the binding of the antisera with additional HIV-1 V3 glycopeptides and free high-mannose N-glycans: (a) ELISA binding of the pretreated sera to A244 N334 high-mannose glycopeptide with different length of the peptide backbone; (b) ELISA binding of the pretreated sera to different V3 N332 high-mannose glycopeptides.
Viral Neutralization.
To examine if the anti-sera elicited by the this A244 V3 glycopeptide possessed any virus neutralizing activity, we next performed a TZM-bl cell-based neutralization assay against tier 1 and tier 2 HIV-1 viruses.32,33 The preliminary experiment showed that the obtained antisera from the short-term immunization in rabbits did not neutralize the tier 1 and tier 2 HIV-1 viruses (data not shown), which is similar to recent immunization studies with the HIV-1 JR-FL N332 high-mannose glycopeptide immunogen in rabbits14 and with a synthetic mini-V3 glycopeptide mixed with an adjuvant in rhesus macaque.15 In general, HIV-1 bNAbs typically have some unusual traits, such as the long heavy-chain third complementarity-determining regions, the high levels of somatic mutations, and the high frequency of insertions and deletions.34,35 After extensive virus Env diversification, most bNAb evolution during HIV-1infection has been observed.36 Currently, there is only one report demonstrating that immunization with a well-ordered Env trimer in cow could elicit broad viral neutralizing activities that target the CD4 binding site.37 In another related study, repetitive vaccination with a special HIV-1 Env trimer carrying high-mannose glycans over a 4-year period resulted in induction of some V3-glycan specific antibodies,38 but the antibodies raised could only neutralize the pseudoviruses bearing a high density of high-mannose N-glycans.
DISCUSSION
As a defense to help the virus evade the immune system, the host cell synthesized N-glycans coated on HIV-1 Env are usually weakly immunogenic. Nevertheless, the glycosylation defense is still vulnerable, as many glycan-directed bNAbs such as PGT128 and 10-1074 have been isolated from infected individuals, and these antibodies can recognize and penetrate the glycan shield of HIV-1. Many of these antibodies target the high-mannose patch centered around the glycan at N332 on V3 loop and demonstrate protection against mucosal SHIV challenge in animal models, thus proving promising template for vaccine design. These bNAbs demonstrate varying degrees of dependency on the N332 glycan site, and a shift of the N332 glycan to the N334 position could result in escape from serum neutralizing antibodies. We and others have demonstrated that V3 glycopeptide bearing a high-mannose glycan at the N332 site could mimic the epitope of some bNAbs and showed the potential to elicit glycan-dependent antibodies responses. However, the immunogenicity of V3 glycopeptide with a N334 high-mannose glycan and its recognition by the PGT bNAbs are not clear. In this study, we sought to systematically investigate the immunogenicity of V3 N334 high-mannose glycopeptide by antibody binding and preliminary rabbit immunization. The 33-mer V3 glycopeptides derived from the A244 strain were efficiently synthesized by chemoenzymatic methods. SPR binding revealed that PGT128 and PGT126 could recognize all the high-mannose glycopeptides, showing promiscuity of glycosylation site, which is similar to the recognition to JR-FL V3 glycopeptides. However, the binding was completely abolished for 10-1074 when the N332 high-mannose glycan was shifted to N334 site. Surprisingly, 10-1074 did not bind to the A244 high-mannose glycopeptide with the N334 being shifted back to N332, suggesting that the precise peptide sequence was also important. These binding results indicated that the N334 high-mannose glycopeptide could mimic the conserved epitope of PGT128 and PGT121 but not 10-1074.
To evaluate the immunogenicity of the A244 V3 glycopeptide, we conjugated the V3 glycopeptide carrying a high-mannose glycan at N334 to a universal T cell epitope and a TLR2 agonist aiming to enhance the immune responses. Preliminary immunization studies in rabbits indicated that the synthetic glycopeptide immunogen elicited antibody responses with broad recognition to the gp120/gp140 carrying N332 or N334 glycan. We found that the antisera induced by the A244 V3 glycopeptide immunogen showed stronger binding to the gp120/gp140 than that by the JR-FL V3 glycopeptide immunogen. This broad recognition was similar to PGT128 but different from 10-1074, where PGT128 also recognized all the gp120/gp140 but 10-1074 only recognized the gp120/gp140 carrying a conserved N332 glycan. Further glycopeptide binding studies revealed that the synthetic A244 V3 glycopeptide immunogen induced substantial glycopeptide specific antibodies. The pretreated antisera exhibited strong binding to all the A244 V3 high-mannose glycopeptides and the V3 high-mannose glycopeptides from other strains, with similar binding profiles. However, the binding of the pretreated antisera to the complex-type glycopeptides was significantly decreased, showing binding preference to the high-mannose glycopeptides. The strong binding to the high-mannose V3 glycopeptides with promiscuity of glycosylation site and weak binbut 10-1074 only recognized the gp120ding to the complex-type glycopeptides were very similar to the PGT128 and PGT126, although the two bNAbs show no binding to the complex-type glycopeptidebut 10-1074 only recognized the gp120s. The antisera raised by the A244 glycopeptide immunogen in a preliminary immunization study in rabbits did not show neutralizing activity against tier 1 and tier 2 HIV-1 viruses, probably due to the lack of somatic mutation of the antibodies in a short-term immunization. Nevertheless, the ability of the A244 glycopeptide immunogen to raise substantial glycopeptide-specific antibodies that are cross-reactive to different HIV-1 gp120/gp140 envelope glycoproteins bearing N-glycans at either the N332 or N334 glycosylation site suggests that the A244 V3 glycopeptide may serve as a valuable component for future HIV-1 vaccine design.
CONCLUSIONS
The antigenicity and immunogenicity of the V3 N334 glycopeptides from the HIV-1 A244 strain that carries a conserved N334 N-glycan were systematically evaluated through chemoenzymatic synthesis, antibody binding analysis, and rabbit immunization studies. The experimental results indicate that the synthetic A244 V3 glycopeptide immunogen with a high-mannose N-glycan at the N334 site could elicit substantial glycopeptide specific antibodies which are similar to PGT128 and PGT126 in terms of their promiscuous recognition of HIV-1 gp120/gp140 envelope glycoproteins as well as synthetic V3 glycopeptides. These results suggest that the N334 glycan in the context of the V3 domain is vulnerable and immunogenic. To further evaluate the immunogenicity of A244 glycopeptide immunogen to raise neutralizing antibody responses, future experiments should be directed to immunization studies in non-human primate models using the A244 glycopeptide as a key vaccine component together with other HIV-1 vaccine candidates.
EXPERIMENTAL SECTION
Purity Assessment.
All synthetic peptides and glycopeptides possessed a purity of at least 95%, which was assessed by reverse phase HPLC and mass spectrometric analysis (see the Supporting Information for details).
General Procedures.
A Waters 626 HPLC system equipped with a dual absorbance UV detector was used for analytical HPLC. The synthetic V3 peptides and glycopeptides were injected into a C18 column (YMC-Triart C18, 4.6 mm × 250 mm, 5 μm) and were run at a flow rate of 1 mL/min using a linear gradient (15–45% MeCN containing 0.1% TFA over 30 min). The peptides containing Pam3CK4 were injected into a CN column (YMC-Pack CN, 4.6 mm × 250 mm, 5 μm) and were run by a linear gradient (20–70% MeCN containing 0.1% TFA over 50 min). All ESI-MS spectra were measured on a Micromass ZQ-4000 single-quadrupole mass spectrometer. Preparative reverse-phase HPLC for V3 peptides and glycopeptides was carried out on a Waters 600 HPLC system equipped with a C18 column (Waters XBridge, Prep Shield RP 10 mm × 250 mm, 5 μm) at a flow rate of 4 mL/min. A CN column (YMC-Pack CN, 10 mm × 250 mm, 5 μm) was used for the preparative HPLC purification of the lipopeptides.
Peptide Synthesis.
The glycopeptides (12, 18, and 19) were synthesized in our previous studies.13,21 A CEM Liberty Blue microwave-assisted peptide synthesizer was used for peptide synthesis. Following the Fmoc chemistry strategy, PAL-PEG-PS resin (0.18 mmol/g) was used on a 0.1 mmol scale. 6 equiv of Fmoc-protected amino acids, 6 equiv of TBTU, and 12 equiv of DIPEA in DMF were used for couplings, which were carried out at 45 °C for 20 min. For the longer full-length V3 peptides, the coupling cycle was repeated. The building block Fmoc-(Ac3GlcNAc)-Asn-OH was placed at the desired glycosylation sites. Fmoc deprotection was done using 20% piperidine in DMF containing 0.1 M HOBt. After completion of the synthesis, the obtained resin was washed with dichloromethane (DCM) (3×) and then reacted with the biotinylated linker NHS-LC-biotin in the presence of an excess of DIPEA. After washing the resin with DMF (3×) and DCM (3×), cleavage was carried out by adding cocktail R (TFA/thioanisole/ethanedithiol/anisole = 90/5/3/2) to the resin. After 2 h, the resin was filtered and the collected solution was added to cold diethyl ether for peptide precipitation. The obtained crude peptide was dissolved in acetic acid and then lyophilized. A 20% aqueous DMSO solution was used for the cyclization of the peptides, and a 5% aqueous hydrazine solution was used for the de-O-acetylation of the acetylated GlcNAc moiety. After lyophilization, the obtained crude peptides were purified by preparative RP-HPLC.
GlcNAc-peptide (1). ESI-MS: calcd, M = 4260.7448; found (m/z) 1066.0159 [M+ 4H]4+, 1421.0188 [M + 3H]3+. RP-HPLC retention time tR = 28.8 min.
Chemoenzymatic Synthesis of A244 V3 Glycopeptide Carrying a High-Mannose Glycan.
Glycopeptide 1 (1.45 mg, 0.34 μmol) was mixed with Man9oxazoline 2 (2.83 mg, 1.7 μmol) and Endo-AN171A (40 μg) in phosphate buffer (100 mM, pH 7, 100 μL), and the mixture was incubated at 30 °C. The enzymatic reaction was monitored by analytical RP-HPLC. Upon completion, the reaction was quenched by adding 0.1% aq TFA. The crude product was then purified by RP-HPLC to give glycopeptide 3 (1.75 mg, 87%). ESI MS: calcd, M = 5923.20; found (m/z) 1185.52 [M + 5H]5+, 1481.65 [M + 4H]4+, 1975.20 [M + 3H]3+. RP-HPLC retention time tR = 26.5 min.
Chemoenzymatic Synthesis of A244 V3 Glycopeptide Carrying a Complex-Type Glycan.
Glycopeptide 1 (1.15 mg, 0.27 μmol), sialylated complex-type oxazoline 4 (2.7 mg, 1.35 μmol), and Endo-MN175Q (40 μg) were mixed in phosphate buffer (100 mM, pH 7, 100 μL) and then incubated at 30 °C. The enzymatic reaction was monitored by analytical RP-HPLC. Upon completion, the reaction was quenched by adding 0.1% aq TFA. The crude product was then purified by RP-HPLC to yield the glycopeptide 5 (1.37 mg, 81%). ESI MS: calcd M = 6263.53; found (m/z) 1253.55 [M + 5H]5+, 1566.68 [M + 4H]4+, 2088.65 [M + 3H]3+. RP-HPLC retention time tR = 25.9 min.
Synthesis of A244 Glycopeptide Immunogen.
The synthesis of lipopeptide (13) containing the Pam3CSK4 and P30 T cell epitope was described in our previous study.14 Glycopeptide (14) carrying an alkyne linker was synthesized using SPPS and chemoenzymatic method, as described in a previous study for the similar JR-FL glycopeptide.14 Finally, lipopeptide (13) (1.5 mg, 0.35 μmol), A244 alkyne glycopeptide 14 (2.5 mg, 0.43 μmol), and CuOAc (4 μg, 0.03 μmol) were dissolved in 100 μL of DMF and then incubated at 40 °C for 18 h. The reaction mixture was diluted by adding 1 mL of water and then lyophilized. After HPLC purification on a CN column, the desired immunogen 15 (2.8 mg, 78%) was obtained.
Surface Plasmon Resonance (SPR) Measurement.
SPR was run on a BIAcore T200 system (GE Healthcare) at 25 °C to evaluate the interactions between the synthetic glycopeptides and the antibodies PGT121, PGT128, and 10-1074. The synthesized glycopeptides and free glycans carrying a biotin tag were immobilized on neutravidin-coated CM5 sensor chips (GE Healthcare) in HBS-P buffer (10 mM HEPES, 150 mM NaCl, P20 surfactant 0.05% v/v, pH 7.4) until 200 response unit (RU) was reached. PGT121, PGT128, and 10-1074 were injected individually over the chip at 2-fold increasing concentration in HBS-P buffer at a flow rate of 40 μL/min for 180 s. Then HBS-P buffer was injected at a flow rate of 40 μL/min for 1210 s to allow for dissociation. Chip regeneration was done by injection of 3 M MgCl2 (50 μL/min for 3 min), followed by injection of HBS-P buffer (50 μL/min for 5 min). SPR data were processed by the BIAcore T200 evaluation software. To obtain the apparent kinetic parameters, appropriate blank references were subtracted and the sensorgrams were fitted globally using a 1:1 Langmuir binding model.
Rabbits Immunization.
New Zealand White rabbits (8 weeks old, female) were fused to evaluate the immunogenicity of the synthetic A244 glycopeptide immunogen. Immunizations were carried out by Spring Valley Laboratories, Inc., licensed by the United States Department of Agriculture (APHIS registration number 51-R-051) and has been accredited fully and continuously by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALA-Ci). All animal experiments were performed according to the NIH Guide for the Care and Use of Laboratory Animal.
The synthetic three-component A244 glycopeptide immunogen 15 was incorporated into liposome following a reported procedure.27 New Zealand White rabbits (n = 3) were immunized by intramuscular and subcutaneous injections of the liposome containing 50 μg of 15. After priming, three booster injections were given at interval of 21 days. Then bleeding was done 7 days after last immunization. The rabbit sera from each group were pooled together and used for ELISA analysis.
ELISA Binding to gp120/gp140.
Gp120/gp140 (2 μg/mL in PBS) was coated on the 96-well ELISA microtiter plates by incubating at 4 °C overnight. After washing the plates with PBS/0.05% Tween-20, the plates were blocked by incubating with 2% sodium caseinate (w/v) in PBS at room temperature for 1 h. After washing three times, the plates were titrated against rabbit antisera diluted in PBS containing 1% sodium caseinate and incubated at 37 °C for 1 h. After washing three times, horseradish peroxidase (HRP)-conjugated goat anti-human IgG (H + L) antibody or horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (H + L) antibody (1:3000 diluted in PBDS) was added to the plates (100 μL/well). The plates were then incubated for 1 h at 37 °C. After washing three times, a substrate solution of 3,3′,5,5′-tetramethylbenidine (TMB) was added. After 5 min for color development, a solution of 1 M H3PO4 was added to quench the reaction, and the readout was recorded at a wavelength of 450 nm.
Treatment of Polyclonal Antisera with Unglycosylated Peptide Beads.
Streptavidin magnetic beads (1 mg, New England BioLabs) were first incubated with the synthetic A244 V3 peptide 10 (0.5 mg/mL, 500 μL) at 37 °C for 30 min. After washing the beads three times with PBS buffer, 1 mL of antisera elicited by glycopeptide immunogen 15 was added and incubated for 30 min at 37 °C to subtract the peptide specific antibodies. The supernatant was then separated by applying magnet and used for ELISA analysis.
ELISA Binding to Synthetic V3 Glycopeptides.
To perform peptide ELISA, NeutrAvidin diluted in PBS (5 μg/mL) was added (100 μL/well) to the 96-well ELISA microtiter plates and incubated at 4 °C overnight. After washing with PBS/0.05% Tween-20, the plates were blocked with 2% sodium caseinate (w/v) dissolved in PBS at room temperature for 1 h. After washing three times, biotinylated A244 glycopeptides dissolved in 1% casein PBS (2 μg/mL) were added to the wells (100 μL/well) and incubated at 37 °C for 1 h.. After washing three times, the plates were titrated against rabbit antisera diluted in 1% sodium caseinate and were incubated at 37 °C for 1 h. The plates were washed three times, and then a solution (100 μL/well) of 1:3000 diluted horseradish peroxidase (HRP)-conjugated goat anti-human IgG (H + L) antibody or horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (H + L) antibody in 1% PBS was added to the wells, and the wells were incubated for 1 h at 37 °C. After washing three times, the TMB substrate solution was added. After 5 min for color development, a solution of 1 M H3PO4 was added to quench the reaction, and the readout was recorded at a wavelength of 450 nm.
Supplementary Material
ACKNOWLEDGMENTS
Antibody 10-1074 was kindly provided by Prof. Pamela Bjorkman. The PGT126, PGT128, and HIV-1 gp120s and gp140s were provided by the NIH AIDS reagent program. This work was supported by the National Institutes of Health (Grant R01 AI113896).
ABBREVIATIONS USED
- HIV
human immunodeficiency virus
- HPLC
high performance liquid chromatography
- SPR
surface plasmon resonance
- SHIV
simian human immunodeficiency virus
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jmedchem.8b01290.
SPR sensor grams of the binding of PGT128, PGT126, 10-1074, and PGT121 to synthetic A244 V3 glycopeptides (Figure S1); SPR analysis of the binding of bNAbs to mutated A244 high-mannose glycopeptide (11) and JR-FL N332 high-mannose glycopeptide (12) (Figure S2); ELISA analysis of the binding of the antisera induced by JR-FL N332 V3 glycopeptide to the JR-FL V3 glycopeptide and A244 V3 glycopeptides (Figure S3); comparison of end point titers of the antisera induced by the JR-FL and A244 V3 glycopeptide immunogens to HIV-1 gp120/gp140s (Table S1); HPLC and mass spectrometry profiles of synthetic glycopeptides (1, 3, 5–11, and 14–17) (PDF)
Molecular formula strings (CSV)
Notes
The authors declare no competing financial interest.
REFERENCES
- (1).Pejchal R; Doores KJ; Walker LM; Khayat R; Huang PS; Wang SK; Stanfield RL; Julien JP; Ramos A; Crispin M; Depetris R; Katpally U; Marozsan A; Cupo A; Maloveste S; Liu Y; McBride R; Ito Y; Sanders RW; Ogohara C; Paulson JC; Feizi T; Scanlan CN; Wong CH; Moore JP; Olson WC; Ward AB; Poignard P; Schief WR; Burton DR; Wilson IA A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science 2011, 334, 1097–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Walker LM; Huber M; Doores KJ; Falkowska E; Pejchal R; Julien JP; Wang SK; Ramos A; Chan-Hui PY; Moyle M; Mitcham JL; Hammond PW; Olsen OA; Phung P; Fling S; Wong CH; Phogat S; Wrin T; Simek MD; Koff WC; Wilson IA; Burton DR; Poignard P Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 2011, 477, 466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Moldt B; Rakasz EG; Schultz N; Chan-Hui PY; Swiderek K; Weisgrau KL; Piaskowski SM; Bergman Z; Watkins DI; Poignard P; Burton DR Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc. Natl. Acad. Sci. U. S. A 2012, 109, 18921–18925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Barouch DH; Whitney JB; Moldt B; Klein F; Oliveira TY; Liu J; Stephenson KE; Chang HW; Shekhar K; Gupta S; Nkolola JP; Seaman MS; Smith KM; Borducchi EN; Cabral C ; Smith JY; Blackmore S; Sanisetty S; Perry JR; Beck M; Lewis MG; Rinaldi W; Chakraborty AK; Poignard P; Nussenzweig MC; Burton DR Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature 2013, 503, 224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Klein F; Halper-Stromberg A; Horwitz JA; Gruell H; Scheid JF; Bournazos S; Mouquet H; Spatz LA; Diskin R; Abadir A; Zang T; Dorner M; Billerbeck E; Labitt RN; Gaebler C; Marcovecchio PM; Incesu RB; Eisenreich TR; Bieniasz PD; Seaman MS; Bjorkman PJ; Ravetch JV; Ploss A; Nussenzweig MC HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature 2012, 492, 118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Shingai M; Nishimura Y; Klein F; Mouquet H; Donau OK; Plishka R; Buckler-White A; Seaman M; Piatak M Jr.; Lifson JD; Dimitrov DS; Nussenzweig MC; Martin MA Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature 2013, 503, 277–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Gautam R; Nishimura Y; Pegu A; Nason MC; Klein F; Gazumyan A; Golijanin J; Buckler-White A; Sadjadpour R; Wang K; Mankoff Z; Schmidt SD; Lifson JD; Mascola JR; Nussenzweig MC; Martin MA A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature 2016, 533, 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Caskey M; Schoofs T; Gruell H; Settler A; Karagounis T; Kreider EF; Murrell B; Pfeifer N; Nogueira L; Oliveira TY; Learn GH; Cohen YZ; Lehmann C; Gillor D; Shimeliovich I; Unson-O’Brien C; Weiland D; Robles A; Kummerle T; Wyen C; Levin R; Witmer-Pack M; Eren K; Ignacio C; Kiss S; West AP; Mouquet H; Zingman BS; Gulick RM; Keler T; Bjorkman PJ; Seaman MS; Hahn BH; Fatkenheuer G; Schlesinger SJ; Nussenzweig MC; Kleine F Antibody 10-1074 suppresses viremia in HIV-1-infected individuals. Nat. Med 2017, 23, 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Garces F; Sok D; Kong L; McBride R; Kim HJ; Saye-Francisco KF; Julien JP; Hua Y; Cupo A; Moore JP; Paulson JC; Ward AB; Burton DR; Wilson IA Structural evolution of glycan recognition by a family of potent HIV antibodies. Cell 2014, 159, 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Sok D; Doores KJ; Briney B; Le KM; Saye-Francisco KL ; Ramos A; Kulp DW; Julien JP; Menis S; Wickramasinghe L ; Seaman MS; Schief WR; Wilson IA; Poignard P; Burton D R. Promiscuous glycan site recognition by antibodies to the high-mannose patch of gp120 broadens neutralization of HIV. Sci. Transl. Med 2014, 6, 236ra63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Mouquet H; Scharf L; Euler Z; Liu Y; Eden C; Scheid JF; Halper-Stromberg A; Gnanapragasam PN; Spencer DI; Seaman MS; Schuitemaker H; Feizi T; Nussenzweig MC; Bjorkman PJ Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc. Natl. Acad. Sci. U. S. A 2012, 109, E3268–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Barnes CO; Gristick HB; Freund NT; Escolano A; Lyubimov AY; Hartweger H; West AP Jr.; Cohen AE; Nussenzweig MC; Bjorkman PJ Structural characterization of a highly-potent V3-glycan broadly neutralizing antibody bound to natively-glycosylated HIV-1 envelope. Nat. Commun 2018, 9, 1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Orwenyo J; Cai H; Giddens J; Amin MN; Toonstra C; Wang LX Systematic synthesis and binding study of HIV V3 glycopeptides reveal the fine epitopes of several broadly neutralizing antibodies. ACS Chem. Biol 2017, 12, 1566–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Cai H; Orwenyo J; Giddens JP; Yang Q; Zhang R; LaBranche CC; Montefiori DC; Wang LX Synthetic three-component HIV-1 V3 glycopeptide immunogens induce glycan-dependent antibody responses. Cell Chem. Biol 2017, 24, 1513–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Alam SM; Aussedat B; Vohra Y; Meyerhoff RR; Cale EM ; Walkowicz WE; Radakovich NA; Anasti K; Armand L; Parks R; Sutherland L; Scearce R; Joyce MG; Pancera M; Druz A; Georgiev IS; Von Holle T; Eaton A; Fox C; Reed SG; Louder M; Bailer RT; Morris L; Abdool-Karim SS; Cohen M; Liao HX; Montefiori DC; Park PK; Fernandez-Tejada A; Wiehe K; Santra S; Kepler TB; Saunders KO; Sodroski J; Kwong PD; Mascola JR; Bonsignori M; Moody MA; Danishefsky S; Haynes BF Mimicry ofan HIV broadly neutralizing antibody epitope with a synthetic glycopeptide. Sci. Transl Med 2017, 9, No. eaai7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Cai H; Zhang R; Orwenyo J; Giddens J; Yang Q; LaBranche CC; Montefiori DC; Wang LX Multivalent antigen presentation enhances the immunogenicity of a synthetic three-component HIV-1 V3 glycopeptide vaccine. ACS Cent. Sci 2018, 4, 582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Kong L; Lee JH; Doores KJ; Murin CD; Julien JP; McBride R; Liu Y; Marozsan A; Cupo A; Klasse PJ; Hoffenberg S; Caulfield M; King CR; Hua Y; Le KM; Khayat R; Deller MC; Clayton T; Tien H; Feizi T; Sanders RW; Paulson JC; Moore JP; Stanfield RL; Burton DR; Ward AB; Wilson IA Supersite of immune vulnerability on the glycosylated face of HIV-1 envelope glycoprotein gp120. Nat. Struct. Mol. Biol 2013, 20, 796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Huang CC; Tang M; Zhang MY; Majeed S; Montabana E ; Stanfield RL; Dimitrov DS; Korber B; Sodroski J; Wilson IA; Wyatt R; Kwong PD Structure of a V3-containing HIV-1 gp120 core. Science 2005, 310, 1025–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Rerks-Ngarm S; Pitisuttithum P; Nitayaphan S; Kaewkungwal J; Chiu J; Paris R; Premsri N; Namwat C; de Souza M; Adams E; Benenson M; Gurunathan S; Tartaglia J; McNeil JG; Francis DP; Stablein D; Birx DL; Chunsuttiwat S; Khamboonruang C; Thongcharoen P; Robb ML; Michael NL ; Kunasol P; Kim JH Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med 2009, 361, 2209–2220. [DOI] [PubMed] [Google Scholar]
- (20).Kim JH; Rerks-Ngarm S; Excler JL; Michael NL HIV vaccines: lessons learned and the way forward. Curr. Opin. HIV AIDS 2010, 5, 428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Cai H; Orwenyo J; Guenaga J; Giddens J; Toonstra C; Wyatt RT; Wang LX Synthetic multivalent V3 glycopeptides display enhanced recognition by glycan-dependent HIV-1 broadly neutralizing antibodies Chem. Commun (Cambridge, U. K.) 2017, 53, 5453–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Huang W; Li C; Li B; Umekawa M; Yamamoto K; Zhang X; Wang LX Glycosynthases enable a highly efficient chemoenzymatic synthesis of N-glycoproteins carrying intact natural N-glycans. J. Am. Chem. Soc 2009, 131, 2214–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Umekawa M; Li C; Higashiyama T; Huang W; Ashida H; Yamamoto K; Wang LX Efficient glycosynthase mutant derived from Mucor hiemalis endo-beta-N-acetylglucosaminidase capable of transferring oligosaccharide from both sugar oxazoline and natural N-glycan. J. Biol. Chem 2010, 285, 511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Gristick HB; von Boehmer L; West AP Jr.; Schamber M; Gazumyan A; Golijanin J; Seaman MS; Fatkenheuer G; Klein F ; Nussenzweig MC; Bjorkman PJ Natively glycosylated HIV-1 Env structure reveals new mode for antibody recognition of the CD4-binding site. Nat. Struct. Mol. Biol 2016, 23, 906–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Cai H; Chen MS; Sun ZY; Zhao YF; Kunz H; Li YM Self-adjuvanting synthetic antitumor vaccines from MUC1 glycopeptides conjugated to T-cell epitopes from tetanus toxoid. Angew. Chem., Int. Ed 2013, 52, 6106–6110. [DOI] [PubMed] [Google Scholar]
- (26).Pett C; Cai H; Liu J; Palitzsch B; Schorlemer M; Hartmann S; Stergiou N; Lu M; Kunz H; Schmitt E; Westerlind U Microarray analysis of antibodies induced with synthetic antitumor vaccines: Specificity against diverse mucin core structures. Chem. -Eur. J 2017, 23, 3875–3884. [DOI] [PubMed] [Google Scholar]
- (27).Ingale S; Wolfert MA; Gaekwad J; Buskas T; Boons GJ Robust immune responses elicited by a fully synthetic three-component vaccine. Nat. Chem. Biol 2007, 3, 663–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Sanders RW; van Gils MJ; Derking R; Sok D; Ketas TJ; Burger JA; Ozorowski G; Cupo A; Simonich C; Goo L; Arendt H; Kim HJ; Lee JH; Pugach P; Williams M; Debnath G ; Moldt B; van Breemen MJ; Isik G; Medina-Ramirez M; Back JW; Koff WC; Julien JP; Rakasz EG; Seaman MS; Guttman M; Lee KK; Klasse PJ; LaBranche C; Schief WR; Wilson IA; Overbaugh J; Burton DR; Ward AB; Montefiori DC; Dean H; Moore JP HIV-1 neutralizing antibodies induced by native-like envelope trimers. Science 2015, 349, aac4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).de Taeye SW; Ozorowski G; de la Pena AT; Guttman M; Julien JP; van den Kerkhof TLGM; Burger JA; Pritchard LK; Pugach P; Yasmeen A; Crampton J; Hu J; Bontjer I; Torres JL; Arendt H; DeStefano J; Koff WC; Schuitemaker H; Eggink D; Berkhout B; Dean H; LaBranche C; Crotty S; Crispin M; Montefiori DC; Klasse PJ; Lee KK; Moore JP; Wilson IA; Ward AB; Sanders RW Immunogenicity of stabilized HIV-1 envelope trimers with reduced exposure of non-neutralizing epitopes. Cell 2015, 163, 1702–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Shivatare SS; Chang SH; Tsai TI; Tseng SY; Shivatare VS; Lin YS; Cheng YY; Ren CT; Lee CC; Pawar S; Tsai CS; Shih HW; Zeng YF; Liang CH; Kwong PD; Burton DR; Wu CY; Wong CH Modular synthesis of N-glycans and arrays for the hetero-ligand binding analysis of HIV antibodies. Nat. Chem 2016, 8, 338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Shivatare VS; Shivatare SS; Lee CD; Liang CH; Liao KS; Cheng YY; Saidachary G; Wu CY; Lin NH; Kwong PD; Burton DR; Wong CH Unprecedented role of hybrid N-glycans as ligands for HIV-1 broadly neutralizing antibodies. J. Am. Chem. Soc 2018, 140, 5202–5210. [DOI] [PubMed] [Google Scholar]
- (32).Bonsignori M; Kreider EF; Fera D; Meyerhoff RR; Bradley T; Wiehe K; Alam SM; Aussedat B; Walkowicz WE; Hwang KK; Saunders KO; Zhang R; Gladden MA; Monroe A ; Kumar A; Xia SM; Cooper M; Louder MK; McKee K; Bailer RT; Pier BW; Jette CA; Kelsoe G; Williams WB; Morris L; Kappes J; Wagh K; Kamanga G; Cohen MS; Hraber PT; Montefiori DC; Trama A; Liao HX; Kepler TB; Moody MA; Gao F; Danishefsky SJ; Mascola JR; Shaw GM; Hahn BH; Harrison SC; Korber BT; Haynes BF Staged induction of HIV-1 glycan-dependent broadly neutralizing antibodies. Sci. Transl. Med 2017, 9, eaai7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Sarzotti-Kelsoe M; Bailer RT; Turk E; Lin CL; Bilska M; Greene KM; Gao H; Todd CA; Ozaki DA; Seaman MS; Mascola JR; Montefiori DC Optimization and validation of the TZM-bl assay for standardized assessments of neutralizing antibodies against HIV-1. J. Immunol. Methods 2014, 409, 131–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Burton DR; Hangartner L Broadly neutralizing antibodies to HIV and their role in vaccine design. Annu. Rev. Immunol 2016, 34, 635–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Kelsoe G; Haynes BF Host controls of HIV broadly neutralizing antibody development. Immunol. Rev 2017, 275, 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Bonsignori M; Liao HX; Gao F; Williams WB; Alam SM; Montefiori DC; Haynes BF Antibody-virus co-evolution in HIV infection: paths for HIV vaccine development. Immunol. Rev 2017, 275, 145–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Sok D; Le KM; Vadnais M; Saye-Francisco KL; Jardine JG; Torres JL; Berndsen ZT; Kong L; Stanfield R; Ruiz J; Ramos A; Liang CH; Chen PL; Criscitiello MF; Mwangi W; Wilson IA; Ward AB; Smider VV; Burton DR Rapid elicitation of broadly neutralizing antibodies to HIV by immunization in cows. Nature 2017, 548, 108–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Saunders KO; Nicely NI; Wiehe K; Bonsignori M; Meyerhoff RR; Parks R; Walkowicz WE; Aussedat B; Wu NR; Cai F; Vohra Y; Park PK; Eaton A; Go EP; Sutherland LL ; Scearce RM; Barouch DH; Zhang R; Von Holle T; Overman RG; Anasti K; Sanders RW; Moody MA; Kepler TB; Korber B; Desaire H; Santra S; Letvin NL; Nabel GJ; Montefiori DC; Tomaras GD; Liao HX; Alam SM; Danishefsky SJ; Haynes BF Vaccine elicitation of high mannose-dependent neutralizing antibodies against the V3-glycan broadly neutralizing epitope in nonhuman primates. Cell Rep. 2017, 18, 2175–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.