Abstract
With the advent of transcatheter aortic valve implantation (TAVI), percutaneous transluminal aortic valvuloplasty (PTAV) has experienced a recent renaissance. The roles of PTAV range widely from bridging to definitive therapy to palliation. In general, PTAV is performed without major trouble, but fatal complications including acute aortic regurgitation (AR) are occasionally encountered. We describe a case of severe acute AR complicating PTAV conducted for palliative purposes. Conversion to salvage TAVI bailed out this critical condition. This case demonstrates a new potential use of TAVI.
<Learning objective: Acute aortic regurgitation is a rare fatal complication of percutaneous transluminal aortic valvuloplasty. Emergent open heart surgery had been the only treatment option for this complication to date. In our case, we bailed out this critical complication by converting to salvage transcatheter aortic valve implantation (TAVI). This case suggests a new potential use of TAVI.>
Keywords: Palliative percutaneous transluminal aortic valvuloplasty, Acute aortic regurgitation, Salvage transcatheter aortic valve implantation
Introduction
In the era of transcatheter aortic valve implantation (TAVI), percutaneous transluminal aortic valvuloplasty (PTAV) is used as a bridging procedure to definitive therapy or a palliative therapy [1]. PTAV is technically simple and usually safe. However, one of the rare serious complications is severe acute aortic regurgitation (AR) [2], [3]. To date, open heart surgery had been the only therapeutic option for this complication. In a hemodynamically unstable patient, it is extremely difficult to perform emergent surgical aortic valve replacement (AVR). Performing TAVI as an emergent procedure can be another option, but there are few reported cases of salvage TAVI to bail out acute AR [4].
We performed palliative PTAV in a 95-year-old woman with severe aortic stenosis (AS), but the procedure was complicated by severe acute AR. We successfully performed salvage TAVI to bail out the situation.
Case report
A 95-year-old woman who had recently experienced two episodes of syncope and five admissions for heart failure caused by severe AS was transferred to our hospital.
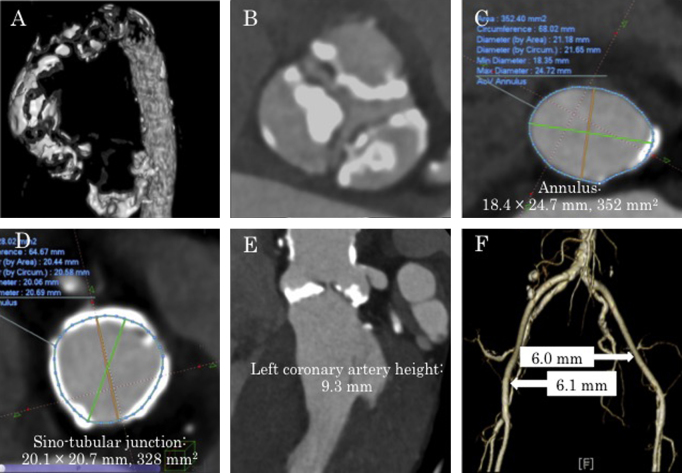
She had a history of hypertension, dyslipidemia, and chronic kidney disease. Her body surface area was only 1.21 m2, but she had maintained daily activities well and her intelligence was normal. Echocardiography and computed tomography (CT) showed severe AS and mild AR due to degenerative changes (aortic valve area by equation of continuity, 0.39 cm2; maximum velocity, 5.4 m/s; mean pressure gradient, 70 mmHg; regurgitant volume, 17 ml; regurgitant fraction, 31%; effective regurgitant orifice area, 0.11 mm2; aortic annulus, 18.4 mm × 24.7 mm, 352 mm2) (Fig. 1, Fig. 2). CT revealed a short distance of 9.3 mm from the aortic annulus to the left coronary artery (Fig. 1E), and severe calcification of all three cusps of the aortic valve (Fig. 1B). The sino-tubular (ST) junction was narrow at 20.1 mm × 20.7 mm (Fig. 1D). The ascending aorta was also severely calcified, with the appearance of porcelain aorta (Fig. 1A). Mean diameter of the right iliac artery was 6.1 mm, and that of left iliac artery was 6.0 mm (Fig. 1F). Our heart team judged that surgical AVR was not indicated for the 95-year-old patient with porcelain aorta, and TAVI was also high risk due to anatomical problems. Eventually, we decided to perform PTAV to palliate her symptoms.
Fig. 1.
Computed tomographic (CT) images. (A) Extremely severe calcification of the ascending aorta (porcelain aorta) is observed. (B) Aortic valve leaflets are severely calcified. (C) Aortic annulus is 18.4 mm × 24.7 mm, 352 mm2. (D) Sino-tubular junction is 20.1 mm × 20.7 mm, 328 mm2. (E) Left coronary artery height is 9.3 mm. (F) Ilio-femoral arteries.
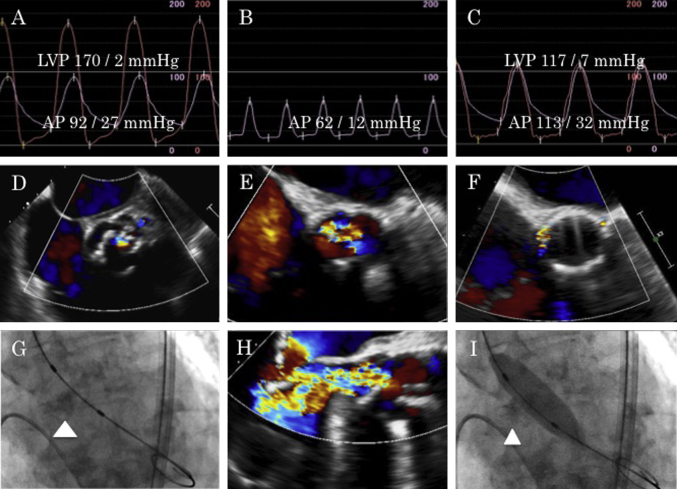
Fig. 2.
(A) Pre-procedural simultaneous aorta and left ventricle pressure curves. (B) Aortic pressure during severe acute aortic regurgitation. (C) Simultaneous aorta and left ventricle pressure curves after valve implantation. (D) Mild aortic regurgitation before percutaneous transluminal aortic valvuloplasty (PTAV). (E, H) Severe acute aortic regurgitation through the non-coronary cusp after PTAV. (F) Trivial peri-valvular leakage after salvage transcatheter aortic valve implantation. (G) Bulky calcification of non-coronary cusp (arrowhead). (I) Balloon aortic valvuloplasty and entrapment of the calcified non-coronary cusp (arrowhead). LVP: left ventricle pressure, AP: aorta pressure.
We performed PTAV under local anesthesia by a retrograde approach from the right common femoral artery. We inserted a 12-French long sheath into the right common femoral artery and placed a stiff wire in the left ventricle apex. Pressure gradient between the left ventricle and ascending aorta (peak to peak) was 78 mmHg (Fig. 2A). We chose a 16-mm balloon based on prior test results and expanded it under rapid ventricular pacing. After the first expansion, hemodynamics suddenly deteriorated and diastolic blood pressure fell to 12 mmHg (Fig. 2B). Echocardiography detected severe AR from the non-coronary cusp (NCC), which was not found before the procedure (Fig. 2E and H). Although echocardiography could not reveal cusp itself, cineangiography showed balloon inflation had entrapped the calcified, bulky NCC in an open (erect) position, and failure of the NCC to close had led to severe AR (Fig. 2G and I). We attempted to reposition the NCC using an 8-French JR guiding catheter, but failed. For life-saving purposes, we converted the procedure to salvage TAVI immediately after obtaining consent from the family.
We replaced the 12-French long sheath with a 16-French e-Sheath (Edwards Lifesciences, Irvine, CA, USA). Under rapid ventricular pacing of 190 per minute, we deployed a 23-mm Edwards SAPIEN XT valve with 3 ml underfilling (Edwards Lifesciences) (Fig. 2C). No significant peri-valvular leakage (Fig. 2F), annulus rupture, coronary obstruction, and aortic dissection were detected. Echocardiography revealed good function of the prosthetic heart valve (effective orifice area, 1.6 cm2; maximum velocity, 2.0 m/s; mean pressure gradient, 7.9 mmHg). Right bundle branch block that had existed before PTAV progressed to complete atrio-ventricular block after valve deployment. Because her iliac arteries were at the lower limit of the recommended vessel diameter and had atherosclerotic changes, the right external iliac artery was ruptured. We surgically replaced the right external iliac artery via a retroperitoneal approach.
On postoperative day 3, we implanted a permanent pacemaker and the patient was transferred to a general ward. After rehabilitation for two weeks, she was discharged and returned home.
Discussion
We performed PTAV in a super-elderly female patient with recurrent heart failure, but the procedure was complicated by severe acute AR. Therefore, we converted the procedure to salvage TAVI, and successfully bailed out the situation.
Before TAVI was available, PTAV had been the only palliative therapy for inoperable patients with severe AS. Even after the introduction of TAVI, PTAV remains a treatment option in patients who are at high risk for TAVI or whose life expectancy is limited due to non-cardiac problems [1]. In a shock state due to AS, emergent PTAV is performed as a bridge to definitive therapies. Generally, PTAV can be performed safely, but serious complications such as acute AR, annulus rupture, and coronary obstruction do occur rarely [2], [3]. Acute AR is fatal if accompanied by sudden hemodynamic aggravation without prompt bail-out. Cusp entrapment or mid-leaflet tear has been reported to be one of the possible mechanisms [5], and the former is suspected to be the cause of acute AR in the present case. Cusp entrapment is caused by interference due to severe calcification of the coronary cusp. In this case, bulky calcification of the NCC resulted in cusp entrapment after aortic valvuloplasty. Repositioning using a guiding catheter is the quickest and the least invasive method, which we attempted but failed. Other than the catheter method, emergent surgical AVR had been the therapeutic option, but it was too invasive to perform for the super-elderly patient in a shock state. This patient had a low coronary height and narrow ST junction. The low coronary height may lead to coronary obstruction following TAVI, which is one of the fatal complications of TAVI [6]. In addition, balloon inflation in the case of narrow ST junction may cause aortic dissection. Although this case was not generally indicated for TAVI, we successfully performed salvage TAVI for bail-out from acute AR. Our heart team is adequately experienced in transcatheter aortic valve interventions including PTAV and TAVI, and is well prepared to perform emergent procedures, which accounts for the excellent outcome of salvage TAVI in this case.
In summary, we performed PTAV as a palliative therapy in a super-old patient with severe AS and recurrent heart failure. The procedure was complicated by severe acute AR after balloon expansion. We converted the procedure to salvage TAVI for lifesaving purposes and succeeded in bailing out the situation. This case demonstrates a new potential use of TAVI. When performing elective PTAV, it is desirable to have backups for TAVI and AVR.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
The authors would like to thank the other members of the TAVI team: Kenichi Hagiya, MD; Yuko Utanohara, MD; Harutoshi Tamura, MD; Keitaro Mahara, MD; Itaru Takamisawa, MD; Makoto Suzuki, MD; and Nobuo Iguchi, MD.
References
- 1.Daly M.J., Monaghan M., Hamilton A., Lockhart C., Kodoth V., Pillai S., Manoharan G., Spence M.S. Short-term efficacy of palliative balloon aortic valvuloplasty in selected patients with high operative risk. J Invasive Cardiol. 2012;24:58–62. [PubMed] [Google Scholar]
- 2.Sgroi C., Gulino S., Attizzani G.F., Immè S., Patanè M., Ohno Y., Cannata S., Giarratana A., Aruta P., Bottari V., Barbanti M., Deste W., Giannazzo D., Tamburino C. Valve rupture after balloon aortic valvuloplasty successfully managed with emergency transcatheter aortic valve implantation. Int J Cardiol. 2013;168:e13–e14. doi: 10.1016/j.ijcard.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 3.Safian R.D., Berman A.D., Diver D.J., McKay L.L., Come P.C., Riley M.F., Warren S.E., Cunningham M.J., Wyman R.M., Weinstein J.S., Grossman W., McKay R.G. Balloon aortic valvuloplasty in 170 consecutive patients. N Engl J Med. 1988;319:125–130. doi: 10.1056/NEJM198807213190301. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Dor, Pichard A.D., Satler L.F., Goldstein S.A., Syed A.I., Gaglia M.A., Jr., Weissman G., Maluenda G., Gonzalez M.A., Wakabayashi K., Collins S.D., Torguson R., Okubagzi P., Xue Z., Kent K.M. Complications and outcome of balloon aortic valvuloplasty in high-risk or inoperable patients. JACC Cardiovasc Interv. 2010;3:1150–1156. doi: 10.1016/j.jcin.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 5.Treasure C.B., Schoen F.J., Treseler P.A., Bittl J.A. Leaflet entrapment causing acute severe aortic insufficiency during balloon aortic valvuloplasty. Clin Cardiol. 1989;12:405–408. doi: 10.1002/clc.4960120711. [DOI] [PubMed] [Google Scholar]
- 6.Ribeiro H.B., Webb J.G., Makkar R.R., Cohen M.G., Kapadia S.R., Kodali S., Tamburino C., Barbanti M., Chakravarty T., Jilaihawi H., Paradis J.M., de Brito F.S., Jr., Cánovas S.J., Cheema A.N., de Jaegere P.P. Predictive factors, management, and clinical outcomes of coronary obstruction following transcatheter aortic valve implantation: insights from a large multicenter registry. J Am Coll Cardiol. 2013;62:1552–1562. doi: 10.1016/j.jacc.2013.07.040. [DOI] [PubMed] [Google Scholar]