Abstract
Fulminant myocarditis (FM) sometimes causes severe left ventricular dysfunction and lethal arrhythmias leading to cardiogenic shock and critical conditions. Thus, mechanical circulation support with intra-aortic balloon pumping and/or a cardiopulmonary support system (CPS) is sometimes needed to save lives. The special recommended therapies for FM for that classified as class I (evidence level C) in the guidelines of the Japanese Circulation Society are intra-aortic balloon pumping, CPS, percutaneous cardiac pacing, and a left ventricular assist device (LVAD), and they are well established in evidence-based medicine. We experienced a case of FM that we were able to save by long-term stable CPS support. Because, unfortunately, the LVAD was not commercially available in Japan at that time, intensive treatments including CPS were continued in our hospital. Finally, a good course of the illness was achieved without any adverse complications. Thus, these intensive treatments in the present case may be one of the optional effective strategies for FM, especially in hospitals and/or countries where the LVAD is not (commercially) available, and when an LVAD may not be suitable because of complications associated with infectious disease.
<Learning objective: Intensive treatment including cardiopulmonary support system as in this case may be one of the optional effective strategies for fulminant myocarditis, especially in hospitals and/or countries where left ventricular assist devices (LVAD) are not (commercially) available, and when an LVAD may not be suitable because of complications associated with infectious disease.>
Keywords: Fulminant myocarditis, Cardiopulmonary support system, Congestive heart failure, Long-term
Introduction
Fulminant myocarditis (FM) sometimes causes severe left ventricular dysfunction and lethal arrhythmias leading to cardiogenic shock and critical conditions [1], [2]. Thus, mechanical circulation support with intra-aortic balloon pumping (IABP) and/or a cardiopulmonary support system (CPS) is needed to save lives. Here, we report a case of FM treated with long-term support, of more than 332 h, by CPS in order to save a patient with FM.
Case report
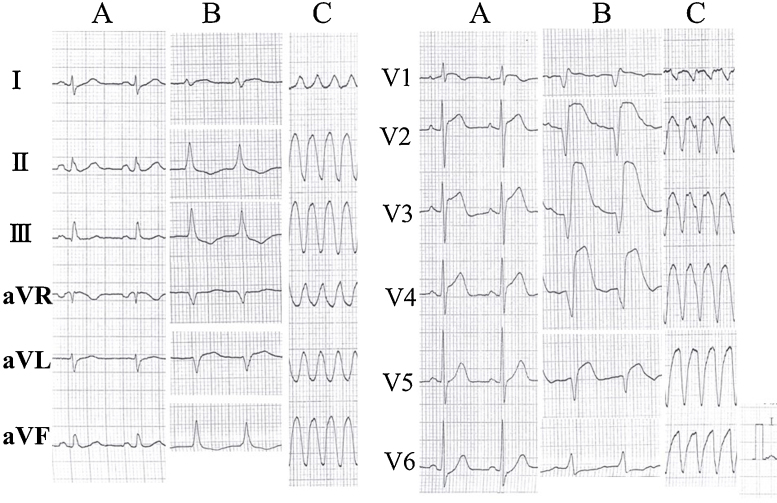
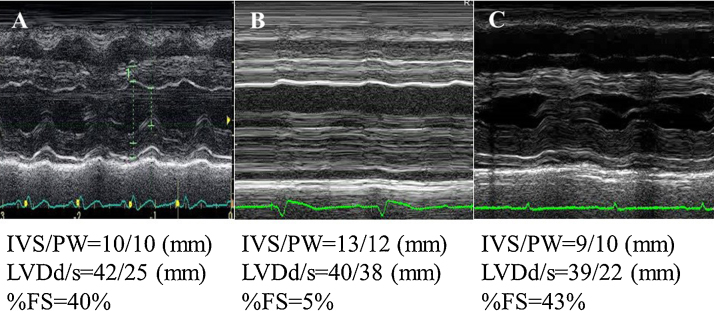
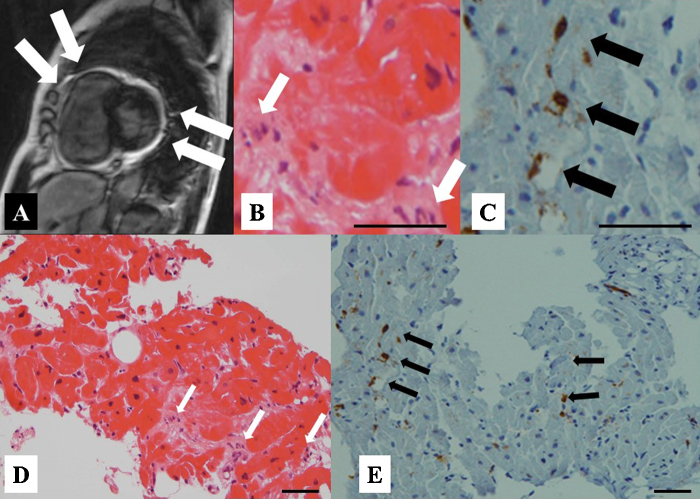
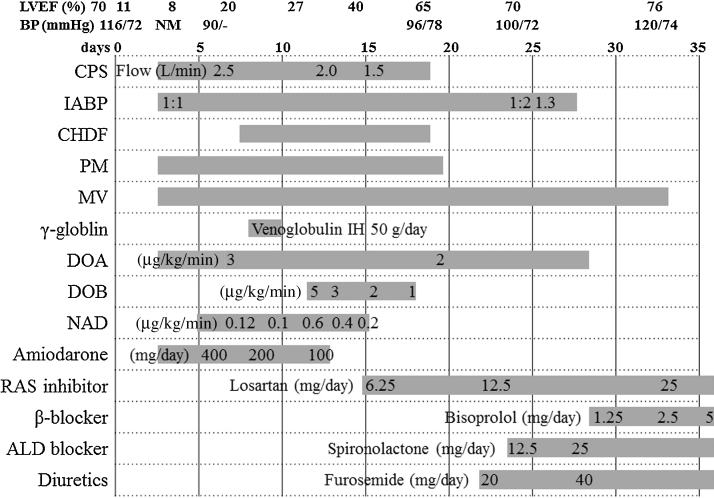
A 65-year-old female (height 156 cm, body weight 56 kg, body surface area 1.51 m2) was admitted to our hospital with a chief complaint of a high fever and general fatigue. She was diagnosed with a common cold by her previous doctor. Her blood pressure was 116/72 mmHg, white cell count 5300/μl with 87% neutrophils, C-reactive protein 13.34 mg/dl, and creatinine kinase (CK) 379 IU/L. The electrocardiogram (ECG) showed mild ST elevation in leads V1–5 (Fig. 1A). The transthoracic echocardiography (TTE) revealed no wall motion abnormalities (Fig. 2A). However, on admission, she complained of chest discomfort with hypotension. Moreover, the ECG exhibited ST elevation in leads V1–5 with complete atrioventricular block and wide QRS intervals (Fig. 1B). Emergent coronary angiography (CAG) revealed normal coronary trees, and the left ventriculogram and TTE showed diffuse severe hypokinesis with a left ventricular (LV) ejection fraction (EF) of 11% (Fig. 2B). Thus, acute myocarditis was diagnosed. Because of her stable hemodynamic condition, we did not insert IABP for this patient then. However, 4 days after the occurrence of clinical symptoms, ventricular tachycardia occurred (Fig. 1C), and she fell into cardiogenic shock. Intensive treatment with intravenous administration of catecholamines and amiodarone, percutaneous cardiac pacing, IABP, CPS, and mechanical ventilation were immediately started to maintain her hemodynamic condition. Moreover, the intravenous administration of gamma globulin (Venoglobulin IH, Welfide, Co., Osaka, Japan; 50 g per day for 2 days) and continuous hemodiafiltration (CHDF) to relieve the cytokines were also performed. Because of her unstable hemodynamic condition, no myocardial biopsies were performed. Furthermore, because she was complicated with a bacteremia and an anemia, the antibiotics and blood transfusion with a total of 32 units of red cell concentrates were needed. After that intensive treatment, her condition gradually, but slowly, improved. Finally, her left ventricular (LV) wall motion normalized with an ejection fraction (EF) of 65% (Fig. 2C), and it was possible to wean her from CPS after long-term CPS support, of more than 332 h. However, because of the oozing bleeding from the wound after CPS was removed, IABP was not removed. After we confirmed discontinuation of the oozing bleeding, IABP was removed after long-term support of more than 524 h. Fortunately, we never needed to exchange the circuit of the artificial lung for the CPS. Before her discharge, her gadolinium-enhanced cardiac magnetic resonance imaging (Fig. 3A) on day 53 and cardiac myocyte biopsies from the LV (Fig. 3B–E) on day 66 showed compatible findings for FM. The maximum CK was 1609 IU/L on day 4, and the eosinophilia in peripheral blood was not seen. She was discharged on day 90 on foot and remains well without any symptoms under medical therapies with renin–angiotensin system inhibitor, β-blocker, aldosterone blocker, and diuretics (Fig. 4).
Fig. 1.
The electrocardiogram received from the previous doctor (A), on admission (B), and on day 3 (C).
Fig. 2.
The M-mode (A–C) transthoracic echocardiogram received from the previous doctor (A), on admission (B), and on day 18 (C). IVS, interventricular septum; PW, posterior wall; LVDd, diastolic left ventricular diameter; LVDs, systolic left ventricular diameter; LVEF, left ventricular ejection fraction.
Fig. 3.
The T2-weighted imaging of the gadolinium-enhanced cardiac magnetic resonance imaging (A) showing a high signal gadolinium late enhancement and stain of the tissue in a part of the right ventricle and left ventricular posterior wall. Histopathological specimens from the inflammatory left ventricular myocardium with hematoxylin–eosin staining (B of 500 and D of 200 magnifications, respectively) showing the difference in the size of the myocardial cellular nuclei and eosinophilic changes in the cytoplasm and the invasion of the mononucleosis in the stroma (white arrows). Immunohistochemical staining (C of 500 and E of 200 magnifications, respectively) showing the monocytes indicating a positive leukocyte common antigen as a brown color (black arrows), positive CD3 of the T cellular marker, and negative CD20 of the B cellular marker. Bars in B, C, D, and E indicate 20 μm.
Fig. 4.
The clinical course of this patient. LVEF, left ventricular ejection fraction; BP, blood pressure; NM, could not be measured; CPS, cardiopulmonary support; IABP, intra-aortic balloon pumping; CHDF, continuous hemodiafiltration; PM, pacemaker implantation; MV, mechanical ventilation; DOA, dopamine; DOB, dobutamine; NAD, noradrenaline; RAS, renin–angiotensin system; ALD, aldosterone.
Discussion
FM is characterized by a critical myocarditis with an acute onset of a severe hemodynamic compromise and the necessity for extracorporeal support of the circulation [1], [2]. Moreover, its frequency and mortality are 10% of acute myocarditis [3] and 43% during hospitalization [4], respectively. Although the efficacies of the supporting treatments for FM, including steroid pulse treatment [1], [5], high-dose intravenous γ-globulin infusions [1], [6], and CHDF [7] are controversial, it appeared to be effective in the present case. The specially recommended therapies for FM in those classified as class I (evidence level C) in the guidelines of the Japanese Circulation Society are IABP, CPS, percutaneous cardiac pacing, and LV assist devices (LVADs) [8], and they are well established in evidence-based medicine. Because, unfortunately, the LVAD was not commercially available in Japan at that time, intensive treatment including CPS was continued in our hospital. The important problems with the long-term treatment with CPS are as follows: (1) insufficient blood flow from the CPS leading to the progression of multiple organ failure (MOF); (2) an increasing afterload leading to LV stress and pulmonary congestion; (3) thrombus formation leading to thromboembolisms; and (4) the span of the life of the artificial lung of the CPS [4]. In order to avoid these problems, a comparably low supporting flow rate of 2.5 L/min for the CPS [9], which was the minimal flow rate to maintain the oxygen supply to the organs, and a minimal increase in the afterload for the LV were performed. Moreover, the strict maintenance of the activated coagulation time at more than 150 s was also performed to prevent thromboembolisms and to protect the artificial lung of the CPS. Finally, she was able to have a good course of the illness without any adverse complications after the long-term support of more than 332 h on the CPS. Several papers have reported cases of FM treated with long-term support, but less than the 332 h in the present case, by CPS [10], [11], [12]. These reports also insisted on the importance of avoiding progression of MOF, thrombus formation, and complications with infectious disease. Moreover, recent reports have shown that the suitable clinical predicting factors to survive after CPS in patients with cardiovascular disease are as follows: (1) age <50 years; (2) diagnosis of FM; (3) no out-of-hospital cardiac arrest; (4) CPS attempted before cardiac arrest; and (5) time from cardiac arrest to CPS <45 min [13], and the importance of the longer interval (more than 4 days) from occurrence of clinical symptoms associated with FM to induction of CPS [12]. Three (2, 3, and 4) of 5 predicting factors were satisfied, and the interval was 4 days in our present case. Finally, IABP was removed after discontinuation of the oozing bleeding for longer-term support, of more than 524 h. The point that we should reflect in the present case is that we should have inserted IABP at an earlier stage, especially when we documented low LVEF of 11%, probably contributing to early recovery of FM. To the best of our knowledge, there have been no reports of long-term support, such as 332 h, using CPS for FM in an old woman as in the present case. In view of these findings, the intensive treatment in the present case may be one of the optional effective strategies for FM, especially in hospitals and/or countries where the LVAD is not (commercially) yet available, and when an LVAD may not be suitable because of complications associated with infectious disease.
Conflict of interest
The authors state that they have no Conflict of Interest (COI).
Acknowledgment
We thank Mr John Martin for his linguistic assistance with this paper.
References
- 1.Kindermann I., Barth C., Mahfoud F., Ukena C., Lenski M., Yilmaz A., Klingel K., Kandolf R., Sechtem U., Cooper L.T., Bohm M. Update on myocarditis. J Am Coll Cardiol. 2012;59:779–792. doi: 10.1016/j.jacc.2011.09.074. [DOI] [PubMed] [Google Scholar]
- 2.Maron B.J., Udelson J.E., Bonow R.O., Nishimura R.A., Ackerman M.J., Estes N.A., 3rd, Cooper L.T., Jr., Link M.S., Maron M.S. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task force 3: hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy and other cardiomyopathies, and myocarditis: a scientific statement from the American Heart Association and American College of Cardiology. Circulation. 2015;132:e273–e280. doi: 10.1161/CIR.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 3.Gupta S., Markham D.W., Drazner M.H., Mammen P.P. Fulminant myocarditis. Nat Clin Pract Cardiovasc Med. 2008;5:693–706. doi: 10.1038/ncpcardio1331. [DOI] [PubMed] [Google Scholar]
- 4.Acker M.A. Mechanical circulatory support for patients with acute-fulminant myocarditis. Ann Thorac Surg. 2001;71:S73–S76. doi: 10.1016/s0003-4975(00)02628-x. [DOI] [PubMed] [Google Scholar]
- 5.Cooper L.T., Jr., Berry G.J., Shabetai R. Idiopathic giant-cell myocarditis – natural history and treatment. Multicenter giant cell myocarditis study group investigators. N Engl J Med. 1997;336:1860–1866. doi: 10.1056/NEJM199706263362603. [DOI] [PubMed] [Google Scholar]
- 6.Drucker N.A., Colan S.D., Lewis A.B., Beiser A.S., Wessel D.L., Takahashi M., Baker A.L., Perez-Atayde A.R., Newburger J.W. Gamma-globulin treatment of acute myocarditis in the pediatric population. Circulation. 1994;89:252–257. doi: 10.1161/01.cir.89.1.252. [DOI] [PubMed] [Google Scholar]
- 7.Furukawa Y., Kobuke K., Matsumori A. Role of cytokines in autoimmune myocarditis and cardiomyopathy. Autoimmunity. 2001;34:165–168. doi: 10.3109/08916930109007380. [DOI] [PubMed] [Google Scholar]
- 8.Guidelines for diagnosis and treatment of myocarditis (JCS 2009): digest version. Circ J. 2011;75:734–743. doi: 10.1253/circj.cj-88-0008. [DOI] [PubMed] [Google Scholar]
- 9.Aoyama N., Izumi T., Hiramori K., Isobe M., Kawana M., Hiroe M., Hishida H., Kitaura Y., Imaizumi T. National survey of fulminant myocarditis in Japan: therapeutic guidelines and long-term prognosis of using percutaneous cardiopulmonary support for fulminant myocarditis (special report from a scientific committee) Circ J. 2002;66:133–144. doi: 10.1253/circj.66.133. [DOI] [PubMed] [Google Scholar]
- 10.Ohkawa M., Nishikawa K., Takazawa T., Hinohara H., Kunimoto F., Goto F. Successful management of a man with fulminant myocarditis using percutaneous cardiopulmonary support. Masui. 2005;54:172–176. [PubMed] [Google Scholar]
- 11.Sugamura K., Sugiyama S., Kawano H., Horio E., Ono S., Kojima S., Kaikita K., Sagishima K., Sakamoto T., Yoshimura M., Kinoshita Y., Ogawa H. Fulminant myocarditis survivor after 56 hours of non-responsive cardiac arrest successfully returned to normal life by cardiac resynchronization therapy: a case report. J Cardiol. 2006;48:345–352. [PubMed] [Google Scholar]
- 12.Oshima K., Kunimoto F., Hinohara H., Hayashi Y., Hirato J., Tajima Y., Kuwano H. Fulminant myocarditis treated with percutaneous cardiopulmonary support system (PCPS) Ann Thorac Cardiovasc Surg. 2008;14:75–80. [PubMed] [Google Scholar]
- 13.Shirakabe A., Nozaki A., Hata N., Kobayashi N., Shinada T., Tomita K., Tsurumi M., Matsushita M., Okazaki H., Yamamoto Y., Yokoyama S., Asai K., Mizuno K. Predictive score for survival after percutaneous cardiopulmonary support in cardiovascular disease patients – evaluation of pre-procedural information. Circ J. 2013;77:2064–2072. doi: 10.1253/circj.cj-12-1326. [DOI] [PubMed] [Google Scholar]