Abstract
Life-threatening arrhythmias are often found in heart diseases, but they are rare as clinical symptoms of Churg-Strauss syndrome. We report a case of a 66-year-old woman with symptomatic monomorphic ventricular tachycardia as the first sign of Churg-Strauss syndrome. Cardiac manifestations were the main clinical symptoms of the disease, and changes in other organs were weakly expressed. Furthermore, increased serum IgG4 level was revealed. It was the reason for the differential diagnosis with IgG4-related diseases. Echocardiography, cardiac magnetic resonance imaging, and histopathological analysis of biopsies had an important role in diagnosis.
<Learning objective: Ventricular arrhythmias are rare clinical symptoms of Churg-Strauss syndrome. This case is interesting because cardiac manifestations were the main clinical symptom of the disease, and changes in other organs were weakly expressed. Echocardiography, cardiac magnetic resonance imaging, and histopathological analysis of biopsies had an important role in diagnosis. Increased serum IgG4 level was the reason for the differential diagnosis with IgG4-related diseases. Churg-Strauss syndrome should be considered as part of the differential diagnosis in patients with noncoronary ventricular arrhythmias.>
Keywords: Ventricular arrhythmia, Churg-Strauss syndrome, IgG4-related disease
Introduction
Churg-Strauss syndrome (CSS) is a systemic small- and medium-vessel necrotizing vasculitis. Heart disorders in CSS can present as severe left ventricular (LV) dysfunction, cardiomyopathy, congestive heart failure, myocardial fibrosis, blood clots, and cardiac tamponade. Sometimes, heart disease is the first clinical manifestation of CSS. There are few described cases of the first manifestations of CSS as an acute coronary syndrome, acute myocarditis and cardiogenic shock, isolated cardiac tamponade, eosinophilic endomyocarditis with rapidly progressive diastolic dysfunction, and the formation of apical thrombosis of both ventricles. Arrhythmias and conduction disturbance are rare clinical symptoms of CSS. Our case report demonstrates clinical onset of CSS with ventricular arrhythmias.
Case report
A 66-year-old woman with history of arterial hypertension, bronchial asthma, diffuse nodular toxic goiter, and chronic sinusitis was referred to our hospital with palpitation, dizziness, and atypical chest pain. On physical examination, her blood pressure was 150/110 mmHg and heart rate was 100 beats per min.
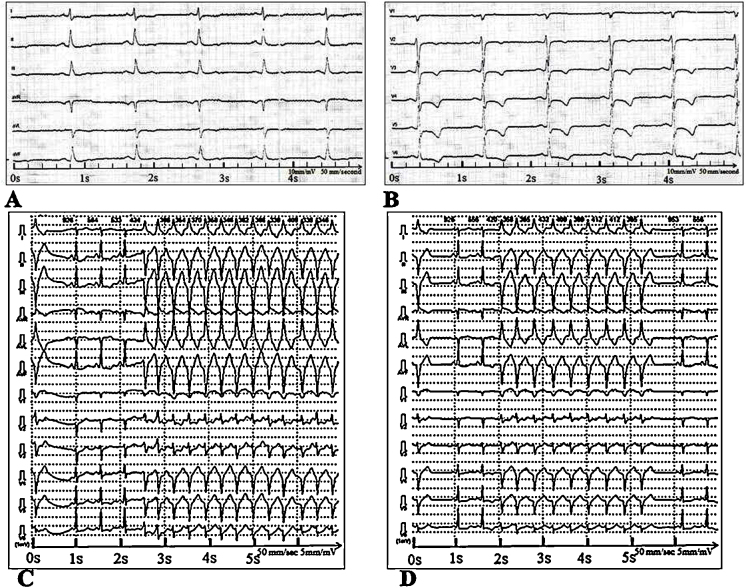
Electrocardiogram (ECG) revealed sinus tachycardia with rapid ectopic ventricular complexes, nonspecific ST-segment changes, and T-wave abnormalities (Fig. 1A and B). During Holter monitoring, frequent single (n = 12,812) and the pair (n = 1580) ectopic ventricular complexes, as well as 140 episodes of nonsustained monomorphic ventricular tachycardia (VT) with a maximum duration of 23 s, were documented. Each paroxysm consisted of 3 or more complexes with a heart rate of 132 to 187 beats per minute (Fig. 1C and D). VT was diagnosed as right ventricular inferoseptal tachycardia.
Fig. 1.
(A, B) Twelve-lead electrocardiography (ECG) showing ST -segment depression in leads V3–V6 and negative T waves in leads V2–V6. Calibration of the ECG is 10 mm/mV. Paper speed is of 50 mm/s. The frequency is of 50 Hz. (C, D) 12-Lead Holter ECG. Episodes of unstable monomorphic ventricular tachycardia with a heart rate of 132 to 187 beats per minute. Filter was 35 Hz, 50 Hz, and isoline; speed was of 50 mm/s and amplitude 5 mm.
Laboratory tests showed eosinophil count range from 2.7% to 29% and slightly elevated troponin I (0.01 ng/ml) and homocysteine (17.3 μmol/l, normal 4.4–13.6 μmol/l). Cytoplasmic pattern antinuclear antibodies (ANA)-HEp2 test detected a titer of 1:320. However, specificity of the ANA and antineutrophil cytoplasm antibodies (ANCA) were not determined. Antibodies to cardiolipin and beta-2-glycoprotein were within normal limits. However, the serum immunoglobulin (Ig) G4 level was slightly elevated (1.59 g/l, normal 0.1–1.35 g/l).
The chest X-ray was normal. But multiple small lesions (up to 5 mm in diameter) of subpleural and perivascular locations were revealed by chest computed tomography scan.
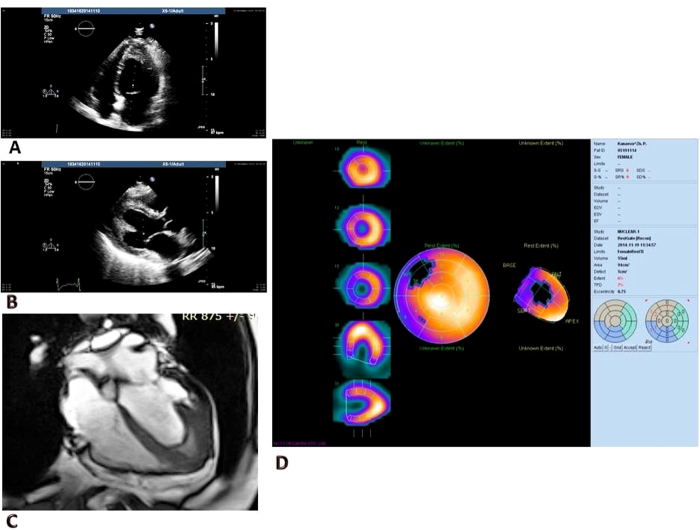
Echocardiography showed a normal-sized LV with regional wall motion abnormalities in apex area. The LV ejection fraction was not impaired (58%). The LV papillary muscle endocardium was thickened, and apical segments of the LV were obliterated by moderate-density heterogeneous masses (Fig. 2).
Fig. 2.
(A, B) Transthoracic echocardiography. Left ventricle with thickening of apical wall and intracavitary thrombus. (C) Cardiac magnetic resonance. Obliteration of the left ventricular apex by thickened endocardium and intracavitary thrombus. (D) Myocardial perfusion gated-single photon emission computed tomography.
Cardiac magnetic resonance showed hypo/akinesis of the LV apical segments with obliteration of the LV apex by thickened endocardium and intracavitary thrombus. Myocardial edema and early and late gadolinium enhancement on T2-weighted images were absent. Myocardial perfusion gated-single photon emission computed tomography revealed a perfusion abnormality at rest in the interventricular septa (middle and basal segments) and large region of hypokinesia in the apical segments. Cardiac malignant tumor was excluded due to normal technetium-99m-labeled methoxyisobutyl isonitrile uptake in the apex. Coronary angiography revealed normal vessels.
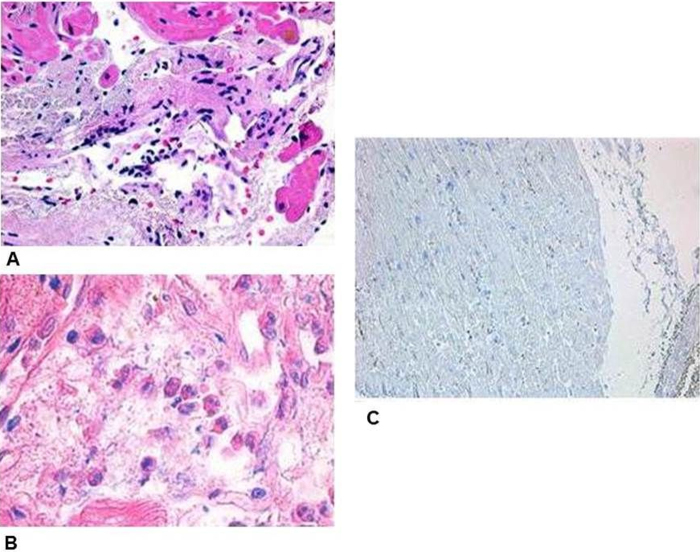
Right ventricular endomyocardial biopsy showed an active myocarditis (CD3+ – 45 cells/mm2) with eosinophilic infiltration and necrotizing granulomatous small-vessel vasculitis (Fig. 3). IgG deposits were revealed, but IgG4 expression was not observed. Skin biopsy showed small-vessel vasculitis and peripheral nerve injury.
Fig. 3.
Endomyocardial biopsy. (A) Necrotizing small-vessel vasculitis with perivascular neutrophilic infiltrates (hematoxylin/eosin staining; original magnification 200×). (B) Myocardial eosinophilic infiltration (hematoxylin/eosin staining; original magnification 400×). (C) Staining for CD38+ IgG4+ cells (Dako, original magnification 100×).
The patient was diagnosed as having ANCA-negative phenotype of CSS with involvement of the heart, respiratory system, and sinuses, according to the American College of Rheumatology criteria of CCS.
The patient received induction therapy with IV cyclophosphamide up to 6 g and medium dose oral steroids. During therapy, the severity of ventricular arrhythmia was reduced from 12,812 single ventricular ectopic complexes to one ventricular ectopic complex per day, with no paired complexes and no paroxysmal VT. Therefore, the patient was switched from initial therapy to azathioprine plus low-dose steroids according to recent recommendations. Standard therapy with warfarin and selective beta-blockers was continued. After 6 months, repeated echocardiography still showed LV with thickening of apical wall and intracavitary thrombus.
Discussion
The echocardiography pattern, which was revealed during the examination, is typical for Loeffler endocarditis. The differential diagnosis for Loeffler endocarditis includes noncompact LV myocardium, asymmetric LV hypertrophy, idiopathic hypereosinophilic syndrome, granulomatous myocarditis (sarcoidosis, tuberculosis), systemic vasculitis, amyloidosis, bronchogenic carcinoma, and T-cell lymphoma. Increased blood eosinophil count has indicated an eosinophilic disorder.
Symptoms such as asthma, elevated serum eosinophils (>10%), mono- or polyneuropathy, pulmonary infiltrates, paranasal sinus abnormalities, and elevated levels of extravasal eosinophils, four of which were present in our patient, are used as diagnostic criteria of CCS according to the American College of Rheumatology.
In our case, the complexity of the clinical diagnosis was due to subtle extracardiac clinical symptoms. There was the controlled asthma, variable eosinophil count, absence of pulmonary infiltrates, and morphological features of vasculitis. Contrariwise, severe symptoms of heart disease such as hypo/akinesis of the LV apical segments with obliteration of the LV apex by thickened endocardium and intracavitary thrombus were present. Life-threatening arrhythmia bedeviled because it is rare for this disease. Absence of ANCA is also less common in CSS, although some studies showed that it was more likely ANCA-negative phenotype of CSS if the heart was affected [1], [2]. Biopsy study of the myocardium and skin flap had an important role in diagnosis.
The most likely mechanism of this VT is abnormal automaticity. All paroxysms of VT occur suddenly in late phase of diastole. They are not induced by extrasystoles and do not depend on the frequency change rate. R-R intervals during paroxysms are relatively regular, but sometimes their duration may vary between 90 and 110 ms. All of these types are more typical for the automatic tachycardia than for trigger or related to re-entry mechanism. The pathologic changes that may cause VT associated with CSS have not been well established. We suggest that arrhythmia can be caused by myocardial ischemia due to necrotizing vasculitis of small- and medium-vessels. We suggest that the VT exit was sited adjacent to the right ventricular lower septum. There was no correlation between the VT origin and structural changes, detected by magnetic resonance imaging. But myocardial perfusion imaging revealed functional abnormalities, such as perfusion defect in interventricular septa.
Due to the increased serum IgG4 level, the differential diagnosis with a group of “IgG4-related diseases” was required. In 2010, Yamamoto and coauthors found an increase of serum IgG4 in patients with CCS [3]. Renal biopsy also revealed infiltration of IgG4-secreting plasmocytes. This finding led to the question whether CSS is an “IgG4-related disease”. On the other hand, it is known that serum IgG4 level is increased in allergies. CSS is associated with bronchial asthma or chronic sinusitis, and therefore allergic reaction is a common feature of CSS and “IgG4-associated diseases”. There are several small reports describing the increase of the IgG subclasses in cases of severe asthma [4], and normal values in other cases [5]. Furthermore, IgG4-Aspergillus antibodies were found in patients with allergic bronchopulmonary aspergillosis complicated by cystic fibrosis [6]. According to the authors, original “IgG4-related diseases” are also characterized by swelling of the affected organ and lack of systemic inflammation. Furthermore, morphological signs such as lymphoplasmacytic infiltrate with IgG4+ plasma cells, storiform fibrosis, and obliterative phlebitis are required to diagnose these diseases. None of the signs were present in our patient. The accurate mechanism of elevated serum IgG4 in CSS and “IgG4-related diseases” is still unknown. It is considered that the Th2 cytokines, such as interleukin-4, 5, and 10, play an important role in allergic reactions, but it is assumed that mechanisms involving regulatory T-cells in the two diseases are different.
Thus, ventricular arrhythmias are one of the main causes of sudden cardiac death. Patients with palpitations and objective signs of heart disease of unknown origin, and also patients with acute coronary syndrome, myocardial infarction, in the absence of coronary atherosclerosis, must be a group requiring special attention. Rapid elucidation of the cause of the heart lesion enables timely use of appropriate treatment. This strategy may improve the survival and quality of life in patients with rare diseases.
Funding source
None.
Conflict of interest
The authors confirm that this article content has no conflict of interest.
Acknowledgments
Solovyova N, MD, PhD, Ignatieva E, MD, Zverev D, MD, PhD.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jccase.2016.10.011.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Vinit J., Bielefeld P., Muller G., Pfitzenmeyer P., Bonniaud P., Lorcerie B., Besancenot J.F. Heart involvement in Churg-Strauss syndrome: retrospective study in French Burgundy population in past 10 years. Eur J Intern Med. 2010;21:341–346. doi: 10.1016/j.ejim.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Neumann T., Manger B., Schmid M., Kroegel C., Hansch A., Kaiser W.A., Reinhardt D., Wolf G., Hein G., Mall G., Schett G., Zwerina J. Cardiac involvement in Churg-Strauss syndrome: impact of endomyocarditis. Medicine (Baltimore) 2009;88:236–243. doi: 10.1097/MD.0b013e3181af35a5. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto M., Takahashi H., Suzuki C., Tabeya T., Ohara M., Naishiro Y., Yamamoto H., Imai K., Shinomura Y. Analysis of serum IgG subclasses in Churg-Strauss syndrome – the meaning of elevated serum levels of IgG4. Intern Med. 2010;49:1365–1370. doi: 10.2169/internalmedicine.49.3532. [DOI] [PubMed] [Google Scholar]
- 4.De Moraes Lui C., Oliveira L.C., Diogo C.L., Kirschfink M., Grumach A.S. Immunoglobulin G subclass concentrations and infections in children and adolescents with severe asthma. Pediatr Allergy Immunol. 2002;13:195–202. doi: 10.1034/j.1399-3038.2002.00058.x. [DOI] [PubMed] [Google Scholar]
- 5.Hoeger P.H., Niggemann B., Haeuser G. Age related IgG subclass concentrations in asthma. Arch Dis Child. 1994;70:179–182. doi: 10.1136/adc.70.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skov M., Pandey J.P., Pressler T., Høiby N., Koch C. Immunoglobulin allotypes and IgG subclass antibody response to Aspergillus fumigatus in cystic fibrosis patients. J Cyst Fibros. 2004;3:173–178. doi: 10.1016/j.jcf.2004.05.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.