Abstract
We report herein a 70-year-old woman, with repeated thromboembolic events, including three cerebral embolisms and two venous thromboembolisms, despite adequate anticoagulant therapy. Trousseau syndrome was suspected, and she was diagnosed as having lung adenocarcinoma. Chemoradiotherapy was started, achieving improvements in the lung cancer, and thrombosis was also brought under control. Ten months later, the lung cancer relapsed, and second-line chemotherapy was performed. D-dimer levels, which had normalized after the first-line therapy, increased together with the relapse, but became negative again following the chemotherapy. In general, the prognosis of Trousseau syndrome is diverse. However, in this case, the course was good following the second lung cancer therapy: D-dimer levels did not increase, and there were no recurrences of thromboembolism. This experience reminds us the prognosis is most affected by whether the underlying disease is being effectively treated, and suggests that for Trousseau syndrome, despite adequate anticoagulant therapy, elevation of D-dimer levels should consider the recurrent cancer.
<Learning objective: We report herein a case with repeated thromboembolic events as a result of Trousseau syndrome due to lung cancer. Chemotherapy achieved improvements, but the cancer relapsed and second-line chemotherapy was done. D-dimer levels, which had normalized, increased with the relapse before again becoming negative. This experience reminds us that prognosis is affected by treating underlying disease and suggests that elevation of D-dimer levels should consider the cancer recurrence.>
Keywords: Trousseau syndrome, Lung cancer, Cerebral embolism, Venous thromboembolism, D-dimer
Introduction
Malignant tumors are often accompanied by complications such as thromboembolism, caused by a hypercoagulable state of the blood, termed Trousseau syndrome. In 1865, Armand Trousseau first reported a comprehensive association between thrombosis and malignancy. He also suggested the importance of screening for malignancy when recurrent or idiopathic thromboembolic disease is encountered [1], [2]. We report herein our experience in treating a patient who had repeated episodes of cerebral embolism and venous thromboembolism (VTE) of the lower extremities despite adequate anticoagulant therapy, and who was ascertained to have Trousseau syndrome as a complication of lung cancer. Treatment of the underlying lung cancer also resulted in long-term control of the refractory thrombosis, with no recurrent events.
Case report
The patient was a 70-year-old woman. She had been previously treated for hypertension, dyslipidemia, and rheumatoid arthritis. She had no family history of thromboembolism, and no history of smoking. In July 14, 2012, she experienced sudden speech difficulty and was examined by the neurosurgery department of a local hospital. Brain magnetic resonance imaging (MRI) showed infarct lesions scattered throughout the right inferior frontal gyrus, left frontal lobe, and right occipital lobe (first cerebral embolism). She was referred to the department of cardiology of another hospital from that neurosurgeon for complete cardiac examination. None of electrocardiography (ECG) or Holter ECG in that hospital yielded any findings of arrhythmia, such as paroxysmal atrial fibrillation, which may lead to cardiogenic embolism. Other systemic examinations of the patient were not performed. However, because of this shower of emboli, the possibility of a cardiac origin was considered, and the patient was started on dabigatran at 220 mg/day. On October 18, 2012, the patient experienced sudden weakness of the left arm. She was examined by the same hospital, and brain MRI showed fresh cerebral infarction in the right frontal lobe and left occipital lobe. Recurrence of cerebral embolism was diagnosed (second cerebral embolism), and the dose of dabigatran was therefore increased to 300 mg/day. On October 26, she was examined at the hospital because of onset of left homonymous hemianopia, and MRI indicated recurrence due to a fresh cerebral infarction in the right occipital lobe (third cerebral embolism). The patient was hospitalized, and dabigatran was replaced by warfarin. At discharge, prothrombin time-international normalized ratio (PT-INR) was 2.14 and activated partial thromboplastin time (APTT) was 46.4 s.
On December 4, 2012, she came to our hospital for consultation for cardiovascular disease, complaining of left lower-limb edema. PT-INR was 2.07, and D-dimer level was 27.6 μg/mL. Ultrasonography revealed fresh thrombus in the left popliteal vein, and she was hospitalized with a diagnosis of VTE. Continuous drip infusion of heparin and an increased dosage of warfarin brought the D-dimer level down to 7.2 μg/mL. Ultrasonography confirmed regression of the thrombus. At discharge, PT-INR was 3.28.
On January 23, 2013, we again examined the patient due to renewed exacerbation of the lower-limb edema. Ultrasonography showed occlusion due to an extensive fresh thrombus in the left superficial femoral vein (Fig. 1a), and the right popliteal vein (Fig. 1b). In addition, T2-weighted brain MRI showed areas of cystic degeneration and atrophic changes (Fig. 1c and d), thought to be associated with past cerebral infarctions. Since chest X-rays had shown a nodular shadow in the right middle lung field (Fig. 2a), chest contrast-enhanced computed tomography (CT) was performed and revealed a nodular shadow, 18 mm in diameter with an irregular margin in segment 3 of the right lung, leading to suspicion of cancer (Fig. 2b). The patient was hospitalized so that VTE therapy and detailed examinations for lung cancer could be carried out.
Fig. 1.
Images of repeated thromboembolic events. Venous ultrasonography of lower extremities indicates an extensive fresh thrombus in the middle portion of the left superficial femoral vein (a) and a 13-mm thrombotic occlusion in the right popliteal vein (b). T2-weighted brain magnetic resonance imaging shows areas of cystic degeneration and atrophic changes are seen in the right frontal lobe (c) and right occipital lobe (d). These were thought to be associated with old cerebral infarctions.
Fig. 2.
Chest X-ray and chest contrast-enhanced computed tomography. A nodular shadow is seen in the right middle lung field (arrow), 18 mm in diameter with an irregular margin, is seen in segment 3 of the right lung (arrow).
On admission, she was alert and physical examination showed no abnormalities other than severe edema in both lower extremities. Body mass index was 21.9. In laboratory findings, no abnormalities of tumor markers or autoantibodies were evident. Coagulation parameters were as follows: PT-INR, 3.17; D-dimer, 15.0 μg/mL; fibrinogen, 135 mg/dL; lupus anticoagulant, 0.91 (normal range, <1.30); protein C activity, 27% (normal range, 64–156%); and protein S activity was 32% (normal range, 60–150%) (while on oral warfarin). Despite PT-INR being within the effective therapeutic range, D-dimer level was increased. Transthoracic echocardiography revealed that left ventricular systolic and diastolic function were maintained, and there were no findings of valvular disease, left atrial enlargement, shunt diseases such as patent foramen ovale or intra-cardiac thrombus. Positron emission tomography revealed a 2-cm diameter accumulation in segment 3 of the right lung and high accumulations in the right supraclavicular, mediastinal, and right hilar lymph nodes. Transbronchial lung biopsy was performed and led to a diagnosis of adenocarcinoma (cT1bN3M0, cStage IIIB). Epidermal growth factor receptor (EGFR) gene mutation testing yielded negative results, while positive results were obtained for anaplastic lymphoma kinase (ALK) fusion gene.
During hospitalization, the warfarin dose was adjusted with a target of 3.0 for PT-INR, and continuous drip infusion of heparin at 12,000–15,000 units/day was added with the objective of decreasing APTT to 1.5- to 2-times the upper limit of normal. However, despite the fact that PT-INR and APTT were within the target ranges, D-dimer level remained high, at 6.7–17.8 μg/mL (Fig. 3), and VTE and lower-limb edema also persisted.
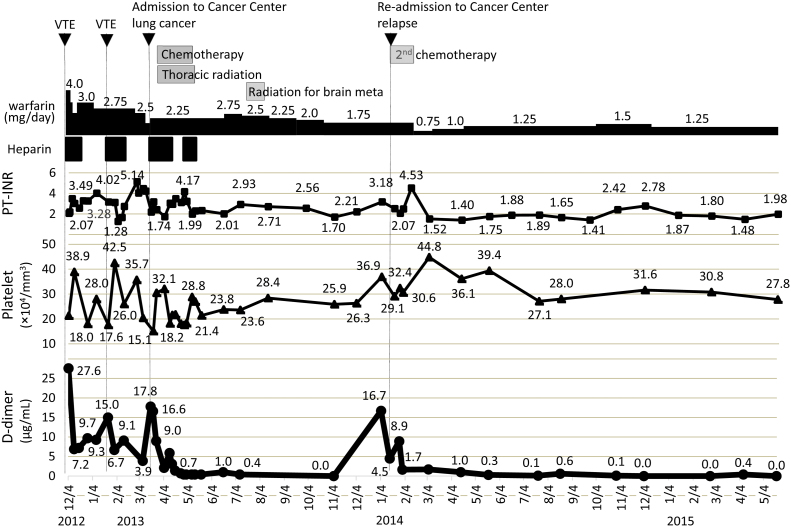
Fig. 3.
Course of lung cancer treatment and anticoagulant treatment and changes in PT-INR, D-dimer, and platelet levels from the time of first admission to our hospital. D-dimer persisted at a high level despite adequate anticoagulant therapy, but then normalized after lung cancer treatment was started. When the lung cancer recurred, D-dimer level also increased again. PT-INR, prothrombin time-international normalized ratio; VTE, venous thromboembolism.
The patient was transferred to the National Hospital Organization Kyushu Cancer Center for treatment of the lung cancer. From March 28 through May 14, 2013, she underwent chemoradiotherapy consisting of carboplatin + vinorelbine + thoracic radiation (60 Gy in 30 fractions). These treatments resulted in rapid clinical improvement, and CT showed stable disease (25.9% shrinkage). D-dimer levels normalized at the same time, showing that the thrombosis had also been brought under control. Brain MRI performed on July 9, 2013, revealed multiple brain metastases, which were treated using stereotactic radiotherapy from July 19 through August 5. At that time, D-dimer levels had not increased. Subsequent imaging showed that chest lesions had increased in size. The lung cancer was diagnosed as having relapsed, and the patient was re-admitted to the same hospital. She was administered second-line chemotherapy comprising 600 mg/day of alectinib, an ALK inhibitor. Chest X-rays showed that the tumors had shrunk in size. D-dimer level, which had previously normalized, increased again to 16.7 μg/mL in concert with the relapse of lung cancer, but normalized again after the second-line chemotherapy was started (Fig. 3).
The course of the patient after the two lines of lung cancer treatment has been good. Chest X-rays showed the lesions had shrunk, and no relapse has been seen during the approximately 2 years since treatment was completed. Cerebral infarction and VTE have also not relapsed.
Discussion
Trousseau syndrome is a paraneoplastic syndrome that produces neurological symptoms associated with latent malignant tumors [3]. In cancer patients, the overall risk of thrombosis is seven times higher than that of non-cancer patients [4]. In addition, cerebrovascular disease is common among cancer patients, with 15% of cancer patients experiencing thromboembolic events during the clinical course [5]. Clearly, Trousseau syndrome is not rare. The stage of cancer along with a number of other factors, such as previous VTE, time from diagnosis of the cancer, and history of surgery and radiation therapy, must be taken into consideration when assessing the risk of thrombosis in a particular patient. Assessing the risk for thrombosis is essential for determining whether prophylactic measures are needed [4].
Trousseau syndrome often occurs with mucinous adenocarcinomas, which secrete abnormally glycosylated mucins and mucin fragment into the bloodstream [6]. Moreover, as in the present case, ALK rearrangements were significantly increased in the predominantly solid tumor with mucin production subtype and in special tissue structures [7].
Regardless of underlying mechanisms, the primary approach to treating Trousseau syndrome is to eliminate the causative tumor, if possible [3]. Meanwhile, heparin is the preferred drug for the treatment of thromboembolism that has developed [3], [4]. Heparin is also known to block P- and L-selectin interactions. Indeed, it has been suggested that most or all of the anti-inflammatory effects of heparin can be explained by selectin blockade [6]. In patients with VTE, heparin was more effective than an oral anticoagulation with warfarin in reducing the risk of recurrent thromboembolism without the risk of bleeding [8]. However, as treatment of recurrent VTE with warfarin has been reported as potentially associated with a reduced risk of cancer [9], we continued oral warfarin in this case.
D-dimer is a direct measure of an activated coagulation and fibrinolysis system and is used in many studies as an indicator of hypercoagulability. In several large series of patients with cancer, an elevated D-dimer level can be identified in 30.5–90%, depending on the presence of metastatic disease [10]. In the present case, coagulability increased when the lung cancer relapsed, but improved when the cancer was suppressed. This train of events is interesting, indicating the strong possibility that the tumor itself was causing the hypercoagulability. In addition, as a lesson learned from this case, in Trousseau syndrome, despite adequate anticoagulant therapy, elevation of D-dimer levels should make physicians consider recurrent cancer. In general, once stroke occurred in a patient with cancer, regardless of etiology, the overall prognosis is poor. Median survival was only 4.5 months [10]. However, fortunately, for the patient we reported, despite repeated thromboembolic events, the prognosis was able to be improved by successful treatment of the causative cancer.
In the present case, when brain metastasis was detected, D-dimer levels had not increased. Buccheri et al. reported that D-dimer level was significantly increased in patients with brain metastatic disease in 826 patients with lung cancer [11]. We could not clarify the reason why D-dimer level did not increase in this patient when brain metastasis was detected. Further studies will be needed for the relationship between D-dimer levels and brain metastatic disease in patients with lung cancer.
In conclusion, we encountered a patient with Trousseau syndrome in whom control of thromboembolism was achieved as a result of treatment for lung cancer. In the case of treatment-resistant thromboembolism, the possibility of the presence of malignant disease should be kept in mind. Our experience suggests that for Trousseau syndrome, despite adequate anticoagulant therapy, elevation of D-dimer level the recurrence of cancer should be considered.
Conflicts of interest
The authors declare that there is no conflict of interest.
References
- 1.Trousseau A. New Sydenham Society; London: 1865. Phlegmasia alba dolens. Clinique Medicale de l’Hotel-Dieu de Paris; pp. 281–332. [Google Scholar]
- 2.Khorana A.A. Malignancy, thrombosis and Trousseau: the case for an eponym. J Thromb Haemost. 2003;1:2463–2465. doi: 10.1111/j.1538-7836.2003.00501.x. [DOI] [PubMed] [Google Scholar]
- 3.Varki A. Trousseau's syndrome: multiple definitions and multiple mechanisms. Blood. 2007;110:1723–1729. doi: 10.1182/blood-2006-10-053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adess M., Eisner R., Nand S., Godwin J., Messmore H.L., Jr., Wehrmacher W.H. Thromboembolism in cancer patients: pathogenesis and treatment. Clin Appl Thromb Hemost. 2006;12:254–266. doi: 10.1177/1076029606291432. [DOI] [PubMed] [Google Scholar]
- 5.Kim S.G., Hong J.M., Kim H.Y., Lee J., Chung P.W., Park K.Y., Kim G.M., Lee K.H., Chung C.S., Bang O.Y. Ischemic stroke in cancer patients with and without conventional mechanisms: a multicenter study in Korea. Stroke. 2010;41:798–801. doi: 10.1161/STROKEAHA.109.571356. [DOI] [PubMed] [Google Scholar]
- 6.Wahrenbrock M., Borsig L., Le D., Varki N., Varki A. Selectin-mucin interactions as a probable molecular explanation for the association of Trousseau syndrome with mucinous adenocarcinomas. J Clin Invest. 2003;112:853–862. doi: 10.1172/JCI18882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qu Y., Che N., Zhao D., Zhang C., Su D., Zhou L., Zhang L., Wang C., Zhang H., Wei L. The clinicopathological significance of ALK rearrangements and KRAS and EGFR mutations in primary pulmonary mucinous adenocarcinoma. Tumour Biol. 2015;36:6417–6424. doi: 10.1007/s13277-015-3331-4. [DOI] [PubMed] [Google Scholar]
- 8.Bang O.Y., Seok J.M., Kim S.G., Hong J.M., Kim H.Y., Lee J., Chung P.W., Park K.Y., Kim G.M., Chung C.S., Lee K.H. Ischemic stroke and cancer: stroke severely impacts cancer patients, while cancer increases the number of strokes. J Clin Neurol. 2011;7:53–59. doi: 10.3988/jcn.2011.7.2.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulman S., Lindmarker P. Incidence of cancer after prophylaxis with warfarin against recurrent venous thromboembolism. Duration of Anticoagulation Trial. N Engl J Med. 2000;342:1953–1958. doi: 10.1056/NEJM200006293422604. [DOI] [PubMed] [Google Scholar]
- 10.Cestari D.M., Weine D.M., Panageas K.S., Segal A.Z., DeAngelis L.M. Stroke in patients with cancer: incidence and etiology. Neurology. 2004;62:2025–2030. doi: 10.1212/01.wnl.0000129912.56486.2b. [DOI] [PubMed] [Google Scholar]
- 11.Buccheri G., Torchio P., Ferrigno D. Plasma levels of D-dimer in lung carcinoma: clinical and prognostic significance. Cancer. 2003;97:3044–3052. doi: 10.1002/cncr.11432. [DOI] [PubMed] [Google Scholar]