Abstract
Introduction
Drug-induced parkinsonism (DIP) and tardive dyskinesia (TD) are stigmatizing movement disorders associated with exposure to dopamine receptor blocking agents such as antipsychotics, but they differ in their pathophysiology and clinical management. Treatment for one may worsen the other, and there are important diagnostic clues that assist in making an accurate assessment and instituting a rational treatment plan.
Methods
A literature review was executed to identify articles relating to the presentation, pathophysiology, epidemiology, and management of DIP and TD.
Results
DIP and TD prevalence estimates range from approximately 20 to 35% among antipsychotic users, but may be higher in select populations. DIP often presents as bradykinesia and rigidity, as well as rhythmic tremor, and the majority of cases appear within hours to weeks of initiation of therapy with an antipsychotic, or if dosage of the antipsychotic is increased. TD onset is delayed, typically appearing after at least 3 months or longer of treatment, and patients will commonly present with involuntary, abnormal facial movements such as lip smacking, puckering, chewing, or tongue protrusion. DIP often resolves with discontinuation of the causative agent, but TD may be permanent. Broadly, proposed mechanisms underlying these adverse events include decreased dopamine concentrations in the nigrostriatal pathway of the striatum and dopamine hypersensitivity, for DIP and TD, respectively. Pharmacologic treatment approaches for DIP have commonly included anticholinergic agents such as benztropine; however, anticholinergic medications can make TD worse. Switching the antipsychotic medication to one with lower propensity for DIP is an option for some patients. Amantadine, a non-anticholinergic agent used for the treatment of DIP, may be preferred in patients with comorbid DIP and TD. In TD, treatment options include the new reversible vesicular monoamine 2 transporter inhibitors, valbenazine and deutetrabenazine.
Conclusions
It is important for clinicians to be able to recognize DIP and TD in patients using antipsychotics so that they can minimize the impact of these adverse events on their patients’ quality of life. Accurate diagnosis will drive the selection of the correct treatment.
Plain Language Summary
Plain language summary available for this article.
Keywords: Antipsychotic, Dyskinesia, Extrapyramidal symptoms, Movement disorders, Parkinsonism
Plain Language Summary
Antipsychotic medications are often used to treat serious mental illnesses, like schizophrenia and bipolar disorder. In some people, these medications cause uncontrollable movements in the face and limbs. Two of the more common movement disorders are called drug-induced parkinsonism (DIP) and tardive dyskinesia (TD). DIP usually starts within days to months after starting an antipsychotic, whereas TD may begin months to years later. The more common signs of DIP are tremor and movements that appear slow and stiff, and TD usually includes face movements like lip puckering or smacking, and chewing. These side effects are often embarrassing for the patient, can get in the way of their daily activities, and may be permanent. It is important for clinicians to watch for these side effects, and to understand the different treatment options because most medications used to treat DIP should not be used to treat TD. There are no medications used regularly to prevent TD. However, there are new medications for the treatment of TD that have significant potential to help patients who develop movement side effects while taking antipsychotics.
Introduction
Movement disorders, or extrapyramidal side effects (EPS), are potential adverse events of antipsychotic use that are often stigmatizing, and can impair patients’ ability to complete activities of daily living. A study assessing quality of life among patients with stable schizophrenia symptoms on clozapine, or a typical antipsychotic, found that less EPS was predictive of better quality of life scores in both groups [1]. Although movement disorders were once thought of primarily as a concern associated with typical, or first-generation, antipsychotic use, increasing recognition is being given to the possibility of most atypical, or second-generation, antipsychotics to precipitate movement disorders. As atypical antipsychotic use is highly prevalent in the treatment of serious mental illness, it is timely to discuss clinical approaches for treating two relatively common antipsychotic-induced movement disorders: drug-induced parkinsonism (DIP) and tardive dyskinesia (TD). The aim of the following discussion is to approach the review of these adverse events with a focus on the similarities and differences of DIP and TD with respect to their epidemiology, presentation, pathophysiology, and management. Understanding the differences between these two movement disorders is particularly important as the treatment approaches are distinct and rely on the accurate identification of the underlying movement disorder.
Methods
On March 29, 2018, the PubMed database was searched with the terms: antipsychotic AND (parkinson* OR “tardive dyskinesia*”), and the results were examined for articles pertaining to the epidemiology, diagnosis, pathophysiology, and treatment of parkinsonism and tardive dyskinesia resulting from treatment with antipsychotics. This search was repeated on April 19, 2018 to identify any new literature published since the original query. Results were notable for sparse primary literature supporting the use of most medications (with the exception of valbenazine and deutetrabenazine) for the treatment of antipsychotic-induced DIP or TD. This article reviews previously published material, and no new information from human participants or animal research conducted by either of the authors was used in this article.
Drug-Induced Parkinsonism
Epidemiology
DIP is difficult to distinguish from Parkinson’s disease, particularly in elderly patients, and is often undiagnosed [2, 3]. These factors make it challenging to understand the scope of antipsychotic-induced parkinsonism. Prevalence rates in studies that include patients taking typical and atypical antipsychotics range from approximately 20 to 35% [4–6], but DIP occurs at higher rates in elderly patients [7]. Several studies have retrospectively assessed medications as causes of identified parkinsonism. In a review focused on the elderly, 46% of those with symptoms due to medications were deemed to be caused by atypical antipsychotics [8]. This is contrasted by a recent report of a 30-year epidemiologic study of parkinsonism, in which typical antipsychotics were identified as the culprit of the majority of all drug-induced cases, with almost no contribution from atypical antipsychotics [9]. These differences may be due to varied populations and study design but, ultimately, they demonstrate that DIP is still a concern of antipsychotic therapy despite the hope that it would abate with increased atypical antipsychotic use.
Pathophysiology
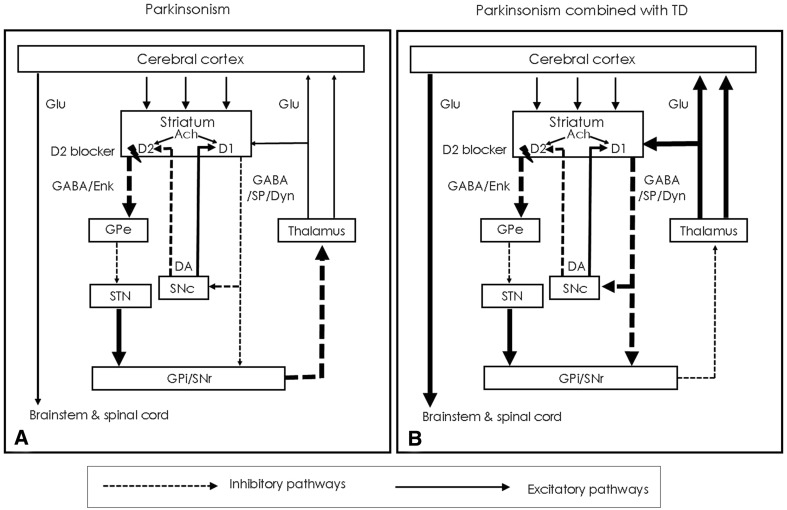
The pathophysiology of DIP is related to drug-induced changes in the basal ganglia motor circuit secondary to dopaminergic receptor blockade [2]. When dopamine D2 receptors in the striatum are blocked, the gamma-aminobutyric acid (GABA)- and encephalin-containing striatal neurons are disinhibited, impacting the indirect pathway, and ultimately leading to a relative decrease in activity in thalamocortical circuitry (see Fig. 1a) [2, 10]. This effect can be moderated by anticholinergic activity of the antipsychotic [11, 12], as supported by observations that clozapine, which is associated with minimal-to-absent propensity to cause DIP [13], also has a high relative affinity for muscarinic cholinergic receptors [11]. Decreased effective dopamine concentrations in the striatum can also be caused by decreased dopamine release into the synapse; as can be seen with the irreversible vesicular monoamine transporter inhibitor, reserpine [12]. Medications whose primary mechanism does not involve direct action on dopamine concentrations (valproic acid, calcium channel blockers) can cause DIP through unclear mechanisms that may involve modulating GABA activity or mitochondrial dysfunction [2, 12, 14]. A summary of the basal ganglia direct and indirect pathways is presented in Box 1.
Fig. 1.
Changes in basal ganglia-thalamocortical motor loop due to blockade of D2 receptors by DRBAs. The blockage of D2 receptors by DRBAs in the striatum leads to disinhibition of GABA- and encephalin-containing striatal neurons at the origin of the indirect pathway, followed by a disinhibition of the subthalamic nucleus. This leads to increased GABAergic inhibition of the thalamocortical projection by facilitation of the inhibitory projection from the GPi/SNr (a). Chronic D2 receptor blockade also induces changes in the direct pathways of the basal ganglia-motor loop to cause orolingual dyskinesia (b). DA dopamine, DRBAs dopamine receptor blocking agents, GABA gamma-aminobutyric acid, GPe globus pallidus pars externa, GPi globus pallidus pars internal, SNc substantia nigra pars compacta, SNr substantia nigra pars reticulata, STN subthalamic nucleus, TD tardive dyskinesia.
Reproduced as per the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) from [2])
Box 1.
Summary of the basal ganglia direct and indirect pathways [74]
|
• The processing of movement in the basal ganglia involves a direct pathway and an indirect pathway • The two pathways originate from distinct populations of striatal medium spiny neurons (MSNs) and project to different output structures • These circuits are believed to have opposite effects on movement: direct pathway MSNs promote movement but activation of indirect pathway MSNs inhibit movement |
| Direct pathway | Indirect pathway | |
|---|---|---|
| Effect on motor thalamus | Disinhibits | Inhibits |
| Effect on thalamo-cortex | Activates | Inhibits |
| Effect on motor cortex | Activates | Inhibits |
| Effect on movement | Facilitates | Inhibits |
Presentation and Diagnosis
Relative to TD, DIP presents earlier in antipsychotic treatment, with approximately 50–75% of cases appearing within the first month, and 90% of cases within the first 3 months [15]. Table 1 highlights select differences between DIP and TD. Acute dystonic reactions can occur relatively soon after administration of intramuscular antipsychotic such as haloperidol [16, 17]. Where tremor is evident, it is rhythmic, occurring at a frequency of 3–6 Hz. Because DIP can be associated with impairments of facial expression, it is important to differentiate between negative symptoms in patients with schizophrenia, or the possibility of untreated depression [18]. As mentioned previously, DIP can be difficult to distinguish from Parkinson’s disease. Characteristic symptoms, like bradykinesia, rigidity, and instable gait are present in both disorders, and while some studies note different prominence of symptoms between DIP and Parkinson’s disease (more bradykinesia, symmetry of symptoms, and rigidity in DIP), others suggest that these clues in presentation are inadequate for an accurate diagnosis [2, 12, 19, 20]. For example, abnormal movement patterns present asymmetrically in a significant minority of cases. Pieters et al. [21] recently noted asymmetrical symptoms in as many as 20% of patients with DIP, as was also observed by Savica et al. [9]. Furthermore, antipsychotic use may unmask Parkinson’s disease, which contributes the difficulty of diagnosing DIP in elderly patients [2].
Table 1.
Differentiating characteristics of drug-induced parkinsonism (DIP) versus tardive dyskinesia (TD)
| Characteristic | DIP | TD |
|---|---|---|
| Onset | Immediate (hours–days–weeks) after initiation of an antipsychotic or after dose is increased | Delayed (months–years) after initiation of an antipsychotic |
| Motor symptoms observed | Rhythmic tremor, rigidity, shuffling gait; akathisia may be present | Arrhythmic movements (generally choreo-athetoid) of the face, trunk and extremities |
| Immediate (hours–days–weeks) effects of increasing antipsychotic dose | Worsens | Improves |
| Immediate (hours–days–weeks) effects of decreasing antipsychotic dose | Improves | Worsens |
| Effects of anticholinergic medications (e.g., benztropine) | Improves | Can worsen |
| Pharmacotherapeutic treatment options | Anticholinergics (for example, benztropine), amantadine | VMAT2 inhibitors (tetrabenazine, valbenazine, deutetrabenazine), Ginkgo biloba, clonazepam, amantadine |
VMAT2 vesicular monoamine transporter 2
Another difference in DIP and Parkinson’s disease that may be useful in forming a diagnosis is improvement when the causative medication’s dose is lowered or discontinued [22]. Finally, dopamine transporter (DAT) imaging approaches have been shown capable of differentiating DIP from Parkinson’s disease by comparing symmetry of radiotracer uptake in the striatum [2, 23, 24].
Akathisia can be observed alongside DIP, or in the absence of overt DIP. Akathisia is defined as a syndrome with subjective symptoms often accompanied by objective findings [25]. Subjective symptoms include inner tension, anxiety, irritability, discomfort, restlessness, or sleeplessness. Objective findings that are often present include movements that are semivolitional, purposeful and suppressible, repetitive, complex, and stereotypical. The pathophysiology of drug-induced akathisia likely differs from that for DIP. Both can improve with reduction of antipsychotic dose; however, anticholinergic medications are generally unhelpful in managing akathisia [26]. Treatment of akathisia includes administration of either a beta-adrenergic antagonist or a serotonergic 5HT2 receptor antagonist.
Risk Factors
A number of risk factors have been associated with DIP development, including: older age, female gender, previous EPS caused by antipsychotics, family history of PD, cognitive impairment, HIV infection, and higher potency and longer duration antipsychotic use [9, 27]. Use of additional medications that may cause DIP include valproic acid, gut motility agents like metoclopramide, antidepressants, and calcium channel blockers like verapamil [12]. Importantly, the vesicular monoamine transporter 2 (VMAT2) inhibitor, tetrabenazine, treats TD but also potentially contributes to DIP risk by decreasing the amount of dopamine released into the synapse, as observed in clinical trials for Huntington’s disease [12]. This is potentially not as concerning with the newer VMAT2 inhibitors, valbenazine and deutetrabenazine, as observed in clinical trials for TD; however, patients with comorbid neurological conditions that could interfere with TD assessment were excluded from these trials [28, 29]. The product label for deutetrabenazine includes a warning regarding Parkinsonism, but this is directed towards persons receiving the medication for Huntington’s disease, not TD [30].
Management
The necessary first step in managing DIP is recognition through monitoring at an appropriate frequency. The American Psychiatric Association (APA) recommends monitoring for acute onset extrapyramidal side effects weekly during initial treatment and until stable for 2 weeks, then at every follow-up visit [18]. Although clinical monitoring for signs and symptoms of DIP may be adequate, a more structured approach is the use of the Simpson–Angus Extrapyramidal Side Effect scale, which is a quick 10-item measure that has demonstrated efficacy in the early detection of DIP [31–33], and is commonly used in clinical trials of antipsychotic medications.
Whenever possible, discontinuation of the causative agent is the recommended treatment strategy for distressing DIP symptoms. Unlike in the management of TD, where this approach results in a transient worsening of symptoms, it will lead to improvement of DIP symptoms within days to months after stopping therapy. However, DIP symptoms have been observed to persist in 10–50% of patients [2]. It is often not possible to discontinue antipsychotics, but the use of any additional agents potentially contributing to DIP risk should be carefully reconsidered. If reasonable in the course of the patient’s treatment, it is appropriate to consider switching to an antipsychotic with less propensity to cause DIP, such as from a typical to an atypical agent. Quetiapine is often an agent of choice in this setting [34], but other agents that have a lower propensity to cause DIP (as well as akathisia) include iloperidone and clozapine [35, 36]. When antipsychotic switching is not a viable management strategy, additional strategies include: (1) gradually lowering the antipsychotic dose if clinically possible, (2) adding an anticholinergic medication, such as benztropine or trihexyphenidyl, or (3) adding a non-anticholinergic agent such as amantadine. The use of potent dopaminergic medications such as levodopa can exacerbate psychotic symptoms [7].
Anticholinergic medications, such as benztropine, are used extensively, and often prophylactically, upon the initiation of antipsychotic medication to manage DIP. However, these medications can increase the risk of developing TD, can worsen comorbid TD, and negatively impact cognition [37]. The deleterious impact of benztropine (and other anticholinergic medication) on memory is not trivial, and can be an iatrogenic factor in the poor cognitive and functional outcomes commonly encountered in persons with schizophrenia [37]. Anticholinergic medication should be avoided in the elderly unless necessary due to an increased risk of delirium [7], especially if already receiving medications with the potential for anticholinergic-induced adverse effects. In addition to altered mentation, peripheral side effects such as blurred vision, dry mouth, constipation, and urinary retention can be encountered. If prescribed, a typical duration of anticholinergic use is 3 months, and they should be periodically stopped to assess the need for continued use [11]. An alternative to anticholinergic medications is amantadine. Amantadine works to improve DIP (and TD, discussed below) through unknown mechanisms that may involve dopamine and N-methyl-d-aspartate (NMDA) receptor antagonism [38]. It may be a more viable option than anticholinergic medications, particularly for elderly patients, due to its reduced propensity for side effects, but having similar efficacy as anticholinergic medications when managing DIP [39]. A study of 44 patients with schizophrenia who were randomized to benztropine or amantadine were found to experience similar improvement in symptoms with less frequent side effects in the amantadine group [39]. Another small (n = 41), blinded, cross-over study comparing amantadine to trihexyphenidyl in the treatment of neuroleptic-induced parkinsonism found similar results [40]. The most common side effects in clinical trials were insomnia, nausea, and dizziness (5–10%), with anticholinergic-like side effects among adverse events noted in 1–5% (anorexia, dry mouth, constipation) [38]. Amantadine is not expected to worsen TD symptoms and there is modest evidence to suggest that it may reduce dyskinetic movements (see below). Extended-release preparations of amantadine have recently become available “for the treatment of dyskinesia in patients with Parkinson’s disease receiving levodopa-based therapy, with or without concomitant dopaminergic medications” [41] and for “Parkinson’s disease and drug-induced extrapyramidal reactions in adult patients” [42]. As with all amantadine-containing products, risk of exacerbating psychosis will also need to be considered [38, 41, 42].
Tardive Dyskinesia (TD)
Epidemiology
Similar to DIP, published TD prevalence and incidence rates may be falsely low [43]. In contrast to DIP, this is likely not due to confusion with an idiopathic disorder as much as to a decreased ability of providers to recognize the insidious development of TD [44]. However, it does appear that TD prevalence and incidence estimates vary with past antipsychotic exposure, antipsychotic class, and age. For example, a large meta-analysis conducted by Carbon et al. found that, in middle-aged patients, most of whom had a schizophrenia-spectrum disorder, the mean prevalence of TD among atypical antipsychotic users was approximately 21%, compared to 30% among current typical antipsychotic users [45]. However, more long-term studies that exclude patients with past typical antipsychotic use will be necessary to develop a clearer picture of the impact of atypical antipsychotics on TD risk [43]. On a cautionary note, in a real-world prospective study, the adjusted TD incidence rate-ratio for subjects treated with atypical antipsychotics alone versus typical antipsychotics alone was 0.68 [46]. This suggests a small advantage for the newer agents, but the 95% confidence interval of 0.29–1.64 implies that there may be no difference [46]. Alarmingly, the severity of the TD was only slightly lower among incident cases of TD appearing after recent atypical antipsychotic exposure versus recent typical antipsychotic exposure.
The rates of TD increase with duration of therapy, as in younger adults taking typical antipsychotics, reported rates of TD were approximately 4–5% annually, but this effect is magnified by age [47]. For example, a longitudinal study of TD in elderly patients noted that 31% (95% CI 20–42) of patients developed TD after 43 weeks [48]. Prevalence rates become more complex when looking at symptom persistence in individuals diagnosed with TD. In patients taking typical antipsychotics, although about 50% appear to have TD symptoms that stagnate, 10–30% will experience remission or improvement of symptoms, and another 10–30% may have a worsening of symptoms [49].
Pathophysiology
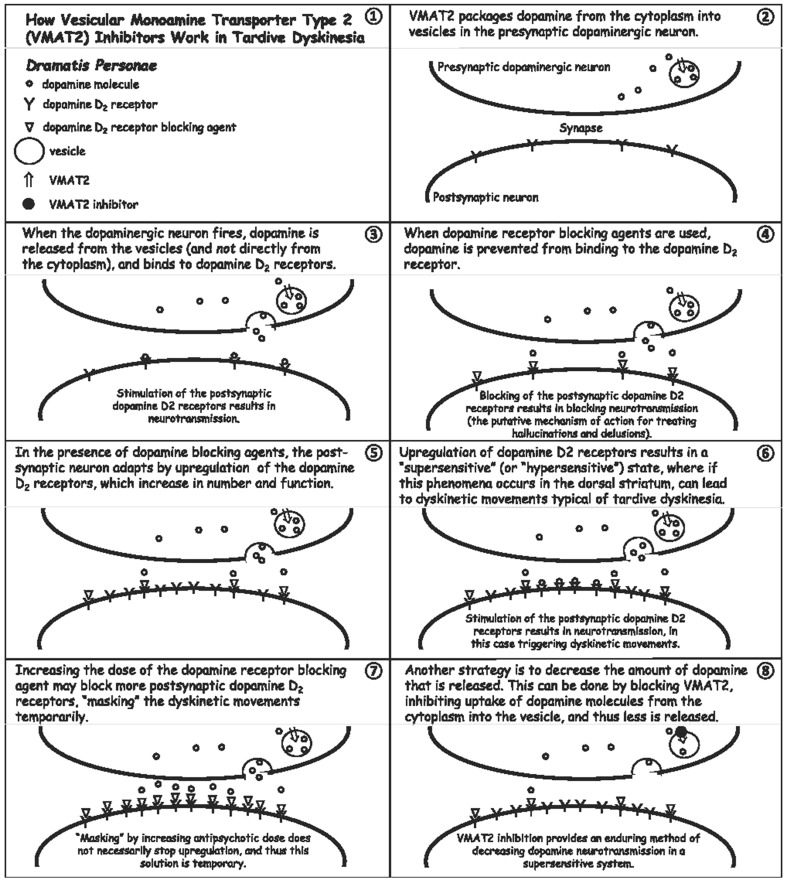
Disruptions in a number of neurotransmitter systems and oxidative damage have been proposed as potential pathways underlying TD. These include dopamine receptor hypersensitivity, altered amino acid metabolism and GABA-containing neuron activity, and NMDA receptor excitotoxicity [50]. Similar to DIP, there is also evidence that genetics are associated with TD susceptibility [51]. Dopamine receptor hypersensitivity is generally accepted as playing a role in TD (Fig. 2) based on several clinical observations, including temporary improvement in symptoms with increased antipsychotic dose or potency, and symptom improvement in some patients when administered VMAT2 inhibitors, such as tetrabenazine, which decrease the amount of dopamine that is ultimately released into the synapse.
Fig. 2.
Hypothesized pathophysiology of tardive dyskinesia and the potential for vesicular monoamine transporter type 2 inhibition to treat the condition (reproduced with permission from [75])
Presentation and Diagnosis
TD is a subset of tardive syndromes associated with antipsychotic drug use and is characterized by involuntary, repetitive movements of the face that includes lip smacking or puckering, chewing, or tongue protrusion, but may also result in uncontrolled movements in the extremities, like contraction or writhing [50]. TD can worsen with stress. In contrast to DIP, the abnormal movements observed with TD are typically arrhythmic. Further information regarding the term tardive syndromes and related issues can be found elsewhere [52].
The Abnormal Involuntary Movement Scale (AIMS) is used clinically and in research to assess the abnormal movements associated with TD [53]. Measuring the AIMS dyskinesia items alone is inadequate, and questions regarding functional impairments attributable to TD need to be asked (e.g., interference with activities such as eating, drinking, speaking, breathing, dressing oneself, writing, working, leisure activities, being with others) [54].
The Schooler–Kane research criteria are commonly used to identify probable antipsychotic-induced TD, and require that three criteria are met: (1) symptoms occur after at least 3 months of treatment with an antipsychotic, (2) abnormal, involuntary movements must occur in 2 or more body regions if mild, or 1 body region if moderate to severe, as determined by a rating scale such as the AIMS, and (3) there are no other conditions that may be causing the abnormal movement patterns [55].
Risk Factors
Risk factors for TD are similar to those for DIP, and primarily include older age and increased antipsychotic medication exposure (particularly typical antipsychotics), but also to some degree female sex, African American ethnicity, preexisting mood disorder, cognitive disturbance, alcohol or substance abuse, use of lithium or antiparkinsonian agents, early occurrence of DIP, diabetes, and HIV [56–58].
Management
The APA recommends monitoring patients with schizophrenia for the development of TD every 3–12 months, depending on the patient’s risk factors and the type of antipsychotic prescribed [18]. Standards include every 6 months for patients on a typical antipsychotic to every 12 months for patients on an atypical antipsychotic, and to monitor twice as frequently for elderly patients and those with early, involuntary, movement patterns after starting an antipsychotic [18]. These guidelines are likely reasonable for most patients being treated with antipsychotics, regardless of diagnosis.
Once TD is diagnosed and treatment is initiated, a baseline assessment should be obtained, and the AIMS examination is recommended for this purpose [54]. Advantages of using the AIMS include its ubiquity both in the clinic and in drug development, and that it can facilitate communication among providers. Follow-up assessments to assess the effectiveness of the intervention(s) should be carried out on a regular basis.
One of the first steps in the management of TD should be to gradually discontinue any anticholinergic medications, as they may worsen current symptoms [7]. In fact, symptoms have been noted to improve in up to 60% of people with TD after discontinuing an anticholinergic [49, 59]. Unlike DIP, discontinuing an antipsychotic or switching from a typical to an atypical antipsychotic does not produce clear evidence of benefit in patients requiring antipsychotic use [60]. However, switching antipsychotics, including a switch to clozapine, is generally supported by older treatment algorithms [61]. A recent review updating the American Academy of Neurology (AAN) TD treatment guidelines ranks treatment options based on available evidence from A to C, where A corresponds to established efficacy, B corresponds to probable efficacy, and C corresponds to potential efficacy [60]. Level A evidence for efficacy exists for the two VMAT2 inhibitors, valbenazine and deutetrabenazine, both recently approved for the treatment of TD by the United States Food and Drug Administration (FDA), with a hypothesized mechanism of action related to dopamine D2 receptor hypersensitivity in the presence of chronic dopamine D2 receptor blockade (Fig. 2).
Valbenazine was the first medication to be approved for TD in the United States, and improves upon tetrabenazine by having a more tolerable side effect profile, potentially through less off-site binding to dopaminergic, serotonergic, adrenergic, histaminergic, and muscarinic receptors, in addition to a more convenient once-daily dosing regimen [62]. In a Phase III, double-blind, randomized, 6-week, placebo-controlled study of 234 participants randomized 1:1:1 to placebo or valbenazine 40 or 80 mg/day, daily doses of 80 mg were shown to reduce AIMS total dyskinesia scores by − 3.2 points (P < 0.001 when compared to the placebo change score of − 0.1 points) and by − 1.9 points in the 40 mg daily group (P = 0.002 when compared to placebo) after 6 weeks of therapy [63]. The most common side effects in the valbenazine group were somnolence (5%), akathisia (3%), and dry mouth (3%), and discontinuation rates due to adverse events were 4% in the valbenazine group versus 3% in the placebo group [63]. A 1-year extension of this study, which included 198 participants, demonstrated continued efficacy and tolerability, with the most common side effects being headache (7%) and urinary retention (7%), with 16% discontinuing treatment due to an adverse drug event [64].
Although the target dose for valbenazine is 80 mg/day (achieved after 1 week at 40 mg/day), 40 mg daily should not be exceeded in those with moderate to severe hepatic impairment, or with concomitant use of strong CYP3A4 inhibitors [62]. This is because valbenazine is primarily metabolized by CYP3A4 to inactive metabolites, although CYP2D6 also plays a role in its metabolism [62]. In the case of strong CYP2D6 inhibitors, or known CYP2D6 poor metabolizers, it is recommended to consider reducing the dose based on tolerability [62]. Valbenazine does not appear to impact the common CYP isoenzymes itself, but does inhibit P-glycoprotein, so digoxin should be monitored carefully in patients requiring both medications [62].
Valbenazine may prolong the electrocardiographic QT interval, but the degree of QT prolongation is not clinically significant at concentrations expected with recommended dosing; nonetheless, valbenazine should be avoided in patients with congenital long QT syndrome or with arrhythmias associated with a prolonged QT interval. For patients at increased risk of a prolonged QT interval, it is suggested that the QT interval be assessed before increasing the dosage (i.e., from 40 to 80 mg/day) [62].
Deutetrabenazine was the second VMAT2 inhibitor approved by the FDA for the treatment of TD. Deutetrabenazine is a deuterated formulation of tetrabenazine; deuterium atoms are substituted for hydrogen atoms at key locations in the molecule, altering its pharmacokinetics because deuterium–carbon chemical bonds are many-fold stronger than hydrogen–carbon bonds. Deuterium is naturally occurring “heavy hydrogen” and is not radioactive. Compared to tetrabenazine, metabolism is slowed, reducing the number of doses required per day, decreasing plasma level variability, and improving tolerability [30]. Recommended dosing for deutetrabenazine is twice daily with food, starting at 6 mg twice daily and increasing by 6 mg weekly up to a maximum dose of 24 mg twice daily, based on tolerability and reduction of TD [30]. In a Phase II, 12-week, clinical trial (n = 117) which allowed titration to optimal efficacy and tolerability, the mean dose achieved was 38.8 mg/day [65]. A Phase III, double-blind, 12-week, placebo-controlled, randomized trial was conducted to compare deutetrabenazine at doses of 12, 24, and 36 mg/day to placebo in 298 patients [66]. The randomization scheme was 1:1:1:1 and the deutetrabenazine dose was increased over 4 weeks. The primary end-point measure was change in AIMS scores from baseline to week 12. At week 12, the treatment difference from baseline (SE) was − 1.4 in the placebo group and − 2.1 (P = 0.217 when compared with placebo), − 3.2 (P = 0.003) and − 3.3 (P = 0.001) for the 12-, 24-, and 36-mg/day treatment groups, respectively [66]. The most common side effects in the deutetrabenazine groups were headache (5%), anxiety (4%), and diarrhea (4%), and discontinuation due to side effects occurred in 4% of the treatment group and 3% of the placebo group [66]. Deutetrabenazine metabolism involves CYP2D6; in the presence of CYP2D6 inhibitors or in patients who are known poor CYP2D6 metabolizers, the total daily dosage of deutetrabenazine should not exceed 36 mg [30]. Deutetrabenazine may prolong the QT interval and use should be avoided in patients with congenital long QT syndrome or with arrhythmias associated with a prolonged QT interval. For patients at increased risk of a prolonged QT interval, it is suggested that the QT interval be assessed before and after increasing the total dose above 12 mg BID [30].
Unlike valbenazine, deutetrabenazine is also approved for the treatment of Huntington’s disease, and contains language in its product label that is specific to tolerability concerns for that population and differs from that for TD (for example, there is a boxed warning for depression and suicidality that has been observed in patients with Huntington’s disease). Other differences between valbenazine and deutetrabenazine are summarized in Table 2.
Table 2.
A comparison of the key characteristics and recommended dosing considerations for valbenazine and deutetrabenazine use in the management of TD.
| Valbenazine | Deutetrabenazine | |
|---|---|---|
| Brand name | Ingrezza | Austedo |
| Available dose formulation | Capsules: 40 and 80 mg | Tablets: 6, 9, and 12 mg |
| Other indications | None | Chorea associated with Huntington’s disease |
| Active metabolites | [+]-α-HTBZ | Deuterated α-HTBZ and β-HTBZ |
| Half-life | Valbenazine and [+]-α-HTBZ: 15–22 h | Total (α + β)-HTBZ from deutetrabenazine: 9–10 h |
| Contraindications relevant to TD | None | Hepatic impairment, use of reserpine, MAOIs, tetrabenazine or valbenazine |
| Warnings and precaution contained in Highlights of Prescribing Information | Somnolence; QT interval prolongation | QT interval prolongation; neuroleptic malignant syndrome; akathisia, agitation, restlessness, and parkinsonism (latter not applicable to TD); sedation/somnolence |
| Dosing frequency | Once daily | Twice daily |
| Recommended dosing | Take with or without food; start at 40 mg daily, increase to 80 mg daily after 1 week | Take with food; start at 12 mg/day, increase by 6 mg/day at weekly intervals up to 48 mg/day, based on tolerability and response |
| CYP2D6 poor metabolizers | Base dose on tolerability | Maximum recommended dose is 36 mg/day |
| Hepatic impairment | Moderate-to-severe hepatic impairment: maximum recommended dose is 40 mg/day | Contraindicated |
| Renal impairment | Avoid in severe renal impairment; no dosing changes are recommended for mild-to-moderate impairment | Package insert does not provide any recommendations (cites a lack of studies in this population), but the metabolites are excreted renally |
| Drug-drug interactions | Valbenazine increases digoxin levels; consider valbenazine dose reduction with strong CYP2D6 inhibitors; with strong CYP3A4 inhibitors the maximum recommended dose is 40 mg daily; use is not recommended with MAOIs or CYP3A4 inducers | Additive sedation may occur with alcohol and other CNS depressants; with strong CYP2D6 inhibitors, the recommended maximum dose is 36 mg/day |
| QT prolongation recommendation | If the patient is at increased risk for QT prolongation, assess QT interval before increasing the dose | If the patient is at increased risk for QT prolongation, assess QT interval before and after increasing the dose above 24 mg/day |
HTBZ dihydrotetrabenazine, TD tardive dyskinesia, MAOIs monoamine oxidase inhibitors, CNS central nervous system
Additional medication interventions for TD are not FDA-approved and carry lower levels of evidence compared to valbenazine and deutetrabenazine in the AAN guidelines. Level B AAN recommendations for the treatment of TD include Ginkgo biloba and clonazepam. Level C options that might be considered include amantadine, tetrabenazine, and pallidal deep brain stimulation [60]. Amantadine is unique among medications used to treat DIP and TD, in the respect that it has evidence supporting its use for both indications. However, the therapeutic effect for TD is modest. When compared to placebo, amantadine has been shown to decrease AIMS scores by approximately 15–22% in small, relatively short-term, placebo-controlled, crossover studies [67, 68]. Drowsiness, fatigue, insomnia, constipation, and dizziness occurred more frequently with amantadine than placebo in these studies [67, 68]. Tetrabenazine is used off-label for the treatment of TD in the United States; however, in other jurisdictions, such as the UK, Canada, New Zealand, Australia, Germany, Italy, Israel, France, and Portugal, it is approved for this purpose [69]. Two small, clinical trials were cited in the AAN recommendation supporting the use of tetrabenazine for the treatment of TD symptoms, as supported by significant reductions in AIMS scores [70, 71]. Although tolerability was not a major issue in these studies, clinical experience with side effects of tetrabenazine that occur in more than 5% of patients in persons with Huntington’s disease (such as sedation, insomnia, depression, akathisia, parkinsonism, instability, irritability) [72], and the requirement to dose the medication up to 3 times per day, and assessing for CYP 2D6 poor/extensive metabolizers if doses greater than 50 mg/day need to be used, led to the development of the two tetrabenazine alternatives, valbenazine and deutetrabenazine, discussed previously.
Co-occurrence of DIP and TD
It is possible for patients treated with antipsychotics to have both DIP and TD, with DIP presumably preceding TD. Although the mechanism is not fully understood, observations regarding the differences in neural pathways between DIP and comorbid DIP and TD are presented in Fig. 1 [2, 10, 73]. As compared to Fig. 1a, Fig. 1b details relatively greater excitatory signaling between the thalamus and the cerebral cortex, potentially explaining TD symptoms occurring in the setting of DIP. For patients experiencing both disorders, treatment options include amantadine (with or without a VMAT2 inhibitor), which may have some positive impact on both disorders. Anticholinergic medication should be avoided or minimized in order to lessen potential adverse impact on TD symptoms. Randomized controlled trials of VMAT2 inhibitors in persons with co-occurring DIP and TD will help answer the question of whether or not the decrease in dopamine release observed with VMAT2 inhibition would have significant clinical impact on the severity of the DIP.
Conclusion
DIP and TD are potential adverse events of antipsychotics that occur at significant rates with both typical and atypical antipsychotic use. DIP can be difficult to recognize and diagnose, especially in the elderly, due to its similarity to Parkinson’s disease, but most often presents within the first 3 months of therapy [27]. TD, however, typically does not develop until after 3 months or longer of antipsychotic drug use (although there may be the occasional exception). Among the agents used to treat DIP, amantadine is expected to be better tolerated than anticholinergics, particularly in the elderly. Valbenazine and deutetrabenazine are reasonable to consider as first-line pharmacotherapy for TD.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
In the past 12 months, Leslie Citrome has served as a consultant to Acadia, Alkermes, Allergan, Intra-Cellular Therapeutics, Janssen, Lundbeck, Merck, Neurocrine, Noven, Otsuka, Pfizer, Shire, Sunovion, Takeda, Teva, Vanda and has served as a speaker for Acadia, Alkermes, Allergan, Janssen, Lundbeck, Merck, Neurocrine, Otsuka, Pfizer, Shire, Sunovion, Takeda, Teva, Vanda, owns stocks (small number of shares of common stock) of Bristol-Myers Squibb, Eli Lilly, J & J, Merck, Pfizer purchased > 10 years ago, and receives royalties from Wiley (Editor-in-Chief, International Journal of Clinical Practice), UpToDate (reviewer), Springer Healthcare (book). Kristen Ward has nothing to disclose.
Compliance with Ethics Guidelines
This article was a review and commentary on previously conducted studies. No information from human participants or animal research conducted by either of the authors was used in this article.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced digital content
To view enhanced digital content for this article go to 10.6084/m9.figshare.6736412.
References
- 1.Strejilevich SA, Palatnik A, Avila R, Bustin J, Cassone J, Figueroa S, et al. Lack of extrapyramidal side effects predicts quality of life in outpatients treated with clozapine or with typical antipsychotics. Psychiatry Res. 2005;133(2–3):277–280. doi: 10.1016/j.psychres.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Shin H-W, Chung SJ. Drug-induced parkinsonism. J Clin Neurol. 2012;8(1):15–21. doi: 10.3988/jcn.2012.8.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wenning GK, Kiechl S, Seppi K, Müller J, Högl B, Saletu M, et al. Prevalence of movement disorders in men and women aged 50–89 years (Bruneck Study cohort): a population-based study. Lancet Neurol. 2005;4(12):815–820. doi: 10.1016/S1474-4422(05)70226-X. [DOI] [PubMed] [Google Scholar]
- 4.Modestin J, Stephan PL, Erni T, Umari T. Prevalence of extrapyramidal syndromes in psychiatric inpatients and the relationship of clozapine treatment to tardive dyskinesia. Schizophr Res. 2000;42(3):223–230. doi: 10.1016/s0920-9964(99)00133-4. [DOI] [PubMed] [Google Scholar]
- 5.Halliday J, Farrington S, Macdonald S, MacEwan T, Sharkey V, McCreadie R. Nithsdale Schizophrenia Surveys 23: movement disorders. 20-year review. Br J Psychiatry. 2002;181:422–427. doi: 10.1192/bjp.181.5.422. [DOI] [PubMed] [Google Scholar]
- 6.van Harten PN, Matroos GE, Hoek HW, Kahn RS. The prevalence of tardive dystonia, tardive dyskinesia, parkinsonism and akathisia The Curaçao Extrapyramidal Syndromes Study: I. Schizophr Res. 1996;19(2–3):195–203. doi: 10.1016/0920-9964(95)00096-8. [DOI] [PubMed] [Google Scholar]
- 7.Mamo DC, Sweet RA, Keshavan MS. Managing antipsychotic-induced parkinsonism. Drug Saf. 1999;20(3):269–275. doi: 10.2165/00002018-199920030-00006. [DOI] [PubMed] [Google Scholar]
- 8.Esper CD, Factor SA. Failure of recognition of drug-induced parkinsonism in the elderly. Mov Disord. 2008;23(3):401–404. doi: 10.1002/mds.21854. [DOI] [PubMed] [Google Scholar]
- 9.Savica R, Grossardt BR, Bower JH, Ahlskog JE, Mielke MM, Rocca WA. Incidence and time trends of drug-induced parkinsonism: a 30-year population-based study. Mov Disord. 2017;32(2):227–234. doi: 10.1002/mds.26839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunne LM, Andrén PE. An animal model for coexisting tardive dyskinesia and tardive parkinsonism: a glutamate hypothesis for tardive dyskinesia. Clin Neuropharmacol. 1993;16(1):90–95. doi: 10.1097/00002826-199302000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Snyder S, Greenberg D, Yamamura HI. Antischizophrenic drugs and brain cholinergic receptors. Arch Gen Psychiatry. 1974;31(1):58. doi: 10.1001/archpsyc.1974.01760130040006. [DOI] [PubMed] [Google Scholar]
- 12.Susatia F, Fernandez H. Drug-induced parkinsonism. Curr Treat Options Neurol. 2009;11:162–169. doi: 10.1007/s11940-009-0019-3. [DOI] [PubMed] [Google Scholar]
- 13.Weiden PJ. EPS profiles: the atypical antipsychotics are not all the same. J Psychiatr Pract. 2007;13(1):13–24. doi: 10.1097/00131746-200701000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Jamora D, Lim S-H, Pan A, Tan L, Tan E-K. Valproate-induced Parkinsonism in epilepsy patients. Mov Disord. 2007;22(1):130–133. doi: 10.1002/mds.21188. [DOI] [PubMed] [Google Scholar]
- 15.Tarsy D. Neuroleptic-induced extrapyramidal reactions: classification, description, and diagnosis. Clin Neuropharmacol. 1983;6(Suppl 1):S9–S26. [PubMed] [Google Scholar]
- 16.van Harten PN, Hoek HW, Kahn RS. Acute dystonia induced by drug treatment. BMJ. 1999;319(7210):623–626. doi: 10.1136/bmj.319.7210.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Satterthwaite TD, Wolf DH, Rosenheck RA, Gur RE, Caroff SN. A meta-analysis of the risk of acute extrapyramidal symptoms with intramuscular antipsychotics for the treatment of agitation. J Clin Psychiatry. 2008;69(12):1869–1879. doi: 10.4088/jcp.v69n1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehman AF, Lieberman JA, Dixon LB, McGlashan TH, Miller AL, Perkins DO, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(2 Suppl):1–56. [PubMed] [Google Scholar]
- 19.Hardie RJ, Lees AJ. Neuroleptic-induced Parkinson’s syndrome: clinical features and results of treatment with levodopa. J Neurol Neurosurg Psychiatry. 1988;51:850–854. doi: 10.1136/jnnp.51.6.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassin-Baer S, Sirota P, Korczyn AD, Treves TA, Epstein B, Shabtai H, et al. Clinical characteristics of neuroleptic-induced parkinsonism. J Neural Transm. 2001;108(11):1299–1308. doi: 10.1007/s007020100006. [DOI] [PubMed] [Google Scholar]
- 21.Pieters LE, Bakker PR, van Harten PN. Asymmetric drug-induced parkinsonism and psychopathology: a prospective naturalistic study in long-stay psychiatric patients. Front Psychiatry. 2018;9:18. doi: 10.3389/fpsyt.2018.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bohlega SA, Al-Foghom NB. Drug-induced Parkinson’s disease. Neurosciences. 2013;18(3):215–221. [PubMed] [Google Scholar]
- 23.Poewe W, Scherfler C. Role of dopamine transporter imaging in investigation of parkinsonian syndromes in routine clinical practice. Mov Disord. 2003;18 Suppl 7(S7):S16–S21. doi: 10.1002/mds.10573. [DOI] [PubMed] [Google Scholar]
- 24.Diaz-Corrales FJ, Sanz-Viedma S, Garcia-Solis D, Escobar-Delgado T, Mir P. Clinical features and 123I-FP-CIT SPECT imaging in drug-induced parkinsonism and Parkinson’s disease. Eur J Nucl Med Mol Imaging. 2010;37(3):556–564. doi: 10.1007/s00259-009-1289-4. [DOI] [PubMed] [Google Scholar]
- 25.Kern D, Lange A. Acute akathisia. In: Friedman J, editor. Medication-induced movement disorders. Cambridge: Cambridge University Press; 2015. pp. 3–19. [Google Scholar]
- 26.Advokat C. A brief overview of iatrogenic akathisia. Clin Schizophr Relat Psychoses. 2010;3(4):226–236. [Google Scholar]
- 27.López-Sendón JL, Mena MA, de Yébenes JG. Drug-induced parkinsonism in the elderly. Drugs Aging. 2012;29(2):105–118. doi: 10.2165/11598540-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 28.Josiassen RC, Kane JM, Liang GS, Burke J, O’Brien CF. Long-term safety and tolerability of valbenazine (NBI-98854) in subjects with tardive dyskinesia and a diagnosis of schizophrenia or mood disorder. Psychopharmacol Bull. 2017;47(3):61–68. [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandez HH, Stamler D, Davis MD, Factor SA, Hauser RA, Jimenez-Shahed J, et al. Confirmed safety of deutetrabenazine for tardive dyskinesia in a 2-year open-label extension study (P4.075). Neurology. 2018;90(15 Supplement). http://n.neurology.org/content/90/15_Supplement/P4.075.abstract. Accessed 11 July 2018.
- 30.AUSTEDO [package insert]. North Wales: Auspex Pharmaceuticals; 2017.
- 31.Sweet RA, Pollock BG, Rosen J, Mulsant BH, Altieri LP, Perel JM. Early detection of neuroleptic-induced parkinsonism in elderly patients with dementia. J Geriatr Psychiatry Neurol. 1994;7(4):251–253. doi: 10.1177/089198879400700411. [DOI] [PubMed] [Google Scholar]
- 32.Sweet RA, DeSensi EG, Zubenko GS. Reliability and applicability of movement disorder rating scales in the elderly. J Neuropsychiatry Clin Neurosci. 1993;5(1):56–60. doi: 10.1176/jnp.5.1.56. [DOI] [PubMed] [Google Scholar]
- 33.Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 34.Blanchet P, Kivenko V. Drug-induced parkinsonism: diagnosis and management. J Park Restless Legs Syndr. 2016;6:83–91. [Google Scholar]
- 35.Volavka J, Citrome L. Oral antipsychotics for the treatment of schizophrenia: heterogeneity in efficacy and tolerability should drive decision-making. Expert Opin Pharmacother. 2009;10(12):1917–1928. doi: 10.1517/14656560903061309. [DOI] [PubMed] [Google Scholar]
- 36.Citrome L. A review of the pharmacology, efficacy and tolerability of recently approved and upcoming oral antipsychotics: an evidence-based medicine approach. CNS Drugs. 2013;27(11):879–911. doi: 10.1007/s40263-013-0105-7. [DOI] [PubMed] [Google Scholar]
- 37.Vinogradov S, Fisher M, Warm H, Holland C, Kirshner MA, Pollock BG. The cognitive cost of anticholinergic burden: decreased response to cognitive training in schizophrenia. Am J Psychiatry. 2009;166(9):1055–1062. doi: 10.1176/appi.ajp.2009.09010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Symmetrel [package insert]. Chadds Ford: Endo Pharmaceuticals; 2009.
- 39.DiMascio A, Bernardo DL, Greenblatt DJ, Marder JE. A controlled trial of amantadine in drug-induced extrapyramidal disorders. Arch Gen Psychiatry. 1976;33(5):599. doi: 10.1001/archpsyc.1976.01770050055008. [DOI] [PubMed] [Google Scholar]
- 40.Fann W, Lake C. Amantadine versus trihexyphenidyl in the treatment of neuroleptic-induced parkinsonism. Am J Psychiatry. 1976;133(8):940–943. doi: 10.1176/ajp.133.8.940. [DOI] [PubMed] [Google Scholar]
- 41.GOCOVRI [package insert]. Emeryville: Adamas Pharma; 2017.
- 42.Osmolex ER [package insert]. Bridgewater: Vertical Pharmaceuticals; 2018.
- 43.Tarsy D, Baldessarini RJ. Epidemiology of tardive dyskinesia: is risk declining with modern antipsychotics? Mov Disord. 2006;21(5):589–598. doi: 10.1002/mds.20823. [DOI] [PubMed] [Google Scholar]
- 44.Weiden PJ, Mann JJ, Haas G, Mattson M, Frances A. Clinical nonrecognition of neuroleptic-induced movement disorders: a cautionary study. Am J Psychiatry. 1987;144(9):1148–1153. doi: 10.1176/ajp.144.9.1148. [DOI] [PubMed] [Google Scholar]
- 45.Carbon M, Hsieh C-H, Kane JM, Correll CU. Tardive dyskinesia prevalence in the period of second-generation antipsychotic use. J Clin Psychiatry. 2017;78(3):e264–e278. doi: 10.4088/JCP.16r10832. [DOI] [PubMed] [Google Scholar]
- 46.Woods SW, Morgenstern H, Saksa JR, Walsh BC, Sullivan MC, Money R, et al. Incidence of tardive dyskinesia with atypical versus conventional antipsychotic medications: a prospective cohort study. J Clin Psychiatry. 2010;71(4):463–474. doi: 10.4088/JCP.07m03890yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kane JM, Woerner M, Lieberman J. Tardive dyskinesia: prevalence, incidence, and risk factors. J Clin Psychopharmacol. 1988;8(4 Suppl):52S–56S. [PubMed] [Google Scholar]
- 48.Saltz BL, Woerner MG, Kane JM, Lieberman JA, Alvir JM, Bergmann KJ, et al. Prospective study of tardive dyskinesia incidence in the elderly. JAMA. 1991;266(17):2402–2406. [PubMed] [Google Scholar]
- 49.Egan MF, Apud J, Wyatt RJ. Treatment of tardive dyskinesia. Schizophr Bull. 1997;23(4):583–609. doi: 10.1093/schbul/23.4.583. [DOI] [PubMed] [Google Scholar]
- 50.Caroff SN, Hurford I, Lybrand J, Campbell EC. Movement disorders induced by antipsychotic drugs: implications of the CATIE schizophrenia trial. Neurol Clin. 2011;29(1):127–48, viii. [DOI] [PMC free article] [PubMed]
- 51.Zai CC, Maes MS, Tiwari AK, Zai GC, Remington G, Kennedy JL. Genetics of tardive dyskinesia: promising leads and ways forward. J Neurol Sci. 2018;15(389):28–34. doi: 10.1016/j.jns.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 52.Savitt D, Jankovic J. Tardive syndromes. J Neurol Sci. 2018;15(389):35–42. doi: 10.1016/j.jns.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 53.Munetz MR, Benjamin S. How to examine patients using the Abnormal Involuntary Movement Scale. Hosp Community Psychiatry. 1988;39(11):1172–1177. doi: 10.1176/ps.39.11.1172. [DOI] [PubMed] [Google Scholar]
- 54.Citrome L. Clinical management of tardive dyskinesia: five steps to success. J Neurol Sci. 2017;15(383):199–204. doi: 10.1016/j.jns.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 55.Schooler NR, Kane JM. Research diagnoses for tardive dyskinesia. Arch Gen Psychiatry. 1982;39(4):486–487. doi: 10.1001/archpsyc.1982.04290040080014. [DOI] [PubMed] [Google Scholar]
- 56.Jankelowitz SK. Treatment of neurolept-induced tardive dyskinesia. Neuropsychiatr Dis Treat. 2013;9:1371–1380. doi: 10.2147/NDT.S30767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miller DD, McEvoy JP, Davis SM, Caroff SN, Saltz BL, Chakos MH, et al. Clinical correlates of tardive dyskinesia in schizophrenia: baseline data from the CATIE schizophrenia trial. Schizophr Res. 2005;80(1):33–43. doi: 10.1016/j.schres.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 58.Solmi M, Pigato G, Kane JM, Correll CU. Clinical risk factors for the development of tardive dyskinesia. J Neurol Sci. 2018;389:21–27. doi: 10.1016/j.jns.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 59.Jeste DV, Lohr JB, Clark K, Wyatt RJ. Pharmacological treatments of tardive dyskinesia in the 1980s. J Clin Psychopharmacol. 1988;8(4 Suppl):38S–48S. [PubMed] [Google Scholar]
- 60.Bhidayasiri R, Jitkritsadakul O, Friedman JH, Fahn S. Updating the recommendations for treatment of tardive syndromes: a systematic review of new evidence and practical treatment algorithm. J Neurol Sci. 2018;15(389):67–75. doi: 10.1016/j.jns.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 61.Margolese HC, Chouinard G, Kolivakis TT, Beauclair L, Miller R, Annable L. Tardive dyskinesia in the era of typical and atypical antipsychotics. Part 2: Incidence and management strategies in patients with schizophrenia. Can J Psychiatry. 2005;50(11):703–714. doi: 10.1177/070674370505001110. [DOI] [PubMed] [Google Scholar]
- 62.Ingrezza [package insert]. San Diego: Neurocrine Biosciences; 2017.
- 63.Hauser RA, Factor SA, Marder SR, Knesevich MA, Ramirez PM, Jimenez R, et al. KINECT 3: a phase 3 randomized, double-blind, placebo-controlled trial of valbenazine for tardive dyskinesia. Am J Psychiatry. 2017;174(5):476–484. doi: 10.1176/appi.ajp.2017.16091037. [DOI] [PubMed] [Google Scholar]
- 64.Factor SA, Remington G, Comella CL, Correll CU, Burke J, Jimenez R, et al. The effects of valbenazine in participants with tardive dyskinesia. J Clin Psychiatry. 2017;78(9):1344–1350. doi: 10.4088/JCP.17m11777. [DOI] [PubMed] [Google Scholar]
- 65.Fernandez HH, Factor SA, Hauser RA, Jimenez-Shahed J, Ondo WG, Jarskog LF, et al. Randomized controlled trial of deutetrabenazine for tardive dyskinesia. Neurology. 2017;88(21):2003–2010. doi: 10.1212/WNL.0000000000003960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anderson KE, Stamler D, Davis MD, Factor SA, Hauser RA, Isojärvi J, et al. Deutetrabenazine for treatment of involuntary movements in patients with tardive dyskinesia (AIM-TD): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Psychiatry. 2017;4(8):595–604. doi: 10.1016/S2215-0366(17)30236-5. [DOI] [PubMed] [Google Scholar]
- 67.Pappa S, Tsouli S, Apostolou G, Mavreas V, Konitsiotis S. Effects of amantadine on tardive dyskinesia: a randomized, double-blind, placebo-controlled study. Clin Neuropharmacol. 2010;33(6):271–275. doi: 10.1097/wnf.0b013e3181ffde32. [DOI] [PubMed] [Google Scholar]
- 68.Angus S, Sugars J, Boltezar R, Koskewich S, Schneider NM. A controlled trial of amantadine hydrochloride and neuroleptics in the treatment of tardive dyskinesia. J Clin Psychopharmacol. 1997;17(2):88–91. doi: 10.1097/00004714-199704000-00004. [DOI] [PubMed] [Google Scholar]
- 69.Prestwick Pharmaceuticals. Tetrabenazine Briefing Document for Peripheral and Central Nervous System Advisory Committee [Internet]. 2007 [cited 2018 May 18]. pp. 1–121. http://wayback.archive-it.org/7993/20180126160441/https://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4328b1-02-Prestwick.pdf. Accessed 11 July 2018.
- 70.Kazamatsuri H, Chien CP, Cole JO. Long-term treatment of tardive dyskinesia with haloperidol and tetrabenazine. Am J Psychiatry. 1973;130(4):479–483. doi: 10.1176/ajp.130.4.479. [DOI] [PubMed] [Google Scholar]
- 71.Ondo WG, Hanna PA, Jankovic J. Tetrabenazine treatment for tardive dyskinesia: assessment by randomized videotape protocol. Am J Psychiatry. 1999;156(8):1279–1281. doi: 10.1176/ajp.156.8.1279. [DOI] [PubMed] [Google Scholar]
- 72.Xenazine [package insert]. Washington, DC: Prestwick Pharmaceuticals, Inc.; 2008.
- 73.Ossowska K. Neuronal basis of neuroleptic-induced extrapyramidal side effects. Pol J Pharmacol. 2002;54(4):299–312. [PubMed] [Google Scholar]
- 74.Calabresi P, Picconi B, Tozzi A, Ghiglieri V, Di Filippo M. Direct and indirect pathways of basal ganglia: a critical reappraisal. Nat Neurosci. 2014;17(8):1022–1030. doi: 10.1038/nn.3743. [DOI] [PubMed] [Google Scholar]
- 75.Citrome L. Tardive dyskinesia: placing vesicular monoamine transporter type 2 (VMAT2) inhibitors into clinical perspective. Expert Rev Neurother. 2018;18(4):323–332. doi: 10.1080/14737175.2018.1455504. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.