Abstract
The rise of sauropodomorphs is still poorly understood due to the scarcity of well-preserved fossils in early Norian rocks. Here, we present an association of complete and exceptionally well-preserved dinosaur skeletons that helps fill that gap. They represent a new species, which is recovered as a member of a clade solely composed of Gondwanan Triassic taxa. The new species allows the definition of a set of anatomical changes that shaped sauropodomorph evolution along a period from 233 to 225 Ma, as recorded in the well dated Late Triassic beds of Brazil. In that time span, apart from achieving a more herbivorous diet, sauropodomorph dinosaurs increased their size in a ratio of 230% and their typical long neck was also established, becoming proportionally twice longer than those of basal taxa. Indeed, the new dinosaur is the oldest-known sauropodomorph with such an elongated neck, suggesting that the ability to feed on high vegetation was a key trait achieved along the early Norian. Finally, the clustered preservation mode of the skeletons represents the oldest evidence of gregarious behaviour among sauropodomorphs.
Keywords: Brazil, Dinosauria, evolution, Norian, Saurischia, Triassic
1. Introduction
The oldest sauropodomorph dinosaurs are represented by relatively rare, small-bodied forms from Carnian strata in southwestern Pangaea [1–5]. Soon after, approaching the end of the Triassic, larger members of the group became dominant faunal components in land ecosystems, with representatives from almost the entire supercontinent [6]. However, this transition is still poorly understood, given the scarce sauropodomorph record from early Norian strata [6,7]. Light was more recently shed on understanding the shift from small faunivorous to large herbivorous sauropodomorphs, with the discovery of new specimens from Brazil [5,8,9]. Nevertheless, well-preserved skeletons of early Norian sauropodomorphs are still lacking, hampering the identification of traits that possibly constrained their evolutionary trajectories along the Mesozoic. Here, we partially fill this gap with the description of an association of three skeletons from Brazil (see electronic supplementary material, figure S1).
2. Systematic palaeontology
Dinosauria Owen, 1842
Saurischia Seeley, 1887
Sauropodomorpha Huene, 1932
Unaysauridae clade nov.
(a). Type genus
Unaysaurus tolentinoi Leal et al., 2004.
(b). Definition
Most inclusive clade including Unaysaurus tolentinoi Leal et al., 2004, but not Plateosaurus engelhardti von Meyer, 1837 nor Saltasaurus loricatus Bonaparte & Powel, 1980.
(c). Diagnosis
Unaysauridae differs from all other sauropodomorphs by a substantial cranial expansion of the medial condyle of the astragalus. In addition, a promaxillary fenestra is also unique for the group of sauropodomorphs, although cranial elements are still unknown for Jaklapallisaurus asymmetrica.
Macrocollum itaquii gen. et sp. nov.
(d). Etymology
The generic name combines the Greek word μακρο (= long) and the Latin word ‘collum’ (= neck), referring to the elongated neck of the new taxon. The specific epithet honours Mr José Jerundino Machado Itaqui, one of the main actors behind the creation of CAPPA/UFSM.
(e). Holotype
CAPPA/UFSM (Centro de Apoio à Pesquisa Paleontológica da Quarta Colônia) 0001a. An almost complete and articulated skeleton.
(f). Paratypes
CAPPA/UFSM 0001b. An almost complete and partially articulated skeleton. CAPPA/UFSM 0001c. An articulated skeleton lacking skull and cervical series.
(g). Locality and horizon
The specimens were collected at the Wachholz site (29°36′46.42″ S; 53°15′54.06″ W), Agudo, Rio Grande do Sul, Brazil; upper portion of the Candelária Sequence, Paraná Basin [10]. Stratigraphically correlated beds from a nearby site were dated as early Norian (ca 225.42 ± 0.37), Late Triassic [11].
(h). Diagnosis
Macrocollum itaquii differs from all other known sauropodomorphs based on a unique combination of characters: antorbital fossa perforated by a promaxillary fenestra; medial margin of the supratemporal fossa with a simple smooth curve at the frontal/parietal suture; proximal articular surface of metacarpal I transversely narrow; acetabulum not fully open; ischiadic longitudinal groove not reaching the caudal half of the ischium; absence of trochanteric shelf on the femur; medial condyle of distal femoral articulation subrectangular in distal view; proximal end of metatarsal II with a straight medial margin (see electronic supplementary material, figure S2 for further details).
3. Description
The skull (figure 1a–c) is gracile and about half the femoral length, which is a usual trait in post-Carnian sauropodomorphs. The snout is slightly elongated, with the internarial fossa bounding a large external naris. It resembles Plateosaurus engelhardti, whereas in Buriolestes schultzi the external naris is narrow. The rostrocaudal length of the antorbital fenestra is subequal to the maximal orbital length. The caudodorsal process of the premaxilla does not contact the rostroventral process of the nasal. A well-developed wall, mediodorsally projecting from the maxilla, forms the extensive antorbital fossa, which is perforated by a promaxillary fenestra. An internarial fenestra is visible in dorsal view and the prefrontal does not contribute to the dorsal orbital rim. There is no marked rostrolateral projection from the frontal/parietal contact at the medial margin of the supratemporal fossa. The craniomandibular articulation is slightly below the tooth line. The rostral tip of the dentary is downturned. The retroarticular process is caudally elongated, but does not strongly fold at its tip. There are four premaxillary, about 23 maxillary, and about 21 dentary teeth. Except for those in the premaxilla, tooth crowns are constricted at their base and bear large denticles forming oblique angles with the main axis of the tooth. In contrast to Carnian sauropodomorphs, there is no evidence of pterygoid teeth.
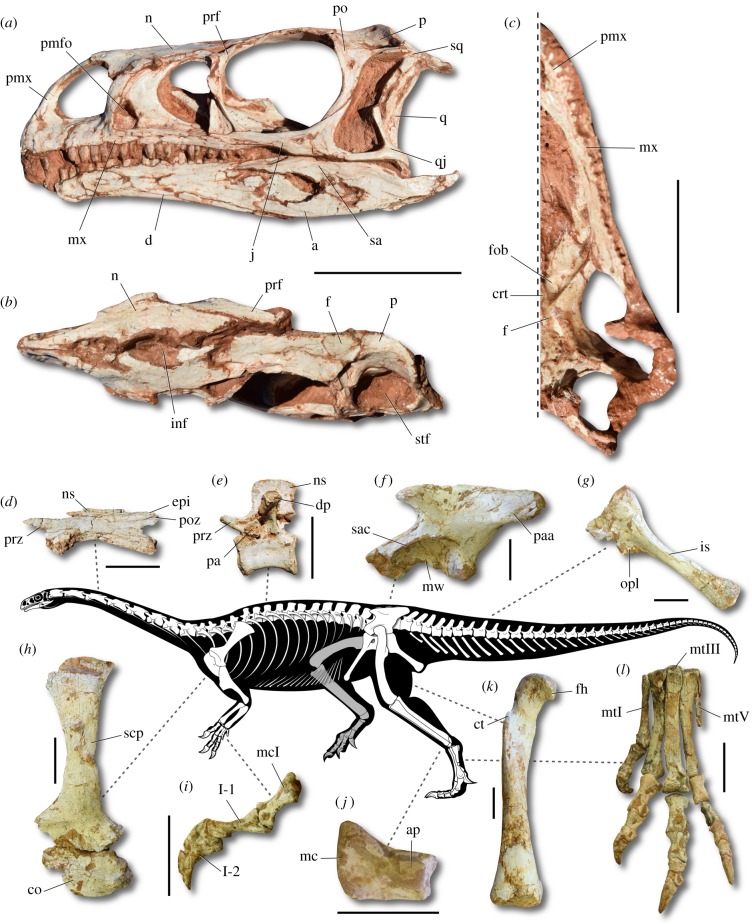
Figure 1.
Reconstructed skeleton and representative elements of Macrocollum itaquii. (a) Skull in left lateral view (CAPPA/UFSM 0001a). (b) Skull in dorsal view (CAPPA/UFSM 0001a). (c) Skull in ventral view (CAPPA/UFSM 0001b). (d) Fourth cervical vertebra in left lateral view (CAPPA/UFSM 0001b). (e) Mid-truncal vertebra in left lateral view (CAPPA/UFSM 0001b). (f) Left ilium in lateral view (CAPPA/UFSM 0001b). (g) Left ischium in lateral view (CAPPA/UFSM 0001b). (h) Right pectoral girdle in lateral view (reversed - CAPPA/UFSM 0001b). (i) Right manual digit I in medial view (CAPPA/UFSM 0001b). (j) Right astragalus in dorsal view (CAPPA/UFSM 0001c). (k) Right femur in cranial view (CAPPA/UFSM 0001b). (l) Left pes in cranial view (CAPPA/UFSM 0001a). I-1, phalanx one of the digit I; I-2, phalanx two of the digit I; a, angular; ap, ascending process; co, coracoid; crt, crest; ct, cranial trochanter; d, dentary; dp, diapophysis; epi, epipophysis; f, frontral; fh, femoral head; fob, fossa for the olfactoy bulbus; inf, internarial fenestra; is, ischium shaft; j, jugal; mc, medial condyle; mcI, metacarpal I; mtI, metatarsal I; mtIII, metatarsal III; mtV, metatarsal V; mw, medial wall; mx, maxilla; n, nasal; ns, neural spine; opl, obturador plate; p, parietal; pa, parapophysis; paa, postacetabular ala; pmfo, promaxillary fenestra; pmx, premaxilla; po, postorbital; poz, postzygapophysis; prf, prefrontal; prz, prezygapophysis; q, quadrate; qj, quadratojugal; sa, surangular; sac, supracetabular crest; scp, scapula; sq, squamosal; stf, supratemporal fenestra. Scale bar = 50 mm. (Online version in colour.)
The cervical vertebrae are remarkably elongated (figure 1d). For instance, the centrum of the fourth cervical vertebra is about six times longer than its cranial height, whereas that ratio is only 2.5 in B. schultzi. The neural spine is craniocaudally short in the cranial trunk vertebrae, but more elongated in the remaining elements (figure 1e). Three vertebrae compose the sacrum: the two primordial elements and one extra added from the trunk series. The chevrons from the cranial half of the tail are quite elongated (more than twice the length of their respective centra). Scapula and coracoid are unfused in all specimens (figure 1h). The proximal articular surface of the humerus is flat, with its distal end just moderately expanded transversely. A deep radial fossa is absent from the proximal end of the ulna and the olecranon is poorly developed. The twisted digit I (figure 1i) is somewhat more robust, but shorter than digits II and III, whereas digits IV and V are reduced, lacking ungual phalanges. The medial acetabular wall of the ilium is well developed (figure 1f), resembling the plesiomorphic condition also present in Jaklapallisaurus asymmetrica. On the other hand, plateosaurian sauropodomorphs, like P. engelhardti and Coloradisaurus brevis have a fully open acetabulum. The supracetabular crest is pronounced and the preacetabular ala is short and triangular. The ischium (figure 1g) is about 80% of the total length of the pubis. Its shaft has a triangular section at midlength, but expands dorsoventrally at the distal end, forming a semicircular outline. The femur is sigmoid in cranial view (figure 1k), but almost straight in lateral view. The proximal articular surface bears a well-developed caudomedial tuber. All the specimens lack a trochanteric shelf. The asymmetrical fourth trochanter is located at the proximal half of the bone. The tibia is nearly 90% the length of the femur. As in J. asymmetrica, the medial condyle of the astragalus is remarkably expanded cranially (figure 1j), whereas this is less pronounced in other sauropodomorphs. The foot is slender (figure 1l), with elongated phalanges, resembling early diverging sauropodomorphs like B. schultzi and Eoraptor lunensis. In contrast, P. engelhardti and massopodans have robust phalanges.
4. Phylogenetic analysis
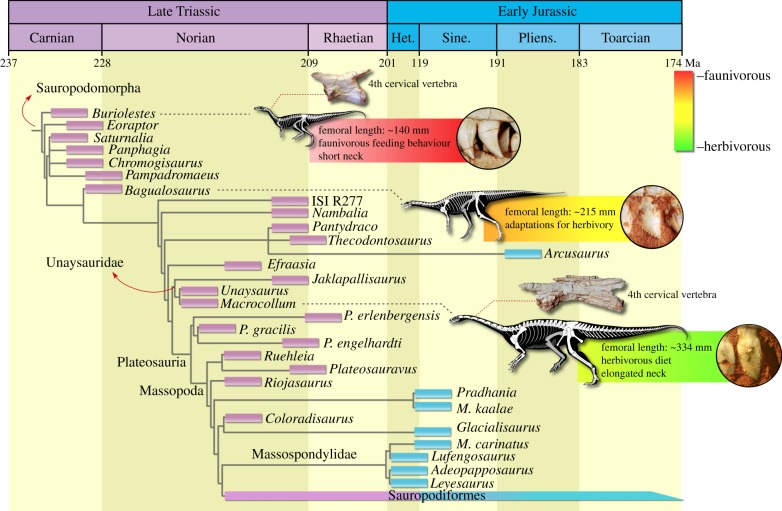
Macrocollum itaquii was scored in a new data matrix constructed from previous studies on Triassic/Jurassic sauropodomorphs, which was the subject of an equally weighted parsimony analysis in TNT v. 1.1 [12] (see electronic supplementary material). The analysis recovered 648 MPTs of 1507 steps each, which reveal a rich (and poorly understood) diversity of non-plateosaurian sauropodomorphs (figure 2). In opposition to previous proposals [13], Unaysaurus tolentinoi does not lie within Plateosauridae. Rather, it is nested in a new clade that encompasses the Indian Jaklapallisaurus asymmetrica [14] as the sister taxon of M. itaquii plus U. tolentinoi. Members of this new clade, Unaysauridae, share a medial condyle of the astragalus 1.6 times the craniocaudal length of the lateral condyle, whereas the sister taxon relationship between M. itaquii and U. tolentinoi is supported by the presence of a longitudinal ventral sulcus in the proximal and middle caudal vertebrae. Unaysauridae is the sister group of Plateosauria, which is composed of Plateosauridae plus Massopoda.
Figure 2.
Time-calibrated reduced strict consensus tree depicting the phylogenetic position of Macrocollum itaquii and some evolutionary trends along the lineage. (Online version in colour.)
5. Discussion
The proposed phylogenetic hypothesis, associated with the precise time calibration of Triassic strata from Brazil [11], allows investigation of the timeline involved in the acquisition of some crucial sauropodomorph traits. Buriolestes schultzi, the sister taxon to all other sauropodomorphs [5,9], was exhumed from beds of about 233 Ma [11], whereas Bagualosaurus agudoensis comes from beds that overlap those yielding Bu. schultzi [8]. Finally, M. itaquii was recovered from rocks belonging to the upper part of the Candelária Sequence [10], constrained as about 225 Ma [11]. This framework allows changes in the sauropodomorph bauplan to be tracked along a ca 8 Ma time interval in Brazil. Indeed, these Brazilian taxa represent stages of early sauropodomorph evolution also recognized in the Ischigualato Formation in Argentina and in Norian deposits of various parts of the world, thus corresponding to general trends within the group. For instance, in contrast to most Carnian members of the group, the teeth of M. itaquii and other Norian taxa are fully adapted to an omnivore/herbivore diet, with coarse tooth serrations, mesiodistally expanded crowns above the root in cheek teeth, and overlap of adjacent crowns [15]. A gradual increase in size accompanies this shift, with femoral length increasing from about 135 mm in Bu. schultzi [9] to 215 in Ba. agudoensis [8], and to over 335 mm in M. itaquii and most mid-late Norian taxa. However, skull proportions reduce from about to 85% of the femoral length in Bu. schultzi, to ca 60% in Ba. agudoensis, and ca 45% in M. itaquii and mid-late Norian taxa, highlighting the presence of allometric processes in the size increase seen in the lineage. Likewise, the neck becomes proportionally two times longer in M. itaquii in comparison to Bu. schultzi. Not only representing one of the most important diagnostic traits of sauropodomorphs, neck elongation may also have provided a competitive advantage for gathering food resources, allowing members of the group to reach higher vegetation [16] compared to other early Norian vertebrates.
The hindlimb of M. itaquii also carries modifications that could be related to the progressive loss of cursorial habits. Its femur is longer than the tibia, whereas earlier forms have the inverse condition [1]. The straight femur of M. itaquii in lateral view also differs from those of older sauropodomorphs, which have a sigmoidal outline, and the absence of a trochanteric shelf also in contrast to the condition in Carnian forms. On the other hand, the gracile construction of the foot (with slender phalanges) resembles the plesiomorphic condition for sauropodomorphs, differing from the robust foot of plateosaurians. Hence, the skeleton of the new dinosaur demonstrates a gradual abandonment of cursoriality combined with the acquisition of traits related to herbivorous feeding strategies.
Finally, the clustered preservation of the three skeletons of Macrocollum itaquii represents the oldest evidence of gregarious behaviour in sauropodomorphs. These corroborate the pattern seen in other Triassic associations, such as the ‘Plateosaurus bonebed’ from Central Europe [17] and the Mussaurus remains from the Laguna Colorada Formation, Argentina [18,19]. Putative older evidences are not so compelling, relying on the skeletons of Bu. schultzi recovered from the same layer in a small area [5,9], as also reported for the coeval Saturnalia tupiniquim, from southern Brazil [2].
Supplementary Material
Acknowledgements
We thank the following people who played a role in the discovery of the specimens presented here: Mr Dilo Wachholz, Mr Olímpio Neu, Mrs Cladis Müller Kobs, Mrs Mariana Kobs, Mr Gercides Müller, and Mrs Estefânia Temp Müller. Handling Editor of Biology Letters, Dr Diego Pol, Dr Fernando Novas, and an anonymous reviewer provided valuable comments that greatly improved this manuscript. We also thank the Willi Henning Society, for the gratuity of TNT software.
Data accessibility
Data are available from the Dryad Digital Repository: http://doi.dx.org/10.5061/dryad.gh4p57r [20].
Authors' contributions
R.T.M. and S.D.-d.-S. conducted the fieldwork. R.T.M. performed the mechanical preparation of specimens and carried out the analyses. R.T.M., M.C.L. and S.D.-d.-S. analysed and discussed the results. R.T.M., M.C.L. and S.D.-d.-S. wrote the paper. All authors approved the final version of the paper and agreed to be accountable for all aspects of the work.
Competing interests
We have no competing interests.
Funding
This work was supported by the Conselho Nacional de Desenvolvimento Científco e Tecnológico (CNPq; research grant to S.D.-d.-S., process number 306352/2016-8).
References
- 1.Sereno PC, Forster CA, Rogers RR, Monetta AM. 1993. Primitive dinosaur skeleton from Argentina and the early evolution of Dinosauria. Nature 361, 64–66. ( 10.1038/361064a0) [DOI] [Google Scholar]
- 2.Langer MC, Abdala F, Richter M, Benton MJ. 1999. A sauropodomorph dinosaur from the Upper Triassic (Carnian) of Southern Brazil. Acad. Sci. Paris Sci. Terre et Planetnèt. 329, 511–517. [Google Scholar]
- 3.Martinez RN, Alcober OA. 2009. A basal sauropodomorph (Dinosauria: Saurischia) from the Ischigualasto Formation (Triassic, Carnian) and the early evolution of Sauropodomorpha. PLoS ONE 4, e4397 ( 10.1371/journal.pone.0004397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ezcurra MD. 2010. A new early dinosaur (Saurischia: Sauropodomorpha) from the Late Triassic of Argentina: a reassessment of dinosaur origin and phylogeny. J. Syst. Palaeontol. 8, 371–425. ( 10.1080/14772019.2010.484650) [DOI] [Google Scholar]
- 5.Cabreira SF, et al. 2016. A unique Late Triassic dinosauromorph assemblage reveals dinosaur ancestral anatomy and diet. Curr. Biol. 26, 3090–3095. ( 10.1016/j.cub.2016.09.040) [DOI] [PubMed] [Google Scholar]
- 6.Müller RT, Langer MC, Dias-da-Silva S. 2017. Biostratigraphic significance of a new early sauropodomorph specimen from the Upper Triassic of southern Brazil. Hist. Biol. 29, 187–202. ( 10.1080/08912963.2016.1144749) [DOI] [Google Scholar]
- 7.Mannion PD, Upchurch P, Carrano MT, Barrett PM. 2011. Testing the effect of the rock record on diversity: a multidisciplinary approach to elucidating the generic richness of sauropodomorph dinosaurs through time. Biol. Rev. 86, 157–181. ( 10.1111/j.1469-185X.2010.00139.x) [DOI] [PubMed] [Google Scholar]
- 8.Pretto FA, Langer MC, Schultz CL. 2018. A new dinosaur (Saurischia: Sauropodomorpha) from the Late Triassic of Brazil provides insights on the evolution of sauropodomorph body plan. Zool. J. Linn. Soc., zly028 ( 10.1093/zoolinnean/zly028) [DOI] [Google Scholar]
- 9.Müller RT, et al. 2018. Early evolution of sauropodomorphs: anatomy and phylogenetic relationships of a remarkably well preserved dinosaur from the Upper Triassic of southern Brazil. Zool. J. Linn. Soc., zly009 ( 10.1093/zoolinnean/zly009) [DOI] [Google Scholar]
- 10.Müller RT, Da-Rosa AAS, Silva LR, Aires ASS, Pacheco CP, Pavanatto AEB, Dias-da-Silva S. 2015. Wachholz, a new exquisite dinosaur-bearing fossiliferous site from the Upper Triassic of southern Brazil. J. South Am. Earth Sci. 61, 120–128. ( 10.1016/j.jsames.2014.10.009) [DOI] [Google Scholar]
- 11.Langer MC, Ramezani J, Da Rosa AA. 2018. U-Pb age constraints on dinosaur rise from south Brazil. Gondwana Res. 57, 133–140. ( 10.1016/j.gr.2018.01.005) [DOI] [Google Scholar]
- 12.Goloboff PA, Farris JS, Nixon KC. 2008. TNT, a free program for phylogenetic analysis. Cladistics 24, 1–13. ( 10.1111/j.1096-0031.2007.00173.x) [DOI] [Google Scholar]
- 13.Leal LA, Azevedo SA, Kellner AW, Da Rosa ÁA. 2004. A new early dinosaur (Sauropodomorpha) from the Caturrita Formation (Late Triassic), Paraná Basin, Brazil. Zootaxa 690, 1–24. [Google Scholar]
- 14.Novas FE, Ezcurra MD, Chatterjee S, Kutty TS. 2011. New dinosaur species from the Upper Triassic Upper Maleri and Lower Dharmaram formations of Central India. Earth Environ. Sci. Trans. R. Soc. Edinb. 101, 333–349. [Google Scholar]
- 15.Nesbitt SJ, Sidor CA, Irmis RB, Angielczyk KD, Smith RM, Tsuji LA. 2010. Ecologically distinct dinosaurian sister group shows early diversification of Ornithodira. Nature 464, 95–98. ( 10.1038/nature08718) [DOI] [PubMed] [Google Scholar]
- 16.Galton PM. 1985. Diet of prosauropod dinosaurs from the late Triassic and early Jurassic. Lethaia 18, 105–123. ( 10.1111/j.1502-3931.1985.tb00690.x) [DOI] [Google Scholar]
- 17.Sander PM. 1992. The Norian Plateosaurus bonebeds of central Europe and their taphonomy. Palaeogeogr. Palaeoclimatol. Palaeoecol. 93, 255–299. ( 10.1016/0031-0182(92)90100-J) [DOI] [Google Scholar]
- 18.Pol D, Powell JE. 2007. Skull anatomy of Mussaurus patagonicus (Dinosauria: Sauropodomorpha) from the late Triassic of Patagonia. Hist. Biol. 19, 125–144. ( 10.1080/08912960601140085) [DOI] [Google Scholar]
- 19.Otero A, Pol D. 2013. Postcranial anatomy and phylogenetic relationships of Mussaurus patagonicus (Dinosauria, Sauropodomorpha). J. Vertebr. Paleontol. 33, 1138–1168. ( 10.1080/02724634.2013.769444) [DOI] [Google Scholar]
- 20.Müller RT, Langer MC, Dias-da-Silva S.2018. Data from: An exceptionally preserved association of complete dinosaur skeletons reveals the oldest long-necked sauropodomorphs. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Müller RT, Langer MC, Dias-da-Silva S.2018. Data from: An exceptionally preserved association of complete dinosaur skeletons reveals the oldest long-necked sauropodomorphs. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository: http://doi.dx.org/10.5061/dryad.gh4p57r [20].