Abstract
Developmental behavioural plasticity is a process by which organisms can alter development of their behavioural phenotype to be better adapted to the environment encountered later in life. This ‘shaping’ process depends on the presence of reliable cues by which predictions can be made. It is now established that cues detected by the mother can be used (primarily via hormones prenatally and maternal behaviour in the early postnatal stage) to shape the behavioural phenotype of her offspring. However, it is becoming increasingly clear that adolescence is another period in which conditions are well-suited for such shaping to occur. We review here how mammalian social behaviour may be shaped in adolescence. We identify limited extant examples, briefly discuss underlying mechanisms, and provide evidence that observed changes are indeed adaptive. We contend that while plasticity diminishes with age, the shaping of the behavioural phenotype in adolescence offers several advantages, including that adolescence is closer to the onset of mating than are earlier phases of life; that unlike earlier phases, information is obtained directly from the environment rather than mediated by the mother; and unlike later in adulthood, there is substantial underlying neural plasticity associated with development to support behavioural change. We also consider conditions that favour the occurrence of social behaviour plasticity during adolescence, including a high degree of sociality and a prolonged developmental period and the implication of these conditions for the occurrence of sex differences in the shaping process.
Keywords: behaviour, development, adolescence, plasticity
1. Behavioural plasticity and development
Even individuals of identical genotype differ in their behaviour [1]. This finding should not surprise since behaviour and its underlying neural structures are constantly modified through interactions with the social and physical environment during the course of life [2,3]. Such malleability, known as behavioural plasticity, enables organisms to adjust their responses across environments [4,5]. Two major forms of behavioural plasticity can be distinguished: activational and developmental [6]. Activational behavioural plasticity refers to the ability of individuals to respond differentially to different environmental conditions through the use of already available underlying neural and endocrine networks. In principle, this ability persists throughout the lifetime. Developmental plasticity, in contrast, is the ability of a given genotype to adopt different developmental trajectories in different environments, and involves the modification of underlying networks. Accordingly, behavioural phenotypes are shaped through genotype by environment interactions during ontogeny, and in this manner, phenotypes can be adjusted to their environment.
To date, most research on developmental behavioural plasticity has focused on early phases of life, in particular, the prenatal and the early postnatal phases, which for mammals encompasses the time from birth until weaning [2]. If environmental cues present during these early phases provide information that is predictive of the quality or nature of the environment during the organism's later life, development may be altered to increase fitness during the later period. Effects can be mediated either directly by environmental cues, or indirectly via the mother, e.g. by maternal hormones transferred to the young or by maternal behaviour [7–10]. The notion that environmental cues early in life can shape the phenotype to be adapted to future environmental conditions is known as the ‘predictive adaptive response’ hypothesis. This hypothesis stipulates that the future environment must match the early prediction in order to augment fitness—a mismatch between the individual's phenotype and the conditions in which it finds itself may have adverse consequences for Darwinian fitness and, later in life, for health [11]. Yet, a process of phenotype–environment matching is not a necessary condition for developmental plasticity to enhance fitness. Many examples exist of individuals born in good conditions having overall fitness or performance advantages in later life—the so-called silver spoon effect—with those exposed to resource-poor early conditions being at a permanent disadvantage [12].
The massive effects of environmental influences on the behavioural phenotype that emerges early in development appear facilitated by the ongoing neural maturation (e.g. synaptogenesis, circuit formation) occurring at this time. However, early development is not the only period in which shaping behaviour for future environmental contingencies might be useful, nor the only period in which the underlying neural conditions are suitable. Indeed, there is increasing evidence that adolescence—that is, the gradual transition from the pre-reproductive phase to adulthood that occurs in many species—also represents a time window (beyond the prenatal and early postnatal periods) in which neural maturation is still in progress and behavioural phenotypes may be routinely shaped by environmental influences [2]. While evidence is mounting for sensitive periods during adolescence, extant examples of environmental shaping during this period are still relatively sparse. In addition, the issue of how specific or limited observed effects are to a sensitive period during adolescence often is not clear [13], and findings providing good evidence for fitness advantages are rare.
The aim of this paper is to summarize evidence for adaptive shaping of mammalian social behaviour phenotypes during adolescence, specifically how mammals may adjust developmental trajectories to match the future social environment. In a first step, we will discuss conditions favouring, and examples of, social behavioural plasticity during adolescence in mammals and draw comparisons with findings in birds. We will then discuss whether observed canalizations of behaviour are adaptive, and whether they provide evidence for environmental matching or for a silver-spoon effect. How the effects of adolescent plasticity might be ‘reshaped’ to meet changing circumstances will also be considered. We will then address what differentiates adaptive shaping during adolescence from that occurring during other phases of life and finally consider the species and environmental conditions under which adaptive shaping of behavioural phenotypes during adolescence should be expected.
2. Behavioural plasticity during adolescence
Adolescence is, of course, a key developmental epoch. The hallmark of adolescence is puberty, involving the activation of the hypothalamic–pituitary–gonadal axis that underlies reproductive maturation and—either immediately or sometime later—the onset of mating behaviour. The activation, per se, of behaviour resulting from pre- and perinatal organization of the brain (as opposed to developmental plasticity of behaviour) is beyond the scope of this paper. However, there are widespread functional and structural alterations in cortical and key subcortical brain regions during adolescence that may support plasticity (e.g. [14–16]), and adolescent neural plasticity may be driven by, or interact with, endocrine changes of puberty to support behavioural change [16,17]. Further, the increase in frequency and importance of interactions with non-family members, particularly peers, that is characteristic of adolescence in social species, affords new cues to potentially augment fitness. These are just the conditions under which behavioural plasticity might be expected to occur [18]. Moreover, in cases in which plasticity during earlier phases of life serves primarily to ensure survival during this vulnerable time, plasticity during adolescence may be needed to allow for adjustments and readjustments to tailor later social behaviour to enhance mating success. But before considering adaptiveness of such changes, there must be evidence that socially relevant behaviour is indeed shaped by adolescent experiences.
A number of relatively isolated findings demonstrate this point. For instance, in adolescent male rats, exposure to defeat by a territorial male [19] and conditions inducing social instability [20] both reduced the tendency to approach conspecifics in adulthood. In addition, social defeat altered later play behaviour and reduced adult aggression [21], and social instability also impaired adult mating competence [20]. In male hamsters, social defeat either increased or decreased later aggression, depending on when in puberty the defeat occurred [22]. In mice, coping with challenge during adolescence in an escapable social defeat paradigm led to an increase in aggression and exploration, and a reduction in anxiety-like behaviour [23]. Among birds, zebra finch males housed in small groups during adolescence showed less courtship and aggressive behaviour in adulthood than did finches housed in adolescence as male–female pairs [24].
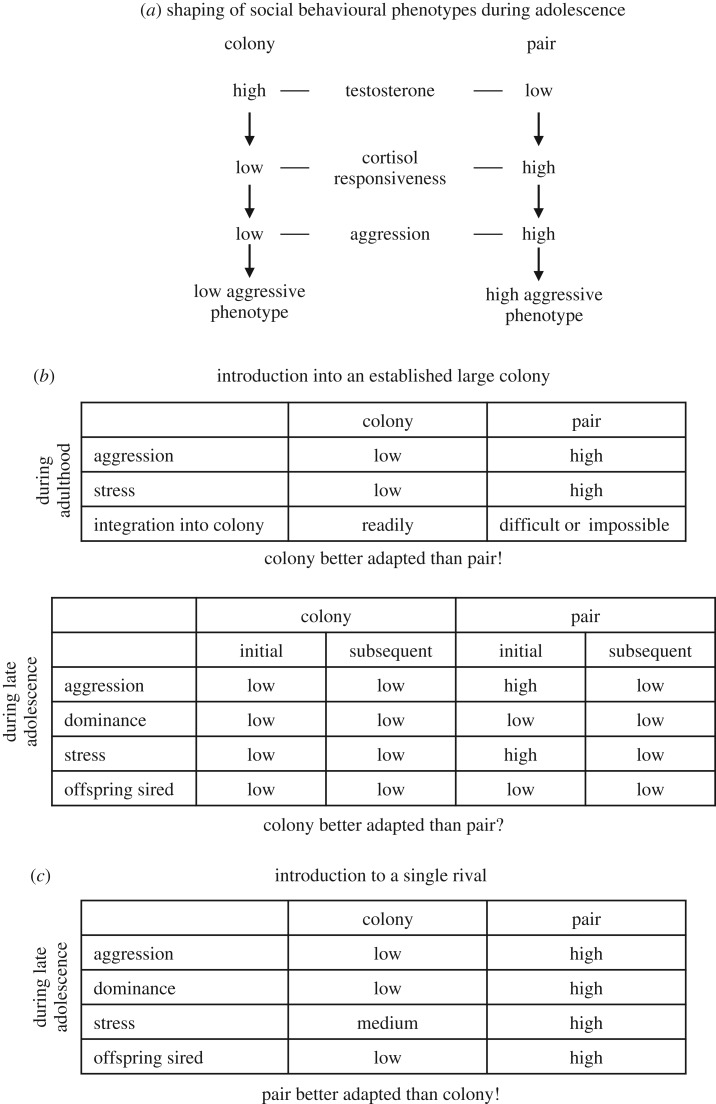
A particularly well-studied instance of the adolescent social environment shaping behaviour occurs in the guinea pig. Males reaching sexual maturity in large, mixed age/sex colonies show little interest in challenging adult breeding males for access to females. Rather, they continue to act much like prepubertal males until, months later, they have grown to a size at which they have a reasonable chance of prevailing in confrontations with the male breeders [25]. This is not a matter of sexual maturation of adolescents being halted or suppressed in some way. In fact, plasma testosterone levels are higher in adolescent males interacting with older conspecifics than in similar-aged males housed only with a like-age female [26,27]. Furthermore, there is strong evidence the heightened testosterone of these socially stimulated adolescent males suppresses their plasma cortisol levels [28], which in turn, appears to suppress their aggression toward other males (figure 1a; [2]). The adolescents then learn social strategies to negotiate interactions with older animals. Adult males that have lived during adolescence in the large colonies can peacefully integrate into new colonies, whereas adult males housed with a single female during adolescence are highly aggressive with unfamiliar males [31]. Unlike colony-raised adult males, adults lacking experience with older animals during adolescence exhibit high levels of stress when introduced to a new colony, as indicated by prolonged elevations of plasma cortisol levels and rapid reductions in body weight (figure 1b; [32]).
Figure 1.
Summary of findings on the adaptive shaping of the social behavioural phenotype during adolescence in male guinea pigs. (a) Neuroendocrine pathways: variation in the adolescent social environment—males living in large mixed/sexed colonies or in pairs of one male and one female—triggers different neuroendocrine responses that shape either low or high aggressive phenotypes (review: [2]). (b) Outcome when males previously living in pairs or colonies are introduced into established large colonies. During adulthood, males previously living in colonies show low levels of aggression and stress and integrate readily. In contrast, adult males previously living in pairs display the opposite pattern in this situation and integration into colonies is difficult or impossible (review: [2]). During late adolescence, males previously living in colonies display low levels of aggression and stress. In contrast, males previously living in pairs respond initially with high aggressiveness and stress. After about a week, these differences disappear and males previously living in pairs and colonies no longer differ. Due to their relatively young age, both classes of males achieve only low dominance ranks and sire few offspring [29]. (c) Outcome when a male previously living in a colony encounters a male previously living in a pair in the presence of two unfamiliar females during late adolescence. Males previously living in pairs are more aggressive and show higher cortisol stress responses. They more frequently become dominant and sire significantly more offspring [30].
3. Adaptiveness of behavioural change
There are different ways in which these guinea pig findings might be interpreted. The results for the two rearing groups could represent either an example of a ‘silver spoon effect’, in which colony-housed males would exhibit adaptive benefits over pair-housed males across adult environments, or as an example of adaptive shaping of the behaviour of the colony-housed males during adolescence to fit their current and future adult environment. That is, the adult colony ‘matched’ the experience of the males housed in colonies during adolescence but not that of males spending adolescence in male–female pairs. Although the matching interpretation is appealing, it is not supported unless the pair-housed males would be found to be more adapted than colony-housed males to a later environment forecasted by pair-housing during adolescence.
It was clear during fieldwork by the first author and colleagues that the population density of the ancestral species of the domestic guinea pig—the wild cavy—undergoes substantial population fluctuations from year to year. When animal numbers are high, the social organization is much like that in large colonies of domestic guinea pigs living in spacious enclosures. That is, dominant males maintain small harems of females and other males adopt a queuing strategy, in which they refrain from challenging dominant males they are unlikely to supplant [25,33,34]. When population density is low, male–female pairs appear to predominate [34,35]. It may be that under these low-density conditions, heightened aggression, such as that shown by male domestic guinea pigs housed in male–female pairs, may be the most effective course of action to ensure breeding success.
To test this hypothesis, males born in the large colonies were moved during early adolescence to either a different large colony or to an enclosure with a single female of comparable age [30]. Then in late adolescence—after males had reached sexual maturity—pairs of one colony-housed and one pair-housed male were transferred to an enclosure together with two reproductively mature females. As expected, pair-housed males directed more aggression towards the male competitor and more often achieved dominance. The males pair-housed during adolescence also directed more courtship and sexual behaviour toward females and, in turn, were more often approached by the females. Of particular importance from a fitness perspective, the previously pair-housed males sired 80% of resulting offspring (figure 1c).
In sum, the above findings provide strong evidence for the adaptive shaping of social behaviour during adolescence. Males encountering cues during adolescence that predict high or low population density shape their behaviour accordingly—either, in high-density conditions, avoiding agonistic interactions they are unlikely to win, and waiting until opportunities to achieve dominance and breed eventually arise or, in low-density conditions, aggressively challenging lone rivals in an apparent ‘early-reproduction’ strategy. These differences in behaviour appear to spring directly from a pattern of endocrine responses that are both specific to adolescence and induced by the social conditions encountered: interactions with adult animals that adolescents are likely to have only in dense population conditions elevate plasma levels of testosterone, which then suppresses the cortisol response, which inhibits the aggression that then allows these animals to successfully negotiate the challenges posed by the dense population (figure 1a; [2]).
Nonetheless, these studies also leave questions unanswered. In the older, original studies examining integration into colonies, the prolonged cortisol elevation and drop in weight indicated adult males spending adolescence in male–female pairs perform less well in this environment. Yet, unlike Zimmerman et al. [30], no measure of paternity was included to more-directly assess fitness. Additionally, while the older work (e.g. [32]) tested outcomes in fully adult animals (approx. 7–9 months), Zimmerman et al. [30] tested post-pubertal adolescents (approx. 4–5 months). Therefore in light of recommendations [36] that predictive adaptive responses be tested in full factorial designs in which animals that differ only in the salient earlier experience are tested in both environments (i.e. those presumably predicted by the two sets of experience), an additional study was conducted [29]. Guinea pigs were of the same age and had experienced the same rearing conditions as in the previous study [30], but rather than testing them in pairs with two breeding females, animals of both conditions were introduced in pairs to large, mixed age/sex colonies. For the first 3 days, results conformed to expectations. Previously pair-housed males were more aggressive, had higher cortisol levels, lost more body weight, and tended to show more courtship/sexual behaviour than did males that had been in a colony during adolescence. However, beginning after about a week, these differences disappeared as previously pair-housed males seemed to switch to the queuing strategy of the previously colony-housed males. Paternity results were inconclusive as few animals of either group sired pups during the four months paternities were traced following rehousing (figure 1b).
While unexpected, these results make the interesting point that a mating strategy shaped by adolescent experience can be reversible. That is, the strategy of males pair-housed during adolescence appeared to be ‘reshaped’ when the environment encountered did not match that which was predicted. Does this imply a silver spoon effect favouring males pair-housed in adolescence? It appears not. Though these males were better adapted to tests of low population density conditions, they appeared no more adapted to colony housing than were males raised in colonies. Moreover, reshaping involved potential costs, including those related to the initially elevated cortisol response, reduced body weight, any injuries from aggressive encounters, and the process of reshaping itself. Further, we would argue that the ability to reshape in this manner is likely a function of age. Plasma cortisol elevations and body weight losses resolved more rapidly in the adolescents lacking colony experience earlier in adolescence [29] than in the adults lacking adolescent colony experience [32,37]. Moreover, it appeared that the aggression elicited by the adolescents was less intense. In early studies with adult males housed in male–female pairs during adolescence, tests frequently had to be terminated after several days to prevent injury [37]. We propose that by adulthood an aggressive strategy may be so canalized and well developed that it is largely impervious to modification; whereas strategies are still malleable in late adolescence, and reshaping remains an option (see figure 1 for summary of results).
How generalizable are these results to other vertebrate species? We are aware of no similar studies in other mammals. However, findings in zebra finches suggest comparable effects could exist in birds. As in guinea pigs, male zebra finches pair-housed during adolescence were more aggressive and showed more courtship in adulthood, and in tests with a single female, were more attractive to her [24]. When introduced to a group, the previously pair-housed males spent less time in proximity to group members and lost more weight [38]. Effects of pair-housing in adolescence could not be reversed with extensive adulthood group-living experience [39]. While fitness benefits remain to be determined, these results together with those in guinea pigs suggest that the phenomenon of adolescent shaping of adult social behaviour and strategies may be a common occurrence, but one that has not been widely investigated.
4. The nature of adaptive shaping during adolescence
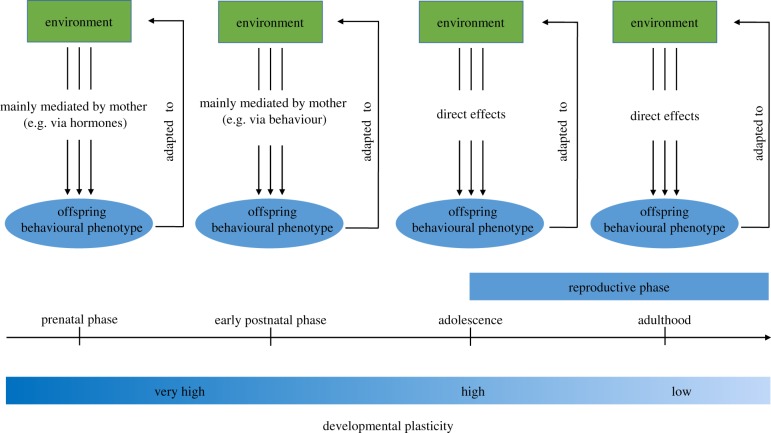
In principle, adaptive shaping of behavioural phenotypes can occur throughout the lifetime (figure 2), but what differentiates behavioural shaping during adolescence from shaping during other phases of life, and how frequently might we expect the shaping of social (and other) behavioural phenotypes during adolescence to occur? Environmental cues act upon the organism from insemination of the ova until death. During the prenatal phase, however, environmental influences are mediated by the mother. In mammals, the mother responds with hormonal changes to environmental stimuli, and hormones cross, at least in part, the placenta to affect and shape fetal brain development and behaviour in later life [9]. After birth, environmental cues directly act upon the offspring. Yet, in many species offspring still depend on their mothers, and it is primarily the mothers that continue to shape offspring brain development and behavioural phenotype, principally through maternal behaviour. In this manner, differences in the environments experienced by mothers are further translated into phenotypic variation in the offspring [2]. Irrespective of whether induced by hormones prenatally or by maternal behaviour postnatally, maternal effects have a fundamental impact on the offspring behavioural phenotype because brain development is highly plastic during these early phases of life. It is frequently argued that maternal effects act to increase offspring fitness and, indeed, there are examples supporting this notion. However, Marshall & Uller [40] have made the important point that natural selection typically acts on maternal effects to maximize maternal, rather than or in addition to offspring, fitness; some maternal effects may even decrease offspring fitness. Indeed much of the information about the environment is mediated by the mother who does not always have the same interests as the offspring. Thus offspring are not necessarily adjusted to the current or future environment in an optimal manner through maternal effects.
Figure 2.
Adaptive shaping of the social behavioural phenotype over the lifespan. The social behavioural phenotype is shaped by the social environment to be adapted to its predicted surroundings. During the prenatal and early postnatal phases environmental influences are mainly mediated by the mother, while during adolescence and adulthood environmental cues act directly upon the individual. Developmental plasticity diminishes progressively from the prenatal phase to adulthood. Adolescence is a unique phase for adaptive shaping of the behavioural phenotype since it is closer to the time of reproduction than are earlier phases of life, environmental information is obtained directly from the environment, and unlike later in adulthood, there is substantial developmental plasticity to support behavioural change. Please note: the environment may not necessarily be the same during the different developmental phases.
During adolescence, the situation is fundamentally different. Sexual maturity emerges and social interactions shift toward a reproductive context. Now, individuals are receiving information about the environment directly, rather than largely indirectly via parental effects, so that social behaviour can be shaped exclusively in the interests of the individual's mating success. Further ‘predictions’ of the future adult environment should be more accurate the closer to adulthood an individual is, which would favour precise adaptive shaping at this time. Due to brain maturation processes, one would expect the range of potential plastic responses to diminish from prenatal life to adolescence. However during adolescence there are substantial changes in neural differentiation, circuit reorganization, and other forms of plasticity in cortical and limbic brain regions which are favourable for long-term behaviour change [14,15]. Thus, adolescence might offer a renewed opportunity for adaptive canalization in a new direction.
In adulthood, just like during adolescence, environmental information is perceived directly by the individual and decisions related to reproductive success could be made even more accurately than during preceding phases. The potential for new adjustments are dependent, however, on the plasticity of the underlying neuronal and endocrine networks. This plasticity appears to greatly subside at this time suggesting that the costs of maintaining developmental plasticity outweigh the benefits. Thus adjustments of the behavioural phenotype become much more bounded by the organisms' activational plasticity. From this line of reasoning one would expect adaptive shaping of the social behavioural phenotype to be a widespread phenomenon during adolescence, but not adulthood. Still, we should not expect adolescent shaping of social behaviour to occur in all species or all environments.
5. Conditions favouring adolescent shaping of adult social behaviour
To begin with, we would expect the degree of adolescent shaping of fitness-relevant social behaviour to increase with the level of socialization of the species in question and the variation in socially relevant cues. For instance, for solitary species in which males and females only interact briefly at the time of mating (e.g. tigers, European hamsters), there is little social behaviour to modify other than mating behaviour itself. Under these conditions, the costs of plasticity are likely to outweigh any benefit plasticity may provide. In contrast, in highly social species (e.g. various primates), effective social engagement is necessary for essentially all life-sustaining and reproductive activities, from predator avoidance to securing a mate, and social interactions are often among the most complex of activities that individuals must negotiate. In this case, proper adjustment of social behaviour is so critical that the benefit of plasticity would be at a premium.
With all else being equal, we would also expect adolescent social plasticity to be greater for species with a longer developmental period. The greater the time span between shaping of behaviour in the early phases of life and the age at mating (and, therefore, the less accurate predictions about the mating environment are likely to be), the more valuable would be later adjustments of that shaping as the individual approaches adulthood. Further, some degree of change in the environment is necessary to make plasticity an effective strategy, but only if that change can be reasonably predicted during the adolescent state, i.e. there must be a changing environment and informative cues of the change that is to occur [18]. Particularly germane for adolescent plasticity are the major changes in the physical and social environment that can occur during adolescence. If an adolescent emigrates to an environment differing in crucial characteristics from that encountered (and predicted) in the prenatal and early postnatal phases, adolescence may be the only opportunity to make accurate behavioural adjustments. These effects may even be greater and more common for unpredicted social changes—which can directly impact mating opportunities—than for unpredicted differences in physical surroundings (e.g. changes in terrain).
Although we have not directly addressed sex differences in adolescent social plasticity, the predictions above have implications for how plasticity for the two sexes would be expected to differ. For instance, in species in which only one sex, typically females, breed immediately upon reaching sexual maturity, little adolescent social plasticity would be expected for that sex, both because the time span between shaping in the early phases of life and the time of mating would be shorter than for the other sex, typically males, but also because of ‘time-lag’ constraints on plasticity—the developmental changes may not have time to occur before they are needed. Likewise, when only members of one sex emigrate at adolescence, we would expect plasticity to be more advantageous for the sex that leaves the natal environment.
We have focused on mammals and, to a small extent, on birds. Yet, complex and varied social behaviour can be seen in other taxa as well. For instance, lizards display an assortment of social organizations and mating patterns, and, as in the case of the wild cavy, patterns can vary with population density [41]. In such cases, we would predict the same principles articulated here to hold, provided there is an adolescent, or adolescent-like, period with underlying neural and/or endocrine flexibility to permit developmental behavioural plasticity to occur.
Data accessibility
This article does not have any additional data.
Authors' contributions
All authors contributed equally to the conceptual design and writing of this paper. All authors gave final approval for publication.
Competing interests
We declare we have no competing interest.
Funding
The writing of this paper was supported by the German Research Foundation (DFG) as part of the SFB TRR 212 (NC3). The second author was supported by a summer fellowship from the Muenster Graduate School of Evolution.
References
- 1.Freund J, Brandmaier AM, Lewejohann L, Kirste I, Kritzler M, Krüger A, Sachser N, Lindenberger U, Kempermann G. 2013. Emergence of individuality in genetically identical mice. Science 340, 756–759. ( 10.1126/science.1235294) [DOI] [PubMed] [Google Scholar]
- 2.Sachser N, Kaiser S, Hennessy MB. 2013. Behavioural profiles are shaped by social experience: when, how and why. Phil. Trans. R. Soc. B 368, 20120344 ( 10.1098/rstb.2012.0344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trillmich F, Müller T, Müller C. 2018. Understanding the evolution of personality requires the study of mechanisms behind the development and life history of personality traits. Biol. Lett. 14, 20170740 ( 10.1098/rsbl.2017.0740) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pigliucci M. 2001. Phenotypic plasticity: beyond nature and nurture. Baltimore, MD: John Hopkins University Press. [Google Scholar]
- 5.West-Eberhard M. 2003. Developmental plasticity and evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 6.Snell-Rood EC. 2013. An overview of the evolutionary causes and consequences of behavioural plasticity. Anim. Behav. 85, 1004–1011. ( 10.1016/j.anbehav.2012.12.031) [DOI] [Google Scholar]
- 7.Mousseau TA, Fox CW. 1998. Maternal effects as adaptations. Oxford, UK: Oxford University Press. [Google Scholar]
- 8.Meaney MJ. 2001. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu. Rev. Neurosci. 24, 1161–1192. ( 10.1146/annurev.neuro.24.1.1161) [DOI] [PubMed] [Google Scholar]
- 9.Kaiser S, Sachser N. 2005. The effects of prenatal social stress on behavior: mechanisms and function. Neurosci. Biobehav. Rev. 29, 283–294. ( 10.1016/j.neubiorev.2004.09.015) [DOI] [PubMed] [Google Scholar]
- 10.Sachser N, Hennessy MB, Kaiser S. 2011. Adaptive modulation of behavioural profiles by social stress during early phases of life and adolescence. Neurosci. Biobehav. Rev. 35, 1518–1533. ( 10.1016/j.neubiorev.2010.09.002) [DOI] [PubMed] [Google Scholar]
- 11.Bateson P, Gluckman P, Hanson M. 2014. The biology of developmental plasticity and the predictive adaptive response hypothesis. J. Physiol. 592, 2357–2368. ( 10.1113/jphysiol.2014.271460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monaghan P. 2008. Early growth conditions, phenotypic development and environmental change. Phil. Trans. R. Soc. B 363, 1635–1645. ( 10.1098/rstb.2007.0011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buwalda B, Geerdink M, Vidal J, Koolhaas JM. 2011. Social behavior and social stress during adolescence: a focus on animal models. Neurosci. Biobehav. Rev. 35, 1713–1721. ( 10.1016/j.neubiorev.2010.10.004) [DOI] [PubMed] [Google Scholar]
- 14.Eiland L, Romeo RD. 2013. Stress and the developing adolescent brain. Neuroscience 249, 162–171. ( 10.1016/j.neuroscience.2012.10.048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patwell SS, et al. 2016. Dynamic changes in neural circuitry during adolescence are associated with persistent attenuation of fear memories. Nat. Commun. 7, 11475 ( 10.1038/ncomms11475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pierkarski DJ, Johnson C, Boivin JR, Thomas AW, Lin WC, Delevich K, Galarce E, Wilbrecht L. 2017. Does puberty mark a transition in sensitive periods for plasticity in the associative neocortex? Brain Res. 1654, 123–144. ( 10.1016/j.brainres.2016.08.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blaustein JD, Ismail N. 2013. Enduring influence of pubertal stressors on behavioral response to hormones in female mice. Horm. Behav. 64, 390–398. ( 10.1016/j.yhbeh.2013.01.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fawcett TW, Frankenhuis WE. 2015. Adaptive explanations for sensitive windows in development. Front. Zool. 12, S3 ( 10.1186/1742-9994-12-S1-S3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vidal J, de Bie J, Granneman RA, Wallenga AE, Koolhaas JM, Buwalda B. 2007. Social stress during adolescence in Wistar rats induces social anxiety in adulthood without affecting brain monoaminergic content and activity. Physiol. Behav. 92, 824–830. ( 10.1016/j.physbeh.2007.06.004) [DOI] [PubMed] [Google Scholar]
- 20.McCormick C, Hodges TE, Simone JJ. 2015. Peer pressures: social instability stress in adolescence and social deficits in adulthood in a rodent model. Dev. Cogn. Neurosci. 11, 2–11. ( 10.1016/j.dcn.2014.04.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buwalda B, Stubbendorff C, Zickert N, Koolhaas JM. 2013. Adolescent social stress does not necessarily lead to a compromised adaptive capacity during adulthood: a study on the consequences of social stress in rats. Neuroscience 249, 258–270. ( 10.1016/j.neuroscience.2012.12.050) [DOI] [PubMed] [Google Scholar]
- 22.Delville Y, David JT, Taravosh-Lahn K, Wommack JC. 2003. Stress and the development of agonistic behavior in golden hamsters. Horm. Behav. 44, 263–270. ( 10.1016/S0018-506X(03)00130-2) [DOI] [PubMed] [Google Scholar]
- 23.Meyer N, Jenikejew J, Richter SH, Kaiser S, Sachser N. 2017. Social experiences during adolescence affect anxiety-like behavior but not aggressiveness in male mice. Behav. Brain Res. 326, 147–153. ( 10.1016/j.bbr.2017.03.017) [DOI] [PubMed] [Google Scholar]
- 24.Ruploh T, Bischof HJ, von Engelhardt N. 2013. Adolescent social environment shapes sexual and aggressive behaviour of adult male zebra finches (Taeniopygia guttata). Behav. Ecol. Sociobiol. 67, 175–184. ( 10.1007/s00265-012-1436-y) [DOI] [Google Scholar]
- 25.Sachser N. 1986. Different forms of social organization at high and low population densities in guinea pigs. Behaviour 97, 253–272. ( 10.1163/156853986X00630) [DOI] [Google Scholar]
- 26.Lürzel S, Kaiser S, Sachser N. 2010. Social interaction, testosterone, and stress responsiveness during adolescence. Physiol. Behav. 99, 40–46. ( 10.1016/j.physbeh.2009.10.005) [DOI] [PubMed] [Google Scholar]
- 27.Lürzel S, Kaiser S, Sachser N. 2011. Social interaction decreases stress responsiveness during adolescence. Psychoneuroendocrinology 36, 1370–1377. ( 10.1016/j.psyneuen.2011.03.010) [DOI] [PubMed] [Google Scholar]
- 28.Lürzel S, Kaiser S, Sachser N. 2011. Inhibiting influence of testosterone on stress responsiveness during adolescence. Horm. Behav. 60, 691–698. ( 10.1016/j.yhbeh.2011.09.007) [DOI] [PubMed] [Google Scholar]
- 29.Zimmermann TD, Kaiser S, Sachser N. 2017. The adaptiveness of a queuing strategy shaped by social experiences during adolescence. Physiol. Behav. 181, 29–37. ( 10.1016/j.physbeh.2017.08.025) [DOI] [PubMed] [Google Scholar]
- 30.Zimmermann TD, Kaiser S, Hennessy MB, Sachser N. 2017. Adaptive shaping of the behavioural and neuroendocrine phenotype during adolescence. Proc. R. Soc. B 284, 20162784 ( 10.1098/rspb.2016.2784) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sachser N. 1998. Of domestic and wild guinea pigs: studies in sociophysiology, domestication, and social evolution. Naturwissenschaften 85, 307–317. ( 10.1007/s001140050507) [DOI] [PubMed] [Google Scholar]
- 32.Sachser N, Renninger S-V. 1993. Coping with new social situations: the role of social rearing in guinea pigs. Ethol. Ecol. Evol. 4, 1–19. ( 10.1080/08927014.1993.9523114) [DOI] [Google Scholar]
- 33.Asher M, Lippmann T, Epplen JT, Kraus C, Trillmich F, Sachser N. 2008. Large males dominate: ecology, social organization, and mating system of wild cavies, the ancestors of the guinea pig. Behav. Ecol. Sociobiol. 62, 1509–1521. ( 10.1007/s00265-008-0580-x) [DOI] [Google Scholar]
- 34.Adrian O, Sachser N. 2011. Diversity of social and mating systems in cavies: a review. J. Mammal. 92, 39–53. (doi.10.1644/09-MAMM-S-405.1) [Google Scholar]
- 35.Asher M, Spinelli de Oliveira E, Sachser N. 2004. Social system and spatial organization of wild guinea pigs (Cavia aperea) in a natural population. J. Mammal. 85, 788–796. ( 10.1644/BNS-012) [DOI] [Google Scholar]
- 36.Groothuis TGG, Taborsky B. 2015. Introducing biological realism into the study of developmental plasticity in behaviour. Front. Zool. 12, S6 ( 10.1186/1742-9994-12-S1-S6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sachser N. 1991. Sozialphysiologische Untersuchungen an Hausmeerschweinchen. Habilitation. University of Bayreuth, Germany.
- 38.Ruploh T, Bischof HJ, von Engelhardt N. 2014. Social experience during adolescence influences how male zebra finches (Taeniopygia guttata) group with conspecifics. Behav. Ecol. Sociobiol. 68, 537–549. ( 10.1007/s00265-013-1668-5) [DOI] [Google Scholar]
- 39.Ruploh T, Bischof HJ, von Engelhardt N. 2015. Effects of social conditions during adolescence on courtship and aggressive behaviour are not abolished by adult social experience. Dev. Psychobiol. 57, 73–82. ( 10.1002/dev.21262) [DOI] [PubMed] [Google Scholar]
- 40.Marshall DJ, Uller T. 2007. When is a maternal effect adaptive? Oikos 116, 1957–1963. ( 10.1111/j.2007.0030-1299.16203.x) [DOI] [Google Scholar]
- 41.Brattstrom BH. 1974. The evolution of reptilian social behavior. Am. Zool. 14, 35–49. ( 10.1093/icb/14.1.35) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article does not have any additional data.