Abstract
Waterfowl undergo an annual simultaneous flight-feather moult that renders them flightless for the duration of the regrowth of the flight feathers. In the wild, this period of flightlessness could restrict the capacity of moulting birds to forage and escape predation. Selection might therefore favour a short moult, but feather growth is constrained and presumably energetically demanding. We therefore tested the hypothesis that for birds that undergo a simultaneous flight-feather moult, this would be the period in the annual cycle with the highest minimum daily heart rates, reflecting these increased energetic demands. Implantable heart rate data loggers were used to record year-round heart rate in six wild barnacle geese (Branta leucopsis), a species that undergoes a simultaneous flight-feather moult. The mean minimum daily heart rate was calculated for each individual bird over an 11-month period, and the annual cycle was divided into seasons based on the life-history of the birds. Mean minimum daily heart rate varied significantly between seasons and was significantly elevated during wing moult, to 200 ± 32 beats min−1, compared to all other seasons of the annual cycle, including both the spring and autumn migrations. The increase in minimum daily heart rate during moult is likely due to feather synthesis, thermoregulation and the reallocation of minerals and protein.
Keywords: avian, energetics, metabolic rate
1. Introduction
Bird flight feathers are continually exposed to a range of degrading factors, such as mechanical fatigue, UV light and parasites [1,2]. As feathers cannot be repaired once growth is completed, flight feathers are typically replaced once a year, during an annual flight-feather moult [3]. The timing and nature of flight-feather moult is a key component of energy allocation during the annual cycle in most birds, and typically takes place either in a sequential fashion (a number of flight feathers at a time) or simultaneously (all flight feathers at once) [3,4]. Given that feather growth rate is constrained [3] (negative allometry of feather growth rate with avian body mass), the only way to quicken the moult process is through increasing the number of flight feathers growing simultaneously, with some very heavy species (such as geese) becoming flightless during moult [4].
During the flightless period of moult, both wild and captive waterfowl undergo extensive changes to their physiology and behaviour [5–12]. These changes include a significant drop in body mass [5,6] and atrophy of the major flight muscles [7,11], with birds often becoming less active, switching to nocturnal feeding, and devoting less time to foraging and preening [7–9,12]. These changes in physiology and behaviour are thought to be in response to the energetic demands of moult, coupled with the increased risk of being predated upon while flightless [2,6,12]. Barnacle geese (Branta leucopsis) are a typical waterfowl species that experience an annual simultaneous flight-feather moult. As archetypal Northern Hemisphere Arctic migrants, barnacle geese spend the summer breeding in the Arctic circle, before migrating south to Western Europe for the winter [13]. Their annual flight-feather moult follows the reproductive period and immediately precedes their autumn migration, thus linking three potentially energetically demanding events close together in the annual cycle.
Measurements of basal metabolic rate (BMR) in captive barnacle geese reveal that BMR in moulting birds is approximately 100% and approximately 55% higher than in non-moulting birds measured in the winter and early summer periods, respectively [6], suggesting, at least in captive birds measured under basal conditions, that moult is an energetically demanding period of the annual cycle. However, long-term and continuous data on energy costs in wild birds that undergo a simultaneous flight-feather moult are lacking, and how simultaneous moult integrates into the annual cycle is poorly understood from a physiological perspective. Since long-term continuous estimates of energy expenditure are difficult to obtain, we aimed to use measurements of heart rate as a qualitative proxy of the rate of energy expenditure [14]. We test the hypothesis that minimum daily heart rate (MDfH) will be significantly higher during the flight-feather moult in wild geese compared with other periods within the annual cycle.
2. Material and methods
(a). Birds and heart rate measurements
Eight wild barnacle geese (five males, three females) were caught at Ny-Ålesund research station on the island of Spitsbergen in the Svalbard archipelago (788550 N, 118560 E, 78.9178 N, 11.9338 E) during the flightless period of wing moult in July 1999. The population of barnacle geese that breed at Ny-Ålesund winter on the Solway Firth (Scotland, 54.981253, −3.489971), and migrate along the Norwegian coast during their annual spring and autumn migrations [13]. Heart rate was used as a qualitative proxy for metabolic rate [14] and was measured continuously throughout the annual cycle by custom-made implantable heart rate data loggers [15,16]. The loggers were programmed to record heart rate (fH) every 5 s. Methods for logger implantation and removal are described in detail elsewhere [13,15]. All implanted geese were colour-ringed to aid recapture the following year. Recordings of fH for a minimum of 10 months were obtained from all six birds (three males, three females) that were recaptured in the summer of 2000; the remaining two birds were sighted but recapture was not possible. Previously, we demonstrated that minimum daily heart rate is correlated with mean daily heart rate, and that minimum daily oxygen consumption is correlated with mean daily oxygen consumption [17], thus an increase in heart rate can be assumed to reflect an increase in daily energy expenditure.
(b). Heart rate data
A custom-written program (QBasic, Microsoft) processed fH data for each goose to extract mean fH values for each 15-min period for the 24 h of each day and the lowest of these was taken as the minimum daily heart rate MDfH [15,16]. The annual cycle was divided into five distinct seasons (figure 1) based on the fH values and prior classifications of barnacle geese behaviour and movements (see [15] for full details): (i) winter, (ii) spring migration, (iii) breeding, (iv) wing moult and (v) autumn migration. The effect of season on MDfH was analysed using a linear mixed model implemented in the nlme package of R v. 3.4.0 [18,19], with season (winter, spring, breeding, moult, autumn) as a fixed factor, individual identity as a random factor and an order 1 temporal autocorrelation structure, with α set at 0.05. Data for fH were log10-transformed for analysis. Subsequent post hoc comparisons between seasons were undertaken using the glht function of the multicomp package v. 1.4–8 [18,19]. Daily means are presented as mean ± s.e.m. Seasonal means are presented as mean ± s.e.m. calculated as the grand mean of individual means in the text and also plotted as violin plots [20] so that the complete data distribution can be visualized.
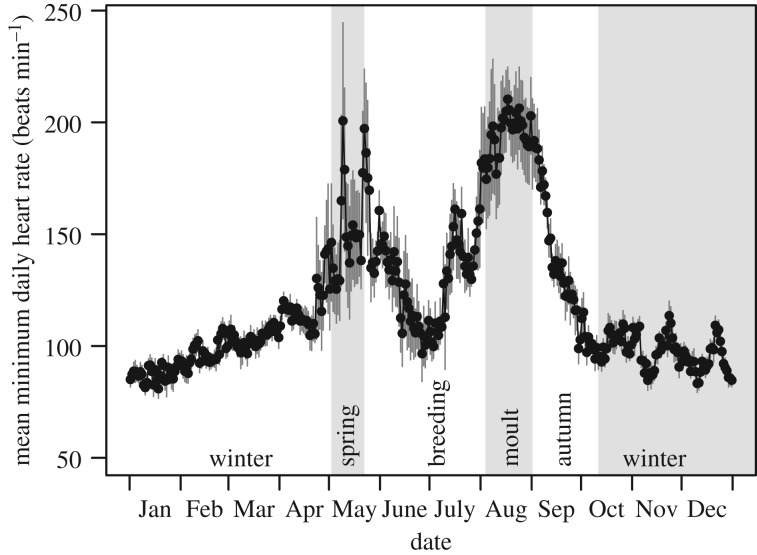
Figure 1.
Mean (± s.e.m.) daily minimum heart rate per day throughout the annual cycle of six wild barnacle geese. The year is split into five distinct seasons: winter, spring migration, breeding, flight-feather moult and autumn migration. Mean minimum heart rate is significantly higher during flight-feather moult than other times of the annual cycle. See the electronic supplementary material, figure S1 for individual heart rate traces per bird.
3. Results
Minimum daily heart rate (MDfH) varied significantly between seasons throughout the annual cycle (figure 1; F4,1996 = 15 357, p < 0.0001). Minimum daily heart rate was significantly elevated during the wing moult period (200 ± 32 beats min−1) compared to all other periods in the annual cycle (figure 2). Breeding had a lower MDfH (133 ± 21 beats min−1) than moult, which is likely due to the six geese being failed breeders; although eggs were laid and incubated, they were not successfully hatched. The MDfH during spring (147 ± 39 beats min−1) and autumn (131 ± 20 beats min−1) migrations were not significantly different from each other, or from that of the breeding season (figure 2). Winter (99 ± 8 beats min−1) had the lowest MDfH which was significantly different from those of all seasons (figure 2).
Figure 2.
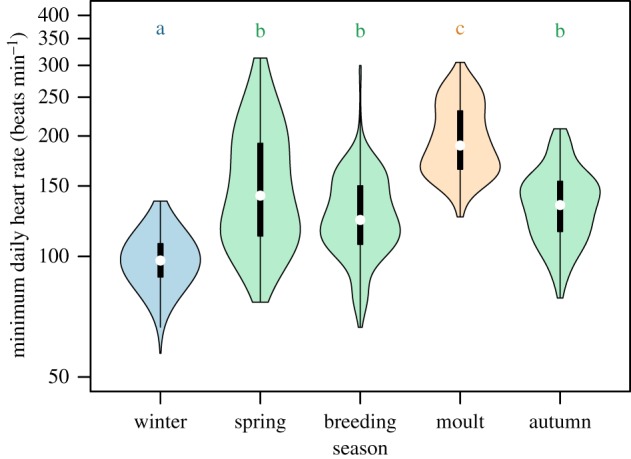
Seasonal differences in minimum heart rate (beats min−1) for six wild barnacle geese. The white dots present the median, the thick vertical bars represent the interquartile range, and the thin vertical lines extend to either 1.5 times the interquartile range (winter, breeding) or the full data range (all other seasons). The density plot width of the violin area presents the frequency distribution. The annual cycle is divided into five seasons: winter, spring migration (spring), breeding, wing moult (moult) and the autumn migration (autumn). Different colours of the violin plot area represent significant differences in minimum daily heart rate between seasons (also categorized by the letters above). (Online version in colour.)
4. Discussion
Minimum daily heart rate (MDfH) was significantly elevated during the annual flight-feather moult of the barnacle geese. Heart rate was approximately 100% higher during moult in comparison to the winter period, and approximately 35% higher than that during the spring migration. This finding corroborates previous observations of captive barnacle geese, where approximately 100% increases in metabolic rates were recorded during moult [7]. Studies with non-simultaneous moulting species showed increases in the metabolic rate of 30% and 58% for common kestrels (Falco tinnunculus) [21] and white-crowned sparrows Zonotrichia leucophrys gambelii [22], respectively, suggesting moult in barnacle geese is more energetically demanding, on a daily basis at least, for this species.
Increased MDfH during moult is presumed to be a result of higher nutrient demands for feather components, augmented amino acid metabolism, changes in water balance, an increase in blood volume and enhanced heat loss [6,22]. Wild moulting common eiders (Somateria mollisima) experienced a daily metabolic rate increase of 12% between the pre-moult and flightless period of moult [23], while increases in metabolic rate of 25% and 35% were recorded in captive common teals (Anas crecca) and northern shovelers (Anas clypeata), respectively, during flight-feather moult [24]; values more similar to those species which undergo a sequential moult [21,22]. Owing to their comparatively larger body size, the pressure to moult quickly [25] may be exacerbated in barnacle geese, while also impairing thermoregulatory function to a greater extent compared with species which moult sequentially, particularly if they are having to rest on water [26]. Flight-feather moult for geese may be costly due to the decrease in foraging [7,8,9] coupled with the constant rate at which feathers grow [3]; the reduction in foraging may result in the geese having to turn over more body protein over a 24-h period to meet the consistent demands of feather growth. Accompanying the regrowth of feathers during moult is a potential reallocation of protein from the flight muscles, which atrophy, to the leg muscles, which hypertrophy [7], suggesting protein availability might be a limiting factor during moult in geese.
It is unlikely that the increase in heart rate observed during flight-feather moult is associated with pre-migratory fattening [27] or increased activity, as both wild and captive barnacle geese lose body mass during the flight-feather moult period, and decrease foraging and general activity levels [6,7,27]. Notably, while individual migratory flights are energetically costly, the regular stops throughout migration [13,15,27] include long periods of rest, thus the MDfH during both migrations are lower than those during moult. Therefore, the flight-feather moult is the period of highest sustained elevated MDfH in the annual cycle, although the full costs of breeding, including any differences between males and females, could not be estimated due to the geese in the study being failed breeders. It is worth restating however, that individual migratory flights can last up to 15 h, with heart rates of over 300 beats min−1 recorded [13], and these flights are likely to be extremely energetically demanding.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Anthony Woakes for assistance with the biologgers and three reviewers for insightful comments.
Ethics
Work in Svalbard was undertaken with full permissions from the Governor of Svalbard, adhering to the Norwegian Animal Welfare Act.
Data accessibility
All data are available in the electronic supplementary material.
Authors' contributions
Conceptualization, design, methodology, writing, reviewing and editing, S.J.P., C.R.W., J.A.G. and P.J.B; resources, data collection, P.J.B.; formal analysis, C.R.W. All authors approve of the final manuscript submission and hold accountability for the accuracy and integrity of its contents.
Competing interests
We declare we have no competing interests.
Funding
Funding was provided by an NERC grant to P.J.B. C.R.W. is funded by the ARC.
References
- 1.Weber TP, Borgudd J, Hedenström A, Persson K, Sandberg G. 2005. Resistance of flight feathers to mechanical fatigues covaries with moult strategy in two warbler species. Biol. Lett. 1, 27–30. ( 10.1098/rsbl.2004.0244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gunderson AR, Frame AM, Swaddle JP, Forsyth MHJ. 2008. Resistance of melanized feathers to bacterial degradation: is it really so black and white? J. Avian Biol. 39, 539–545. ( 10.1111/j.0908-8857.2008.04413.x) [DOI] [Google Scholar]
- 3.Rohwer S, Ricklefs RE, Rohwer VG, Copple MM. 2009. Allometry of the duration of flight feather molt in birds. PLoS Biol. 7, e1000132 ( 10.1371/journal.pbio.1000132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woolfenden GE. 1967. Selection for a delayed simultaneous wing molt in loons (Gaviidae). Wilson Bull. 70, 416–420. [Google Scholar]
- 5.Kahlert J. 2006. Effects of feeding patterns on body mass loss in moulting greylag geese Anser anser. Bird Study 53, 20–31. ( 10.1080/00063650609461412) [DOI] [Google Scholar]
- 6.Portugal SJ, Green JA, Butler PJ. 2007. Annual changes in body mass and resting metabolism in captive barnacle geese: the importance of wing moult. J. Exp. Biol. 210, 1391–1397. ( 10.1242/jeb.004598) [DOI] [PubMed] [Google Scholar]
- 7.Portugal SJ, Thorpe SKS, Green JA, Myatt JP, Butler PJ. 2009. Testing the use/disuse hypothesis: pectoral and leg muscle changes in captive barnacle geese during wing moult. J. Exp. Biol. 212, 2403–2410. ( 10.1242/jeb.021774) [DOI] [PubMed] [Google Scholar]
- 8.Portugal SJ, Quinton K, Isaac R, Reynolds SJ. 2010. Do captive waterfowl respond to the flightless period of wing moult in the same manner as their wild counterparts? J. Ornithol. 151, 443–448. ( 10.1007/s10336-009-0474-3) [DOI] [Google Scholar]
- 9.Portugal SJ, Green JA, Piersma T, Eichhorn G, Butler PJ. 2011. Energy stores enable flightless moulting geese to maintain cryptic behaviour. Ibis 153, 868–874. ( 10.1111/j.1474-919X.2011.01167.x) [DOI] [Google Scholar]
- 10.Portugal SJ, Butler PJ, Green JA, Cassey P. 2011. Indications of phenotypic plasticity in moulting birds: captive geese reveal adaptive changes in mineralisation of their long bones during wing moult. J. Ornithol. 152, 1055–1061. ( 10.1007/s10336-011-0699-9) [DOI] [Google Scholar]
- 11.Piersma T. 1988. Breast muscle atrophy and constraints on foraging during the flightless period of wing moulting great crested grebes. Ardea 76, 96–106. [Google Scholar]
- 12.Kahlert J, Fox AD, Ettrup H. 1996. Nocturnal feeding in moulting Greylag Geese Anser anser—an anti-predator response? Ardea 84, 15–22. [Google Scholar]
- 13.Butler PJ, Woakes AJ, Bishop CM. 1998. Behaviour and physiology of Svalbard barnacle geese Branta leucopsis during their autumn migration. J. Avian. Biol. 29, 536–545. ( 10.2307/3677173) [DOI] [Google Scholar]
- 14.Green JA. 2011. The heart rate method for estimating metabolic rate: review and recommendations. Comp. Biochem. Physiol. 158, 287–304. ( 10.1016/j.cbpa.2010.09.011) [DOI] [PubMed] [Google Scholar]
- 15.Portugal SJ, Green JA, White CR, Guillemette M, Butler PJ. 2013. Wild geese do not increase flight behaviour prior to migration. Biol. Lett. 8, 469–472. ( 10.1098/rsbl.2011.0975) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White CR, Butler PJ, Grémillet D, Martin GR. 2008. Behavioural strategies of predation in cormorants Phalacrocoracidae foraging under challenging light conditions. Ibis 150, 231–239. ( 10.1111/j.1474-919X.2008.00837.x) [DOI] [Google Scholar]
- 17.Portugal S.J., et al. 2016. Associations between resting, activity and daily metabolic rate in free-living endotherms: no universal rule for birds and mammals. Physiol. Biochem. Zool. 89, 251–261. ( 10.1086/686322) [DOI] [PubMed] [Google Scholar]
- 18.Pinheiro JD, Bates S, DebRoy S, Sarkar D, R Core Team. 2017. nlme: Linear and nonlinear mixed effects models. R package version 3.1−131.
- 19.R Core Team. 2017. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 20.Adler D.2005. vioplot: Violin plot. R package version 0.2. http://wsopuppenkiste.wiso.uni-goettingen.de/~dadler .
- 21.Dietz MW, Daan S, Masman D. 1992. Requirements for molt in the kestrel Falco tinnunculus. Physiol. Zool. 65, 1217–1235. ( 10.1086/physzool.65.6.30158276) [DOI] [Google Scholar]
- 22.Murphy ME, King JR. 1992. Energy and nutrient use during molt by white-crowned sparrows Zonotrichia leucophrys gambelii. Ornis Scand. 23, 304–313. ( 10.2307/3676654) [DOI] [Google Scholar]
- 23.Guillemette M, Butler PJ. 2012. Seasonal variation in energy expenditure is not related to activity level or water temperature in a large diving bird. J. Exp. Biol. 215, 3161–3168. ( 10.1242/jeb.061119) [DOI] [PubMed] [Google Scholar]
- 24.Guozhen Q, Hongfa X. 1986. Moult and resting metabolic rate in the common teal Anas crecca and the shoveller Anas clypeata. Acta Zool. Sin. 32, 73–84. [Google Scholar]
- 25.Hedenstrom A. 2006. Scaling of migration and the annual cycle of birds. Ardea 94, 399–408. [Google Scholar]
- 26.de Vries J, van-Eerden MR. 1995. Thermal conductance in aquatic birds in relation to the degree of water contact, body mass and body fat: energetic implications of living in a strong cooling environment. Physiol. Zool. 68, 1143–1163. ( 10.1086/physzool.68.6.30163797) [DOI] [Google Scholar]
- 27.Owen M, Ogilvie MA. 1979. Wing moult and weights of barnacle geese in Spitsbergen. Condor 81, 42–52. ( 10.2307/1367855) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the electronic supplementary material.