Abstract
The factors contributing to the maintenance of sex over asexuality in natural populations remain unclear. Ecological divergences between sexual and asexual lineages could help to maintain reproductive polymorphisms, at least transiently, but the consequences of asexuality for the evolution of ecological niches are unknown. Here, we investigated how niche breadths change in transitions from sexual reproduction to asexuality. We used host plant ranges as a proxy to compare the realized feeding niche breadths of five independently derived asexual Timema stick insect species and their sexual relatives at both the species and population levels. Asexual species had systematically narrower realized niches than sexual species, though this pattern was not apparent at the population level. To investigate how the narrower realized niches of asexual species arise, we performed feeding experiments to estimate fundamental niche breadths but found no systematic differences between reproductive modes. The narrow realized niches found in asexual species are therefore probably a consequence of biotic interactions such as predation or competition, that constrain realized niche size in asexuals more strongly than in sexuals.
Keywords: degree of specialization, herbivorous insect, host plant range, realized versus fundamental niche, sexual versus asexual reproduction, Timema stick insects
1. Introduction
The maintenance of obligate sex in natural populations, despite numerous disadvantages compared to other reproductive systems, is a major evolutionary paradox. Although there is a rich body of theory proposing potential benefits of sex, empirical studies evaluating such benefits under natural conditions remain scarce [1]. A simple mechanism that could contribute to the maintenance of reproductive polymorphisms is niche differentiation between sexual and asexual species [2–7]. Such niche differentiation could result from a difference in ecological optima between sexuals and asexuals [3–5], or from situations where sexual species cover larger fractions of the available niche space than their asexual counterparts [6].
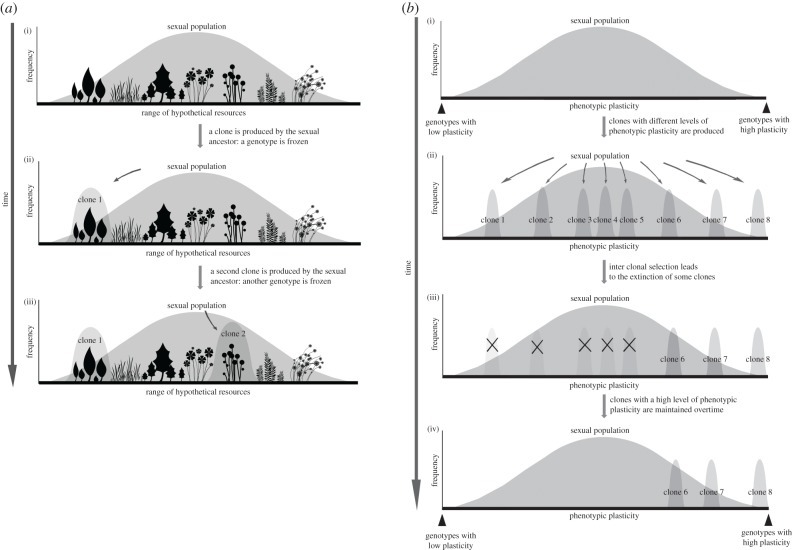
Because asexual species derive from sexual ancestors, fundamental niches (i.e. the range of environmental conditions that allow for survival, growth and reproduction) in new asexual species should depend directly upon the fundamental niche found in the ancestral sexual species. However, how the fundamental niche in an ancestral sexual population translates to that found in an asexual population is unclear. For example, the frozen niche variation (FNV) model predicts that the phenotypic distribution of a new, recently derived asexual would be narrower than that of its genetically variable sexual ancestor, because a single sexual genotype will be ‘frozen’ in the new asexual lineage [3,8–10]. This may result in different fundamental niche breadths between sexual and asexual species, with the new asexual species being more specialized and able to exploit fewer niches than the sexual species from which it is derived from ([8,11]; figure 1a). By contrast, the ‘general-purpose genotype’ (GPG) hypothesis [12–14] proposes that asexual lineages should generally have broader environmental tolerances than sexual individuals because of strong selection for plasticity in asexuals. Indeed, a temporally and spatially variable environment should favour, among all the independently derived asexual clones, those with the broadest environmental tolerances. Under this scenario, we would expect asexual populations to have broader ecological niches than sexual ones (figure 1b). The two hypotheses are non-mutually exclusive. For example, the FNV model can be applied to sexual genotypes with different levels of plasticity—a specific plasticity level will be ‘frozen’ in the new asexual lineage, depending on the sexual genotype it derives from [11]. Furthermore, by combining the FNV and GPG, we can suggest that young asexual lineages would feature, on average, narrow niches, while old ones would feature broad niches.
Figure 1.
(a) The frozen niche variation model. (i) A sexual population (broad curve) exhibits genetic variation for the niche use (here symbolized by a range of hypothetical plants); (ii) a new asexual clone is produced, comprising a subset of the genotypic diversity contained in its sexual ancestor; (iii) a second clone is produced from a different sexual genotype characterized by a different ecological niche. The niche breadth of the sexual population as a whole is larger than the one of each individual clone. Adapted from Vrijenhoek & Parker [11]. (b) General-purpose genotype. (i) Individuals in a sexual population vary in the range of their environmental tolerances (narrow to broad plasticity); (ii) clones are produced from different genotypes in the sexual population with different levels of plasticity; (iii,iv) natural selection favours clones with broader tolerances such that clones may feature higher levels of plasticity than the sexual population as a whole (e.g. extreme case of clone 5). Figure adapted from Vrijenhoek & Parker [11].
Regarding the breadth of the realized niche (i.e. the fraction of the fundamental niche used by organisms under natural conditions), there is currently no specific theory predicting similarities or differences between sexuals and asexuals. There are, however, several theories predicting that sex can accelerate the rate of adaptation compared to asexuality [15–18]. Sexual organisms therefore may be able to evolve adaptations to competitors, pathogens or predators more rapidly than asexuals. As a consequence, the realized niche in asexual organisms may be smaller than in sexual organisms owing to a reduced ability to respond to these biotic pressures.
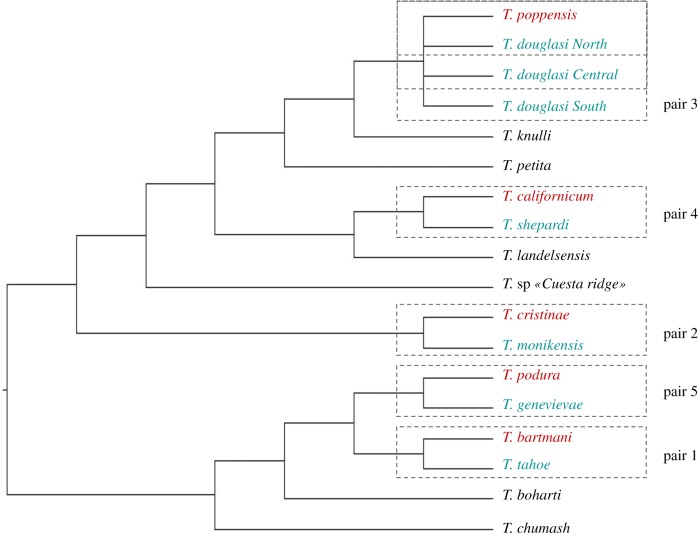
Here, we evaluate whether asexuality is associated with different niches and niche sizes than sexual reproduction, using herbivorous stick insects of the genus Timema as a model system and different host plants as a proxy for different niches. Seven independently derived asexual lineages have been identified in this genus, each with a closely related sexual counterpart ([19]; figure 2). This allows us to perform replicate comparisons between sexual and asexual lineages. Moreover, the asexual Timema lineages vary in age [19,22], allowing us to assess the possible consequences of asexuality on niche breadth over a range from recently derived to long-term asexuality.
Figure 2.
Timema species phylogeny. Phylogeny redrawn from Riesch et al. [20] with the seven asexual lineages added from Schwander et al. [19] (in blue). The used sexual species are labelled in red. Pair numbers correspond to the most recent (i.e. pair 1) to the most ancient (i.e. pair 5) transition to asexuality (ranking from Bast et al. [21]).
We first estimated the size of the realized feeding niches of sexuals and asexuals both at the species and at the population level in five sexual–asexual Timema sister species pairs, using occurrences on different host plants in natural populations. Timema feed on the leaves or the needles of very diverse host plants, comprising both angiosperms and conifers, and the quality of these plants as a food source is highly variable [23]. We then conducted feeding experiments with species from four sexual–asexual species pairs to estimate the size of their fundamental feeding niches. Finally, we evaluated the contribution of predation to shaping realized niches in sexuals and asexuals. Timema are characterized by different cryptic morphs on different host plants, both within and between species [24–26]. Previous studies have shown that the combination of selection imposed by predators and Timema host preference maintain a correlation between morph frequency and host plant frequency between populations [24,27,28], indicating that colour polymorphism and predation may be of key importance for realized niches in Timema.
2. Material and methods
(a). Realized feeding niche breadths
Different host plants are generally considered to reflect different ecological niches for herbivorous insects [29] and we therefore chose to study the host plants used by Timema under natural conditions as a proxy for their realized niches.
Data from a previous study that collected information on host plant use across all 23 known Timema species [23] allowed us to estimate the size of the realized feeding niche of sexuals and asexuals at the species level. To estimate the realized niche at the population level, we further performed a count of the number of individuals collected on each potential host plant across 30 populations from five species pairs (between two and six populations per species; electronic supplementary material, table S2). The size of the realized feeding niche per population was then quantified with the inversed Tau (τ) specialization index [30], which ranges from 0 (pure specialist) to 1 (complete generalist), calculated as follows:
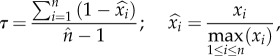 |
where n corresponds to the number of Timema host plants found in a given location, xi represents the frequency of occurrence on plant i and max (xi) is the highest frequency of occurrence for the focal population in this location.
(b). Degree of colour polymorphism
Realized feeding niches depend on multiple factors, including the fundamental niches as well as species interactions (notably predation and competition). Timema are characterized by different cryptic colour morphs and previous studies have shown that selection imposed by predators favours different colour morph sets on different plants [24,28,31]. To develop insights into the contribution of predators to the sizes of the realized feeding niches in Timema, we thus quantified colour polymorphism at the species and population levels.
Colour phenotypes vary broadly in several Timema species but can be separated into a total of 14 discrete morphs across all species (range 1–8 per species; electronic supplementary material, table S1). We recorded colour morph frequencies from all sampling locations (electronic supplementary material, table S2) and used the Simpson diversity index to quantify the level of polymorphism [32]. This index varies between 0 (here indicating colour monomorphism) and 1 (indicating the diversity of equally frequent colour morphs). We then estimated the correlation between the degree of colour polymorphism and the size of the realized feeding niche, both at the species and at the population levels with phylogenetic generalized least squares (PGLS) to account for phylogenetic non-independence among Timema species. These analyses were conducted using the ape [33] and nlme [34] R packages [35] using a Brownian motion model for trait evolution.
(c). Fundamental feeding niche breadths
To estimate the fundamental feeding niche breadths of sexual and asexual Timema species, we performed a feeding experiment and measured insect performance on different host plants. We chose seven plants known to be commonly used by several Timema species while trying to cover the phylogenetic diversity of the host plants [23]. Specifically, we chose four angiosperms: (Ceanothus thyrsiflorus (lilac, lil), Adenostoma fasciculatum (chamise, cha), Quercus agrifolia (oak) and Arctostaphylos glauca (manzanita, mz)), and three conifers: (Pseudotsuga menziesii (douglas fir, df), Abies concolor (white fir, wf) and Sequoia sempervirens (redwood, rdw)). Stick insects from eight Timema species (four sexual–asexual species pairs) were collected from multiple field sites in California (electronic supplementary material, table S3). We only used fourth-instar juvenile females for feeding experiments to minimize age-related effects on insect performance during our experiments. Between 10 and 20 such females were used per host plant for a total of 70–105 females per population (635 insects in total; electronic supplementary material, table S3). The females were installed individually in tubes closed with a net, each containing a fresh branch from one of the seven plants of the experiment, as described in [23], to measure survival and weight gain after 10 days.
We first used a generalized linear model (GLM) with a binomial error to compare survival and an ANOVA to compare the weight gain of all stick insects species on the different plants using R [35]. We then compared for each Timema species pair separately, the survival and weight gain of the sexual and asexual individuals, testing specifically for an interaction between reproductive mode and plant species because a significant interaction between these two factors would indicate a difference in fundamental feeding niche between sexuals and asexuals. Finally, we estimated the breadth of the fundamental feeding niche of the eight Timema species using again the inversed Tau index (calculated as described above; but where xi represents the survival or weight gain on plant i, and max (xi) represents the best survival or weight gain for a given Timema species). We could not compare the fundamental feeding niche of the Timema bartmani/Timema tahoe species pair because T. tahoe individuals of the appropriate developmental stage could not be collected in sufficient numbers for the feeding experiment.
3. Results
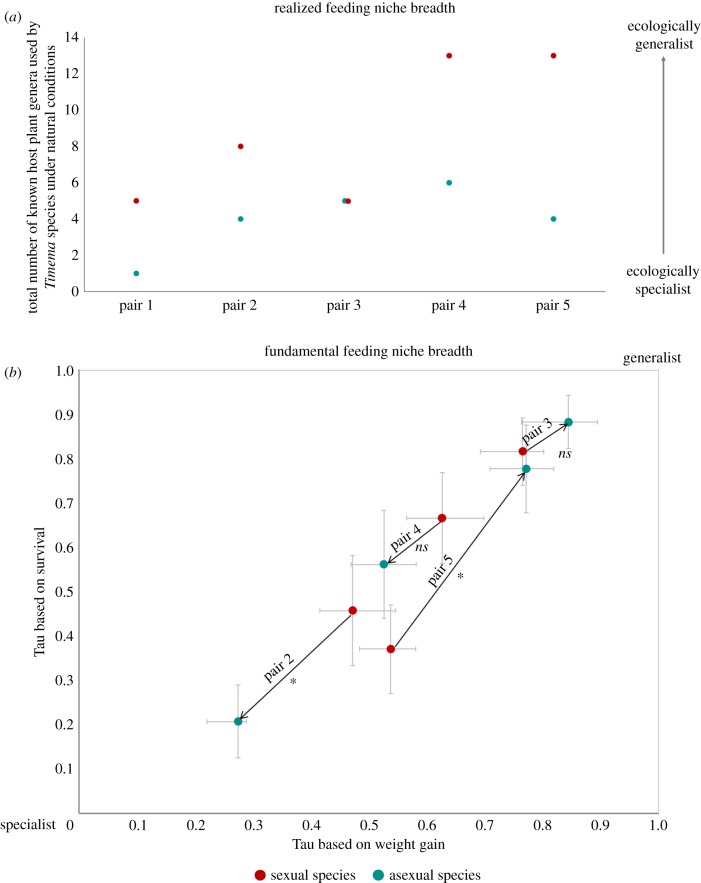
For realized niches measured at the species level, the sexuals are more ecologically generalist in four out of five cases, as they used at least twice as many different plant genera as their asexual relatives (figure 3a). In the remaining case (Timema poppensis/Timema douglasi), the sexual and the asexual species used the same number of host plants in the wild (figure 3a). For realized niches measured at the population level, all 10 species are specialized, feeding typically on one or two host plants species even when additional species are available (Tau indices varying between 0 and 0.48; electronic supplementary material, figure S1B). There were no significant differences in the degree of specialization between sexual and asexual populations (GLM; p-value = 0.19). However, we did find that (within species) sexual populations vary more than asexual ones in their degree of specialization (Levene's test, F1,27 = 12.2, p-value < 0.002; electronic supplementary material, figure S1B).
Figure 3.
Realized (a) and fundamental (b) feeding niche breadths of sexual and asexual Timema stick insects. The pairs are listed from the most recent to the most ancient transition to asexuality (ranking from Bast et al. [21]). Stars indicate significant differences of the Tau indices of the sexual and asexual species of a pair. For species pair numbers, see figure 2.
To assess potential interactions between colour polymorphism and the number of different host plant species used, and thus study the contribution of predation to the realized feeding niches, we compared the degree of colour polymorphism within Timema species and populations with their degree of ecological specialization. At the species level, the size of the realized niche was correlated with the number of morphs of these species (correlation corrected with PGLS; r = 0.57, p-value < 0.003; electronic supplementary material, figure S1). Similar to the size of the species level realized niche, the asexuals contain two to five times fewer morphs than their sexual relatives, with the exception of T. poppensis/T. douglasi, in which both species have only a single morph (electronic supplementary material, table S1). By contrast, at the population level, we did not detect any link between colour polymorphism and the size of the realized feeding niche (Pearson's correlation; r = 0.14, p-value = 0.46; electronic supplementary material, figure S2B).
Survival and weight gain vary widely among the different studied Timema species when fed with different plants (p < 2.2 × 10−16 for survival and F7,292 = 8.94, p < 5.5 × 10−10 for weight gain; electronic supplementary material, figure S1A and figure 3b). We first tested whether sexual and asexual species feature different fundamental feeding niches by modelling, in each species pair, the survival and weight gain as functions of the species' reproductive mode and of the experimental feeding treatments (with interaction term). A significant interaction would indicate that sexual and asexual species have different fundamental feeding niches. We found a significant interaction for the pair Timema californicum/Timema shepardi; however, this was only the case for survival and only a trend for weight gain (table 1). We also found a significant interaction for the pair T. poppensis/T. douglasi, but only for weight gain, not survival (table 1). These results suggest that in at least two species pairs, asexuals and sexuals may have diverged in their fundamental niches.
Table 1.
Effect of experimental feeding treatments and reproductive mode on survival and weight gain of insects.(***p < 0.001; *p < 0.05; •p < 0.1.)
| Timema species pair | factors tested in the statistical models | survival | weight gain |
|---|---|---|---|
| pair 2: T. cristinae/T. monikensis |
[reproductive mode] | 1.1 × 10−05*** | F(1,34) = 3.9, p = 0.054• |
| [feeding treatment] | 2.9 × 10−09*** | F(5,34) = 14.8, p = 10.0 × 10−08*** | |
| [reproductive mode: feeding treatment] interaction | 0.59 | F(2,34) = 3.9, p = 0.222 | |
| pair 3: T. poppensis/T. douglasi |
[reproductive mode] | 0.33 | F(1,107) = 4.9, p = 0.03* |
| [feeding treatment] | 0.20 | F(6,107) = 13.1, p = 4.6 × 10−11*** | |
| [reproductive mode: feeding treatment] interaction | 0.44 | F(6,107) = 5.5, p = 5.4 × 10−05*** | |
| pair 4: T. californicum/T. shepardi |
[reproductive mode] | 0.009*** | F(1,71) = 13.7, p = 0.0004*** |
| [feeding treatment] | 4.8 × 10−05*** | F(6,71) = 19.4, p = 2.9 × 10−13*** | |
| [reproductive mode: feeding treatment] interaction | 0.0009*** | F(6,71) = 1.9, p = 0.09• | |
| pair 5: T. podura/T. genevievae |
[reproductive mode] | 0.0004*** | F(1,80) = 4.4, p = 0.04* |
| [feeding treatment] | 6.4 × 10−19*** | F(6,80) = 22.1, p = 3.5 × 10−15*** | |
| [reproductive mode: feeding treatment] interaction | 0.35 | F(5,80) = 2.1, p = 0.08• |
We then used the Tau index to test whether the breadth of the sexual and asexual species’ fundamental feeding niches also differ. Tau indices based on survival or weight gain were strongly correlated (Pearson's correlation, r = 0.96, p < 0.0001; figure 3b). We found significant differences in the fundamental niche breadths of sexuals compared to asexual species in two species pairs (Timema cristinae/Timema monikensis and Timema podura/Timema genevievae; electronic supplementary material, figure S1A and figure 3b). The remaining two pairs (T. poppensis/T. douglasi and T. californicum/T. shepardi) showed no significant difference (figure 3). Interestingly, T. monikensis and T. genevievae, which represent the most recent asexual lineage and oldest asexual lineage tested, respectively, were characterized by an opposite result. Timema monikensis was significantly more specialist (Tau based on weight gain = 0.27, 95% CI 0.22–0.29; survival = 0.21, 95% confidence interval (CI) 0.13–0.29) than its sexual relative T. cristinae (Tau based on weight gain = 0.47, 95% CI 0.41–0.55; survival = 0.46, 95% CI 0.34–0.58; figure 3b). On the contrary, the ancient asexual T. genevievae was significantly more generalist (Tau based on weight gain = 0.77, 95% CI 0.71–0.82; survival = 0.78, 95% CI 0.68–0.88) than its sexual sister species T. podura (Tau based on weight gain = 0.54, 95% CI 0.48–0.58; survival = 0.37, 95% CI 0.27–0.47; figure 3b). Finally, we found that the fundamental feeding niche breadths were not correlated with the sizes of their realized feeding niche, neither at the species level (Pearson's correlation; r = 0.13, p = 0.77; electronic supplementary material, figure S1A), nor at the population level (r = −0.14, p = 0.50; electronic supplementary material, figure S1B).
4. Discussion
We investigated if sexual and asexual Timema species differ in their realized feeding niches, i.e. the plant species they use as hosts under natural conditions, and how such differences come about. We find that asexual species generally feature smaller realized feeding niches than their sexual counterparts. Specifically, in four out of five sexual–asexual Timema species pairs, sexuals use about twice as many plants as asexuals in nature. In the fifth species pair, T. poppensis/T. douglasi, sexuals and asexuals use the same number of host plants. This species pair is probably an exception to the general pattern in Timema because of their ability to use the host plant redwood. We have shown in a previous study that sexual Timema species adapted to this specific host plant are ecologically highly specialized, perhaps because of reduced biotic pressures such as predation, parasitism and competition, on redwood [23]. This high level of ecological specialization in the sexual species T. poppensis makes a further specialization in the related asexual relatively unlikely.
To develop insights into how the narrower realized niches of asexual versus sexual Timema species come about, we quantified the size of their fundamental feeding niches, i.e. the range of plants Timema are able to use in the absence of the biotic pressures they normally face in nature. This allowed us to test if the size of the fundamental niche constrains the size of the realized niche, i.e. whether the reduced realized niche size in asexuals results from a reduced intrinsic ability to use different host plants. Fundamental feeding niche sizes varied significantly among all Timema species; however, there was no overall difference between reproductive modes. Fundamental niche sizes therefore do not explain why sexuals have broader realized niches than asexuals in Timema. Specifically, in two species pairs, the estimated fundamental niche size was very similar for sexuals and asexuals. In the other two pairs, the fundamental niche differed between sexuals and asexuals, however in opposite directions; in one species pair (T. cristinae/T. monikensis), the asexual species had a narrower fundamental niche than the sexual one, while in the other (T. podura/T. genevievae) the asexual species had a broader fundamental niche than the sexual one. The latter case is particularly interesting because T. genevievae is a very old asexual lineage (approx. 1.5–2 myr) and the oldest asexual Timema known (Schwander et al. [19]). The broad fundamental feeding niche in T. genevievae is consistent with predictions from the GPG theory, which posits that clones with broad environmental tolerances (i.e. broad fundamental niches) should be selectively favoured as such clones would be characterized by low variance in fitness across environments ([12]; figure 1b). General-purpose genotypes are also believed to contribute to the persistence of one of the oldest known asexual species, the darwinulid ostracod Darwinula stevensoni. This species has probably existed as an obligate asexual for at least 25 million years and shows almost no morphological [36] or genetic [37] variability, yet it is a very common and cosmopolitan species [38] with broad tolerances for salinity and temperature [39].
In contrast to the old asexual T. genevievae, our findings in the youngest studied Timema asexual, T. monikensis, are consistent with the FNV model. This model suggests that the phenotypic distribution (i.e. fundamental niche) of a young, recently derived asexual lineage will be narrower than that of its genetically variable sexual ancestor ([8]; figure 1a). Indeed, T. monikensis is the only studied asexual that features a narrower fundamental niche than its sexual relative T. cristinae (figure 3b and electronic supplementary material, figure S1A).
Given that asexual Timema do not generally have narrower fundamental niches than sexual Timema, the narrow realized niches in asexuals are probably a consequence of biotic interactions that affect realized niche size in asexuals more strongly than in sexuals. A likely biotic factor affecting realized niches in Timema is selection imposed by predators (e.g. [24,25,28,40]. Several Timema species feature a natural colour polymorphism conferring crypsis on different host plants [24] and we therefore tested for links between colour polymorphism, realized niche size and reproductive mode in Timema. The sister species T. douglasi and T. poppensis do not feature any colour polymorphism, but in the four remaining species pairs, intra-population colour polymorphism is always higher in the sexual than asexual species. However, the level of polymorphism was only correlated to the size of the realized niche at the species level, not at the population level. Nevertheless, this higher degree of colour polymorphism in sexuals may allow for reduced predation rates on a larger number of plants relative to asexuals, potentially explaining the narrower realized niche size in asexual species.
In conclusion, we provide, to our knowledge, the first comparative study of realized and fundamental niches in replicated asexual–sexual species pairs. We found that sexual Timema species have a larger realized niche than asexual ones, but this difference is not explained by a similar difference in fundamental niche size. Thus, the smaller realized niches in asexuals are probably a consequence of biotic interactions that constrain asexuals more strongly than sexuals. Verifying potential links between population level colour polymorphism, realized feeding niche size and biotic interactions (especially predation and competition) will be a challenge for future studies. Finally, our finding that the oldest asexual Timema lineage is more generalist than its sexual relative could help explain its unusually long maintenance in the absence of sex.
Supplementary Material
Acknowledgements
We thank Armand Yazdani and Ian S. Ford for their help in the field, and Giacomo Bernardi at UC Santa-Cruz for labspace.
Data accessibility
All raw data is available from the Dryad Digital Repository at: http://dx.doi.org/10.5061/dryad.4j1h61t [41].
Authors' contributions
C.L. and T.S. designed the study. C.L. and D.J.P. conducted fieldwork and performed experiments. C.L. analysed data with input from T.S. and D.J.P. C.L. and T.S. wrote the manuscript with input from D.J.P.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by grant nos PP00P3_139013 and PP00P3_170627 of the Swiss FNS to T.S.
References
- 1.Neiman M, Meirmans PG, Schwander T, Meirmans S. 2018. Sex in the wild: why field-based studies play a critical role in resolving the problem of sex. Evolution 72, 1194–1203. [DOI] [PubMed] [Google Scholar]
- 2.Meirmans S, Meirmans PG, Kirkendall LR. 2012. The costs of sex: facing real-world complexities. Q. Rev. Biol. 87, 19–40. ( 10.1086/663945) [DOI] [PubMed] [Google Scholar]
- 3.Case TJ, Taper ML. 1986. On the coexistence and coevolution of asexual and sexual competitors. Evolution 40, 366–387. ( 10.1111/j.1558-5646.1986.tb00478.x) [DOI] [PubMed] [Google Scholar]
- 4.Halkett F, Kindlmann P, Plantegenest M, Sunnucks P, Simon JC. 2006. Temporal differentiation and spatial coexistence of sexual and facultative asexual lineages of an aphid species at mating sites. J. Evol. Biol. 19, 809–815. ( 10.1111/j.1420-9101.2005.01055.x) [DOI] [PubMed] [Google Scholar]
- 5.Lehto MP, Haag CR. 2010. Ecological differentiation between coexisting sexual and asexual strains of Daphnia pulex. J. Anim. Ecol. 79, 1241–1250. ( 10.1111/j.1365-2656.2010.01726.x) [DOI] [PubMed] [Google Scholar]
- 6.Bell G. 1982. The masterpiece of nature: the evolution and genetics of sexuality. Cambridge, UK: CUP Archive. [Google Scholar]
- 7.Doncaster CP, Pound GE, Cox SJ. 2000. The ecological cost of sex. Nature 404, 281–285. ( 10.1038/35005078) [DOI] [PubMed] [Google Scholar]
- 8.Vrijenhoek RC. 1984. Ecological differentiation among clones: the frozen niche variation model. In Population biology and evolution (eds Wohrmann K, Loeschcke V), pp. 217–231. Berlin, Germany: Springer. [Google Scholar]
- 9.Case TJ. 1990. Pattern of coexistence in sexual and asexual species of Cnemidophorus lizards. Oecologia 83, 220–227. ( 10.1007/BF00317756) [DOI] [PubMed] [Google Scholar]
- 10.Weeks SC. 1993. The effects of recurrent clonal formation on clonal invasion patterns and sexual persistence: a Monte Carlo simulation of the frozen niche-variation model. Am. Nat. 141, 409–427. ( 10.1086/285481) [DOI] [PubMed] [Google Scholar]
- 11.Vrijenhoek RC, Parker ED Jr. 2009. Geographical parthenogenesis: general purpose genotypes and frozen niche variation. In Lost sex. The evolutionary biology of parthenogenesis (eds Schon I, Martens K, Dijk P), pp. 99–131. Berlin, Germany: Springer. [Google Scholar]
- 12.Lynch M. 1984. Destabilizing hybridization, general-purpose genotypes and geographic parthenogenesis. Q. Rev. Biol. 59, 257–290. ( 10.1086/413902) [DOI] [Google Scholar]
- 13.Baker HG. 1965. Characteristics and mode of origin of weeds. In The genetics of colonizing species (eds Baker HG, Stebbins GL), pp. 147–172. New York, NY: Academic Press. [Google Scholar]
- 14.Parker ED, Selander RK, Hudson RD, Lester LJ. 1977. Genetic diversity in colonizing parthenogenetic cockroaches. Evolution 31, 836–842. ( 10.1111/j.1558-5646.1977.tb01076.x) [DOI] [PubMed] [Google Scholar]
- 15.Hill WG, Robertson A. 1966. The effect of linkage on limits to artificial selection. Genet. Res. (Camb.) 8, 269–294. ( 10.1017/S0016672300010156) [DOI] [PubMed] [Google Scholar]
- 16.Kondrashov AS. 1988. Deleterious mutations and the evolution of sexual reproduction. Nature 336, 435–440. ( 10.1038/336435a0) [DOI] [PubMed] [Google Scholar]
- 17.Barton NH, Charlesworth B. 1998. Why sex and recombination? Science 281, 1986–1990. ( 10.1126/science.281.5385.1986) [DOI] [PubMed] [Google Scholar]
- 18.Otto SP, Lenormand T. 2002. Resolving the paradox of sex and recombination. Nat. Rev. Genet. 3, 252 ( 10.1038/nrg761) [DOI] [PubMed] [Google Scholar]
- 19.Schwander T, Henry L, Crespi BJ. 2011. Molecular evidence for ancient asexuality in Timema stick insects. Curr. Biol. 21, 1129–1134. ( 10.1016/j.cub.2011.05.026) [DOI] [PubMed] [Google Scholar]
- 20.Riesch R, et al. 2017. Transitions between phases of genomic differentiation during stick-insect speciation. Nat. Ecol. Evol. 1, 1–13. ( 10.1038/s41559-017-0082) [DOI] [PubMed] [Google Scholar]
- 21.Bast J, Parker DJ, Dumas Z, Jalvingh K, Tran Van P, Jaron K, Figuet E, Galtier N, Schwander T. 2018. Consequences of asexuality in natural populations: insights from stick insects. Mol. Biol. Evol. 35, 1668–1677. ( 10.1093/molbev/msy058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Law JH, Crespi BJ. 2002. Recent and ancient asexuality in Timema walking sticks. Evolution 56, 1711–1717. ( 10.1111/j.0014-3820.2002.tb01484.x) [DOI] [PubMed] [Google Scholar]
- 23.Larose C, Rasmann S, Schwander T. 2018. Evolutionary dynamics of specialization in herbivorous stick insects. bioXriv ( 10.1101/367706) [DOI] [PubMed] [Google Scholar]
- 24.Sandoval CP. 1994. Differential visual predation on morphs of Timema cristinae (Phasmatodeae: Timemidae) and its consequences for host-range. Biol. J. Linn. Soc. 52, 341–356. ( 10.1111/j.1095-8312.1994.tb00996.x) [DOI] [Google Scholar]
- 25.Sandoval CP. 1994. The effects of the relative geographic scales of gene flow and selection on morph frequencies in the walking-stick Timema cristinae. Evolution 48, 1866–1879. ( 10.1111/j.1558-5646.1994.tb02220.x) [DOI] [PubMed] [Google Scholar]
- 26.Nosil P. 2007. Divergent host plant adaptation and reproductive isolation between ecotypes of Timema cristinae walking sticks. Am. Nat. 169, 151–162. ( 10.1086/510634) [DOI] [PubMed] [Google Scholar]
- 27.Sandoval CP, Nosil P. 2005. Counteracting selective regimes and host preference evolution in ecotypes of two species of walking-sticks. Evolution 59, 2405–2413. ( 10.1111/j.0014-3820.2005.tb00950.x) [DOI] [PubMed] [Google Scholar]
- 28.Nosil P. 2004. Reproductive isolation caused by visual predation against migrants between divergent environments. Proc. R. Soc. Lond. B 271, 1521–1528. ( 10.1098/rspb.2004.2751) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaenike J. 1990. Host specialization in phytophagous insects. Annu. Rev. Ecol. Syst. 21, 243–273. ( 10.1146/annurev.es.21.110190.001331) [DOI] [Google Scholar]
- 30.Yanai I, et al. 2004. Genome-wide midrange transcription profiles reveal expression level relationships in human tissue specification. Bioinformatics 21, 650–659. ( 10.1093/bioinformatics/bti042) [DOI] [PubMed] [Google Scholar]
- 31.Sandoval CP. 2000. Persistence of a walking-stick population (Phasmatoptera : Timematodea) after a wildfire. Southwest. Nat. 45, 123–127. ( 10.2307/3672452) [DOI] [Google Scholar]
- 32.Simpson EH. 1949. Measurement of diversity. Nature 163, 688 ( 10.1038/163688a0) [DOI] [Google Scholar]
- 33.Paradis E, Claude J, Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290. ( 10.1093/bioinformatics/btg412) [DOI] [PubMed] [Google Scholar]
- 34.Pinheiro JDB, DebRoy S, Sarkar D.2009. nlme: linear and nonlinear mixed effects models. R Packag. version 3. 1–137. See https://CRAN.R-project.org/package=nlme .
- 35.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 36.Rossetti G, Martens K.. 1998. Taxonomic revision of the Recent and Holocene representatives of the Family Darwinulidae (Crustacea, Ostracoda), with a description of three new genera. Bull. Inst. R. des Sci. Nat. Belgique. Meded. K. Belgisch Inst. voor Natuurwetenschappen 68, 55–110. [Google Scholar]
- 37.Schön I, Butlin RK, Griffiths HI, Martens K, Schon I, Butlin RK, Griffiths HI, Martens K. 1998. Slow molecular evolution in an ancient asexual ostracod. Proc. R. Soc. Lond. B 265, 235–242. ( 10.1098/rspb.1998.0287) [DOI] [Google Scholar]
- 38.Griffiths HI, Butlin RK. 1994. Darwinula stevensoni: a brief review of the biology of a persistent parthenogen. In The evolutionary ecology of reproductive modes in non-marine ostracoda (eds Horne DJ, Martens K), pp. 27–36. London, UK: Greenwich University Press. [Google Scholar]
- 39.Van Doninck K, Schön I, De Bruyn L, Martens K. 2002. A general purpose genotype in an ancient asexual. Oecologia 132, 205–212. ( 10.1007/s00442-002-0939-z) [DOI] [PubMed] [Google Scholar]
- 40.Nosil P, Crespi BJ, Sandoval CP. 2003. Reproductive isolation driven by the combined effects of ecological adaptation and reinforcement. Proc. R. Soc. Lond. B 270, 1911–1918. ( 10.1098/rspb.2003.2457) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larose C, Parker DJ, Schwander T. 2018. Data from: Fundamental and realized feeding niche breadths of sexual and asexual stick insects Dryad Digital Repository. ( 10.5061/dryad.4j1h61t) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Larose C, Parker DJ, Schwander T. 2018. Data from: Fundamental and realized feeding niche breadths of sexual and asexual stick insects Dryad Digital Repository. ( 10.5061/dryad.4j1h61t) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
All raw data is available from the Dryad Digital Repository at: http://dx.doi.org/10.5061/dryad.4j1h61t [41].