Abstract
The trade-off between reproductive investment and survival is central to life-history theory, but the relative importance and the complex interactions among the physiological mechanisms mediating it are still debated. Here we experimentally tested whether baseline glucocorticoid hormones, the redox system or their interaction mediate reproductive investment–survival trade-offs in wild great tits (Parus major). We increased the workload of parental males by clipping three feathers on each wing, and 5 days later determined effects on baseline corticosterone concentrations (Cort), redox state (reactive oxygen metabolites, protein carbonyls, glutathione peroxidase [GPx], total non-enzymatic antioxidants), body mass, body condition, reproductive success and survival. Feather-clipping did not affect fledgling numbers, chick body condition, nest provisioning rates or survival compared with controls. However, feather-clipped males lost mass and increased both Cort and GPx concentrations. Within feather-clipped individuals, GPx increases were positively associated with reproductive investment (i.e. male nest provisioning). Furthermore, within all individuals, males that increased GPx suffered reduced survival rates. Baseline Cort increases were related to mass loss but not to redox state, nest provisioning or male survival. Our findings provide experimental evidence that changes in the redox system are associated with the trade-off between reproductive investment and survival, while baseline Cort may support this trade-off indirectly through a link with body condition. These results also emphasize that plastic changes in individuals, rather than static levels of physiological signals, may mediate life-history trade-offs.
Keywords: reaction norm, trade-off, survival, glutathione peroxidase, corticosterone, workload
1. Introduction
Life-history theory centres on the concept that trade-offs exist between reproductive investment and survival [1–4]. Trade-offs are mediated by processes that compete with each other for resources that are potentially limited [1,4,5]. However, the physiological systems that mediate such trade-offs are still debated [6,7]. At present, two major physiological processes are being discussed as potential mediators: the endocrine and the redox systems [6,8–10]. Among endocrine signals, glucocorticoid hormones (GCs) have emerged as systemic regulators of life-history trade-offs [11–14]. GCs are often referred to as ‘metabolic hormones’ because one of their main functions is the mobilization of energy sources to support increased energetic needs of tissues [15–18]. These functions render GCs prime candidates for mediating trade-offs that are based on energy allocation. Indeed, high circulating concentrations of GCs, such as induced by the endocrine stress response, can directly inhibit reproductive processes through actions on the brain and the hypothalamic–pituitary–gonadal axis [13,15,16,19–21]. Hence, at high stress-induced concentrations, GCs reduce investment in current reproduction and prioritize processes that promote survival behaviours like escape and foraging to restore internal resources [19]. However, whether GCs play a role in mediating such trade-offs at low baseline concentrations is less clear, although their involvement has been hypothesized [22]. Baseline GCs are elevated when individuals experience increased energetic demands [11,12,17,18,22–25], for example, in the reproductive season when parents provision their offspring [18,26–31]. These findings prompted the hypothesis that baseline GCs may mediate trade-offs, but in an opposite way to stress-induced concentrations: baseline GCs would support investment in current reproduction at the expense of survival [22,32,33]. However, thus far the evidence for GCs supporting reproductive investment [22,26,34–37] or impairing survival is mixed [22].
According to the ‘oxidative stress life-history theory’, trade-offs can be a direct consequence of oxidative damages induced by exposure to reactive oxygen species (ROS) that have significant pro-oxidant effects [8,38–41]. If the concentrations of ROS cannot be counterbalanced by antioxidant defences, oxidative stress will ensue, which can generate cellular oxidative damage [42–44]. ROS can damage vital molecules such as DNA and proteins, impair cellular functionality and impact longevity [44–46]. Oxidative costs can arise both from an increase in oxidative damages and a change in antioxidant concentrations [47–50]. Antioxidants can be upregulated to cope with a pro-oxidant challenge or can be depleted when ROS cannot be buffered, thus exposing the organism to oxidative damage [46]. The central concept of the ‘oxidative stress life-history theory’ is that ROS are produced proportionally to metabolic rate. Since metabolic rate typically increases during reproduction, more resources have to be allocated to antioxidant protection [38–41,51]. While there is some evidence that reproductive investment does entail oxidative costs [52,53], the postulated positive association between metabolic rate and the production of ROS has not been widely supported [47,54,55]. At least partly, such conflicting results could arise from the correlative approaches that have been used in most investigations thus far, where individuals are allowed to decrease their reproductive effort to minimize exposure to pro-oxidants [47]. Therefore, experimental studies in which the metabolic factors that cause cellular oxidative stress are manipulated are essential to properly test this hypothesis [54]. Moreover, we still need to understand whether changes in the redox system that are within the normal physiological range, and not only acute oxidative damage, are linked to life-history trade-offs.
Increasing the workload of parents during the reproductive period in free-ranging individuals has been shown to be an effective method to elevate their costs and two recent experimental studies in swallows suggest an involvement of baseline Cort in the resulting increased parental effort [56,57]. However, neither of these studies investigated the existence of costs like impaired survival or health state. Thus, the question of whether baseline Cort mediates life-history trade-offs has not been conclusively answered. Likewise, a study that feather-clipped great tits (Parus major) prior to the nesting phase did not reveal oxidative costs of reproduction because manipulated parents decreased their reproductive investment by laying smaller clutches [58]. A recent study raised the workload of reproducing European starling (Sturnus vulgaris) females by mounting a backpack radio transmitter, feather-clipping three wing feathers or combining the two handicaps [59]. Cort concentrations were higher in individuals carrying a radio transmitter but lower in individuals with both handicaps, rendering the interpretation of the findings difficult. Females subjected to both handicaps did show evidence of costs by having increased concentrations of ROS and lower return rates in the following breeding season [59].
The majority of the studies reviewed above conducted their analyses at the population level, which can obscure processes occurring at the individual level. For example, individuals can differ in trade-off strategies depending on their body condition or ecological circumstances, such that individuals in optimal condition or living in high-quality environments can maximize both survival and reproductive success [4,60–62]. Hence, analyses of individual variation are required to truly understand life-history trade-offs and to test for direct links between physiological measures and fitness.
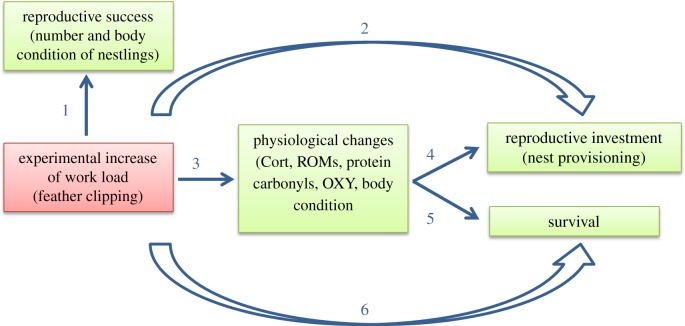
Here, we experimentally increased energetic costs during reproduction in a free-living bird species to test for effects on fitness variables like reproductive investment and survival as well as physiological mechanisms like baseline Cort concentrations and the redox system. We designed our experiment along the lines of the ‘ideal manipulation’ concept [62] by avoiding any direct effects of the experimental manipulation on reproductive success (which in turn would influence the reproductive investment of the parents) or on survival (figure 1, arrows 1, 2 and 6). Instead, we aimed at inducing physiological changes in experimental individuals to test for consequences on reproductive investment and survival. Specifically, we clipped three primary feathers on both wings of male great tits (Parus major) during the offspring provisioning phase to increase wing load and thus energetic costs of flight and nestling provisioning (our measure of reproductive investment) [57,63–65]. With this experiment, we aimed at testing whether baseline Cort, the redox system or both may mediate the trade-off between reproductive investment and survival. In general, we expected that the increase in wing load, an unexpected worsening of conditions for parental individuals, will lead to an upregulation in baseline Cort concentrations, to an increase in oxidative damage and to a change in antioxidant concentrations (either increase or decrease) [48]. We analysed the resulting changes in male great tits both at the population and the individual level.
Figure 1.
Conceptual representation of the statistical analyses used in the study. Our main aim was to assess the physiological changes induced by an experimentally increased workload (arrow 3), and how changes in physiology affected reproductive investment and survival (arrows 4 and 5). One major assumption for the success of this experiment was the lack of a direct effect of the manipulation on reproductive success (arrow 1), reproductive investment (arrow 2) and survival (arrow 6). This design represents the ‘ideal manipulation’ for studying the physiological costs of parental care [62]. For further explanations, see text.
2. Material and methods
The study was carried out between March 2015 and November 2017, in a mixed forest located in the district of Starnberg, southern Germany (47°99′ N–11°39′ E; for further details on field work, see electronic supplementary material). Adult great tits were captured two times in their nest-box between 08.00 and 15.00 h by triggering a remote-controlled flap at the nest-box entrance (for a time line, see electronic supplementary material, figure S1). During the first trapping, when chicks were 7 days old (electronic supplementary material, figure S1), we clipped three primary feathers on both wings in experimental males (FC-males), leaving approximately 1.5 cm of the feather shaft intact. Control males (C-males) and all females were caught and handled similarly, but had no feathers cut. To assess the effect of the treatment, on chick day 12 males were re-trapped and re-measured as described above. Following each capture, a blood sample of maximally 80 µl was taken from the wing vein within less than 3 min (see electronic supplementary material) from the remote-controlled closure of the nest-box entrance. The number of 15-day-old nestlings and their body mass were recorded as proxies for fledging success.
One day before each trapping, we video-recorded the nest provisioning rate of both parents, our proxy for reproductive investment. Apparent survival was estimated by the presence/absence of an individual at the study site in the following breeding season or during fall recaptures in the year after the experiment [26]. Previous studies have suggested that presence/absence data closely match actual survival rates of great tits [66]. Plasma corticosterone concentrations were determined using enzyme immunoassay following a double diethyl ether extraction (see electronic supplementary material). We measured changes in the redox system by quantifying its two main components: the potential oxidative damage incurred as well as the antioxidant defences (both enzymatic and non-enzymatic antioxidants). Potential oxidative damage was quantified by measuring the plasma levels of reactive oxygen metabolites (ROMs; d-ROM test kit, Diacron International). ROMs are organic hydroperoxides, end-products of the oxidation of lipids, proteins and nucleic acids that are generated by hydrogen peroxides produced primarily during mitochondrial respiration [67]. The non-enzymatic antioxidants (OXY) present in the plasma, which can be either produced by the organism or acquired through the diet were quantified with a colorimetric assay (OXY-Adsorbent test kit, Diacron International) [67]. The intra-cellular antioxidant enzymatic activity was quantified through glutathione peroxidase (GPx; kit Randox Laboratories) concentrations. GPx has the unique function to enzymatically convert hydrogen peroxides into water. We quantified protein carbonyl concentrations in red blood cells (kit Cayman Chemical Company), which indicates terminal oxidative damage to proteins [46], following a validated protocol for birds [49]. All samples were measured in duplicates (number of plates run for each assay and data on assay quality are provided in the electronic supplementary material).
We were able to sample 43 males twice (23 FC-males and 20 C-males; n = 86 repeated measures, but total n = 112 including individuals trapped only once). Before the onset of the experiment, FC- and C-males did not differ in any of the variables measured (see electronic supplementary material). To verify that the treatment had no direct effects on reproductive success (figure 1, arrow 1), we ran two separate general linear models with number of fledglings and mean body condition of fledglings as response variables. The body condition of nestlings (and that of adults, see below) was calculated as body mass scaled by wing length [68]. These models included treatment group as a fixed factor while controlling for provisioning rate recorded on day 15, year and hatching date. We next tested for direct effects of feather-clipping on reproductive investment (figure 1, arrow 2) by using a linear mixed model with nest provisioning (number of nest visits × h–1) standardized for brood size as the response variable, including treatment and day of sampling (two levels: day 7 and day 12) as fixed factors in a full factorial model. Individual identity was nested into treatment and included as random factor [69]. We used similar models to determine whether the feather-clipping manipulation affected aspects of male physiology (figure 1, arrow 3), specifically using Cort concentrations (ng ml–1), body mass (g) and body condition as body mass standardized for wing length (g) [68], d-ROMs (mM H2O2 equivalents), Oxy (mM HOCl), GPx (Units—U l−1) and protein carbonyls (nmol mg−1 of proteins) as response variables.
To investigate whether the physiological variables that changed following the treatment could predict male reproductive investment (figure 1, arrow 4), we ran a repeated measure model with provisioning rate per chick as the response variable and Cort, body condition and GPx as predictors. Among the predictors, we also included year because in 2015 Cort was higher, but GPx lower and nestling body condition worse. The two groups were analysed separately because they showed divergent changes in physiological variables (treatment × time interactions; see Results).
Model selection was carried out using the Akaike information criterion [70] for small sample sizes (AICc). We considered the models with the lowest AICc value as well as those with an AICc difference lower than 2 (electronic supplementary material, tables S1–S3) [70]. The final model included the predictors that were present in the majority of the best models and its AICc was compared to the AICc of a null model that did not include any predictors (intercept-only model).
To assess whether the physiological changes observed during the experiment explained annual survival (figure 1, arrow 5; 0 = not survived, 1 = survived), we included change in GPx, Cort and standardized body mass (i.e. all variables that were affected by the treatment; changes were calculated as the difference between days 12 and 7) into a logistic model, together with year and treatment. This model also allowed us to test for a direct effect of feather-clipping on survival (figure 1, arrow 6). For reasons of sample size, the two treatment groups were pooled for this analysis. Also, changes in physiological variables were calculated because repeated measurements of individuals were not available due to the nature of the survival data. To provide a similarly structured analysis for reproductive investment, we used changes in GPx, Cort and standardized body mass as predictors, together with year and treatment in a model run for both treatment groups combined.
In addition to the analyses depicted in figure 1, we assessed whether the Cort concentrations explained variations in the redox system (i.e. whether Cort could potentially be a mediator of trade-offs). For this, we ran separate models for FC- and C-males with the response variables being GPx, OXY, ROM and protein carbonyl, respectively, and Cort being the predictor. A similar model was used to assess the relationship between body condition and Cort.
All analyses were performed using JMP v. 12.2.0 (SAS Institute Inc. Cary, NC, USA), which provides a p-value for each fixed effect in the model based on F- or χ2-statistics by testing the null hypothesis that the parameters associated with that effect are zero. In line with this approach, we analysed post hoc within-individual differences with the Tukey's HSD test. For all models, we used z-score normalized variables. All data are given as means ± s.e.m. Further statistical details are provided in the electronic supplementary material.
3. Results
(a). Treatment effects on reproductive success and parental provisioning rate
Feather-clipping male great tits did not affect their reproductive success (figure 1, arrow 1). Our final model for the number of fledglings as a function of the feather-clipping included hatching date and year as predictors and showed that FC- and C-males did not differ in number of fledglings produced (electronic supplementary material, table S1). Supporting this finding, this model had a lower fit than the intercept-only model. Similarly, the final model for the body condition of fledglings as a function of feather-clipping showed that fledgling condition did not differ for treated versus control males (electronic supplementary material, table S1). Only year explained the body condition of nestlings (F(1,43)=6.91, p = 0.01), with condition being worse in 2015. Again, the null model had a better AICc value than the final model.
The treatment did not affect the rate at which nestlings were fed by their parents (figure 1, arrow 2). The final model selected for nest provisioning as a function of the feather-clipping included year, mate nest provisioning and hatching date, but treatment did not significantly explain nest provisioning and this model had a similar fit than an intercept-only model (electronic supplementary material, table S2). Overall, the provisioning rate of males was positively associated with that of their female partners (β = 0.48 ± 0.14, F1,85 = 12.45, p < 0.001; electronic supplementary material, table S2).
(b). Treatment effects on body mass, corticosterone and redox system
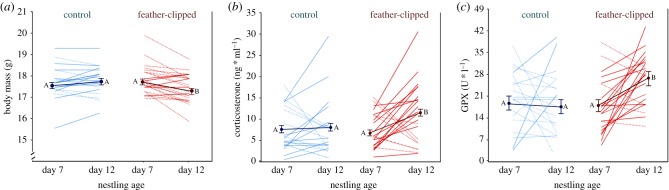
Feather-clipping induced changes in three physiological parameters of male great tits (figure 1, arrow 3). First, body mass was significantly affected by the treatment, as FC-males decreased body mass (change: −0.36 ± 0.10 g, p = 0.004) while C-males did not (0.13 ± 0.11 g, p = 0.59; figure 2a; table 1; electronic supplementary material, table S3A). The final model also included number of fledglings, with heavier males producing more fledglings (table 1; electronic supplementary material, table S3). Male body condition was similarly affected by the treatment, as was body mass (table 1; electronic supplementary material, table S3B). However, in the final model for body condition, the number of fledglings was not retained (electronic supplementary material, table S1B).
Figure 2.
(a) Body mass (C-males n = 20, FC-males n = 23), (b) corticosterone concentrations (C-males n = 19, FC-males n = 22) and (c) glutathione peroxidase (GPx; C-males n = 19, FC-males n = 22) concentrations of males on chick days 7 and 12. Different capital letters indicate a significant difference between days 7 and 12 (Tukey's HSD post hoc tests). Thin solid lines: positive individual changes, thin dotted lines: negative individual changes, thick lines: group least squared means (±1 s.e.m.).
Table 1.
Significant predictors of the best-supported repeated-measure models for physiological variables (for chick days 7 and 12). Model selection, whole model and variance analysis of random effects are reported in electronic supplementary material, table S3A–F.
| estimate | s.e | d.f. | F | p-value | |
|---|---|---|---|---|---|
| body mass (R2 = 0.89) | |||||
| time × treatment | −0.03 | 0.009 | 142.26 | 11.45 | <0.01 |
| number of fledglings | 0.27 | 0.12 | 147.31 | 5.03 | 0.03 |
| body condition (R2 = 0.98) | |||||
| time × treatment | −0.12 | 0.03 | 142.64 | 13.80 | <0.001 |
| corticosterone (R2 = 0.51) | |||||
| time [day 7] | −0.04 | 0.02 | 147.41 | 5.21 | 0.03 |
| time × treatment | 0.04 | 0.02 | 147.48 | 3.93 | 0.05 |
| year [2015] | 0.06 | 0.02 | 156.23 | 9.23 | <0.01 |
| bleeding duration | 0.24 | 0.11 | 181.4 | 4.72 | 0.03 |
| GPx (R2 = 0.16) | |||||
| treatment [C] | −0.05 | 0.02 | 136.84 | 4.47 | 0.04 |
| time × treatment | 0.05 | 0.02 | 141.23 | 4.92 | 0.03 |
| year [2015] | −0.07 | 0.02 | 141.93 | 41.92 | <0.01 |
| ROMs (R2 = 0.65) | |||||
| GPx | 0.18 | 0.07 | 1.74.69 | 5.80 | 0.02 |
| OXY (R2 = 0.55) | |||||
| body mass | 0.32 | 0.17 | 167.11 | 4.24 | 0.04 |
Second, variation in baseline Cort concentrations was significantly explained by the treatment (figure 2b; table 1; electronic supplementary material, table S3C), as FC-males increased Cort over time after feather-clipping (change: 4.53 ± 1.44 ng ml−1, p = 0.015), while C-males did not (0.31 ± 1.56 ng ml−1, p = 0.99; figure 2; electronic supplementary material, table S1C). The final model for Cort also included bleeding duration, which increased with longer blood sampling times (table 1; electronic supplementary material, table S3C).
Third, red blood cell GPx concentrations were affected by the treatment and increased from day 7 to day 12 in FC-males (change: 8.14 ± 2.74 U l−1, p = 0.02) but not in C-males (0.69 ± 2.89 U l−1, p = 0.99; figure 2c; table 1; electronic supplementary material, table S3D). Year was also included in the final model (table 1, electronic supplementary material, table S3D).
By contrast, feather-clipping did not affect ROM concentrations (electronic supplementary material, table S3E) or protein carbonyl concentrations (electronic supplementary material, table S3G), and the null model had the best fit for ROMs and equal fit for protein carbonyl model. We then included GPx in the models for ROMs, because hydrogen peroxides are produced by this enzyme, and obtained a better fit of the final model compared to the null model (electronic supplementary material, table S3F). Treatment still had no effect on ROM concentrations, but ROMs were positively related to GPx concentrations (table 1; electronic supplementary material, table S3F). Finally, feather-clipping did not change plasma OXY concentrations (electronic supplementary material, table S1H), but males with higher body mass had higher OXY levels (table 1; electronic supplementary material, table S3H).
(c). Effects of physiological variables on reproductive investment and survival rate
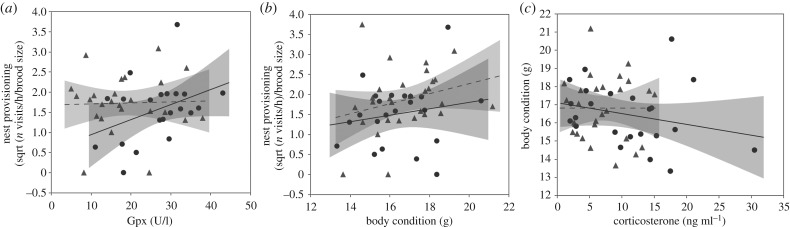
Reproductive investment (i.e. provisioning rate) of FC-males was positively associated with variation in GPx between days 7 and 12 (figure 3a; table 2; electronic supplementary material, table S4), but was not explained by Cort variation (arrow 4 in figure 1; electronic supplementary material, table S4). FC-males in better body condition provided more parental care (figure 3b; table 2; electronic supplementary material, table S4). In a separate analysis, we found that in FC-males, body condition was negatively related to circulating levels of Cort (ß = −0.14, F(1,22.91) = 6.74, p = 0.016; figure 3c), but not to GPx (FC-males: F1,21 = 1.81, p = 0.19). The provisioning rate of C-males was not explained by changes in Cort, GPx or body condition (electronic supplementary material table S4). Body condition of C-males was not explained by Cort levels (F(1,20.48) = 0.55, p = 0.55) or GPx (F(1,20.79) = 0.0005, p = 0.98). Since the model for survival (see below) was run by pooling the two treatment groups and including the changes in physiological variables from chick days 7 to 12 as predictors, we ran the same model for reproductive investment as well. None of Cort, GPx or body condition changes explained male provisioning rates (electronic supplementary material, table S5).
Figure 3.
Relationships in FC-males between (a) nest provisioning rate and glutathione peroxidase (GPx) concentrations (day 7: n = 25, day 12: n = 21), (b) nest provisioning rate and corticosterone concentrations (day 7: n = 28, day 12: n = 22), and (c) body condition and corticosterone concentrations (day 7: n = 28, day 12: n = 23). Grey triangles and dotted lines for chick day 7; black circles and solid lines for chick day 12.
Table 2.
Only significant predictors of the best supported repeated measure model on provisioning rate as response variable for the two groups separately. Model selection and variance analysis of random effects are reported in the electronic supplementary material, table S4.
| standardized provisioning rate | estimate | s.e | d.f. | F | p-value |
|---|---|---|---|---|---|
| feather-clipped (R2 = 0.40) | |||||
| year [2015] | 0.08 | 0.034 | 124.77 | 5.91 | 0.022 |
| body condition | 0.30 | 0.13 | 119.54 | 5.50 | 0.030 |
| GPx | 0.34 | 0.15 | 139.89 | 5.17 | 0.028 |
| controls (R2 = 0.55) | |||||
| none | |||||
In the year after each experimental manipulation, we recorded the presence of 9 FC-males (39% survived) and 6 C-males (30% survived) out of the 43 birds for which we have repeated measures (figure 1, arrows 5 and 6). Irrespective of the treatment (which did not affect survival), individuals that increased GPx more were less likely to survive (electronic supplementary material, table S6; β = 4.84 ± 2.28, F1,37 = 6.18, p < 0.01; figure 4). Neither changes in Cort or in body condition were related to survival (electronic supplementary material, table S5). Change in Cort concentrations also did not explain any redox variable in either group (electronic supplementary material, table S7).
Figure 4.
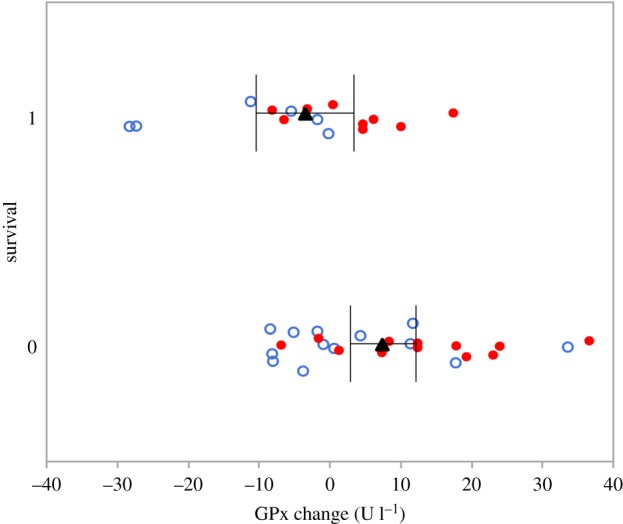
Apparent survival (1 = yes, 0 = no) versus change in glutathione peroxidase concentrations (GPx; chick day 12—chick day 7). Red filled circles indicate FC-males, blue open circles indicate C-males, black triangles show mean values (±95% confidence intervals).
4. Discussion
Increasing the workload of male great tits during nestling provisioning by feather-clipping did not directly alter their nestling provisioning rates, reproductive success or survival. These were three important requirements for our study (figure 1, arrows 1, 2 and 6) that enabled us to assess how increased workload affects the physiological mechanisms that mediate the life-history trade-off between reproductive investment and survival. In response to the experimental treatment, feather-clipped males upregulated both Cort and GPx, and lost 3% of their body mass (similar results were obtained for body condition). Furthermore, the increase in GPx in feather-clipped males was positively related to their nest provisioning rates, while individuals that upregulated GPx suffered lower survival rates across treatment groups. We could not detect similar physiological changes in control males and, in both groups, individual changes in Cort concentrations did not explain male provisioning rate or overwinter survival. These results indicate a role of the redox system, in particular of GPx concentrations, in the life-history trade-off between reproductive investment and survival. While they cannot discount an involvement of baseline Cort, the relationships observed in feather-clipped males between body condition, parental care and Cort suggest that its effect may be indirect, perhaps through a link with body condition.
As predicted, baseline Cort concentrations increased in feather-clipped male great tits. Our experimental manipulation was successful, as Cort concentrations of FC-males at the end of our experiment were within the range of baseline levels observed for this species [26]. However, we found no association between changes in Cort and nest provisioning rate of males, our measure for post-hatching reproductive investment. This finding suggests that baseline Cort does not mediate investment in current reproduction. We cannot exclude an indirect relationship between Cort and nest provisioning rates via Cort's link with body condition, because FC-males that lost more body mass (standardized for body size) provisioned their offspring less often, but showed higher levels of Cort (figure 2).
An indirect involvement of Cort in regulating reproductive investment via body condition may in fact explain some inconsistencies in the current literature. While some studies show that absolute levels, as well as plastic changes, of baseline Cort are positively associated with the degree of parental care [26,27,36,71,72], other investigations disagree. For example, two recent correlative studies showed a negative association between chick feeding rates and baseline Cort concentrations [30,73]. Thus, the relationship between Cort and reproductive investment (or success) appears to vary across species and/or environmental context. This is not surprising, because if baseline Cort is intrinsically related to the energetic state of an individual [16], variation in body condition or environmental settings during the breeding season are expected to shape the link between this hormone and fitness traits [74]. Indeed, a recent three-year study in blue tits (Cyanistes caeruleus) demonstrated that the relationship between circulating levels of Cort during the parental phase and number of fledglings varied among years with contrasting environmental conditions [75]. Likewise, a multi-year study in parental tree swallow females showed that Cort increased after feather-clipping in females that also decreased body mass [76]. Together with our present data, these studies highlight that only by incorporating measures of individual state (such as body condition) and environmental context into our analyses can we achieve a full understanding of the role of Cort in the regulation of life-history trade-offs (see also [74]). The present study focused on baseline Cort, but other Cort traits like stress-induced levels (see Introduction), the strength of the negative feedback to normalize stress-induced concentrations production and the maximal capacity to secrete Cort may also all play a role in regulating life-history trade-offs [23].
Our experimental treatment did not reduce male nest visits, demonstrating that we provided a metabolic challenge during the reproductive season that allowed males to maintain their initial degree of parental care [62]. This finding also demonstrates that male great tits can cope with a deterioration in conditions during parental care rather well, probably aided by the observed changes in physiology. It is plausible that the mass loss in FC-males, which has been observed in other species subjected to a similar treatment [57,59], helped the birds to decrease wing load and save energy [59]. Strikingly, the mass loss recorded in our study was similar to that shown by female starlings (3%), irrespective of the severity of their experimental handicap [59]. However, mass loss did not attenuate oxidative costs associated with feather-clipping as we did not find any relationship between changes in mass and GPx concentrations.
We did not identify a link between Cort and survival in the present study. Even though our sample size was limited, the finding that survival rate was predicted by GPx changes demonstrates that we had sufficient power to detect links between physiological variables and this fitness-relevant trait. The lack of an association between Cort and survival casts further doubt on the idea that baseline Cort mediates reproductive investment–survival trade-offs, at least in this species.
However, as suggested by the oxidative stress life-history hypothesis [8,38,40], our study did identify an involvement of the redox system in the reproductive investment–survival trade-off. Feather-clipped males that invested more in parental provisioning also upregulated GPx more strongly, and an increase in GPx was associated with a lower survival rate across treatment groups (figure 3). This pattern, however, did not emerge in a statistical model in which we pooled the two groups and included the calculated changes in the physiological variables between the nestling days 7 and 12. This discrepancy in results could be due to the fact that only FC-males, but not C-males, showed changes in GPx and body condition, which probably masked any association with a labile trait such as nest provisioning. For this reason, we drew our conclusions from the repeated measure model, which better represents the real-time variation in the physiological and behavioural traits of individuals in our experiment. The enzyme GPx is directly involved in detoxifying intra-cellular hydrogen peroxides by oxidizing glutathione [46], and its increase is generally associated with an increase in metabolic rate [77]. For example, in model species ranging from rats to humans GPx can be upregulated by 20–177% during low–medium endurance exercise [78]. Likewise, GPx was also elevated in female great tits caring for experimentally enlarged broods [79], in migratory European robins (Erithacus rubecula) during nocturnal endurance flight [80], and in short-tailed field voles (Microtus agrestius) thermoregulating at low ambient temperatures [77]. However, high workload can also result in decreased levels of GPx, as observed in zebra finches raising experimentally increased brood sizes [50]. Indeed, GPx synthesis is expected to be inhibited when the oxidative challenge is too high to be buffered, because oxidative stress can damage the genes responsible for encoding GPx expression [81].
The link between metabolic rate and GPx reviewed above could be the cause of the observed GPx upregulation in our feather-clipped male great tits (figure 3). Feather-clipping increases the costs of flight [33,57,63–65], and because feather-clipped males maintained provisioning rates at levels similar to those of control males (table 1), they probably had to elevate metabolic rate. The GPx increase apparently buffered any workload-induced production of ROMs, as plasma ROM concentrations did not change following feather-clipping. However, we observed a positive association between GPx and ROMs, suggesting that individuals that increased GPx more also experienced a greater oxidative challenge. Moreover, individual males that upregulated GPx had decreased chances to survive to the subsequent year. Mounting an antioxidant defence can be a costly process in terms of allocated resources, especially when it involves the re-establishment of original levels of reduced gluthatione (which are oxidized by GPx to buffer the production of hydrogen peroxides [46]). While this is the first study to report a survival cost of increases in GPx concentrations, breeding Seychelles warblers (Acrocephalus sechellensis) that upregulated total non-enzymatic capacity (OXY) during reproduction also had a lower probability to survive to the next year [82].
The negative association between GPx concentrations and survival rate may represent a delayed cost, induced in two potential ways: via cumulative damage caused by the initiation of a costly process involving this enzyme [46,54] or, alternatively, by a cell-signalling role of ROS [41,54,83,84]. Studies on intra-cellular signalling of ROS, especially of peroxide molecules, show that they are able to regulate several processes related to cell growth [84]. We did not find an increase in plasma ROMs (i.e. extracellular organic hydrogen peroxides), but in our study red blood cell GPx levels were positively related to plasma ROM concentrations. We cannot exclude the possibility that ROS concentrations increased inside the cells of diverse tissues. Trade-offs could be mediated by just small alterations of the redox system, and low ROS levels can activate signalling pathways that regulate complex biological processes [85]. Furthermore, not only ROS but also antioxidants can have signalling effects [86]. For example, besides its role as antioxidant, GPx can block the biosynthesis of prostaglandins, lipids with extensive vital roles including the regulation of the vascular system, cell growth and inflammatory processes, or affect apoptosis [86]. Thus, similar to hormones, specific components of the redox system could function as active mediators of life-history trade-offs.
5. Conclusion
Our results are in line with general predictions from life-history theory but are the first experimental data that indicate a role of the redox system in the reproduction–survival trade-off. The current study also highlights the importance of experimental approaches and individual-level analyses in investigations of trade-offs. Furthermore, our findings emphasize the need for further theoretical work. Thus far, the vast majority of hypotheses that have considered the physiological processes underlying life-history trade-offs propose associations between absolute values of physiological traits measured at a single point in time with those of life-history traits also measured once in an individual. However, the physiological system is crucial for responding to an array of external and internal challenges, and therefore analysing individual changes (or reaction norms) are perhaps as important as absolute values, or even more important [23,31,87–91]. The field is now ripe for the development of quantitative predictions for the links between changes in physiology and life-history decisions of individuals.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgement
We are grateful to Wolfgang Goymann for providing valuable feedback on a previous version of the manuscript, Julia Cramer for expert field help, Alessandro Candelari for developing a remote-controlled trap, and Lucia Mentesana and Gary Burness for excellent insights during laboratory meeting discussions. We are also grateful to two anonymous reviewers for their valuable comments.
Ethics
All experimental procedures were conducted according to the legal requirements of Germany and were approved by the governmental authorities of Oberbayern, Germany.
Data accessibility
This article has no additional data.
Authors' contributions
M.H. and S.C. conceived and designed the study; S.C. carried out the fieldwork, laboratory work and statistical analysis; M.H. participated in statistical analysis; S.C. wrote a first version of the manuscript; M.H. contributed in drafting its final version. Authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
We appreciate financial and logistical support of the Max Planck Society for this study.
References
- 1.Stearns SC. 1992. The evolution of life histories. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Zera AJ, Harshman LG. 2001. The physiology of life history trade-offs in animals. Annu. Rev. Ecol. Syst. 32, 95–126. ( 10.1146/annurev.ecolsys.32.081501.114006) [DOI] [Google Scholar]
- 3.Roff DA, Fairbairn DJ. 2007. The evolution of trade-offs: where are we? J. Evol. Biol. 20, 433–447. ( 10.1111/j.1420-9101.2006.01255.x) [DOI] [PubMed] [Google Scholar]
- 4.van Noordwijk AJ, De Jong G. 1986. Acquisition and allocation of resources: their influence on variation in life history tactics. Am. Nat. 128, 137–142. ( 10.1086/284547) [DOI] [Google Scholar]
- 5.Roff DA. 2002. Life history evolution. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 6.Ricklefs RE, Wikelski M. 2002. The physiology/life-history nexus. Trends Ecol. Evol. 17, 462–468. ( 10.1016/S0169-5347(02)02578-8) [DOI] [Google Scholar]
- 7.Folstad I, Karter AJ. 1992. Parasites, bright males, and the immunocompetence handicap. Am. Nat. 139, 603–622. ( 10.1086/285346) [DOI] [Google Scholar]
- 8.Alonso-Alvarez C, Bertrand S, Devevey G, Prost J, Faivre B, Sorci G. 2004. Increased susceptibility to oxidative stress as a proximate cost of reproduction. Ecol. Lett. 7, 363–368. ( 10.1111/j.1461-0248.2004.00594.x) [DOI] [Google Scholar]
- 9.Zera AJ, Harshman LG, Williams TD. 2007. Evolutionary endocrinology: the developing synthesis between endocrinology and evolutionary genetics. Annu. Rev. Ecol. Evol. Syst. 38, 793–817. ( 10.1146/annurev.ecolsys.38.091206.095615) [DOI] [Google Scholar]
- 10.Ketterson ED, Nolan JV. 1999. Adaptation, exaptation and constraint: a hormonal perspective. Am. Nat. 154, S4–S25. ( 10.1086/303280) [DOI] [PubMed] [Google Scholar]
- 11.Crespi EJ, Williams TD, Jessop TS, Delehanty B. 2013. Life history and the ecology of stress: how do glucocorticoid hormones influence life-history variation in animals? Funct. Ecol. 27, 93–106. ( 10.1111/1365-2435.12009) [DOI] [Google Scholar]
- 12.Hau M, Ricklefs RE, Wikelski M, Lee KA, Brawn JD. 2010. Corticosterone, testosterone and life-history strategies of birds. Proc. R. Soc. B 277, 3203–3212. ( 10.1098/rspb.2010.0673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wingfield JC, Sapolsky RM. 2003. Reproduction and resistance to stress: when and how. J. Neuroendocrinol. 15, 711–724. ( 10.1046/j.1365-2826.2003.01033.x) [DOI] [PubMed] [Google Scholar]
- 14.Vitousek MN, Taff CC, Hallinger KK, Zimmer C, Winkler DW. 2018. Hormones and fitness: evidence for trade-offs in glucocorticoid regulation across contexts. Front. Ecol. Evol. 6, 1–14. ( 10.3389/fevo.2018.00042) [DOI] [Google Scholar]
- 15.McEwen BS, Wingfield JC. 2003. The concept of allostasis in biology and biomedicine. Horm. Behav. 43, 2–15. ( 10.1016/S0018-506X(02)00024-7) [DOI] [PubMed] [Google Scholar]
- 16.Romero LM, Wingfield J. 2016. Tempests, poxes, predators, and people. New York, NY: Oxford University Press. [Google Scholar]
- 17.Jimeno B, Hau M, Verhulst S. 2017. Strong association between corticosterone and temperature dependent metabolic rate in individual zebra finches. J. Exp. Biol. 220, 4426–4431. ( 10.1242/jeb.166124) [DOI] [PubMed] [Google Scholar]
- 18.Romero M. 2002. Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen. Comp. Endocrinol. 128, 1–24. ( 10.1016/S0016-6480(02)00064-3) [DOI] [PubMed] [Google Scholar]
- 19.Wingfield JC, Maney DL, Breuner CW, Jacobs JD, Lynn S, Ramenofsky M, Richardson RD. 1998. Ecological bases of hormone–behavior interactions: the ‘emergency life history stage’. Integr. Comp. Biol. 38, 191–206. ( 10.1093/icb/38.1.191) [DOI] [Google Scholar]
- 20.Angelier F, Shaffer SA, Weimerskirch H, Chastel O. 2006. Effect of age, breeding experience and senescence on corticosterone and prolactin levels in a long-lived seabird: the wandering albatross. Gen. Comp. Endocrinol. 149, 1–9. ( 10.1016/j.ygcen.2006.04.006) [DOI] [PubMed] [Google Scholar]
- 21.Breuner CW, Patterson SH, Hahn TP. 2008. In search of relationships between the acute adrenocortical response and fitness. Gen. Comp. Endocrinol. 157, 288–295. ( 10.1016/j.ygcen.2008.05.017) [DOI] [PubMed] [Google Scholar]
- 22.Bonier F, Martin PR, Moore IT, Wingfield JC. 2009. Do baseline glucocorticoids predict fitness? Trends Ecol. Evol. 24, 634–642. ( 10.1016/j.tree.2009.04.013) [DOI] [PubMed] [Google Scholar]
- 23.Hau M, Casagrande S, Ouyang JQ, Baugh AT. 2016. Glucocorticoid-mediated phenotypes in vertebrates: multilevel variation and evolution. In Advances in the study of behavior (eds Naguib M, Mitani JC, Simmons LW, Barrett L, Healy S, Zuk M), pp. 41–115. New York, NY: Academic Press. [Google Scholar]
- 24.Angelier F, Wingfield JC. 2012. Importance of the glucocorticoid stress response in a changing world: theory, hypotheses and perspectives. Gen. Comp. Endocrinol. 190, 118–128. ( 10.1016/j.ygcen.2013.05.022) [DOI] [PubMed] [Google Scholar]
- 25.Vitousek MN, Jenkins BR, Safran RJ. 2014. Stress and success: individual differences in the glucocorticoid stress response predict behavior and reproductive success under high predation risk. Horm. Behav. 66, 812–819. ( 10.1016/j.yhbeh.2014.11.004) [DOI] [PubMed] [Google Scholar]
- 26.Ouyang JQ, Sharp P, Quetting M, Hau M. 2013. Endocrine phenotype, reproductive success and survival in the great tit, Parus major. J. Evol. Biol. 26, 1988–1998. ( 10.1111/jeb.12202) [DOI] [PubMed] [Google Scholar]
- 27.Bonier F, Moore IT, Robertson RJ. 2011. The stress of parenthood? Increased glucocorticoids in birds with experimentally enlarged broods. Biol. Lett. 7, 944–946. ( 10.1098/rsbl.2011.0391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Love OP, Madliger CL, Bourgeon S, Semeniuk CAD, Williams TD. 2014. Evidence for baseline glucocorticoids as mediators of reproductive investment in a wild bird. Gen. Comp. Endocrinol. 199, 65–69. ( 10.1016/j.ygcen.2014.01.001) [DOI] [PubMed] [Google Scholar]
- 29.Altmann J, Lynch JW, Nguyen N, Alberts SC, Gesquiere LR. 2004. Life-history correlates of steroid concentrations in wild peripartum baboons. Am. J. Primatol. 64, 95–106. ( 10.1002/ajp.20064) [DOI] [PubMed] [Google Scholar]
- 30.Goymann W, Trappschuh M, Urasa F. 2017. Corticosterone concentrations reflect parental expenditure in contrasting mating systems of two coucal species. 5, 1–11. ( 10.3389/fevo.2017.00015) [DOI] [Google Scholar]
- 31.Casagrande S, et al. 2018. Do seasonal glucocorticoid changes depend on reproductive investment? A comparative approach in birds. Integr. Comp. Biol. 58, 739–750. ( 10.1093/icb/icy022) [DOI] [PubMed] [Google Scholar]
- 32.Love OP, Breuner CW, Vezina F, Williams TD. 2004. Mediation of a corticosterone-induced reproductive conflict. Horm. Behav. 46, 59–65. ( 10.1016/j.yhbeh.2004.02.001) [DOI] [PubMed] [Google Scholar]
- 33.Love OP, Williams TD. 2008. Plasticity in the adrenocortical response of a free-living vertebrate: the role of pre- and post-natal developmental stress. Horm. Behav. 54, 496–505. ( 10.1016/j.yhbeh.2008.01.006) [DOI] [PubMed] [Google Scholar]
- 34.Sorenson GH, Dey CJ, Madliger CL, Love OP. 2017. Effectiveness of baseline corticosterone as a monitoring tool for fitness: a meta-analysis in seabirds. Oecologia 183, 353–365. ( 10.1007/s00442-016-3774-3) [DOI] [PubMed] [Google Scholar]
- 35.Jaatinen K, Seltmann MW, Hollmén T, Atkinson S, Mashburn K, Öst M. 2013. Context dependency of baseline glucocorticoids as indicators of individual quality in a capital breeder. Gen. Comp. Endocrinol. 191, 231–238. ( 10.1016/j.ygcen.2013.06.022) [DOI] [PubMed] [Google Scholar]
- 36.Ouyang JQ, Sharp PJ, Dawson A, Quetting M, Hau M. 2011. Hormone levels predict individual differences in reproductive success in a passerine bird. Proc. R. Soc. B 278, 2537–2545. ( 10.1098/rspb.2010.2490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crossin GT, Love OP, Cooke SJ, Williams TD. 2016. Glucocorticoid manipulations in free-living animals: considerations of dose delivery, life-history context and reproductive state. Funct. Ecol. 30, 116–125. ( 10.1111/1365-2435.12482) [DOI] [Google Scholar]
- 38.Costantini D. 2008. Oxidative stress in ecology and evolution: lessons from avian studies. Ecol. Lett. 11, 1238–1251. ( 10.1111/j.1461-0248.2008.01246.x) [DOI] [PubMed] [Google Scholar]
- 39.Metcalfe NB, Alonso-Alvarez C. 2010. Oxidative stress as a life-history constraint: the role of reactive oxygen species in shaping phenotypes from conception to death. Funct. Ecol. 24, 984–996. ( 10.1111/j.1365-2435.2010.01750.x) [DOI] [Google Scholar]
- 40.Monaghan P, Metcalfe NB, Torres R. 2009. Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol. Lett. 12, 75–92. ( 10.1111/j.1461-0248.2008.01258.x) [DOI] [PubMed] [Google Scholar]
- 41.Isaksson C, Sheldon BC, Uller T. 2011. The challenges of integrating oxidative stress into life-history biology. Bioscience 61, 194–202. ( 10.1525/bio.2011.61.3.5) [DOI] [Google Scholar]
- 42.Costantini D, Marasco V, Møller AP. 2011. A meta-analysis of glucocorticoids as modulators of oxidative stress in vertebrates. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 181, 447–456. ( 10.1007/s00360-011-0566-2) [DOI] [PubMed] [Google Scholar]
- 43.Haussmann MF, Longenecker AS, Marchetto NM, Juliano SA, Bowden RM. 2012. Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proc. Biol. Sci. 279, 1447–1456. ( 10.1098/rspb.2011.1913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Halliwell B, Whiteman M. 2004. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br. J. Pharmacol. 142, 231–255. ( 10.1038/sj.bjp.0705776) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Finkel T, Holbrook NJ. 2000. Oxidants, oxidative stress and the biology of ageing. Nature 408, 239–247. ( 10.1038/35041687) [DOI] [PubMed] [Google Scholar]
- 46.Halliwell B, Gutteridge JMC. 2007. Free radicals in biology and medicine. Oxford, UK: Oxford University Press. [Google Scholar]
- 47.Speakman JR, et al. 2015. Oxidative stress and life histories: unresolved issues and current needs. Ecol. Evol. 5, 5745–5757. ( 10.1002/ece3.1790) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Costantini D, Verhulst S. 2009. Does high antioxidant capacity indicate low oxidative stress? Funct. Ecol. 23, 506–509. ( 10.1111/j.1365-2435.2009.01546.x) [DOI] [Google Scholar]
- 49.Costantini D, Casasole G, Eens M. 2014. Does reproduction protect against oxidative stress? J. Exp. Biol. 217, 4237–4243. ( 10.1242/jeb.114116) [DOI] [PubMed] [Google Scholar]
- 50.Wiersma P, Selman C, Speakman J, Verhulst S. 2004. Birds sacrifice oxidative protection for reproduction. Proc. R. Soc. Lond. B 271, S360–S363. ( 10.1098/rsbl.2004.0171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blount JD, Vitikainen EIK, Stott I, Cant MA. 2016. Oxidative shielding and the cost of reproduction. Biol. Rev. 91, 483–497. ( 10.1111/brv.12179) [DOI] [PubMed] [Google Scholar]
- 52.Bergeron P, Careau V, Humphries MM, Réale D, Speakman JR, Garant D. 2011. The energetic and oxidative costs of reproduction in a free-ranging rodent. Funct. Ecol. 25, 1063–1071. ( 10.1111/j.1365-2435.2011.01868.x) [DOI] [Google Scholar]
- 53.Fletcher QE, Selman C, Boutin S, McAdam AG, Woods SB, Seo AY, Leeuwenburgh C, Speakman JR, Humphries MM. 2013. Oxidative damage increases with reproductive energy expenditure and is reduced by food-supplementation. Evolution (NY) 67, 1527–1536. ( 10.1111/evo.12014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Speakman JR, Garratt M. 2013. Oxidative stress as a cost of reproduction: beyond the simplistic trade-off model. Bioessays 36, 93–106. ( 10.1002/bies.201300108) [DOI] [PubMed] [Google Scholar]
- 55.Salin K, et al. 2015. Individuals with higher metabolic rates have lower levels of reactive oxygen species in vivo. Biol. Lett. 11, 20150538 ( 10.1098/rsbl.2015.0538) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patterson SH, Hahn TP, Cornelius JM, Breuner CW. 2014. Natural selection and glucocorticoid physiology. J. Evol. Biol. 27, 259–274. ( 10.1111/jeb.12286) [DOI] [PubMed] [Google Scholar]
- 57.Rivers JW, Newberry GN, Schwarz CJ, Ardia DR. 2017. Success despite the stress: violet-green swallows increase glucocorticoids and maintain reproductive output despite experimental increases in flight costs. Funct. Ecol. 31, 235–244. ( 10.1111/1365-2435.12719) [DOI] [Google Scholar]
- 58.Wegmann M, Voegeli B, Richner H. 2015. Oxidative status and reproductive effort of great tits in a handicapping experiment. Behav. Ecol. 26, 747–754. ( 10.1093/beheco/arv006) [DOI] [Google Scholar]
- 59.Fowler MA, Williams TD. 2017. A Physiological signature of the cost of reproduction associated with parental care. Am. Nat. 190 ( 10.1086/694123) [DOI] [PubMed] [Google Scholar]
- 60.Mathot KJ, Frankenhuis WE. 2018. Models of pace-of-life syndromes (POLS): a systematic review. Behav. Ecol. Sociobiol. 72, 41 ( 10.1007/s00265-018-2459-9) [DOI] [Google Scholar]
- 61.Barnes AI, Partridge L. 2003. Costing reproduction. Anim. Behav. 66, 199–204. ( 10.1006/anbe.2003.2122) [DOI] [Google Scholar]
- 62.Williams TD, Fowler MA. 2015. Individual variation in workload during parental care: can we detect a physiological signature of quality or cost of reproduction? J. Ornithol. 156, 441–451. ( 10.1007/s10336-015-1213-6) [DOI] [Google Scholar]
- 63.Patterson SH, Winkler DW, Breuner CW. 2011. Glucocorticoids, individual quality and reproductive investment in a passerine bird. Anim. Behav. 81, 1239–1247. ( 10.1016/j.anbehav.2011.03.012) [DOI] [Google Scholar]
- 64.Tomotani MB, Muijres FT, Koelman J, Casagrande S, Visser ME. 2017. Simulated moult reduces flight performance but overlap with breeding does not affect breeding success in a long-distance migrant. Funct. Ecol. 38, 42–49. ( 10.1111/1365-2435.12974) [DOI] [Google Scholar]
- 65.Sanz JJ, Kranenbarg S, Tinbergen JM. 2000. Differential response by males and females of partner in the great tit manipulation contribution (Parus major). J. Anim. Ecol. 69, 74–84. ( 10.1046/j.1365-2656.2000.00373.x) [DOI] [Google Scholar]
- 66.Bauchau V, VanNoordwijk AJ. 1995. Comparison of survival estimates obtained from three different methods of recapture in the same population of the great tit. J. Appl. Stat. 22, 1031–1037. ( 10.1080/02664769524775) [DOI] [Google Scholar]
- 67.Costantini D, Casagrande S, De Filippis S, Brambilla G, Fanfani A, Tagliavini J, Dell'Omo G. 2006. Correlates of oxidative stress in wild kestrel nestlings (Falco tinnunculus). J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 176, 329–337. ( 10.1007/s00360-005-0055-6) [DOI] [PubMed] [Google Scholar]
- 68.Peig J, Green AJ. 2009. New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118, 1883–1891. ( 10.1111/j.1600-0706.2009.17643.x) [DOI] [Google Scholar]
- 69.Schielzeth H, Nakagawa S. 2013. Nested by design: model fitting and interpretation in a mixed model era. Methods Ecol. Evol. 4, 14–24. ( 10.1111/j.2041-210x.2012.00251.x) [DOI] [Google Scholar]
- 70.Burnham KP, Anderson DR, Burnham KP. 2002. Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn, p. 66 New York, NY: Springer-Verlag. [Google Scholar]
- 71.Angelier F, Bost CA, Giraudeau M, Bouteloup G, Dano S, Chastel O. 2008. Corticosterone and foraging behavior in a diving seabird: the Adélie penguin, Pygoscelis adeliae. Gen. Comp. Endocrinol. 156, 134–144. ( 10.1016/j.ygcen.2007.12.001) [DOI] [PubMed] [Google Scholar]
- 72.Crossin GT, Trathan PN, Phillips R, Gorman K, Dawson A, Sakamoto Q, Williams T. 2012. Corticosterone predicts foraging behavior and parental care in Macaroni penguins. Am. Nat. 180, E31–E41. ( 10.1086/666001) [DOI] [PubMed] [Google Scholar]
- 73.Schoenle LA, Dudek AM, Moore IT, Bonier F. 2017. Red-winged blackbirds (Agelaius phoeniceus) with higher baseline glucocorticoids also invest less in incubation and clutch mass. Horm. Behav. 90, 1–7. ( 10.1016/j.yhbeh.2017.02.002) [DOI] [PubMed] [Google Scholar]
- 74.Bonier F, Martin PR. 2016. How can we estimate natural selection on endocrine traits? Lessons from evolutionary biology. Proc. R. Soc. B 283, 20161887 ( 10.1098/rspb.2016.1887) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Henderson L, Heidinger B, Evans N, Arnold K. 2017. Do glucocorticoids predict fitness? Linking foraging conditions, corticosterone and reproductive success in the blue tit, Cyanistes caeruleus. R. Soc. open sci. 4, 170875 ( 10.1098/rsos.170875) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Madliger CL, Semeniuk CAD, Harris CM, Love OP. 2015. Assessing baseline stress physiology as an integrator of environmental quality in a wild avian population: implications for use as a conservation biomarker. Biol. Conserv. 192, 409–417. ( 10.1016/j.biocon.2015.10.021) [DOI] [Google Scholar]
- 77.Selman C, McLaren JS, Himanka MJ, Speakman JR. 2000. Effect of long-term cold exposure on antioxidant enzyme activities in a small mammal. Free Radic. Biol. Med. 28, 1279–1285. ( 10.1016/S0891-5849(00)00263-X) [DOI] [PubMed] [Google Scholar]
- 78.Powers SK, Jackson MJ. 2010. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol. Rev. 88, 1243–1276. ( 10.1152/physrev.00031.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Norte AC, Ramos JA, Sampaio HL, Sousa JP, Sheldon BC. 2010. Physiological condition and breeding performance of the great tit. Condor 112, 79–86. ( 10.1525/cond.2010.080071) [DOI] [Google Scholar]
- 80.Jenni-Eiermann S, Jenni L, Smith S, Costantini D. 2014. Oxidative stress in endurance flight: an unconsidered factor in bird migration. PLoS ONE 9, e97650 ( 10.1371/journal.pone.0097650) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sahin E, DePinho RA. 2012. Axis of ageing: telomeres, p53 and mitochondria. Nat. Rev. Mol. Cell Biol. 13, 397–404. ( 10.1038/nrm3352) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van de Crommenacker J, Hammers M, van der Woude J, Louter M, Santema P, Richardson DS, Komdeur J. 2017. Oxidative status and fitness components in the Seychelles warbler. Funct. Ecol. 31, 1210–1219. ( 10.1111/1365-2435.12861) [DOI] [Google Scholar]
- 83.Alonso-Alvarez C, Canelo T, Romero-Haro AÁ. 2017. The oxidative cost of reproduction: theoretical questions and alternative mechanisms. Bioscience 67, 176 ( 10.1093/biosci/biw176) [DOI] [Google Scholar]
- 84.Jay FH, Maiorino M, Ursini F. 2010. Signaling functions of reactive oxygen species. Biochemistry 49, 835–842. ( 10.1021/bi9020378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schieber M, Chandel NS. 2014. Review ROS function in redox signaling and oxidative stress. CURBIO 24, R453–R462. ( 10.1016/j.cub.2014.03.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brigelius-Flohé R, Flohé L. 2003. Is there a role of glutathione peroxidases in signaling and differentiation? Biofactors 17, 93–102. ( 10.1002/biof.5520170110) [DOI] [PubMed] [Google Scholar]
- 87.Williams TD. 2008. Individual variation in endocrine systems: moving beyond the ‘tyranny of the Golden Mean’. Phil. Trans. R. Soc. B 363, 1687–1698. ( 10.1098/rstb.2007.0003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dingemanse NJ, Edelaar P, Kempenaers B. 2010. Why is there variation in baseline glucocorticoid levels? Trends Ecol. Evol. 25, 261–262. ( 10.1016/j.tree.2010.01.008) [DOI] [PubMed] [Google Scholar]
- 89.Lema SC, Kitano J. 2013. Hormones and phenotypic plasticity: implications for the evolution of integrated adaptive phenotypes. Curr. Zool. 59, 506–525. ( 10.1093/czoolo/59.4.506) [DOI] [Google Scholar]
- 90.Hau M, Goymann W. 2015. Endocrine mechanisms, behavioral phenotypes and plasticity: known relationships and open questions. Front. Zool. 12, S7 ( 10.1186/1742-9994-12-S1-S7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Taff CC, Vitousek MN. 2016. Endocrine flexibility: optimizing phenotypes in a dynamic world? Trends Ecol. Evol. 31, 476–488. ( 10.1016/j.tree.2016.03.005) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.