Abstract
Coal mining and extraction of methane from coal beds generate effluent with elevated salinity or major ion concentrations. If discharged to freshwater systems, these effluents may have adverse environmental effects. There is a growing body of work on freshwater invertebrates that indicates variation in the proportion of major ions can be more important than salinity when determining toxicity. However, it is not known if saline toxicity in a subset of species is representative of toxicity across all freshwater invertebrates. If patterns derived from a subset of species are representative of all freshwater invertebrates, then we would expect a correlation in the relative sensitivity of these species to multiple saline waters. Here, we determine if there is a correlation between the acute (96 h) lethal toxicity in freshwater invertebrates to synthetic marine salts (SMS) and sodium bicarbonate (NaHCO3) added to dechlorinated Sydney tap water. NaHCO3 is a major component of many coal bed effluents. However, most salinization in Australia exhibits ionic composition similar to seawater, which has very little HCO3−. Across all eight species tested, NaHCO3 was 2–50 times more toxic than SMS. We also observed strong correlations in the acute toxicity of seven of the tested species to SMS and NaHCO3. The strongest relationship (LC50 r2 = 0.906) was dependent on the exclusion of one species, Paratya australiensis (Decopoda: Atyidae), which was the most sensitive species tested to NaHCO3, but the second-most tolerant of SMS. We conclude that differences in the toxicity of different proportions of major ions can be similar across a wide range of species. Therefore, a small subset of the invertebrate community can be representative of the whole. However, there are some species, which based on the species tested in the current study appear to be a minority, that respond differently to saline effluent and need to be considered separately. We discuss the implications of this study for the management of saline coal bed waters.
This article is part of the theme issue ‘Salt in freshwaters: causes, ecological consequences and future prospects'.
Keywords: ecotoxicology, freshwater, invertebrates, bicarbonate, salinity, Australia
1. Introduction
The concentrations of major ions or salinity are increasing in freshwaters from a range of human actives [1], including the disposal of waste water from coal bed methane extraction and coal mining (hereafter coal bed waters) [2–6]. High salinity levels are toxic to freshwater organisms and have other ecological effects [7,8]. There is a concern that disposal of coal bed waters has had and will have adverse effects on organisms in a stream, their populations, communities and ecosystems.
The majority of studies in which salinity sensitivity of species has been measured have used sodium chloride (NaCl), or synthetic marine salts (SMS)—which are approximately 85% NaCl—as the salt source [9–14]. These salts have been widely studied because they are generally representative of salinization from road de-icing with NaCl in cold regions in North America, and dryland salinity on the east coast of Australia, which tends to have ionic proportions more similar to seawater [15]. However, coal bed waters contain high proportions of other major ions, and they do not have proportions of ions similar to seawater [16]. In particular, these waters typically have significant concentrations (20–80% of anions) of bicarbonate (HCO3−), which is much less abundant in seawater (about 0.3% of anions) [17,18]. Relatively few studies have looked into the toxicity of HCO3−-based waters, so little is understood about the potential impact that a release of these waters might have on the invertebrate community within a stream. Furthermore, it would take significant resources to explore the toxicity of HCO3−-based waters to all the relevant species.
Here, we use linear regression analysis to determine if the effects of one combination of major ions to a group of species can predict the effects of another combination of major ions to the same group of species. If regression equations can describe the relationship between the toxicity of SMS and bicarbonate in multiple species, then it shows that the effects of altering the ionic composition observed in a small number of species are broadly representative of the effect on many species. If this is the case, then it may be possible to use a regression equation to estimate the toxicity of bicarbonate to untested species. For example, the toxicity of SMS to 377 freshwater invertebrates from eastern Australia, France, Israel and South Africa has been published [8]. So, if a statistical relationship can be established between a sample of species' toxicity to SMS versus their toxicity to bicarbonate, it could be possible to estimate the toxicity of novel saline waters high in bicarbonate to these 377 species, even if none of them has been tested directly. This method would allow the estimation of toxicity to a large number of species, where it is logistically and financially impossible to test all species directly.
Typically, studies of the effects of specific ion compositions have been conducted on a small number of species, often three or fewer [16,19]. This common approach has several advantages, including a more detailed understanding of the effects of multiple ion compositions on these species. However, this approach has the disadvantage that it remains uncertain whether the effect of altering the proportions of ions on a small number of species is representative of the effect of this alteration on other (un-studied) species. This issue is analogous to toxicity testing of species to a single substance, whereas it is impractical to test all species. Consequently, species sensitivity distributions (SSDs) are used to estimate parameters such as concentrations that will protect a high proportion of species (most of which are untested) [20]. Alternatively, if regression equations cannot describe such relationships, measuring the effect of altering the ionic composition of water on a few species would not be predictive for other species.
In this paper, we determine if there is a direct linear relationship between the toxicity (as indicated by acute LC lethal concentration) values of SMS and that of sodium bicarbonate (NaHCO3) in a sample of freshwater species. NaHCO3 was chosen because it represents the primary compositional difference between natural surface salinity observed in many streams and the saline waters associated with coal beds in Australia [16]. If a relationship is found then, we will explore the possibility of using the extensive SMS toxicity database [8] in the literature to make effective management predictions.
2. Material and methods
(a). Relationships between LC50 values for NaCl and NaHCO3 in existing literature
We extracted all LC50 values of NaCl and NaHCO3 for a range of freshwater animals from the USEPA Ecotoxicology Database (ECOTOX; https://cfpub.epa.gov/ecotox/). These LC50 values were acute (48 h for Ceriodaphnia dubia, Gambusia affinis and Chironomus dilutus, 96 h for other species); comparable toxicity data were found for nine freshwater species. We then conducted a linear regression analysis, to determine if regression equations might adequately describe the relationship between the acute toxicities of NaCl and NaHCO3 to these animals.
(b). Animal collection and transport
Four of the eight tested species (Austrophlebioides pusillus [Ephemeroptera: Leptophlebiidae], Jappa sp., Heloccabus sp. and Dinotoperla sp.), were collected from Rouchel Brook (32° 8'15.40″ S, 150°59'51.16″ E) and the Hunter River (31°55'33.12″ S, 151°14'12.82″ E) in the Hunter Region of New South Wales (NSW), Australia. Paratya australiensis was collected from South West Arm Creek (34°6'54.34″ S, 151°4'5.75″ E) within the Royal National Park, NSW. These five field-collected species were obtained using hand nets (500 µm mesh) after disturbing the bankside vegetation or stream substrate. Water temperature, pH and electrical conductivity (EC) were measured at the site during each sampling event using a multiprobe (Hydrolab Scout 2 model). Total alkalinity and bicarbonate alkalinity of the site and test waters site water were determined by a double-indicator titration method [21]. Bicarbonate alkalinity was converted into a bicarbonate concentration through multiplication using a conversion derived from Kehew [22]. This conversion gives the theoretical maximum value of the bicarbonate anion (HCO3−), and can be considered the HCO3− concentration in the synthetic waters owing to the absence of other anions that contribute to bicarbonate alkalinity, such as phosphate, borate and silicate.
The invertebrates were transported to the laboratory in the water from their collection site, in a portable cooler containing nylon mesh (60 µm) and conditioned leaves collected at the site, to provide substrate and food. The water was kept cool using ice placed outside the coolers and was aerated. On arrival at the laboratory, the invertebrates were placed in the control water: dechlorinated Sydney tap water, at the test temperature (20 ± 1°C), with light cycle 14 h day : 10 h night and light intensity 600–1000 lux. The animals were acclimatized in these conditions for 72 h before commencing toxicity testing. An additional three species were obtained from non-field sources. Isidorella newcombi [Hygrophila:Planorbidae] was collected from uncontaminated plots at the Yanco Agricultural Institute, in Yanco, NSW. Cherax destructor was obtained from a farm located in Karuah, NSW. Ceriodaphnia dubia was obtained from laboratory bred stock at the Office of Environment and Heritage ecotoxicology laboratory in Lidcombe, NSW; original stock animals were collected from the Parramatta River, NSW.
(c). Production of test waters
NaHCO3 treatments were produced by adding reagent grade (Ajax Finechem, UNIVAR) sodium hydrogen carbonate to dechlorinated Sydney tap water. Synthetic Marine Salts water was produced by adding desired amounts of Ocean Nature (Aquasonic) to dechlorinated Sydney tap water. Prior to use, all solutions were pumped through a 0.45 µm filter (Air-Met Scientific, Sydney, NSW) in order to remove any particulates or precipitate that may have formed.
(d). Acute toxicity tests
Ceriodaphnia dubia was tested against both SMS and NaHCO3, as per USEPA method [23]. The remaining seven species of freshwater invertebrate species were tested (against both SMS and NaHCO3) with 96 h of exposure. The test vessels were 1000 ml glass beakers each containing 900 ml of test water, which was not renewed. Each treatment, including the control, consisted of two vessels per test, with A. pusillus tested against NaHCO3 three times, C. destructor, Jappa sp. and P. australiensis tested against NaHCO3 twice and C. dubia and C. destructor tested against SMS twice, increasing the effective replication for these experiments. This level of replication of treatments/controls (per test) is common in toxicity testing [24]. Ten animals of each species were randomly allocated to each of the test vessels. These containers were then randomly placed on a bench, covered with plastic to minimize evaporation, and aerated. Invertebrates were provided with conditioned leaves for food, shelter and substrate. Mortality was defined as the cessation of all visible signs of movement or activity when gently prodded with a probe. Mortality was recorded daily and any dead animals were removed. An experiment would have been considered valid if mortality in the control group (pooled two replicates) did not exceed 10% or one individual, whichever was greater, at the end of the test. However, zero mortality was recorded in every control conducted during this study.
(e). Analyses
Major ion concentrations in the test waters were determined by ion chromatography, in accordance with APHA 4110-B, using a commercial laboratory (Envirolab Services, Chatswood, NSW, Australia). Total alkalinity and bicarbonate alkalinity of control and test waters were determined by a double-indicator titration method at the beginning and end of experiments using the same method as with the collection waters.
All statistical analysis was conducted using SPSS 20. Dose–response curves were generated using probit regression, with mortality as the dependent variable and EC or total salinity concentration the independent variable. The conductivities lethal to 10% of individuals (LC10), 50% of individuals (LC50) and their 95% confidence intervals (CI) were determined from this regression. While similar, both EC and total salinity concentration were used, to provide relevant data to readers that use one or the other. Standard linear regressions were conducted to determine the relationships between acute mortality due to SMS and NaHCO3. We considered the potential for nonlinear relationships by log transforming the LC50 values. The best relationship was determined in terms of r2 as the same number of predictor variables was used in all regressions considered.
3. Results
(a). Relationships between LC50 values for NaCl and NaHCO3 in existing literature
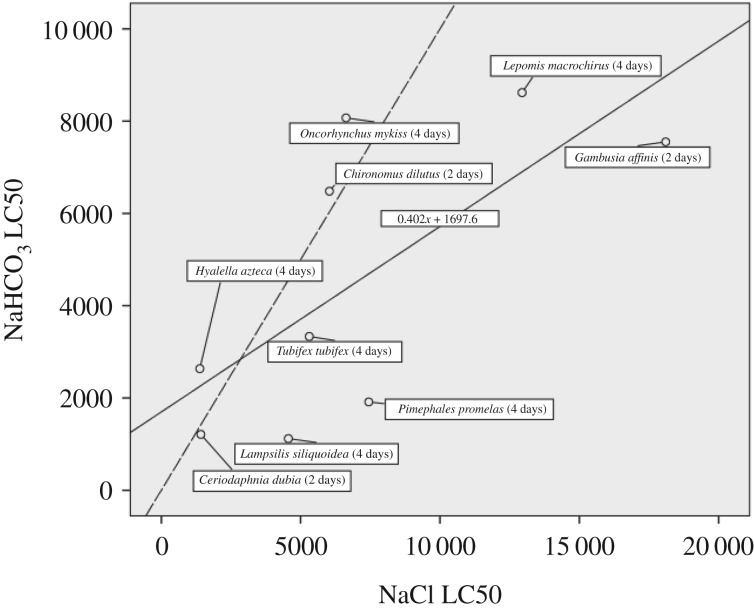
There was a statistically significant linear regression relationship (p = 0.037) between LC50 values for freshwater animals (n = 9 species) for NaCl and NaHCO3 from the ECOTOX database (n = 9 species), with an r2 of 0.487 (figure 1).
Figure 1.
Relationship between LC50 values for NaCl and NaHCO3 in terms of EC (mS cm−1) for freshwater animals in the ECOTOX database. The solid line is the least-squares regression line; the dashed line is 1 : 1 line for reference. The least-squares regression line is described by: y = 0.402x + 1697.6.
(b). Chemistry of test waters
After the 96 h duration of the experiments, measured pH ranged from 7.6 to 8.3 in SMS treatments and 8.2 to 9.6 in NaHCO3 treatments. No carbonate alkalinity was ever detected in any of the controls or treatments at either the beginning or end of each experiment. Bicarbonate alkalinity of each treatment solution did not change over the 96 h test period.
(c). Acute toxicity of NaHCO3 and SMS
Probit regressions were used to produce LC values and dose–response curves on the basis of EC and total salinity concentration values after 96 h. NaHCO3 water was observed to be the most acutely toxic, at 2–50 times more toxic to the invertebrates than SMS water (tables 1 and 2).
Table 1.
Lethal concentration (LC) values with 96 h exposure for 10 and 50% of the test populations in terms of electrical conductivity (mS cm−1 at 25°C) to three significant figures for taxa tested in this study. Numbers in parentheses indicate 95% confidence intervals. Values predicted using probit regression. SMS, synthetic marine salts; n.r., no result. See electronic supplementary material, table S1 for LC values for 72 h exposure.
| test animal | NaHCO3 LC10 | SMS LC10 | NaHCO3 LC50 | SMS LC50 |
|---|---|---|---|---|
| Austrophlebioides pusillus | 2.48 (1.8–2.9) | 5.65 (1.2–8.0) | 3.49 (3.2–3.9) | 11.3 (9.0–14.4) |
| Cerodaphnia dubia | 1.75 (1.5–1.9) | 4.62 (3.7–5.6) | 2.31 (2.2–2.4) | 8.93 (7.3–13.9) |
| Cherax destructor | 7.61 (5.6–8.6) | 49.8 (47.2–51.0) | 10.1 (9.2–11.0) | 52.5 (51.5–53.7) |
| Dinotoperla sp. | 2.51 (1.4–3.2) | n.r. | 4.25 (3.7–4.8) | 12.3 (9.3–16.9) |
| Heloccabus sp. | 7.06 (n.r.) | 13.4 (n.r.) | 7.55 (n.r.) | 16.9 (n.r.) |
| Isidorella newcombi | 1.28 (0.3–1.9) | 4.43 (0.7–6.5) | 3.48 (2.9–4.1) | 10.4 (8.4–13.0) |
| Jappa sp. | 2.84 (2.1–3.2) | 5.62 (1.0–8.1) | 4.12 (3.7–4.8) | 11.3 (8.9–14.1) |
| Paratya australiensis | 0.67 (0.6–0.8) | 33.9 (29.5–35.8) | 1.01 (0.9–1.1) | 38.6 (36.9–40.7) |
Table 2.
Lethal concentration (LC) values with 96 h exposure for 10 and 50% of the test populations in terms of total salinity concentration (g l−1) for taxa tested in this study. Numbers in parentheses indicate 95% confidence intervals. Values predicted using probit regression. SMS, synthetic marine salts; n.r., no result.
| test animal | NaHCO3 LC10 | SMS LC10 | NaHCO3 LC50 | SMS LC50 |
|---|---|---|---|---|
| Austrophlebioides pusillus | 2.4 (1.8–2.7) | 3.4 (0.4–5.0) | 3.4 (3.1–3.7) | 7.3 (5.8–9.4) |
| Cerodaphnia dubia | 1.4 (1.1–1.6) | 2.7 (2.1–3.3) | 2.0 (1.9–2.2) | 5.6 (4.5–8.9) |
| Cherax destructor | 8.0 (5.6–9.3) | 38.1 (35.8–39.1) | 11.2 (10.1–12.4) | 40.4 (39.6–41.5) |
| Dinotoperla sp. | 2.3 (0.3–3.3) | n.r. | 4.3 (3.4–5.2) | 8.0 (6.0–11.3) |
| Heloccabus sp. | 6.6 (n.r.) | 9.1 (n.r.) | 8.0 (n.r.) | 11.5 (n.r.) |
| Isidorella newcombi | 1.1 (0.0–1.7) | 2.7 (0.2–4.1) | 3.5 (2.9–4.3) | 6.8 (5.4–8.6) |
| Jappa sp. | 2.5 (1.1–3.1) | 3.8 (0.4–5.5) | 3.8 (3.3–4.7) | 7.5 (5.9–9.4) |
| Paratya australiensis | 0.6 (0.4–0.6) | 24.4 (20.1–26.0) | 0.8 (0.8–0.9) | 28.3 (26.9–30.1) |
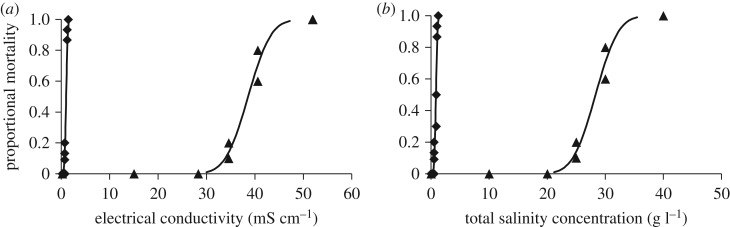
Paratya australiensis (figure 2) was the most sensitive species tested to NaHCO3 with a 96-h LC50 value of 1.0 mS cm−1 (tables 1 and 2). Conversely, P. australiensis was the second-most tolerant species tested to SMS (tables 1 and 2) resulting in a 51- and 38-fold increase in the LC10 and LC50 values for these salts (figure 2).
Figure 2.
Dose–response curves showing the 96 h toxicity of SMS (triangles) and NaHCO3 (diamonds) to Paratya australiensis in terms of measured electrical conductivity (a) and total salinity concentration (b).
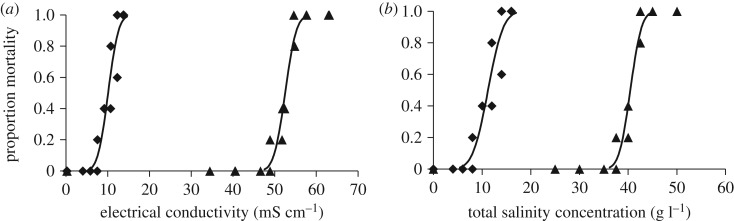
Cherax destructor, another decapod, was the most tolerant to NaHCO3 water and the most tolerant species to SMS (table 1) resulting in 5-fold difference in LC50 values figure 3).
Figure 3.
Dose–response curves showing the 96 h toxicity of SMS (triangles) and NaHCO3 (diamonds) to Cherax destructor in terms of measured (a) electrical conductivity (mS cm−1) and (b) total salinity concentration (g l−1).
Other species tested (tables 1 and 2) had well-fitted concentration response curves, which for reasons of space are given in the electronic supplementary material, figures S8–S15.
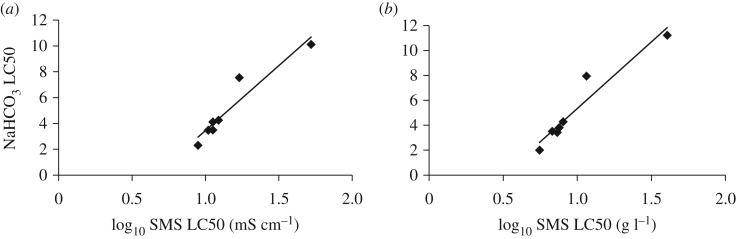
Considering all test species examined (n = 8), acute toxicity to NaHCO3 was not statistically significantly correlated with acute toxicity to SMS, when comparing the LC50 values (r2 = 0.221, p = 0.240). However, P. australiensis was an extreme outlier, being the most sensitive species tested to NaHCO3, and the second-most tolerant of SMS (tables 1 and 2). The regression analyses conducted excluding P. australiensis (n = 7) resulted in statistically significant correlations between acute LC50 values for the two salts (r2 = 0.794, p = 0.007). We explored nonlinear correlations by implementing log10 transformations and found the best relationships occurred when using log10 SMS LC50 values and untransformed NaHCO3 LC50 values (relationships for EC (figure 4a), n = 7, r2 = 0.906, p = 0.001 and total salinity concentration (figure 4b), n = 7, r2 = 0.923, p = 0.001)). Similar relationships were observed with 72 h LC values (electronic supplementary material, figures S16–S21).
Figure 4.
Relationship between log10 LC50 value for SMS and LC50 value for NaHCO3. Straight lines are least-squares regression lines. The relationship for (a) electrical conductivity (mS cm−1) is described by: y = 10.051x − 6.600 and the relationship for (b) total salinity concentration (g l−1) is described by y = 10.686 × x − 5.342.
4. Discussion
Although there is a variability in the hydrochemistry of groundwater within coal seams, concentrations of the major ions within the groundwater can be categorized as primarily fitting two water types: either sodium–bicarbonate, where sodium and bicarbonate are the dominant cation and anion, respectively, or sodium–chloride–bicarbonate where sodium is the dominant cation and chloride and bicarbonate together are the dominant anions [5,25]. Both of these coal bed waters have low calcium, magnesium and sulfate concentrations [5,25–27]. By comparison, inland surface waters on the east coast of Australia salinized by dryland salinity tend to have ionic proportions more similar to seawater, which is sodium chloride-dominated with low bicarbonate concentrations [15]. The primary difference in ionic proportion between CBM waters and the local salinized surface water is the increased concentration of bicarbonate within CBM water.
Given CBM waters have a different major ion composition to surface waters in the study area, the specific ionic composition of these waters has the potential to have adverse environmental effects, aside from total salinity, if discharged into surface water systems. Freshwater organisms may not be equipped to process the ionic compositions prevalent in coal bed waters. Several studies have looked at the acute toxicity of NaCl [14,28] or SMS [8,29,30] to freshwater invertebrates. While some studies conducted on HCO3− have found that HCO3− is toxic to freshwater invertebrates [16,31–34], relatively few of these studies exist; so little is understood about the potential impact of such a release on the invertebrate community within the stream. Furthermore, it would take significant resources to explore the toxicity of HCO3−-based waters to the number of species that have been tested against NaCl and SMS.
In this study, we examined the toxicity of NaHCO3 to eight native freshwater invertebrate species. This allowed us to determine the acute toxicity of NaHCO3 to the animals most likely to be affected by CBM water discharge. We also determined the acute toxicity of SMS to the same species. Acute LC50 values showed NaHCO3 was considerably more toxic than SMS to the species tested (tables 1 and 2). The LC10 and LC50 concentrations of HCO3− determined in these experiments closely match the bicarbonate concentrations commonly found in coal bed waters of Australia and the USA, with a concentration of up to 3.5 g l−1 measured in some cases [26,27,31]. Furthermore, chronic and sub-lethal toxicity to these species and indirect effects [35] are likely to occur at lower concentrations. This finding supports the premise that the specific ionic composition of coal bed waters will have a different toxicity from those more commonly found in surface waters and provides toxicity data for eight species common in receiving streams.
Using linear regressions, we showed a strong direct relationship between the acute toxicity of seven of the tested species to SMS and NaHCO3 (with the best relationship LC50 r2 = 0.906). Given the high r2, we determined there was a potential for a predictive relationship between the toxicity of HCO3−-based waters associated with CBM discharge, and the more frequently studied NaCl-based waters found on the surface in eastern Australia. This means that relatively few species could be examined for each ionic composition in order to broadly understand the tolerances of species within the system. After which, the toxicity database for SMS [8] could be used to predict the toxicity to HCO3−-based waters to the remaining species.
As P. australiensis was the most sensitive species tested to NaHCO3 and the second-most tolerant to SMS, it was an extreme outlier to the regression. The reason for the different response exhibited by P. australiensis is unclear. This response was not repeated in two other crustaceans, C. dubia and C. destructor, the latter belonging to Decapoda, the same order as P. australiensis. So, the response of P. australiensis appears to be unlikely to be a consequence of being a (decapod) crustacean. It is also unlikely a consequence of being extremely tolerant of SMS, as C. destructor was more tolerant of SMS and had the same response as all species other than P. australiensis. Paratya australiensis did not fit the curve and was one in eight species tested or 12.5%. It is plausible that we picked one of the very few species that are such outliers. Alternatively, such species could be quite common and it was chance that we only picked one. Regardless, it is apparent that some species will not fit the predictive regression model. We cannot tell if this frequency is indicative of the proportion of species that do not fit the regression in nature, based on the results of this study alone.
While the current study is the first we are aware of that uses regression analysis to show a relationship between the toxicities of two saline waters of which one is not principally composed of the other (i.e. SMS and NaHCO3). Kefford et al. [28] observed a significant relationship between the LC50 values of NaCl and SMS to freshwater invertebrates (r2 = 0.828). However, as seawater is predominantly NaCl (approximately 85%), this relationship is likely due to NaCl driving the toxicity of seawater in these animals. In order to see if a significant correlation might exist between data found in the literature, we ran a regression analysis using LC50 values of freshwater animals for NaCl and NaHCO3 available in the ECOTOX database. In this case, some 49% of the NaHCO3 LC50 values were explained by the LC50 for NaCl. While this relationship is weaker than the one observed in our study, the relationship for the ECOTOX database is across a wider range of taxonomic groups, including fish, insects, oligochaetes, molluscs and crustaceans. It was also derived using different salts, across multiple laboratories, base waters and testing methods. Regardless, the positive correlation seen in the ECOTOX data supports the findings of this paper.
(a). Limitations
The experiments were conducted with only two saline waters, where many other ionic constituents exist. K+, Mg2+ and Ca2+ are prevalent in many coal bed waters; furthermore, hardness has been shown to modify the toxicity of other ions [16,19,31,36–38]. While there is a strong relationship between SMS and NaHCO3 (the current study) and SMS and NaCl [28], it is yet to be tested if similar relationships exist between other key salt compositions. If relationships between the toxicities of other salt compositions associated with freshwater salinization and more established toxicity data such as those for SMS cannot be found, then the task of setting guidelines for these compositions cannot build on existing toxicity knowledge, but will instead have to start from scratch with each unique saline water composition. Given that NaHCO3 is a key component of coal bed waters [16], the observed correlation here presents a valuable starting point for future research and shows potential for this avenue of regulation and management.
A further concern is that the regression analyses were conducted on a relatively small number of species (9), and one species (P. australensis) was an extreme outlier. It is plausible that we picked one of the very few species that is such an outlier. Alternatively, such species could be quite common and it was chance that we only picked one. Regardless, it is apparent that some species will not fit the predictive regression model. Based on the results of this study alone, we cannot tell if this frequency is indicative of the proportion of species that do not fit the regression in nature. Again, this paper represents a worthwhile starting point for future investigations.
Finally, the results of this study have been expressed against EC and total salinity concentration, rather than osmolality or other measures that might be directly related to toxicity. It is logically plausible that the toxicities of various saline waters are driven by a single variable, such as osmolality [39] or ionic strength [40]. However, the results of this study indicate that osmolality cannot be the only driver of the toxicity of SMS and NaHCO3. If the relationship between the toxicities of SMS and NaHCO3 could be attributed only to osmolality, then we would expect a linear relationship to have been observed as EC and the total salinity concentration are linearly correlated with osmolality [41]. However, the strongest relationship was described using a regression between NaHCO3 and log-transformed SMS, indicating a nonlinear relationship. The toxicity of NaHCO3 to the outlier P. australensis was an order of magnitude greater than the toxicity of SMS, indicating that osmolality could not be a driver for this species. Furthermore, recent work suggests the standard physiological model where toxicity is based on osmolality does not describe the toxicity of saline solutions to salt-sensitive mayflies [29,42]. So while osmolality may be a contributing factor to our ability to fit a regression to the toxicity of SMS and NaHCO3, it appears to be unlikely to be the only factor covering all the species considered.
(b). Potential for confounding factors
The NaHCO3 treatments (pH 8.2–9.6) tended to have higher pH values than the SMS treatments (pH 7.6–8.3) after 96 h, which were not likely to cause damage to the ionocytes (also referred to as chloride cells or mitochondria-rich cells) in the short exposure time. For example, Peters et al. [43] observed no ultrastructural changes in the ionocytes of the mayfly Isonychia bicolor exposed to sub-lethal alkaline levels of pH less than or equal to 10 for 96 h. High pH has been observed to reduce SMS salinity tolerance [44]. Regardless, even if some of the mortality in the NaHCO3 treatments was the result of increased pH, increased pH is a likely consequence of CBM waters with high HCO3− concentrations. So, the rise in pH may represent some of the effects of NaHCO3 toxicity. However, if pH was a strong driver of the toxicity we see in these waters, then we would not expect to see such a strong relationship.
In the current study, we observed that, in terms of EC and total salinity, NaHCO3 was more toxic than SMS, to all species tested; similarly data from the ECOTOX database showed that NaHCO3 was always more toxic to freshwater animals than NaCl. This is in contrast to the effects observed on leaf litter breakdown by microbes [15]. That study observed that both NaCl-based waters with ionic proportions similar to seawater and coalmine discharge waters with high NaHCO3 reduced the rate of leaf litter breakdown by microbes in nature. However, of the two, NaCl-based waters had the stronger effect. Subsequent experiments showed that SMS and NaCl caused a greater reduction in leaf litter breakdown by microbes than NaHCO3 at the same EC. The reason for the contradictory results between the relative toxicity of NaHCO3 versus SMS for freshwater invertebrates and leaf litter breakdown by microbes is not known. This contradiction might suggest complications in comparing the ecological effects of SMS and NaHCO3 on populations and communities of invertebrates in natural systems. On one hand, direct toxicity to invertebrates is greater from NaHCO3 than SMS. On the other hand, a significant proportion of food for stream invertebrates can be provided by the breakdown of leaf litter, which is facilitated by microbes. So it is plausible that in nature some of the toxic effects of NaHCO3-based waters could be (partially) reduced through their having a lesser effect on food resources than NaCl-based waters. Field or mesocosm studies could be conducted to determine if the relative toxicity observed here can be reproduced in field (-like) conditions.
Supplementary Material
Acknowledgements
We are particularly thankful to the following people who served on a steering committee: Christopher Doyle, Moreno Julli, Georgina Kelly, Klaus Koop and Keith Osborne. We are grateful to the DPI Yanco Agricultural Institute for supply of snails. We thank Renee Dowse, Sherri Lehmann, Amanda McDonald and Fleur Pablo for helping with finding sites, laboratory work, field work, advice and moral support. We also thank the peer reviewers and Miguel Cañedo Argüelles for their helpful comments that improved the paper.
Data accessibility
Data available as part of the electronic supplementary material.
Authors' contributions
Overall design concept: B.J.K.; detailed design of the experiment: K.A.H., R.V.H., B.J.K.; provision of funding: B.J.K., R.V.H.; provision of equipment and resources: R.V.H.; collection of data: K.A.H., R.V.H.; data analysis: K.A.H.; production of first draft: K.A.H.; editing of the manuscript and approval of final version: all.
Competing interests
We declare we have no competing interests.
Funding
The work reported here was funded by Australian Research Council Linkage Projects LP130100100 awarded to B.J.K. and R.V.H. from which K.A.H. derived a scholarship. Cash funding and in-kind contributions were also made from the NSW Office of Environment and Heritage, including equipment, resources and staff time.
References
- 1.Cañedo-Argüelles M, Kefford BJ, Piscart C, Prat N, Schäfer RB, Schulz CJ. 2013. Salinisation of rivers: an urgent ecological issue. Environ. Pollut. 173, 157–167. ( 10.1016/j.envpol.2012.10.011) [DOI] [PubMed] [Google Scholar]
- 2.Patz MJ, Ready KJ, Skinner QD. 2004. Chemistry of coalbed methane discharge water interacting with semi-arid ephemeral stream channels 1. J. Am. Water Resour. Assoc. 40, 1247–1255. ( 10.1111/j.1752-1688.2004.tb01583.x) [DOI] [Google Scholar]
- 3.Veil JA, Puder MG, Elcock D, Redweik RJ. Jr. 2004. A white paper describing produced water from production of crude oil, natural gas, and coal bed methane. Lemont, IL: Argonne; National Lab. [Google Scholar]
- 4.Jackson R, Reddy KJ. 2007. Geochemistry of coalbed natural gas (CBNG) produced water in Powder River Basin, Wyoming: salinity and sodicity. Water Air Soil Pollut. 184, 49–61. ( 10.1007/s11270-007-9398-9) [DOI] [Google Scholar]
- 5.Hamawand I, Yusaf T, Hamawand SG. 2013. Coal seam gas and associated water: a review paper. Renew. Sustain. Energy Rev. 22, 550–560. ( 10.1016/j.rser.2013.02.030) [DOI] [Google Scholar]
- 6.Wright IA. 2012. Coal mine ‘dewatering'of saline wastewater into NSW streams and rivers: a growing headache for water pollution regulators. In Proc. 6th Australian Stream Management Conf. Managing for Extremes (eds Grove JR, Rutherford ID), pp. 1–8. Canberra, Australia: River Basin Management Society. [Google Scholar]
- 7.Kefford BJ, Buchwalter D, Canedo-Argüelles M, Davis J, Duncan RP, Hoffmann A, Thompson R. 2016. Salinized rivers: degraded systems or new habitats for salt-tolerant faunas? Biol. Lett. 12, 20151072 ( 10.1098/rsbl.2015.1072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kefford BJ, Hickey GL, Gasith A, Ben-David E, Dunlop JE, Palmer CG, Allan K, Choy SC, Piscart C. 2012. Global scale variation in the salinity sensitivity of riverine macroinvertebrates: eastern Australia, France, Israel and South Africa. PloS ONE 7, e35224 ( 10.1371/journal.pone.0035224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kefford B, Dunlop JD, Choy S. 2007. Understanding salinity thresholds in freshwater biodiversity: freshwater to saline transition. In Salt, nutrient, sediment and interactions: findings from the national river contaminants program (eds Lovett S, Price P, Edgar B), pp. 9–28. Canberra, Australia: Land & Water Australia. [Google Scholar]
- 10.Kefford BJ. 2014. Rapid tests for community level risk assessments. In Encyclopedia of aquatic ecotoxicology (ed. Blaise CAF.), pp. 957–966. Dordrecht, The Netherlands: Springer Publishers. [Google Scholar]
- 11.Batterton JC, Baalen CV. 1971. Growth response of blue-green algae to sodium chloride concentration. Arch. Mikrobiol. 76, 151–165. ( 10.1007/BF00411789) [DOI] [PubMed] [Google Scholar]
- 12.Dickman MD, Gochnauer MB. 1978. Impact of sodium chloride on the microbiota of a small stream. Environ. Pollut. 17, 109–126. ( 10.1016/0013-9327(78)90044-7) [DOI] [Google Scholar]
- 13.Environment Canada. 2001. Priority substances list assessment report – road salts, p. 171 Gatineau, QC: Environment Canada. [Google Scholar]
- 14.Gillis PL. 2011. Assessing the toxicity of sodium chloride to the glochidia of freshwater mussels: implications for salinization of surface waters. Environ. Pollut. 159, 1702–1708. ( 10.1016/j.envpol.2011.02.032) [DOI] [PubMed] [Google Scholar]
- 15.Sauer FG, Bundschuh M, Zubrod JP, Schäfer RB, Thompson K, Kefford BJ. 2016. Effects of salinity on leaf breakdown: dryland salinity versus salinity from a coalmine. Aquat. Toxicol. 177, 425–432. ( 10.1016/j.aquatox.2016.06.014) [DOI] [PubMed] [Google Scholar]
- 16.Vera CL, Hyne RV, Patra R, Ramasamy S, Pablo F, Julli M, Kefford BJ. 2014. Bicarbonate toxicity to Ceriodaphnia dubia and the freshwater shrimp Paratya australiensis and its influence on zinc toxicity. Environ. Toxicol. Chem. 33, 1179–1186. ( 10.1002/etc.2545) [DOI] [PubMed] [Google Scholar]
- 17.Gros N, Camões MF, Oliveira C, Silva MCR. 2008. Ionic composition of seawaters and derived saline solutions determined by ion chromatography and its relation to other water quality parameters. J. Chromatogr. A 1210, 92–98. ( 10.1016/j.chroma.2008.09.046) [DOI] [PubMed] [Google Scholar]
- 18.Cormier SM, Wilkes SP, Zheng L.. 2013. Relationship of land use and elevated ionic strength in Appalachian watersheds. Environ. Toxicol. Chem. 32, 296–303. ( 10.1002/etc.2055) [DOI] [PubMed] [Google Scholar]
- 19.Mount DR, Gulley DD, Hockett JR, Garrison TD, Evans JM. 1997. Statistical models to predict the toxicity of major ions to Ceriodaphnia dubia, Daphnia magna and Pimephales promelas (flathead minnows). Environ. Toxicol. Chem. 16, 2009–2019. ( 10.1897/1551-5028(1997)016%3C2009:SMTPTT%3E2.3.CO;2) [DOI] [Google Scholar]
- 20.Posthuma L, Suter GW, Traas T. 2001. Species sensitivity distributions in ecotoxicology, p. 587 Boca Raton, FL: CRC Press. [Google Scholar]
- 21.Hach. 2007–2017 Phenolphthalein and total alkalinity. Method 8221. Buret titration. DOC316.53.01151, 9th edn Loveland, CO: Hach Company; See https://www.hach.com/asset-get.download-en.jsa?id=7639983916. [Google Scholar]
- 22.Kehew AE. 2000. Applied chemical hydrogeology. Englewood Cliffs, NJ: Prentice Hall. [Google Scholar]
- 23.USEPA. 2002. Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms, 5th edn Washington, DC: United States Environmental Protection Agency. [Google Scholar]
- 24.Slaughter A, Palmer C, Muller W. 2008. A chronic toxicity test protocol using Caridina nilotica (Decapoda: Atyidae) and the generation of salinity toxicity data. Afr. Aquat. Sci. 33, 37–44. ( 10.2989/AJAS.2007.33.1.4.388) [DOI] [Google Scholar]
- 25.Van Voast WA. 2003. Geochemical signature of formation waters associated with coalbed methane. AAPG Bull. 87, 667–676. ( 10.1306/10300201079) [DOI] [Google Scholar]
- 26.Kinnon ECP, Golding SD, Boreham CJ, Baublys KA, Esterle JS. 2010. Stable isotope and water quality analysis of coal bed methane production waters and gases from the Bowen Basin, Australia. Int. J. Coal Geol. 82, 219–231. ( 10.1016/j.coal.2009.10.014) [DOI] [Google Scholar]
- 27.Papendick SL, Downs KR, Vo KD, Hamilton SK, Dawson GKW, Golding SD, Gilcrease PC. 2011. Biogenic methane potential for Surat Basin, Queensland coal seams. Int. J. Coal Geol. 88, 123 ( 10.1016/j.coal.2011.09.005) [DOI] [Google Scholar]
- 28.Kefford BJ, Palmer CG, Pakhomova L, Nugegoda D. 2004. Comparing test systems to measure the salinity tolerance of freshwater invertebrates. Water SA 30, 499–506. ( 10.4314/wsa.v30i4.5102) [DOI] [Google Scholar]
- 29.Dowse R, Palmer CG, Hills K, Torpy F, Kefford BJ. 2017. The mayfly nymph Austrophlebioides pusillus Harker defies common osmoregulatory assumptions. R. Soc. Open Sci. 4, 160520 ( 10.1098/rsos.160520) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kefford BJ, Fields EJ, Nugegoda D, Clay C. 2007. The salinity tolerance of riverine microinvertebrates from the southern Murray Darling Basin. Mar. Freshw. Res. 58, 1019–1031. ( 10.1071/MF06046) [DOI] [Google Scholar]
- 31.Hoke RA, Gala WR, Drake JB, Giesy JP, Flegler S. 1992. Bicarbonate as a potential confounding factor in cladoceran toxicity assessments of pore water from contaminated sediments. Can. J. Fish. Aquat. Sci. 49, 1633–1640. ( 10.1139/f92-182) [DOI] [Google Scholar]
- 32.Palmer MA, et al. 2010. Mountaintop mining consequences. Science 327, 148–149. ( 10.1126/science.1180543) [DOI] [PubMed] [Google Scholar]
- 33.Farag AM, Harper DD (eds). 2012. The potential effects of sodium bicarbonate, a major constituent of produced waters from coalbed natural gas production, on aquatic life. Reston, VA: U.S. Geological Survey. [Google Scholar]
- 34.Cormier SM, Suter GW, Zheng L, Pond GJ. 2013. Assessing causation of the extirpation of stream macroinvertebrates by a mixture of ions. Environ. Toxicol. Chem. 32, 277–287. ( 10.1002/etc.2059) [DOI] [PubMed] [Google Scholar]
- 35.Bray JP, Reich J, Nichols SJ, Kon Kam King G, Mac Nally R, Thompson R, O'Reilly-Nugent A, Kefford BJ.. 2019. Biological interactions mediate context and species-specific sensitivities to salinity. Phil. Trans. R. Soc. B 374, 20180020 ( 10.1098/rstb.2018.0020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soucek DJ, Linton TK, Tarr CD, Dickinson A, Wickramanayake N, Delos CG, Cruz LA. 2011. Influence of water hardness and sulfate on the acute toxicity of chloride to sensitive freshwater invertebrates. Environ. Toxicol. Chem. 30, 930–938. ( 10.1002/etc.454) [DOI] [PubMed] [Google Scholar]
- 37.Soucek DJ. 2007. Comparison of hardness- and chloride-regulated acute effects of sodium sulfate on two freshwater crustaceans. Environ. Toxicol. Chem. 26, 773–779. ( 10.1897/06-229R.1) [DOI] [PubMed] [Google Scholar]
- 38.Soucek DJ, Kennedy AJ. 2005. Effects of hardness, chloride, and acclimation on the acute toxicity of sulfate to freshwater invertebrates. Environ. Toxicol. Chem. 24, 1204–1210. ( 10.1897/04-142.1) [DOI] [PubMed] [Google Scholar]
- 39.Mount DR, et al. 2016. The acute toxicity of major ion salts to Ceriodaphnia dubia: I. Influence of background water chemistry. Environ. Toxicol. Chem. 35, 3039–3057. ( 10.1002/etc.3487) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pond GJ, Passmore ME, Borsuk FA, Reynolds L, Rose CJ. 2008. Downstream effects of mountaintop coal mining: comparing biological conditions using family- and genus-level macroinvertebrate bioassessment tools. J. N. Am. Benthol. Soc., 27, 717–737. ( 10.1899/08-015.1) [DOI] [Google Scholar]
- 41.Kefford BJ, Papas PJ, Nugegoda D. 2003. Relative salinity tolerance of macroinvertebrates from the Barwon River, Victoria, Australia. Mar. Freshwater Res. 54, 755–765. ( 10.1071/MF02081) [DOI] [Google Scholar]
- 42.Kefford BJ. 2019. Why are mayflies (Ephemeroptera) lost following small increases in salinity? Three conceptual osmophysiological hypotheses. Phil. Trans. R. Soc. B 374, 20180021 ( 10.1098/rstb.2018.0021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peters GT, Cherry DS, Cairns J Jr. 1985. Responses of Isonychia bicolor to alkaline pH: an evaluation of survival, oxygen consumption, and chloride cell ultrastructure. Can. J. Fish. Aquat. Sci. 42, 1088–1095. ( 10.1139/f85-135) [DOI] [Google Scholar]
- 44.Zalizniak L, Kefford BJ, Nugegoda D. 2009. Effects of pH on salinity tolerance of selected freshwater invertebrates. Aquatic Ecology 43, 135–144. ( 10.1007/s10452-007-9148-5) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available as part of the electronic supplementary material.