Abstract
Recent discoveries have documented evolutionary responses to freshwater salinization. We investigated if evolutionary responses to salinization exhibit life-history trade-offs or if they can mitigate ecological impacts such as cascading effects through mechanisms of tolerance and cross-tolerance. We conducted an outdoor mesocosm experiment using populations of Daphnia pulex—a ubiquitous algal grazer—that were either naive or had previously experienced selection to become more tolerant to sodium chloride (NaCl). During the initial phase of population growth, we discovered that evolved tolerance comes at the cost of slower population growth in the absence of salt. We found evolved Daphnia populations maintained a tolerance to NaCl approximately 30 generations after the initial discovery. Evolved tolerance to NaCl also conferred cross-tolerance to a high concentration of CaCl2 (3559 µS cm−1) and a moderate concentration of MgCl2 (967 µS cm−1). A higher concentration of MgCl2 (2188 µS cm−1) overwhelmed the cross-tolerance and killed all Daphnia. Tolerance to NaCl did not mitigate NaCl-induced cascades leading to phytoplankton blooms, but cross-tolerance at moderate concentrations of MgCl2 and high concentrations of CaCl2 mitigated such cascading effects caused by these two salts. These discoveries highlight the important interplay between ecology and evolution in understanding the full impacts of freshwater salinization.
This article is part of the theme issue ‘Salt in freshwaters: causes, ecological consequences and future prospects’.
Keywords: contemporary adaptation, deicing, global change, salinization, road salt, trophic cascades
1. Introduction
Salinization of freshwater ecosystems worldwide threatens ecological diversity, community structure and ecosystem services [1–5]. Salinization occurs from many sources, including agriculture, resource mining, climate warming and the application of deicing salts [6,7]. Every type of freshwater ecosystem has been affected by salinization, from small streams to large rivers and small wetlands to large lakes [3,6,8–10]. Since most freshwater organisms do not have a recent evolutionary history with high salinity, it is critically important to identify the ecological and evolutionary impacts of freshwater salinization.
Scientific attention on salinization resulting from the application of road-deicing salts has increased exponentially from the 1970s to the present. This has occurred because road salt concentrations in freshwater systems can be quite severe. For instance, the chloride (Cl−) concentration in stream ecosystems can reach 25% of the concentration of seawater [11]. A recent study estimated that in the next 50 years 27% of temperate lake ecosystems in the United States may exceed the 230 mg Cl− l−1 threshold to protect freshwater biota established by the United States Environmental Protection Agency, owing primarily to the use of road salts [12]. Such instances of elevated salinity occur in freshwater systems because of the use of inorganic deicing salts. The most widely used salt is sodium chloride (NaCl, ‘rock salt’), but others such as calcium chloride (CaCl2) and magnesium chloride (MgCl2) are becoming more common in road deicing operations [13].
Road salt salinization has negative ecological impacts. Recent studies show that road salts can substantially reduce the abundance of zooplankton grazers [14–16] and interact with natural stressors like predation [17,18]. Such direct and synergistic effects trigger cascading effects leading to phytoplankton blooms [18,19]. Road salts can also reduce the abundance or growth of multiple freshwater vertebrates such as amphibians and fish [20–22]. Over time, these effects can generate communities comprised of salt-tolerant species [23–26]. Moreover, salinization alters ecosystem functions, such as decomposition and nutrient cycling [27–29]. While road salts can dramatically change the ecology of freshwater ecosystems, less known are the evolutionary impacts in fresh waters.
In a few cases, researchers have discovered that freshwater organisms can exhibit evolutionary responses to road salt. For example, roadside populations of amphibians can be locally adapted to high salinity resulting from road salt applications [30,31]. Recently, researchers demonstrated the experimental evolution of a ubiquitous species of zooplankton (Daphnia pulex), resulting in higher NaCl tolerance after multi-generational exposure to NaCl [32]. This was an important discovery because Daphnia are a critical component of freshwater food webs, responsible for transferring energy from primary producers to higher trophic levels [33]. Given this evolved tolerance in populations, key questions are whether the NaCl-evolved tolerance: (i) comes with trade-offs that affect Daphnia population growth in the absence of NaCl, (ii) buffers Daphnia from subsequent NaCl exposures and varies in magnitude depending on past exposures to different NaCl concentrations, (iii) provides Daphnia with cross-tolerance to other salts and varies in magnitude depending on past exposures to different NaCl concentrations and (iv) mitigates salt-induced cascades leading to phytoplankton blooms. Given that high concentrations of NaCl can decimate zooplankton populations and indirectly cause phytoplankton blooms, the evolution of salt tolerance poses the possibility of mitigating these salt-induced cascades. We addressed these questions using one naive population and two NaCl-evolved populations of Daphnia in an outdoor mesocosm experiment. Two Daphnia populations used in our study evolved a tolerance to NaCl road salt in a prior mesocosm experiment [18] and we confirmed this increased tolerance in a follow-up experiment [32]. We maintained Daphnia from the original experiment [18] in low-salt (approx. 125 µS cm−1 or 15 mg Cl− l−1) laboratory cultures for approximately 1 year until we began the present study.
2. Methods
Our experiment at the Rensselaer Aquatic Laboratory (Troy, New York, USA) was a completely randomized factorial design that crossed three populations of Daphnia pulex with the three road salt types (NaCl, MgCl2 and CaCl2) that were each added at moderate and high concentrations and included a no-salt control for each population (figure 1 and table 1). Concentrations were chosen based on published literature values on the relative toxicity of the three salts to zooplankton. Of the three salts, MgCl2 appears to be the most toxic to zooplankton, followed by NaCl then CaCl2 [34–36], but some tests suggest that NaCl is less toxic than CaCl2 [37]. Our goal was to include one concentration that was sublethal and another that approached a lethal level. The Daphnia population types were a naive population with no prior exposure to elevated NaCl concentrations and the two other Daphnia populations that evolved tolerance to 833 µS cm−1 (250 mg Cl− l−1; Population 1 [P1]) and 2662 µS cm−1 (1000 mg Cl− l−1; Population 2 [P2]) of NaCl [18,32]. The 21 treatment combinations were replicated four times for a total of 84 experimental mesocosms. Each mesocosm was also populated with two snail species (Physella acuta and Helisoma trivolvus) and one species of amphipod (Hyalella sp.). These species co-occur naturally with D. pulex and they consume periphyton, thereby maintaining a more natural ecological community. Please see the electronic supplementary material for further methods and results for periphyton and macroconsumers.
Figure 1.
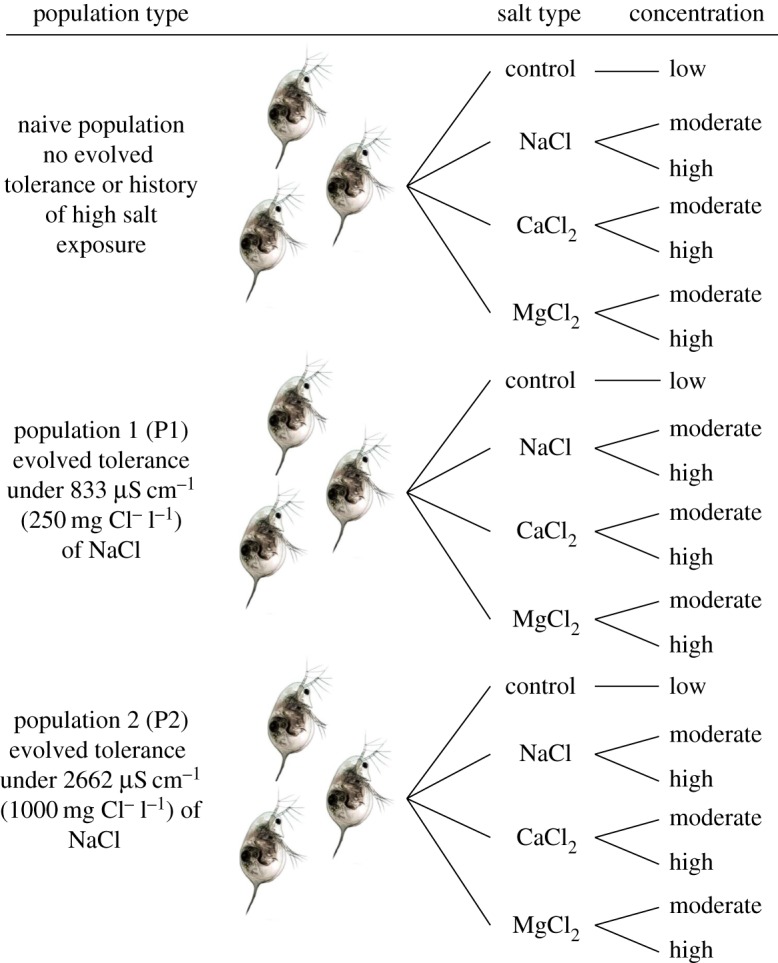
Experimental design to evaluate if evolved tolerance to NaCl confers life-history trade-offs in the absence of NaCl, tolerance to moderate and high NaCl concentrations, cross-tolerance to moderate and high concentrations of MgCl2 and CaCl2, and whether such tolerance and cross-tolerance can mitigate salt-induced cascades. Each treatment was replicated four times. See table 1 for the conductivities of each salt type. (Online version in colour.)
Table 1.
Measured conductivities for the treatments used to test cross-tolerance in Daphnia pulex and if tolerance and cross-tolerance could mitigate salt-induced cascading effects leading to phytoplankton blooms.
| treatment | conductivity (µS cm−1) ± 95% CI |
|---|---|
| control | 250 ± 55 |
| moderate NaCl | 1813 ± 55 |
| high NaCl | 4367 ± 135 |
| moderate CaCl2 | 1501 ± 43 |
| high CaCl2 | 3559 ± 106 |
| moderate MgCl2 | 967 ± 29 |
| high MgCl2 | 2188 ± 65 |
The 84 experimental tanks (58.4 cm × 49.5 cm Rubbermaid BRUTE™ trash cans) were filled with 65 l of aged tap water on 17 May 2016. On 20 May, we added 20 g of mostly oak leaf litter (Quercus spp.) and 1.5 g of rabbit chow to each mesocosm to serve as basal nutrient sources. On 23 May, we added a 450 ml aliquot of phytoplankton from Lake George, New York (USA). The aliquot was filtered four times through a 53 µm sieve and treated with carbonated water to ensure no additional zooplankton were introduced to the experimental tanks with the phytoplankton aliquots. Examination of the aliquot revealed no zooplankton. On 10 June, we added 30 Daphnia from the three evolved populations to their respective mesocosms. We added the three road salts to the mesocosms on 14 June, which was day 1 of the experiment. We conducted the experiment for 60 days. Additional details on water quality parameters for the water sources used in the experiment and culturing can be found in the electronic supplementary material.
We measured abiotic conditions multiple times throughout the experiment to examine whether abiotic conditions remained similar among treatments. We sampled abiotic conditions six times during the experiment (28 June; 6, 12 and 27 July; 4 and 12 August). We used a calibrated YSI ProPlus multiparameter instrument to measure temperature (°C), dissolved oxygen (mg O2 l−1), conductivity (µS cm−1) and pH (YSI, Yellow Springs, Ohio, USA). Summary tables of the mean, standard deviation and variance for temperature, dissolved oxygen and pH are presented in the electronic supplementary material, tables S1–S3.
We sampled Daphnia and phytoplankton abundance on 5 July (day 21), 28 July (day 44) and 12 August (day 60). For zooplankton, we collected two 450 ml samples from the west side and centre of each mesocosm from the benthos to the water surface. The pooled 900 ml sample was filtered through 64 µm Nitex screening and Daphnia were preserved in Lugol's solution for later enumeration. We estimated phytoplankton biomass by filtering 700 ml of water collected from the centre of each mesocosm at 50% of the depth through glass microfibre filters (1.2 µm pore size; Whatman). The glass microfibre filters were frozen after filtration until fluorometry with acid correction chlorophyll a analysis [38], which was done within two weeks of freezing.
(a). Statistical analyses
We used analysis of variance (ANOVA) within each sampling date to determine the effects of population type, salt type and concentration and their interactions on Daphnia and phytoplankton abundance. If we found interactive or main effects in the ANOVAs, we examined pairwise comparisons with a Holm–Sidak multiple comparisons procedure. As noted above, we had specific hypotheses and therefore conducted an a priori subset of all possible mean comparisons. To determine if NaCl-evolved tolerance comes at the cost of reduced Daphnia population growth, we compared the abundance of the three Daphnia populations in the absence of any salt using a one-way ANOVA.
To determine if NaCl-evolved tolerance buffers Daphnia from subsequent NaCl exposures and whether this tolerance varies in magnitude depending on past exposures to different NaCl concentrations, we conducted a two-way ANOVA using the no-salt and elevated NaCl treatments. In this analysis, evidence of increased tolerance would be (a) the presence of a population-by-salt interaction and (b) larger proportional declines in the abundance of the naive population than in the evolved population when comparing the no-salt and elevated NaCl environments.
To determine if NaCl-evolved tolerance provides Daphnia with cross-tolerance to other salts and if the cross-tolerance varies in magnitude depending on past exposures to different NaCl concentrations, we conducted a two-way ANOVA using the no-salt and either the elevated CaCl2 or MgCl2 concentrations. Similar to the analysis of tolerance, evidence of cross-tolerance would be (a) the presence of a population-by-salt interaction and (b) larger proportional declines in the abundance of the naive population than in the evolved population when comparing the no-salt and elevated salt environments.
To determine if NaCl-evolved tolerance and cross-tolerance mitigate cascading effects in the presence of each salt, we conducted three, two-way ANOVAs; each ANOVA used the no-salt control and the two concentrations of a given salt. We then used mean comparisons to determine whether naive Daphnia populations that experienced a decline in abundance owing to salt exposure caused an indirect positive effect on phytoplankton (i.e. a salt-induced cascade). Given a cascading effect with naive Daphnia populations, evidence for its mitigation with NaCl-evolved Daphnia populations would be (a) a significant population-by-salt interaction and (b) larger proportional increases in phytoplankton when the naive Daphnia population was exposed to elevated salt environments compared to the evolved populations that were exposed to the elevated salt environments.
There was intense variability in the responses of Daphnia and phytoplankton abundance throughout the experiment. However, the differences between sample means among many of the group comparisons was high. Given the signal to noise ratio in our data and in accordance with the American Statistical Association's statement on p-values [39], we did not use the binary decision rule where p ≤ 0.05 was deemed statistically and hence ecologically significant. However, we did limit our designation of statistical significance. We evaluated statistical significance when p < 0.10 and we report exact p-values and the associated effect sizes (i.e. differences between sampled means).
Additional details of the statistical analyses can be found in the electronic supplementary material.
3. Results
(a). Tolerance trade-offs in Daphnia abundance
On day 21, we found an effect of population type on the abundance of Daphnia in the absence of added salt (i.e. the control; F2,9 = 4.2, p = 0.052). Compared to the naive population, Daphnia abundance was 65% and 67% lower in populations P1 (t = 2.6, p = 0.089) and P2 (t = 2.6, p = 0.074), respectively. On days 44 and 60, we found no effect of NaCl tolerance on Daphnia abundance (F2,9 ≤ 1.9, p ≥ 0.202).
(b). Tolerance to subsequent exposures of NaCl
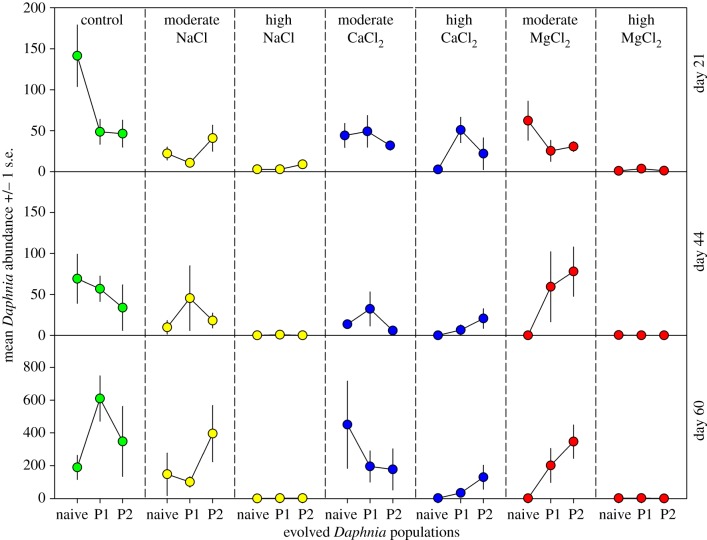
To test for evidence of evolved tolerance in Daphnia populations that were re-exposed to NaCl, we examined whether there was a population-by-salt interaction and whether the naive population showed a larger proportional decline in abundance compared to P1 and P2. On day 21, we found a population-by-salt interaction (F4,27 = 3.8, p = 0.014; figure 2). For the naive population, compared to the low-salt control, Daphnia abundance declined by 84% in the moderate salt concentration (t = 4.8, p < 0.001) and 98% in the high concentration (t = 7.0, p < 0.001). For Daphnia P1, compared to the low-salt control, abundance declined by 78% in the moderate salt concentration (t = 2.5, p = 0.041) and 93% in the high concentration (t = 3.4, p = 0.007). For Daphnia P2, compared to the low-salt control, there was no decline in the moderate concentration (t = 0.9, p = 0.365) while abundance declined by 78% in the high concentration (t = 2.5, p = 0.062). In short, there was evidence of tolerance in the moderate NaCl concentrations for P2. However, the highest NaCl concentration caused near complete death across all populations, which overwhelmed any amounts of evolved tolerance.
Figure 2.
Abundance of three Daphnia pulex populations after exposure to either no salt (control), or two elevated concentrations of either NaCl, CaCl2 or MgCl2. The naive population had no prior history of exposure to high salt concentrations. In a prior experiment, population 1 (P1) evolved a tolerance to 833 µS cm−1 (250 mg Cl− l−1) of NaCl and population 2 (P2) evolved a tolerance to 2662 µS cm−1 (1000 mg Cl− l−1) of NaCl. Abundances were quantified on days 21, 44 and 60.
As the experiment progressed, we no longer observed differences in abundance among Daphnia populations. We only found a main effect of NaCl concentration on Daphnia abundance on days 44 (F2,26 = 13.0, p < 0.001) and 60 (F2,26 = 24.0, p < 0.001). On these days, Daphnia abundance in the high concentration was only about 1% of that in the moderate and control concentrations (t ≥ 3.0, p ≤ 0.011).
(c). Cross-tolerance to CaCl2 and MgCl2
(i). Responses to CaCl2
To test for evidence of evolved cross-tolerance in Daphnia populations that were exposed to CaCl2 or MgCl2, we examined whether there was a population-by-salt interaction and whether the naive population showed a larger proportional decline in abundance compared to P1 and P2. For CaCl2 on day 21, we found a population-by-salt interaction (F4,27 = 3.3, p = 0.024). For the naive population, abundance declined by 69% in the moderate concentration (t = 2.7, p = 0.025) and 98% in the high concentration (t = 4.9, p < 0.001). For P1 and P2, we found no differences in abundance between the control and elevated CaCl2 concentrations (t ≤ 1.6, p ≥ 0.342). Thus, P1 and P2 exhibited cross-tolerance to moderate and high concentrations of CaCl2.
On day 44, we only found a main effect of concentration on Daphnia abundance (F2,27 = 5.8, p = 0.008). Compared to the low-salt control, Daphnia abundance declined by 68% in the moderate concentration (t = 2.6, p = 0.029) and 83% in the high concentration (t = 3.2, p = 0.010).
On day 60, we also only found a main effect of concentration on Daphnia abundance (F2,27 = 7.0, p = 0.003). Compared to the low-salt control, we found no decline in abundance in the moderate concentration (t = 1.0, p = 0.351), but an 85% decline in the high concentration (t = 3.6, p = 0.004).
(ii). Responses to MgCl2
For MgCl2, on day 21 we found main effects of concentration (F2,27 = 26.7, p < 0.001) and tolerance (F2,27 = 3.7, p = 0.037; described in §3a), but no interaction (F4,27 = 1.4, p = 0.226). Compared with the control concentration, Daphnia abundance declined by 50% in the moderate concentration (t = 2.6, p = 0.017) and 97% in the high concentration (t = 7.2, p < 0.001). Daphnia abundance also declined by 94% between the moderate and high concentrations (t = 4.7, p < 0.001). Thus, we observed no cross-tolerance with MgCl2 on day 21 and the high concentration eliminated all Daphnia.
On day 44, we found a population-by-salt interaction (F4,27 = 5.8, p = 0.002). For the naive population, Daphnia abundance declined by 100% the moderate and high MgCl2 concentrations compared to the control concentration (t ≥ 3.7, p ≤ 0.002). For Daphnia P1, compared to the control, there was no effect of the moderate concentration (t = 0.9, p = 0.353), but abundance declined by 100% in the high concentration (t = 4.6, p < 0.001). For Daphnia P2, compared to the control, abundance was 2.3 times higher in the moderate concentration (t = 2.1, p < 0.041). For Daphnia P2, abundance declined by 100% in the high concentration compared to control and moderate concentrations (t ≥ 2.8, p ≤ 0.020). Thus, P1 and P2 exhibited cross-tolerance to moderate concentrations of MgCl2 on day 44, but the highest MgCl2 concentration caused complete death across all populations, which overwhelmed any amounts of evolved tolerance.
On day 60, we also found a population-by-salt interaction (F4,27 = 3.5, p = 0.021). For the naive population, abundance was reduced by 99% in both the moderate and high concentrations of MgCl2 (t ≥ 2.7, p ≤ 0.028). For Daphnia P1, compared to the control, there was a 66% decline in abundance in the moderate concentration (t = 2.0, p = 0.060) and a 99% decline in the high concentration (t = 2.8, p = 0.021). For Daphnia P2, compared to the control, there was no reduction in abundance in the moderate concentration (t = 1.0, p = 0.336), but abundance declined by 99% in the high concentration compared with the control and moderate concentrations (t ≥ 3.6, p ≤ 0.003). Thus, P2 exhibited cross-tolerance to moderate concentrations of MgCl2 on day 60, but the highest MgCl2 caused complete death across all populations, which overwhelmed any amounts of evolved tolerance.
(d). The mitigation of salt-induced cascades by evolved Daphnia
(i). Responses to NaCl
To test for the mitigation of cascades owing to evolved tolerance and cross-tolerance, we examined whether the naive populations caused a larger proportional increase in phytoplankton abundance compared to P1 and P2. For NaCl on day 21, we found no main effects of Daphnia population, salt concentration, or their interaction on phytoplankton abundance (F2–4,27 ≤ 1.4, p ≥ 0.260).
On day 44, we only detected a main effect of salt concentration on phytoplankton abundance (F2,27 = 7.3, p = 0.003). Compared to the low-salt control, there was no increase in the moderate NaCl concentration (t = 0.1, p = 0.892), but there was a 76% increase in the high NaCl concentration (t = 3.3, p = 0.006).
On day 60, we only found a main effect of salt concentration on phytoplankton abundance (GLM: t = 2.0, p = 0.053). Compared to the no-salt control, phytoplankton was not different in the moderate concentration (t = 0.2, p = 0.850), but 99% more abundant in the high concentration (t = 3.9, p = 0.001). In short, we found evidence for NaCl-induced cascades, but we did not find evidence that Daphnia P1 and P2 mitigated these cascades.
(ii). Responses to CaCl2
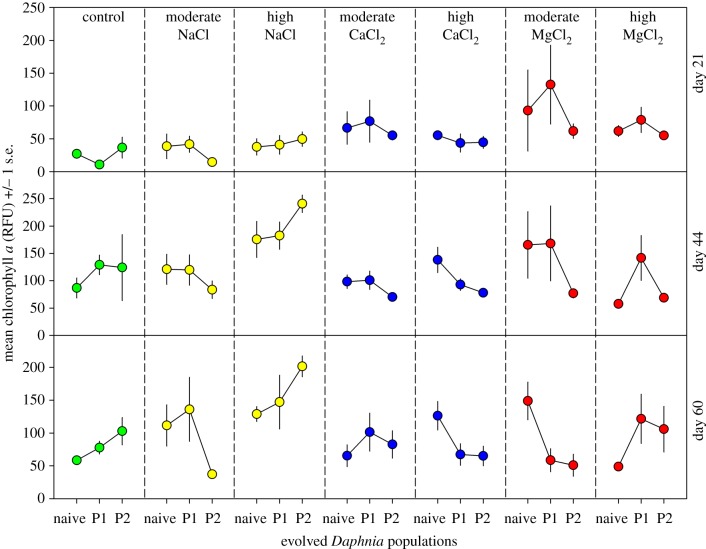
For CaCl2 on days 21 and 44, we found no main effects or a population-by-salt interaction on phytoplankton abundance (F2–4,27 ≤ 1.6, p ≥ 0.213). On day 60, however, we found an interaction (figure 3; F4,27 = 3.0, p = 0.037). For the naive Daphnia population, compared to the low-salt control, phytoplankton abundance was not different in the moderate concentration (t = 0.3, p = 0.789), but was 2.2 times higher in the high concentration (t = 2.6, p = 0.044). For P1 and P2, there were no differences in phytoplankton abundance across the CaCl2 concentrations (t ≤ 1.4, p ≥ 0.407). Thus, CaCl2 caused a cascade in the naive Daphnia population. However, this impact appeared to be reduced by Daphnia left over from P1 and P2 at high concentrations by the end of the experiment (day 60).
Figure 3.
Abundance of phytoplankton (measured as chlorophyll a relative fluorescence units; RFU) in ecological communities with three different Daphnia pulex populations exposed to either no salt (control), or two elevated concentrations of either NaCl, CaCl2 or MgCl2. The naive Daphnia population had no prior history of exposure to high salt concentrations. In a prior experiment, population 1 (P1) evolved a tolerance to 833 µS cm−1 (250 mg Cl− l−1) of NaCl and population 2 (P2) evolved a tolerance to 2662 µS cm−1 (1000 mg Cl− l−1) of NaCl. Phytoplankton abundance was quantified on days 21, 44 and 60.
(iii). Responses to MgCl2
For MgCl2 on days 21 and 44, we detected no main effects or a population-by-salt interaction on the abundance of phytoplankton (F2–4,27 ≤ 1.6, p ≥ 0.218). On day 60, however, we found a population-by-salt interaction (F4,26 = 4.7, p = 0.005). For the naive Daphnia population, compared to the low-salt control, phytoplankton abundance was 2.5 times higher in the moderate MgCl2 concentration (t = 2.8, p = 0.018) but was not different in the high concentration (t = 0.3, p = 0.776). For P1 and P2, there were no differences in phytoplankton abundance compared to the control (t ≤ 1.8, p ≥ 0.221). Thus, MgCl2 caused a cascade when the naive Daphnia population was present, but this was mitigated by the cross-tolerance of P1 and P2 by day 60 in the moderate concentration.
On day 60, Daphnia grazers were absent in the high concentrations of MgCl2 and NaCl. Yet, phytoplankton abundance appeared to vary substantially among the three population types. However, we focused an analysis using a one-way ANOVA on day 60 for the high concentrations of MgCl2 and NaCl and we found no main effects or interactions (F2,9 ≤ 2.1, p ≥ 0.181) or pairwise differences (t ≤ 1.9, p ≥ 0.225) between population types. Thus, even marginally significant differences were not detected in phytoplankton abundance between the populations on day 60.
4. Discussion
Freshwater organisms exhibit evolutionary responses to multiple contaminants [40–43]. In the case of freshwater salinization, evolutionary responses have recently been discovered [30,31,44]. Because salinization can alter the structure and function of freshwater ecosystems [2,3,46], it is essential to understand the ecological implications of evolved responses. Our study shows how evolutionary responses to salinization by a ubiquitous freshwater organism—critically important in freshwater food webs—to one salt type confers cross-tolerance to other salt types and can mitigate salt-induced cascades. These discoveries were dependent on salt type, concentration, and were highly variable through time. Further, the cross-tolerance we discovered also occurred at lower salinities compared to multiple studies of salinity tolerance in Daphnia [47,48]. This suggests that evolutionary responses may occur at much lower salinities that are perhaps more relevant to those resulting from various sources of freshwater salinization.
Trade-offs are often associated with evolutionary responses to anthropogenic chemicals and contaminants [49–51]. Such responses to freshwater salinization can be either adaptive or maladaptive [30,31]. We discovered that an evolved tolerance to NaCl road salt by Daphnia comes at the cost of slower population growth during the early phases of population growth. During the first 21 days of the experiment, there were nearly three times more Daphnia in the naive population compared to the two NaCl-tolerant populations in control conditions. Although impossible to track in our semi-natural mesocosm experiment, slower population growth could have resulted from reduced brood size or number, reduced offspring survival, or increased time to reproduction owing to the costs of maintaining an evolved tolerance. Freshwater contaminants including salinity can alter life-history characteristics of Daphnia after multi-generational exposure [52–55]. If, at the population level, reductions in life-history traits became constitutive in our NaCl-evolved populations, this would explain why we observed slower population growth. Another possibility could be a metabolic cost of maintaining evolved tolerance that decreases energy allocation to reproduction and growth [56]. The abundances of P1 and P2 compared to the naive population at the end of the experiment may indicate that slow initial population growth associated with evolved tolerance is not reflected in overall population size through time or after carrying capacity is reached. The much higher abundances of Daphnia among the populations at the end of the experiment likely indicate that juveniles were no longer competitively suppressed by adults in our mesocosms [57]. While we cannot identify which life-history characteristic contributed to slower growth in the two populations with evolved tolerance during the early stages of population growth, this could certainly be the focus of future research.
We found that an evolved tolerance to NaCl can buffer Daphnia from subsequent NaCl exposures. Evidence for this buffering capacity emerged during the early stages of the experiment. These results suggest that the evolved tolerance was conserved for a year (approx. 30 generations) after it evolved in a prior experiment [32]. A corollary to being buffered from subsequent NaCl exposures is the magnitude of past NaCl exposure. Our results indicate that selection under high NaCl concentrations is needed to buffer Daphnia from subsequent NaCl exposures. Daphnia from P2 were not reduced in moderate NaCl concentrations like the naive and P1 populations. For P1, it is unlikely that culturing in low-salt water over multiple generations prior to the present experiment selected against the original evolved tolerance. This is supported by our discovery of cross-tolerance by P1 to another salt type (discussed below). Ultimately, we do not know if factors like epigenetic change or, although unlikely, a mutation from genetic reversion occurred in the Daphnia genome for P1 [58,59]. Further work on the genetic dynamics of evolved road salt tolerance is necessary to elucidate such mechanisms.
We found that evolved responses to NaCl, one of the most common salts contributing to freshwater salinization, can confer cross-tolerance to another common salt, MgCl2. The variable responses of Daphnia from both evolved populations to MgCl2 showed the most consistent patterns of cross-tolerance, which was evident towards the end of the experiment. This indicates that cross-tolerance to MgCl2 may take multiple generations to appear, perhaps owing to suppressed life-history traits that take time to generate patterns in population abundance [16]. Prior selection history (i.e. P1 versus P2) did not seem to matter regarding cross-tolerance to MgCl2 but did matter regarding persistence of the cross-tolerance. By our operational definition, we did not find evidence of cross-tolerance to MgCl2 in P1 at the end of the experiment, which may have occurred in part because of the high abundance of P1 in the control. It could also signal a limitation of cross-tolerance in populations with historical exposure to lower salt concentrations. Furthermore, cross-tolerance to MgCl2 occurred at moderate concentrations, which were low compared to the moderate concentrations of NaCl and CaCl2. High concentrations of MgCl2, again relatively low compared to the high NaCl and CaCl2 concentrations, were lethal to all Daphnia populations regardless of tolerance level. This highlights that the ecological implications of cross-tolerance are specific to salt type and concentration. An evolved response to one salt type at any given concentration does not necessarily confer cross-tolerance to another salt type at a similar concentration.
In the moderate MgCl2 concentration, the naive Daphnia population crashed after 44 days but was higher on day 21 than the initial stocked densities, a pattern that may reveal a potential mechanism of cross-tolerance. That is, the naive adults in the moderate MgCl2 concentrations likely released offspring that were already developed in the brood pouch prior to their introduction to the mesocosms. The instars survived to the adult phase because the abundance of the naive population in the 900 ml samples on day 21 was higher on average than the stocked Daphnia density. Neither the naive parental generation nor the F1 generation survived until day 44 because no Daphnia were found. This crash that occurred by day 44 in the naive population suggests that (i) offspring produced by the parental and the F1 generations did not survive or (ii) that no offspring were produced by the parental and F1 generations. The latter explanation is supported by our data because there were more Daphnia on day 21 in the naive population than were originally stocked into the experiment—indicating survival of the F1 generation after they were released. Thus, a potential mechanism of cross-tolerance is that the tolerant populations were still able to reproduce successfully (via cyclical parthenogenesis) in moderate MgCl2 concentrations but moderate MgCl2 concentrations inhibited reproduction in the naive population, perhaps owing to salinity stress [17].
Cross-tolerance to CaCl2 was only evident at the beginning of our experiment. Prior selection history did not matter regarding cross-tolerance to CaCl2, as both evolved Daphnia populations maintained a similar abundance from control to high CaCl2 concentrations. It does not appear that the concentrations of CaCl2 used in our experiment limited cross-tolerance in Daphnia, which occurred with high MgCl2 concentrations. However, the lack of a stronger signal of cross-tolerance on days 44 and 60 suggests that the high CaCl2 concentration used in our experiment may eventually overwhelm cross-tolerance. Further, the high CaCl2 concentration was much higher than the high MgCl2 concentration, which indicates that MgCl2 is far more lethal to Daphnia than CaCl2. Ultimately, cross-tolerance to CaCl2 and MgCl2 may confirm a generalizable pattern of cross-tolerance to contaminants occurring in freshwater systems as cross-tolerance has also been described among multiple pesticides [41,60,61]. In general, cross-tolerance to contaminants in freshwater ecosystems may be important for environmental conservation because it could provide resistance and stability in ecological communities [60,62].
While our operational definition of cross-tolerance was met, we must acknowledge that the possibility exists that tolerant Daphnia populations might be predisposed to evolving a tolerance to MgCl2. If this were the case, rather than the hypothesized mechanism of cross-tolerance, Daphnia in the moderate MgCl2 concentration and high CaCl2 concentration may have evolved a new tolerance to these salt types. Although this scenario seems unlikely given the short duration of this study and we cannot identify if this is the case from our data, it certainly merits further investigation.
Our study illustrates that all three road salt types trigger cascading effects that lead to phytoplankton blooms with naive Daphnia populations, which has previously been shown for NaCl [18,19]. Moreover, despite the variable nature of the phytoplankton data, we found that cross-tolerance can reduce cascading effects triggered by road salts. Cross-tolerance in the evolved populations prevented phytoplankton blooms that occurred in naive populations of Daphnia exposed to MgCl2 and to an extent CaCl2. Similar studies using pesticides have shown that zooplankton with an evolved tolerance can also mitigate cascading effects that lead to phytoplankton blooms [61,62]. Here, we demonstrate how evolved responses may affect the ecological outcomes among different salts contributing to freshwater salinization. Cross-tolerance to MgCl2 conferred the most striking reduction in phytoplankton. Further, both evolved Daphnia populations reduced phytoplankton similarly, suggesting prior selection history did not alter the ability of Daphnia to mitigate a salt-induced cascade resulting from MgCl2 contamination.
Based on our analyses, evolved tolerance to NaCl did not mitigate a NaCl-induced cascade. We found no interaction between Daphnia populations and NaCl concentration on phytoplankton abundance. However, it is worth noting that P2 Daphnia abundance in the moderate NaCl concentration was the same as in the control, which also occurred with the naive population. On day 60, phytoplankton abundance in P2 was 64% lower in the moderate concentration than in the low-salt control. In a parametric ANOVA, the interaction was significant (F4,26 = 4.7, p = 0.005) and post hoc comparison for P2 between the control and moderate NaCl concentrations was significant (t = 3.1, p = 0.009). We ultimately did not use the parametric ANOVA model because no transformation met the assumption of constant variance. We therefore proceeded with GLM, which indicated that the interaction was not significant. In short, there appears to be a signal of mitigation in Daphnia P2, but we were unable to pick this up in our statistical model because of the high variability in phytoplankton in the naive and P1 populations. Nevertheless, the mitigating effect of cascades seems probable for the P2 Daphnia, but further study is needed to confirm this conclusion.
5. Conclusion
Our study stresses that an evolutionary perspective is critical in the study of freshwater salinization [44,49] and underscores the importance of integrating evolutionary and ecological impacts. Although it is unknown if evolved tolerance to road salts is occurring in natural populations of zooplankton, our work shows that evolutionary responses have the potential to buffer Daphnia from exposure to road salts. As alternative salts like MgCl2 and CaCl2 become more popular in road deicing operations, it is possible that evolved tolerance could reduce the impacts of road salts on ecosystem services such as water clarity. Importantly, the evolutionary responses we have discovered here could be occurring in many other freshwater organisms with fast generation times. However, the scale and magnitude at which these evolutionary processes are occurring and operating is severely understudied in wild populations [44]. Identifying the ecological importance of evolutionary responses in a broader range of freshwater organisms would help our understanding of how rapid evolution and cross-tolerance can mitigate the impacts of salinization.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Justin Rappold, Jeevan Narendran, Skylar Carter and Erika Yates for assistance with data collection, experimental set-up and take down. We thank Brian Mattes and Kayla Coldsnow for culturing the Daphnia used in the experiment. We also thank Laurel Dean for her efforts processing zooplankton samples.
Data accessibility
Data available as part of the electronic supplementary material.
Authors' contributions
W.D.H. and R.A.R. designed the experiment. W.D.H. and D.K.J. set up and took down the experiment. All authors contributed to the writing of the manuscript. R.A.R. provided financial and logistical support.
Competing interests
We declare we have no competing interests.
Funding
This research was supported by the Jefferson Project at Lake George, a collaboration between IBM, the FUND for Lake George and Rensselaer Polytechnic Institute.
References
- 1.Cañedo-Argüelles M, Bundschuh M, Gutierrez-Canovas C, Kefford BJ, Prat N, Trobajo R, Schaefer RB. 2014. Effects of repeated salt pulses on ecosystem structure and functions in a stream mesocosm. Sci. Total Environ. 476, 634–642. ( 10.1016/j.scitotenv.2013.12.067) [DOI] [PubMed] [Google Scholar]
- 2.Cañedo-Argüelles M, et al. 2016. Saving freshwater from salts. Science 351, 914–916. ( 10.1126/science.aad3488) [DOI] [PubMed] [Google Scholar]
- 3.Jeppesen E, Sondergaard M, Pedersen AR, Jurgens K, Strzelczak A, Lauridsen TL, Johansson LS. 2007. Salinity induced regime shift in shallow brackish lagoons. Ecosystems 10, 47–57. ( 10.1007/s10021-006-9007-6) [DOI] [Google Scholar]
- 4.Berger E, Frör O, Schäfer RB. 2019. Salinity impacts on river ecosystem processes: a critical mini-review. Phil. Trans. R. Soc. B 374, 20180010 ( 10.1098/rstb.2018.0010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venâncio C, Castro BB, Ribeiro R, Antunes SC, Abrantes N, Soares AMVM, Lopes I. 2019. Sensitivity of freshwater species under single and multigenerational exposure to seawater intrusion. Phil. Trans. R. Soc. B 374, 20180252 ( 10.1098/rstb.2018.0252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herbert ER, Boon P, Burgin AJ, Neubauer SC, Franklin RB, Ardon M, Hopfensperger KN, Lamers LPM, Gell P. 2015. A global perspective on wetland salinization: ecological consequences of a growing threat to freshwater wetlands. Ecosphere 6, 206 ( 10.1890/es14-00534.1) [DOI] [Google Scholar]
- 7.Schuler MS, Cañedo-Argüelles M, Hintz WD, Dyack B, Birk S, Relyea RA. 2019. Regulations are needed to protect freshwater ecosystems from salinization. Phil. Trans. R. Soc. B 374, 20180019 ( 10.1098/rstb.2018.0019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mueller B, Gaechter R. 2012. Increasing chloride concentrations in Lake Constance: characterization of sources and estimation of loads. Aquat. Sci. 74, 101–112. ( 10.1007/s00027-011-0200-0) [DOI] [Google Scholar]
- 9.Hill AR, Sadowski EK. 2016. Chloride concentrations in wetlands along a rural to urban land use gradient. Wetlands 36, 73–83. ( 10.1007/s13157-015-0717-4) [DOI] [Google Scholar]
- 10.Chapra SC, Dove A, Rockwell DC. 2009. Great Lakes chloride trends: long-term mass balance and loading analysis. J. Great Lakes Res. 35, 272–284. ( 10.1016/j.jglr.2008.11.013) [DOI] [Google Scholar]
- 11.Kaushal SS, Groffman PM, Likens GE, Belt KT, Stack WP, Kelly VR, Band LE, Fisher GT. 2005. Increased salinization of fresh water in the northeastern United States. Proc. Natl Acad. Sci. USA 102, 13 517–13 520. ( 10.1073/pnas.0506414102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dugan HA, et al. 2017. Salting our freshwater lakes. Proc. Natl Acad. Sci. USA 114, 4453–4458. ( 10.1073/pnas.1620211114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuler MS, Hintz WD, Jones DK, Lind LA, Mattes BM, Stoler AB, Sudol KA, Relyea RA. 2017. How common road salts and organic additives alter freshwater food webs: in search of safer alternatives. J. Appl. Ecol. 54, 1353–1361. ( 10.1111/1365-2664.12877) [DOI] [Google Scholar]
- 14.Petranka JW, Doyle EJ. 2010. Effects of road salts on the composition of seasonal pond communities: can the use of road salts enhance mosquito recruitment? Aquat. Ecol. 44, 155–166. ( 10.1007/s10452-009-9286-z) [DOI] [Google Scholar]
- 15.Petranka JW, Francis RA. 2013. Effects of road salts on seasonal wetlands: poor prey performance may compromise growth of predatory salamanders. Wetlands 33, 707–715. ( 10.1007/s13157-013-0428-7) [DOI] [Google Scholar]
- 16.Searle CL, Shaw CL, Hunsberger KK, Prado M, Duffy MA. 2016. Salinization decreases population densities of the freshwater crustacean, Daphnia dentifera. Hydrobiologia 770, 165–172. ( 10.1007/s10750-015-2579-4) [DOI] [Google Scholar]
- 17.Hintz WD, Relyea RA. 2017. A salty landscape of fear: responses of fish and zooplankton to freshwater salinization and predatory stress. Oecologia 185, 147–156. ( 10.1007/s00442-017-3925-1) [DOI] [PubMed] [Google Scholar]
- 18.Hintz WD, Mattes BM, Schuler MS, Jones DK, Stoler AB, Lind LA, Relyea RA. 2017. Salinization triggeres a trophic cascade in experimental freshwater communities with varying food-chain length. Ecol. Appl. 27, 833–844. ( 10.1002/eap.1487) [DOI] [PubMed] [Google Scholar]
- 19.Jones DK, Mattes BM, Hintz WD, Schuler MS, Stoler AB, Lind LA, Cooper RO, Relyea RA. 2017. Investigation of road salts and biotic stressors on freshwater wetland communities. Environ. Pollut. 221, 159–167. ( 10.1016/j.envpol.2016.11.060) [DOI] [PubMed] [Google Scholar]
- 20.Hintz WD, Relyea RA. 2017. Impacts of road deicing salts on the early-life growth and development of a stream salmonid: salt type matters. Environ. Pollut. 223, 409–415. ( 10.1016/j.envpol.2017.01.040) [DOI] [PubMed] [Google Scholar]
- 21.Karraker NE, Gibbs JP. 2011. Road deicing salt irreversibly disrupts osmoregulation of salamander egg clutches. Environ. Pollut. 159, 833–835. ( 10.1016/j.envpol.2010.11.019) [DOI] [PubMed] [Google Scholar]
- 22.Karraker NE, Gibbs JP, Vonesh JR. 2008. Impacts of road deicing salt on the demography of vernal pool-breeding amphibians. Ecol. Appl. 18, 724–734. ( 10.1890/07-1644.1) [DOI] [PubMed] [Google Scholar]
- 23.Van Meter RJ, Swan CM. 2014. Road salts as environmental constraints in urban pond food webs. PLoS ONE 9, e0090168 ( 10.1371/journal.pone.0090168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Meter RJ, Swan CM, Leips J, Snodgrass JW. 2011. Road salt stress induces novel food web structure and interactions. Wetlands 31, 843–851. ( 10.1007/s13157-011-0199-y) [DOI] [Google Scholar]
- 25.Van Meter RJ, Swan CM, Snodgrass JW. 2011. Salinization alters ecosystem structure in urban stormwater detention ponds. Urban Ecosyst. 14, 723–736. ( 10.1007/s11252-011-0180-9) [DOI] [Google Scholar]
- 26.Bridgeman TB, et al. 2000. A limnological survey of Third Sister Lake Michigan with historical comparisons. J. Lake Reservoir Manage. 16, 253–267. ( 10.1080/07438140009354234) [DOI] [Google Scholar]
- 27.Duan S, Kaushal SS. 2015. Salinization alters fluxes of bioreactive elements from stream ecosystems across land use. Biogeosciences 12, 7331–7347. ( 10.5194/bg-12-7331-2015) [DOI] [Google Scholar]
- 28.Hale RL, Groffman PM. 2006. Chloride effects on nitrogen dynamics in forested and suburban stream debris dams. J. Environ. Qual. 35, 2425–2432. ( 10.2134/jeq2006.0164) [DOI] [PubMed] [Google Scholar]
- 29.Kim SY, Koretsky C. 2013. Effects of road salt deicers on sediment biogeochemistry. Biogeochemistry 112, 343–358. ( 10.1007/s10533-012-9728-x) [DOI] [Google Scholar]
- 30.Brady SP. 2012. Road to evolution? Local adaptation to road adjacency in an amphibian (Ambystoma maculatum). Sci. Rep. 2, 235 ( 10.1038/srep00235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brady SP. 2017. Environmental exposure does not explain putative maladaptation in road-adjacent populations. Oecologia 184, 931–942. ( 10.1007/s00442-017-3912-6) [DOI] [PubMed] [Google Scholar]
- 32.Coldsnow KD, Mattes BM, Hintz WD, Relyea RA. 2017. Rapid evolution of tolerance to road salt in zooplankton. Environ. Pollut. 222, 367–373. ( 10.1016/j.envpol.2016.12.024) [DOI] [PubMed] [Google Scholar]
- 33.Carpenter SR, Kitchell JF, Hodgson JR. 1985. Cascading trophic interactions and lake productivity. Bioscience 35, 634–639. ( 10.2307/1309989) [DOI] [Google Scholar]
- 34.Erickson RJ, Mount DR, Highland TL, Hockett JR, Hoff DJ, Jenson CT, Norberg-King TJ, Peterson KN. 2017. The acute toxicity of major ion salts to Ceriodaphnia dubia: II. Empirical relationships in binary salt mixtures. Environ. Toxicol. Chem. 36, 1525–1537. ( 10.1002/etc.3669) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mount DR, et al. 2016. The acute toxicity of major ion salts to Ceriodaphnia dubia: I. Influence of background water chemistry. Environ. Toxicol. Chem. 35, 3039–3057. ( 10.1002/etc.3487) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evans M, Frick C.. 2001. The effects of road salts on aquatic ecosystems. Saskatchewan, Canada: Environment Canada—Water Science and Technology Directorate. [Google Scholar]
- 37.Mount DR, Gulley DD, Hockett JR, Garrison TD, Evans JM. 1997. Statistical models to predict the toxicity of major ions to Ceriodaphnia dubia, Daphnia magna and Pimephales promelas (fathead minnows). Environ. Toxicol. Chem. 16, 2009–2019. ( 10.1002/etc.5620161005) [DOI] [Google Scholar]
- 38.Arar EJ, Collins GB. 1997. In vitro determination of chlorophyll a and pheophytin in a marine and freshwater algae by fluorescence. (ed. U. E. P. Agency). Cincinnati, OH: U.S. Environmental Protection Agency. [Google Scholar]
- 39.Wasserstein RL, Lazar NA. 2016. The ASA's statement on p-values: context, process, and purpose. Am. Stat. 70, 129–131. ( 10.1080/00031305.2016.1154108) [DOI] [Google Scholar]
- 40.Hua J, Jones DK, Mattes BM, Cothran RD, Relyea RA, Hoverman JT. 2015. The contribution of phenotypic plasticity to the evolution of insecticide tolerance in amphibian populations. Evol. Appl. 8, 586–596. ( 10.1111/eva.12267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hua J, Jones DK, Relyea RA. 2014. Induced tolerance from a sublethal insecticide leads to cross-tolerance to other insecticides. Environ. Sci. Technol. 48, 4078–4085. ( 10.1021/es500278f) [DOI] [PubMed] [Google Scholar]
- 42.Hua J, Morehouse NI, Relyea R. 2013. Pesticide tolerance in amphibians: induced tolerance in susceptible populations, constitutive tolerance in tolerant populations. Evol. Appl. 6, 1028–1040. ( 10.1111/eva.12083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cothran RD, Brown JM, Relyea RA. 2013. Proximity to agriculture is correlated with pesticide tolerance: evidence for the evolution of amphibian resistance to modern pesticides. Evol. Appl. 6, 832–841. ( 10.1111/eva.12069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brady SP, Richardson JL, Kunz BK. 2017. Incorporating evolutionary insights to improve ecotoxicology for freshwater species. Evol. Appl. 10, 829–838. ( 10.1111/eva.12507) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arribas P, Gutiérrez-Cánovas C, Botella-Cruz M, Cañedo-Argüelles M, Antonio Carbonell J, Millán A, Pallarés S, Velasco J, Sánchez-Fernández D. 2019. Insect communities in saline waters consist of realized but not fundamental niche specialists. Phil. Trans. R. Soc. B 374, 20180008 ( 10.1098/rstb.2018.0008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cañedo-Argüelles M, Kefford BJ, Piscart C, Prat N, Schaefer RB, Schulz C-J. 2013. Salinisation of rivers: an urgent ecological issue. Environ. Pollut. 173, 157–167. ( 10.1016/j.envpol.2012.10.011) [DOI] [PubMed] [Google Scholar]
- 47.Latta LC, Weider LJ, Colbourne JK, Pfrender ME. 2012. The evolution of salinity tolerance in Daphnia: a functional genomics approach. Ecol. Lett. 15, 794–802. ( 10.1111/j.1461-0248.2012.01799.x) [DOI] [PubMed] [Google Scholar]
- 48.Loureiro C, Castro BB, Claro MT, Alves A, Pedrosa MA, Goncalves F. 2012. Genetic variability in the tolerance of natural populations of Simocephalus vetulus (Muller, 1776) to lethal levels of sodium chloride. Ann. De Limnologie-Int. J. Limnol. 48, 95–103. ( 10.1051/limn/2012002) [DOI] [Google Scholar]
- 49.Semlitsch RD, Bridges CM, Welch AM. 2000. Genetic variation and a fitness tradeoff in the tolerance of gray treefrog (Hyla versicolor) tadpoles to the insecticide carbaryl. Oecologia 125, 179–185. ( 10.1007/s004420000443) [DOI] [PubMed] [Google Scholar]
- 50.Andersson DI, Hughes D. 2010. Antibiotic resistance and its cost: is it possible to reverse resistance? Nat. Rev. Microbiol. 8, 260–271. ( 10.1038/nrmicro2319) [DOI] [PubMed] [Google Scholar]
- 51.Bennett AF, Lenski RE. 2007. An experimental test of evolutionary trade-offs during temperature adaptation. Proc. Natl Acad. Sci. USA 104, 8649–8654. ( 10.1073/pnas.0702117104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jansen M, Coors A, Stoks R, De Meester L. 2011. Evolutionary ecotoxicology of pesticide resistance: a case study in Daphnia. Ecotoxicology 20, 543–551. ( 10.1007/s10646-011-0627-z) [DOI] [PubMed] [Google Scholar]
- 53.Coors A, De Meester L. 2008. Synergistic, antagonistic and additive effects of multiple stressors: predation threat, parasitism and pesticide exposure in Daphnia magna. J. Appl. Ecol. 45, 1820–1828. ( 10.1111/j.1365-2664.2008.01566.x) [DOI] [Google Scholar]
- 54.Arnér M, Koivisto S. 1993. Effects of salinity on metabolism and life history characteristics of Daphnia magna. Hydrobiologia 259, 69–77. ( 10.1007/bf00008373) [DOI] [Google Scholar]
- 55.Miracle MR, Serra M. 1989. Salinity and temperature influence rotifer life-history characteristics. Hydrobiologia 186, 81–102. ( 10.1007/bf00048900) [DOI] [Google Scholar]
- 56.Guillemaud T, Lenormand T, Bourguet D, Chevillon C, Pasteur N, Raymond M. 1998. Evolution of resistance in Culex pipiens: allele replacement and changing environment. Evolution 52, 443–453. ( 10.2307/2411080) [DOI] [PubMed] [Google Scholar]
- 57.Goitom E, Kilsdonk LJ, Brans K, Jansen M, Lemmens P, De Meester L. 2018. Rapid evolution leads to differential population dynamics and top-down control in resurrected Daphnia populations. Evol. Appl. 11, 96–111. ( 10.1111/eva.12567) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Latta LC, Morgan KK, Weaver CS, Allen D, Schaack S, Lynch M. 2013. Genomic background and generation time influence deleterious mutation rates in Daphnia. Genetics 193, 539 ( 10.1534/genetics.112.146571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee J-Y, Park YK, Chung ES, Na IY, Ko KS. 2016. Evolved resistance to colistin and its loss due to genetic reversion in Pseudomonas aeruginosa. Sci. Rep. 6, 25543 ( 10.1038/srep25543) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hua J, Cothran R, Stoler A, Relyea R. 2013. Cross-tolerance in amphibians: wood frog mortality when exposed to three insecticides with a common mode of action. Environ. Toxicol. Chem. 32, 932–936. ( 10.1002/etc.2121) [DOI] [PubMed] [Google Scholar]
- 61.Bendis RJ, Relyea RA. 2016. If you see one, have you seen them all? Community-wide effects of insecticide cross-resistance in zooplankton populations near and far from agriculture. Environ. Pollut. 215, 234–246. ( 10.1016/j.envpol.2016.05.020) [DOI] [PubMed] [Google Scholar]
- 62.Bendis RJ, Relyea RA. 2016. Wetland defense: naturally occuring presticide resistance in zooplankton populations protects the stability of aquatic communities. Oecologia 181, 487–498. ( 10.1007/s00442-016-3574-9) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available as part of the electronic supplementary material.