Abstract
Anthropogenic salinization of freshwater is a global problem with largely unknown consequences for stream functions. We compared the effects of salt addition (6 g l−1 NaCl) in microcosms on leaf mass loss and microbial parameters in single- and multispecies assemblages of fungal strains (Heliscus lugdunensis, HELU; Tetracladium marchalianum, TEMA; Flagellospora curta, FLCU) isolated from a reference (R) or salinized (S) stream. Fungal growth and interactions were also assessed. Salinization inhibited leaf decomposition and fungal biomass, but no differences were observed between species, strains or species combinations. Sporulation rates in monocultures were not affected by added salt, but differed among species (FLCU > HELU > TEMA), with S strains releasing more conidia. Fungal assemblages did not differ significantly in total conidia production (either between strains or medium salt concentration). HELU was the dominant species, which also had highest growth and most pronounced antagonistic behaviour. Fungal species, irrespective of origin, largely maintained their function in salinized streams. Strains from salt-contaminated streams did not trade-off conidial production for vegetative growth at high salt levels. The expected reduction of fungal diversity and potential changes in nutritional litter quality owing to salinization may impact leaf incorporation into secondary production in streams.
This article is part of the theme issue ‘Salt in freshwaters: causes, ecological consequences and future prospects’.
Keywords: salt, aquatic hyphomycetes, decomposition, interactions, antagonism
1. Introduction
Small-forested streams are light-limited systems where decomposition of allochthonous leaf litter is the pivotal ecosystem-level process, encompassing recycling of terrestrially derived organic matter and energy transfer [1]. Microbial decomposers, mainly aquatic hyphomycetes (AHs), are intermediaries between the dominant food source in streams (leaf litter) and leaf consumers. Their crucial importance arises from efficient and species-specific enzymatic activities towards the cell wall's structural polysaccharides [2,3] and from interactions among themselves and with other microorganisms, primarily with bacteria. Both decomposer groups shape detritus palatability to detritivores and its incorporation into secondary production [3,4]. AHs are distributed globally and have been classified as having a predominantly ruderal life strategy [5–7]. They are adapted to large fluctuations in resource availability (allochthonous leaves) interpreted as disturbance [6] and make major investments in reproduction. Combining high levels of disturbance and stress is supposed to preclude a viable strategy for plants [5]; ruderal fungi may overcome stress by surviving (as resistant mycelia) or escaping (producing long-lasting spores or colonizing other resources) [6]. Stressors generally inhibit most members of a guild; in stressed environments, interspecies competition may therefore be less severe and antagonistic behaviour less beneficial [5].
While clearly impacted, ecological functions of AHs are remarkably resilient in the face of adverse anthropogenic conditions (organic pollution, heavy metals, etc.; [8]). Fungal responses to environmental stressors include changes in the diversity, identity, biomass and/or activity of leaf-associated fungal assemblages, which frequently affect the rate and efficacy of their ecological function [8].
Salinization of streams and rivers is of global concern and is expected to intensify owing to climate change, growing water demand and other anthropogenic activities [9–12]. Secondary salinization results from clearing of native vegetation, agricultural irrigation, rising groundwater, mining activity, industrial discharge or the use of highly soluble salts (sodium chloride, NaCl) as chemical deicing and anti-icing agents to maintain safe winter roadways [10,13–17]. There is a general consensus that salinization has deleterious effects on stream structure and function [9,18–22]. Previous studies have been biased towards assessing structure (species composition and diversity) and have largely focused on macroinvertebrate communities, while studies on the consequences of salt contamination for leaf litter decomposition have been limited [9,10,19,22]. It seems clear that less abundant and species-impoverished communities of detritivores result in reduced leaf decay rates (e.g. [11,14,21–27]). The effects of salinization on fungal degradation remain unclear and results have been contradictory. Existing information indicates that AHs generally have a high but species-specific tolerance of salt (NaCl), which ensures microbially mediated leaf degradation at much higher salt concentrations than values tolerated by most invertebrates [22,27,28]. Species-specific trade-offs between maintaining cellular integrity and vegetative growth versus reproduction may allow continued mycelial colonization at increasing salt concentrations by distinct, gradually impoverished and less effective AH communities. In fact, AH reproduction seems to be the most salt-sensitive parameter [22,29,30], while biomass, microbial respiration/activity or microbial richness do not show consistent, monotonically declining responses, especially when compared with leaf mass loss [9,19–22]. Such discrepancies between decomposition and other measures of fungal performances have been attributed to different experimental approaches (e.g. microcosm, mesocosm or field) but also to abiotic (e.g. salt chemistry) and biotic factors [9,14,19,29–31].
AH responses in the presence of some stressors (e.g. metals) suggest differences in tolerance/resistance between conspecifics originating from non-stressed or stressed streams [32,33]. Such changes have frequently been associated with diverging morphological traits, distinct growth behaviour and modified competitive interactions, which are all important modulators of fungal community structure and its ecological functions [32–34].
In an earlier study, Canhoto et al. [22] assessed the effects of an environmentally relevant range of salt additions (0, 2, 4, 8 and 16 g l−1 NaCl) on the behaviour of aquatic hyphomycetes species isolated from a reference stream. Results indicated that tolerant fungal assemblages composed of a few sporulating species were able to maintain decomposition of leaf litter at high (up to 2 g l−1 NaCl) salt levels. Based on these results, our main goal in the present study was directed at evaluating potential adaptive changes of fungal species. We used microcosms to investigate whether long-standing stream salinization affects leaf decomposition and related parameters (fungal biomass and sporulation rates) of resident strains (S, salinized stream) compared to reference strains (R) from a non-polluted stream. Pairs (S, R) of three AH species were chosen. We also assessed fungal colony morphology, growth rates and interactions between strains in reference and salted media. We expected fungal strains from the salted stream to perform better in a high salt microcosm than strains from a reference stream, and vice versa. Since adaptation to high salinity is likely to entail metabolic costs, we hypothesize that S strains exhibit a combination of reduced growth, sporulation or antagonistic behaviour. Finally, we tested the hypothesis that owing to functional redundancies, multispecies assemblages induce greater mass loss than individual species.
2. Experimental procedure
(a). Microcosm experiment set-up
Two strains each (R and S) of three AH species—Heliscus lugdunensis (HELU), Tetracladium marchalianum (TEMA) and Flagellospora curta (FLCU)—were isolated from single conidia released from submerged leaf litter collected from two different sites. S strains were collected in the Pontével stream (Cartaxo; 39°08′40″ N; 8°50′04.4″ W), which has had high salinity (6 ± 0.3 g l−1 salinity and 12.72 ± 2.3 mS cm−1 conductivity) for more than 3 years owing to surrounding agricultural activities (A.L.G. 2016, personal communication). R strains were collected from the Candal stream (Lousã; 40°4′44″ N, 8°12′10″ W), a reference headwater (≤0.01 ± 0.01 g l−1 salinity and 26.80 ± 2.1 µS cm−1 conductivity). Pure cultures of both strains of all fungal species were grown on malt extract agar (MEA; 2%) medium at the salt concentration corresponding to the stream of origin (0 or 6 g l−1 NaCl) for 14 days.
To evaluate degradation rates by reference (R) and saline (S) AHs, sets of 20 dried and weighed discs (ø = 12 mm) of senescent poplar leaves (Populus nigra L.) were immersed in Erlenmeyer flasks (microcosms) filled with 40 ml of nutrient solution (75.5 mg of CaCl2, 10 mg of MgSO4·7H2O, 0.5 g of 3-morpholinopropanesulfonic acid, 5.5 mg of K2HPO4 and 100 mg of KNO3 per litre of sterile distilled water; pH 7; [35]). For all assemblages, reference (R; no added salt) and salted (S; 6 g l−1 NaCl added) conditions were used. A total of 48 microcosms were inoculated with different strains of the three AH species: six replicates were individually inoculated with each strain of the three AH species, and another 12 microcosms were inoculated with all three R or all three S strains. All treatments received the same total inoculum, based on a 12 mm agar plug for single cultures. In mixed cultures, the sizes of the plugs from the three strains were adjusted accordingly. All microcosms were incubated on orbital shakers at 120 rpm under a 12 L : 12 D photoperiod.
After 7 days, nutrient solutions in all microcosms were renewed and agar plugs were removed. The medium was subsequently replaced every 48 h. After 21 days (sporulation peak according to the study of Graça et al. [36]), discs from each microcosm were used to evaluate leaf mass loss, ergosterol concentration (as proxy of fungal biomass) and sporulation rates.
Dry mass loss (% DM) after incubation was estimated from the difference between initial and final leaf dry mass of the 20 discs from each microcosm.
To determine fungal biomass, ergosterol concentrations [37,38] were measured from five leaf discs per microcosm. Discs were frozen, lyophilized and weighed. Ergosterol was then extracted and measured by liquid chromatography. Ergosterol concentrations were converted into fungal biomass using the conversion factor of 5.5 µg ergosterol per mg fungal dry mass [37]. The results were expressed as mg of fungal biomass per g of leaf disc dry mass.
For sporulation, five discs were placed on a shaker in 25 ml of the same medium after the end of the incubation period to induce sporulation for 48 h. Discs were dried and weighed, suspensions were collected and conidia were fixed with 2 ml of formalin (37%). To ensure even distribution of the conidia, the suspension was mixed with 100 µl of Triton X-100 (0.5%), an aliquot was filtered (Millipore SMWP filters, 5 µm pore size) and spores were stained with 0.05% cotton blue in lactic acid (60%). Spores released by each species in the various microcosms were counted under a microscope (250×). Sporulation rates were expressed as the number of conidia produced per mg of leaf dry mass per day.
(b). Morphology and growth rate of R and S strains
Morphology and growth of the three fungal species were analysed by observing R and S strains on MEA: an agar plug (ø = 4 mm) was cut from the edge of 14-day-old pure cultures and placed in the centre of Petri dishes with R and S MEA. In total, 36 Petri dishes were used: each strain of each species was exposed to both R and S medium, with three replicates for each treatment (three species×two strains×two treatments×three replicates). The colony morphology was assessed visually in terms of colour, shape and growth. The diameter of each colony was measured every second day until one of the colonies grew to within 1 cm from the wall of the Petri dish. Radial growth rate was calculated as the slope of the linear regression obtained between the diameter of the colony and time, and expressed as mm d∼1.
(c). Intraspecific and interspecific interactions
To evaluate intra- and interspecific interactions among AHs, one agar plug (ø = 4 mm) from a pure culture of each strain (R, S) of the three AH species grown in R and S MEA medium was placed in a Petri dish, maintaining equal distances between agar plugs and the wall of the Petri dish.
Interactions, including paired and triplet groups, were investigated in both media (five replicates per treatment, table 1). The interaction was evaluated when one of the colonies was close to the edge of the Petri dish or when the interaction between mycelia was clear [32]. At that time, two distances from the centre of each colony of each Petri dish were measured: one to the edge of the colony towards the edge of the Petri dish (R1) and the other towards the challenger strain (R2). R1 and R2 were used to calculate the percentage inhibition of each strain by the opposing strain, using the formula [(R1 − R2)/R1] × 100 [39,40].
Table 1.
Experimental design of interactions test, including intra- and interspecific (in pairs and triplets) interactions between two strains (R, reference; S, saline) of three AH species—Heliscus lugdunensis (HELU), Tetracladium marchalianum (TEMA) and Flagellospora curta (FLCU) growing on MEA medium with (S, 6 g l−1 NaCl) or without added salt (R).
| medium | interaction | species | strain | species | strain | species | strain |
|---|---|---|---|---|---|---|---|
| reference and salted | intraspecific (pairs) | HELU | R | HELU | R | — | — |
| HELU | S | HELU | S | — | — | ||
| TEMA | R | TEMA | R | — | — | ||
| TEMA | S | TEMA | S | — | — | ||
| FLCU | R | FLCU | R | — | — | ||
| FLCU | S | FLCU | S | — | — | ||
| interspecific (pairs) | HELU | R | TEMA | R | — | — | |
| HELU | S | TEMA | S | — | — | ||
| HELU | R | FLCU | R | — | — | ||
| HELU | S | FLCU | S | — | — | ||
| TEMA | R | FLCU | R | — | — | ||
| TEMA | S | FLCU | S | — | — | ||
| interspecific (triplet) | HELU | R | TEMA | R | FLCU | R | |
| HELU | S | TEMA | S | FLCU | S |
The type of interactions and categories were evaluated according to Yuen et al. [41]. These authors considered three general types of interaction: (i) mutual intermingling, including five categories without inhibition (category A: the hyphae of both species freely intermingle; B1 or E1: response or challenge species, respectively, overgrows the other; B2 or E2: response or challenge species, respectively, grows up or around the other), (ii) mutual inhibition at contact (C: when both response and challenge species grow until almost in contact and stop growing) and (iii) mutual inhibition at a distance (D: the inhibition of both response and challenge species occurs without contact). In addition, the index of antagonism (IA) was calculated for all interactions based on different assigned points for each category. The total number of interactions of each category that a particular strain exhibited in each medium was counted. The following formula was used: IA = B1(n × 1) + B2(n × 1) + C(n × 2) + D(n × 3) + E1(n × 4) + E2(n × 4), where the capital letters indicate the interaction type and n is the number of times that the strain exhibited this type of interaction.
(d). Statistical analysis
The effects of the three factors (species composition, four levels: three pure cultures and mixture of all three; strains, two levels; salt addition, two levels) on mass loss, fungal biomass and sporulation rate were analysed by a three-way ANOVA. To compare growth (linear extensions of colonies) among species, between strains and treatments, we used a repeated-measures ANOVA. Before using ANOVA, the Levene's variances homogeneity test was performed, and data were transformed when necessary. When significant statistical differences were found (p < 0.05), Tukey's test was applied.
Paired t-tests were used to compare the percentages of induced inhibition between opposing strains or species.
3. Results
(a). Mass loss, fungal biomass and sporulation
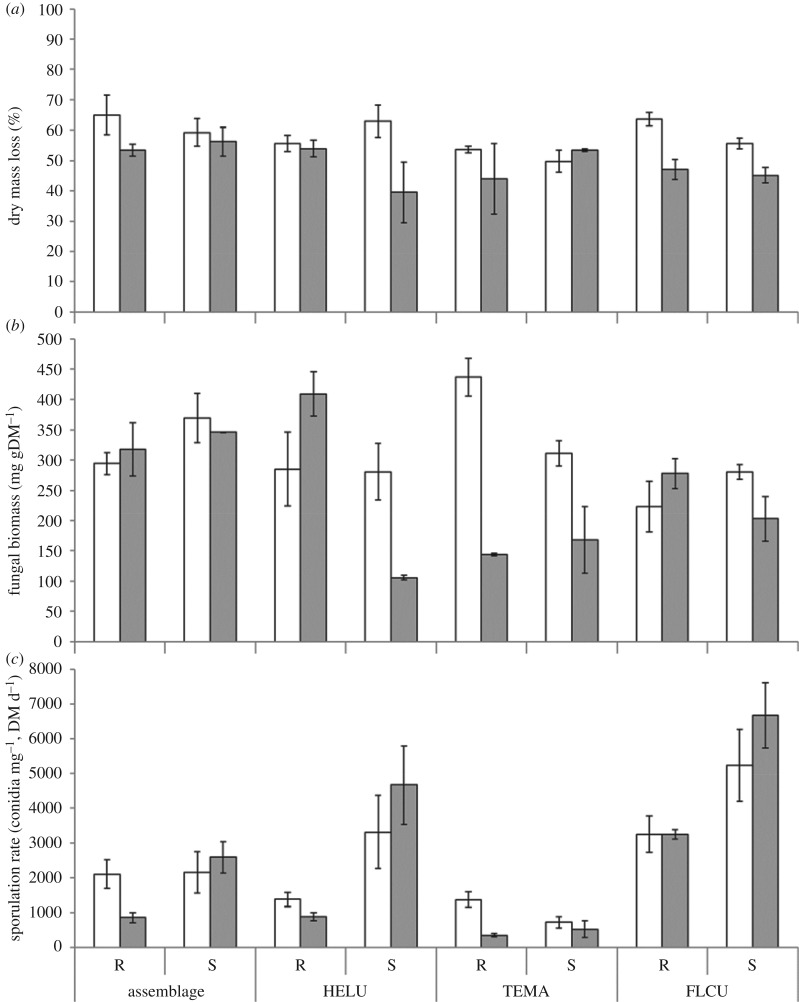
Mass loss was significantly higher in media without added salt (three-way ANOVA, F = 9.11, p = 0.005; figure 1a). Neither species composition (F = 1.98, p = 0.137) nor the origin of fungal strains (R or S; F = 0.30, p = 0.585) had a significant effect, and none of the interactions between factors were significant (three-way ANOVA F > 0.10, p > 0.178).
Figure 1.
(a) Dry mass loss of poplar leaf discs and associated (b) fungal biomass and (c) sporulation rates, incubated in microcosms with mono- or multicultures of two strains (R, reference; S, saline) of three aquatic hyphomycete species—Heliscus lugdunensis (HELU), Tetracladium marchalianum (TEMA) and Flagellospora curta (FLCU). All treatments were exposed to a reference (white bars) and a salted (with 6 g l−1 NaCl; grey bars) solution for 21 days (mean ± s.e.; n = 3).
Ergosterol build-up was also significantly reduced by salt addition (F = 19.28; p < 0.005; figure 1b). In HELU-S and TEMA-R, it was negatively affected in salt-contaminated medium (Tukey's test, p = 0.001 and 0.002, respectively) in contrast with their counterparts (HELU-R and TEMA-S, Tukey's test, NS).
Sporulation rates differed between species composition (F = 42.48; p < 0.001) and strains (F = 28.91; p < 0.001; figure 1c), but were not affected by the presence of salt in the medium (F = 0.98; p = 0.33). Only the interactions between strains with each of the other factors separately were significant (F > 7.03; p < 0.005). Globally, S strains sporulated more profusely than the R strains (Tukey's test, p < 0.001). Sporulation rates were highest in FLCU in individual microcosms, followed by treatments inoculated with all three species and HELU individually, regardless of salt concentration in the medium (Tukey's test, p = 0.55). TEMA released the lowest number of spores (Tukey's test, p < 0.001). Of the three species, only HELU showed significant differences between strains, with S releasing over three times the number of spores of the R strain in the salt concentrations corresponding to their origin (Tukey's test, p = 0.02). Exposure to salt did not affect the R strain, which maintained a much lower sporulation rate than the S strain in the same medium (Tukey's test, p = 0.001).
Mixed fungal assemblages did not show significant differences in total conidia production, either between strains or media (Tukey's test, p > 0.25; figure 1c). All species strains were able to produce spores across treatments. However, the relative contribution of each species was differently affected by salt addition in the medium: without salt, the mixture of R strains was dominated by TEMA conidia (59%), followed by HELU (38%), while in the mixture of S strains FLCU contributed almost 63% of the spores; in the presence of salt, the reproductive output of HELU dominated, contributing 86 and 87% of the spores in the mixtures of R and S strains, respectively.
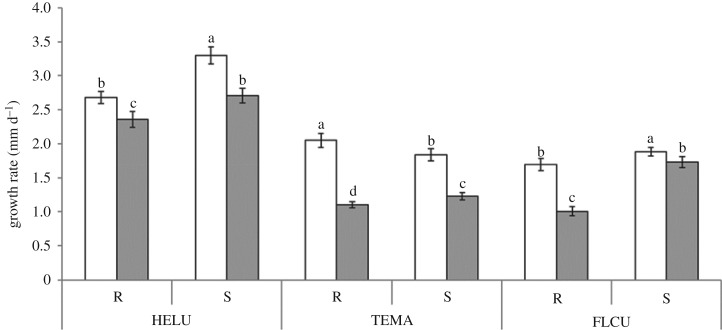
Figure 3.
Linear growth rate (mm d−1) of two strains (R, reference; S, saline) of Heliscus lugdunensis (HELU), Tetracladium marchalianum (TEMA) and Flagellospora curta (FLCU) in solid MEA medium with (S, 6 g l−1 NaCl; grey bars) or without added salt (R; white bars) (mean ± s.e.; n = 3). Significant differences (p < 0.05) within the same species are indicated by different letters.
(b). Morphology and growth rate of R and S fungal species strains
(i). Morphology
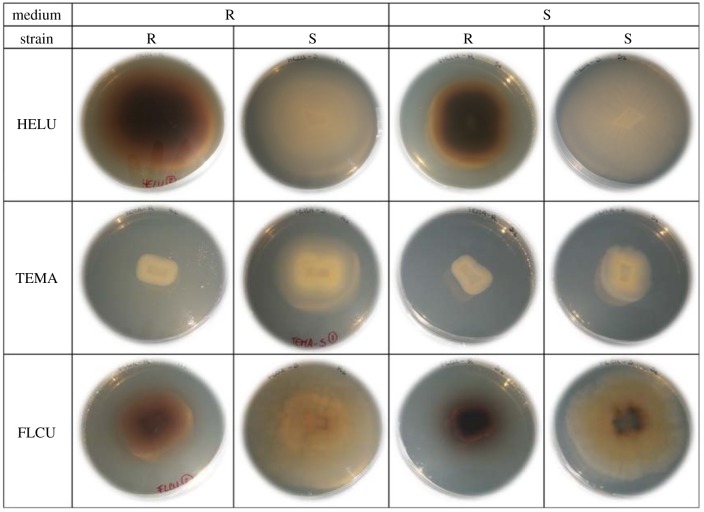
All species and strains formed circular colonies in both media, except FLCU-S strain that consistently had irregular boundaries (figure 2).
Figure 2.
Colony morphology of two strains (R, reference; S, saline) of three AH species—Heliscus lugdunensis (HELU), Tetracladium marchalianum (TEMA) and Flagellospora curta (FLCU) growing in MEA medium with (6 g l−1) or without added NaCl. All colonies after 21 days of growth. (Online version in colour.)
The colour of fungal colonies on MEA varied considerably. The HELU-R strain was brown and opaque, and HELU-S white and translucent. Both TEMA strains were white and transparent, while FLCU-R colonies were opaque brown and FLCU-S translucid yellow. In summary, the shape and colour of strains of all three species were unaffected by the presence of salt in the medium.
(ii). Growth rate
Growth was significantly affected by species composition, strain and salt concentration (factorial repeated-measures ANOVA, F = 4788.60, 733.8, 2725.8, p < 0.001). All fungal strains grew significantly faster in the unsalted medium (Tukey's test, p < 0.001; figure 3), with HELU growing fastest (Tukey's test, p < 0.001; figure 3). In most cases, S strains grew faster, regardless of species or medium salt concentration (Tukey's test, p < 0.001; figure 3). HELU and FLCU behaved similarly, with both strains growing at the same rate when exposed to the original salt concentration. Where the S strain was stimulated in the absence of salt, the correspondending R strain was negatively affected by its presence. By contrast, TEMA grew faster in media without added salt, with R outpacing the S strain.
(c). Interactions
In more than half of the tested interactions and independent of species, strains or number of colonies per Petri dish, ‘mutual intermingling’ was the dominant interaction type (table 2). Mutual inhibition was less frequent and was observed at a distance (three cases) or when the mycelium of both colonies touched each other (mutual inhibition at contact; 11 cases).
Table 2.
Growth inhibition for interacting species and interaction type for each combination of two strains (R, reference; S, saline) of three AH species—Heliscus lugdunensis (HELU), Tetracladium marchalianum (TEMA) and Flagellospora curta (FLCU) growing on MEA medium with (S, 6 g l−1 NaCl) or without added salt (R) (mean ± s.e.; n = 5). (n.a., not available).
| medium | interaction | species | strain | induced inhibition (%) | species | strain | induced inhibition (%) | paired t-test (P) | interaction type |
|---|---|---|---|---|---|---|---|---|---|
| reference | intraspecific (pairs) | HELU | R | 20.1 ± 1.6 | HELU | R | 24.2 ± 1.3 | 0.078 | mutual intermingling |
| HELU | S | 35.3 ± 0.3 | HELU | S | 35.9 ± 1.2 | 0.401 | mutual intermingling | ||
| TEMA | R | 33.9 ± 0.9 | TEMA | R | 36.4 ± 0.7 | 0.050 | mutual intermingling | ||
| TEMA | S | 30.2 ± 2.6 | TEMA | S | 32.2 ± 2.1 | 0.565 | mutual inhibition at contact | ||
| FLCU | R | 28.5 ± 1.9 | FLCU | R | 31.2 ± 1.4 | 0.315 | mutual inhibition at contact | ||
| FLCU | S | 30.5 ± 0.9 | FLCU | S | 31.5 ± 1.4 | 0.617 | mutual inhibition at contact | ||
| interspecific (pairs) | HELU | R | 35.1 ± 3.5 | TEMA | R | 0.0 ± 0.0 | <0.001 | mutual intermingling | |
| HELU | S | 26.4 ± 0.5 | TEMA | S | 0.0 ± 0.0 | <0.001 | mutual intermingling | ||
| HELU | R | 38.8 ± 2.2 | FLCU | R | 14.8 ± 1.5 | <0.001 | mutual intermingling | ||
| HELU | S | 18.2 ± 2.8 | FLCU | S | 30.6 ± 1.4 | <0.001 | mutual inhibition at contact | ||
| TEMA | R | 30.4 ± 2.6 | FLCU | R | 33.8 ± 1.4 | 0.274 | mutual intermingling | ||
| TEMA | S | 32.2 ± 2.1 | FLCU | S | 27.1 ± 3.5 | 0.256 | mutual intermingling | ||
| interspecific (triplet) | HELU | R | 33.1 ± 3.9 | TEMA | R | 0.0 ± 0.0 | <0.001 | mutual intermingling | |
| HELU | S | 23.3 ± 2.3 | TEMA | S | 21.6 ± 4.2 | 0.732 | mutual intermingling | ||
| HELU | R | 10.9 ± 3.6 | FLCU | R | 0.0 ± 0.0 | 0.024 | mutual intermingling | ||
| HELU | S | 33.2 ± 2.2 | FLCU | S | 19.2 ± 3.2 | 0.007 | mutual inhibition at contact | ||
| TEMA | R | 8.0 ± 5.1 | FLCU | R | 7.6 ± 4.8 | 0.950 | mutual inhibition at contact | ||
| TEMA | S | 9.7 ± 1.7 | FLCU | S | 13.7 ± 3.6 | 0.378 | mutual intermingling | ||
| salted | intraspecific (pairs) | HELU | R | 28.5 ± 1.1 | HELU | R | 27.4 ± 1.3 | 0.543 | mutual inhibition at contact |
| HELU | S | 30.6 ± 0.8 | HELU | S | 28.1 ± 1.1 | 0.816 | mutual intermingling | ||
| TEMA | R | 23.9 ± 3.1 | TEMA | R | 22.0 ± 3.2 | 0.666 | mutual inhibition at contact | ||
| TEMA | S | 26.2 ± 2.1 | TEMA | S | 27.5 ± 1.8 | 0.660 | mutual inhibition at a distance | ||
| FLCU | R | 18.1 ± 3.4 | FLCU | R | 24.1 ± 1.9 | 0.168 | mutual inhibition at a distance | ||
| FLCU | S | 27.1 ± 1.4 | FLCU | S | 27.0 ± 1.8 | 0.975 | mutual inhibition at contact | ||
| interspecific (pairs) | HELU | R | 32.4 ± 2.5 | TEMA | R | 0.0 ± 0.0 | <0.001 | mutual intermingling | |
| HELU | S | 31.4 ± 2.6 | TEMA | S | 0.0 ± 0.0 | <0.001 | mutual intermingling | ||
| HELU | R | 38.4 ± 0.8 | FLCU | R | 0.0 ± 0.0 | <0.001 | mutual intermingling | ||
| HELU | S | 38.9 ± 2.0 | FLCU | S | 23.4 ± 0.9 | <0.001 | mutual inhibition at contact | ||
| TEMA | R | 16.0 ± 3.8 | FLCU | R | 30.0 ± 1.7 | 0.015 | mutual inhibition at contact | ||
| TEMA | S | 0.0 ± 0.0 | FLCU | S | 19.2 ± 2.7 | <0.001 | mutual intermingling | ||
| interspecific (triplet) | HELU | R | 28.2 ± 3.8 | TEMA | R | 0.0 ± 0.0 | <0.001 | mutual intermingling | |
| HELU | S | 18.3 ± 3.8 | TEMA | S | 20.7 ± 2.1 | 0.592 | mutual intermingling | ||
| HELU | R | 23.9 ± 8.1 | FLCU | R | 0.0 ± 0.0 | 0.026 | mutual intermingling | ||
| HELU | S | 37.8 ± 2.0 | FLCU | S | 26.8 ± 4.7 | 0.063 | mutual intermingling | ||
| TEMA | R | n.a. | FLCU | R | n.a. | n.a. | n.a. | ||
| TEMA | S | 11.4 ± 5.1 | FLCU | S | 20.2 ± 6.3 | 0.307 | mutual inhibition at a distance |
(i). Intraspecific interactions
Reference medium. Interactions between the S and the R strain of each species were not highly aggressive—inhibition varied between 20.1 and 36.4% in reference medium—and no significant difference of inhibition occurred between the colonies (paired t-test, p > 0.05; table 2). In both cases, the type of interaction in HELU or TEMA was mutual intermingling (except in TEMA-S × TEMA-S, which showed mutual inhibition at contact). FLCU intraspecific interactions indicated mutual inhibition upon contact.
Salt-rich medium. In the presence of salt, aggressiveness between the S and the R strain of each species was lower than in reference medium; however, the difference was not significant (paired t-test, p > 0.17). Additionally, high salinity seems to inhibit intermingling between strain colonies, with growth ceasing as soon as they reached each other (HELU and TEMA) or even before contact.
(ii). Interspecific interactions
Reference medium. Significant growth inhibition occurred in all combinations of species, regardless of strain (paired t-test, p < 0.001). HELU colonies imposed an inhibition of 26.4–38.8% in all cases, except when S strain interacted with FLCU-S. In that case, the growth of HELU-S was inhibited by 30.6% and caused a 18.2% inhibition in its opponent (table 2). None of the TEMA or FLCU strains reacted significantly with each other (paired t-test, p > 0.256). In general, the triple interactions pattern was consistent with the results of pair combinations, with the exception of the inhibition between HELU-S and TEMA-S that was nullified in the presence of FLCU-S.
Salt-rich medium. Salt addition to the medium increased aggressiveness between fungal species of the same origin: HELU consistently inhibited all other species (between 31.4 and 38.9%; paired t-test, p < 0.001), and FLCU became more competitive when facing TEMA (paired t-test, p < 0.015). However, in the presence of salt, the response between species underwent some changes when all species were simultaneously exposed to each other (i.e. triplets): when all strains were from the salt-rich stream, there was no significant interaction (paired t-test, p > 0.063) and mycelium of each colony grew towards the other without inhibiting or being inhibited by the opposing colony. HELU-R consistently dominated the other two species. In this triple situation, no interaction occurred between TEMA-R and FLCU-R, and there was slight though non-significant mutual inhibition between the S strains before contact (paired t-test, p = 0.307).
(iii). Antagonism index
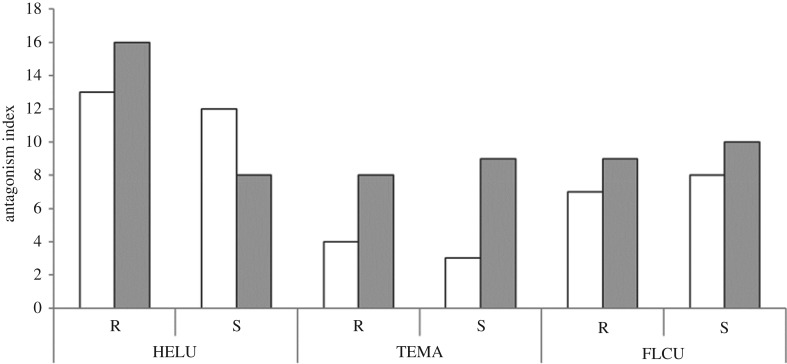
The IA measures the ability of each fungal colony to compete and dominate opponents (figure 4).
Figure 4.
IA of two strains (R, reference; S, saline) of Heliscus lugdunensis (HELU), Tetracladium marchalianum (TEMA) and Flagellospora curta (FLCU) growing on MEA without (R; white bars) or with added salt (S, 6 g l−1 NaCl; grey bars).
Reference medium. Clear differences were registered among IA values of the three species in the reference medium (HELU >> FLCU > TEMA); there were no differences between S and R strains.
Salt-rich medium. Salt addition tends to standardize the competitiveness of all strains, except in the case of HELU-R that had IA values about 2× higher than the other strains. Regardless of the strain's origin, all AHs (except HELU) became more antagonistic when exposed to salt, with TEMA strains having similar levels of antagonism to the other strains.
4. Discussion and conclusion
Overall, our results suggest that the direct impact of salinization on the ecological functions of AHs is relatively low. Nevertheless, salt contamination represents a stress on individual AHs, to which they are expected to respond by trade-offs between growth and reproduction and possibly antagonistic responses within fungal community competitors. Ecological functions are commonly related to the trophic roles of a species or a group of species [42]. Environmental conditions, e.g. the level of anthropogenic stress, including nutrients, modulate the shape of this relationship [43,44]. In a recent analysis, Baert et al. [45] conclude that the biodiversity effect is greatest at a low-to-intermediate level of stress. The salinity level in our study was high; the thresholds at which biological communities are significantly affected by salinity are relatively low at between 1 and 2 g l−1 according to different authors [24,46]. Compared with invertebrates, algae or macrophytes, AHs are remarkably resilient. At 6 g l−1, leaf decomposition rates owing to pure fungal cultures declined by an average of 21% and fungal biomass by 15% (figure 1). By contrast, release of conidia (which might benefit filter-feeders; [47]) was not affected (figure 1).
Contrary to our expectations, the S fungal strains were not more effective in salt-rich media than their reference counterparts, either alone or in combination. These results tend to corroborate previous findings that indicate that all tested species (HELU, TEMA and FLCU) naturally tolerate high levels of salt [22], and suggest that no genetic degradative advantage in terms of superior degradation potential or biomass accumulation was acquired by strains in the salinized stream. A reduction in mass loss was consistently associated with lower fungal biomass concentrations in leaves, colonized by S or R strain communities, in salt-rich media. This supports a fungal-mediated, strain-independent, effect of salinity on leaf degradation. Such results are in agreement with previous studies reporting an overall negative relationship between salt addition to the stream, leaf mass loss and/or associated fungal biomass (e.g. [9,18–22]).
Conidial production was not affected by salinization. This was surprising considering the generally high sensitivity of fungal reproductive output to stressors [48–50], including salt [22,29,30]. Here, it seems that the total sporulation rate variability was most probably related to species identity and origin: in microcosms inoculated with individual strains, FLCU had the highest sporulation rate. The halotolerance and high sporulating capacity of FLCU-R, in reference to salt-rich media (up to 8 g l−1), were previously reported by Canhoto et al. [22]. Overall, S strains tended to release more conidia than R strains, singly or in mixtures. In fact, a general tendency to produce more spores paralleled a (non-significant) reduction in biomass in most S strains in the salinized (versus reference) media treatment. If confirmed, this might indicate that in salt-rich environments, these strains tend to guarantee reproduction and dispersal rather than mycelial growth. This contrasts with the growth versus reproduction trade-off previously found in R species grown in NaCl-rich medium [22].
The total number of spores produced by the two mixed assemblages was not different, which indicates that the origin of strains had no effect. Ferreira et al. [34] observed the same response by another three AH species from a reference and a metal-contaminated stream inoculated on alder leaves in microcosms. However, while dominated by the same species, HELU, overall evenness in the two fungal assemblages differed between salt treatments. When present in colonized leaves, this species frequently exhibits high resilience, being one of the most profusely sporulating aquatic fungi, regardless of identities of co-occurring fungi or environmental stressors [49,51].
The degradative capacity of the multispecies assemblage did not differ significantly from those of single species cultures, regardless of salt exposure. This lack of diversity effect suggests considerable functional redundancy among the tested AH species [52]. Assuming that species-specific conidial production is a reliable indicator of fungal activity on leaves, the fact that reduced sporulation of some strains (TEMA, FLCU) in salt-rich medium was compensated for by the more tolerant HELU [24] reflects a change of their relative contributions to leaf decomposition. This follows the pattern observed in other stream microcosms assessing leaf decomposition under different stressors [50,51,53–55].
AH colonies differed in shape and/or pigmentation among species and, within each species, between strains; the presence of salt seems to induce phenotypic changes in the colonies (i.e. pigmentation), suggesting that potential tolerance of contamination was translated, as frequently observed, into morphological vegetative differences [32,56].
Exposure to salt clearly inhibited growth of all strains (figure 3). This suggests that coping with salt diverts some energy from growth. Previous studies in terrestrial [57] and aquatic environments suggest that this may involve the synthesis of osmoprotective compounds [22], such as polyols [58,59], which are able to stabilize cellular protein function and to regulate and possibly detoxify high concentrations of saline intracellular macromolecules [60–62]. The higher growth rates observed in most S (over R) strains in salinized medium point to possible metabolic adaptations to deal with osmotic stress triggered by salt.
In salt-enriched medium, intraspecific interactions tended to be weaker, while among interspecific strains, antagonism and aggressiveness were accentuated. This contradicts our hypothesis that in stressed environments, interspecific competition is weakened and mutual inhibition less beneficial. It remains to be seen if the observed pattern favours intraspecific gene flow [40] and shifts the interspecific competitive balance. In our study, AH antagonisms are strain-specific but dependent on the number of species involved and vary in intensity with salt level. HELU was the most antagonistic species, particularly the S strain in reference conditions. Although some studies claim stronger allelopathic interactions for TEMA than for HELU, a clear inhibitory activity of this latter towards other AH species was previously recognized [40,63]. This trait, combined with high growth rates, may be responsible for its dominance in both mixed assemblages in the decomposition test (S medium). While the importance of competition for AH succession and community structure in salinized environments seems clear (see [32,39,64]), our results also underline its unpredictability under natural conditions.
Overall, our results corroborate the hypothesis that AHs are highly halotolerant [22], which may largely guarantee leaf litter processing in temporary or historically salt-contaminated streams. We hypothesize that different strategies will be adopted by communities exposed to temporary versus long-term exposures: temporary/discrete exposure to salt (R strains) may favour investment in mycelial integrity and growth, while extended exposure may trigger physiological adjustments that continue to safeguard cellular viability, but also provide energy for conidium production. Both strategies may result in continued fungal ecological functions that guarantee leaf decomposition across a wide range of salinities. Considering the importance of resource quality for conidia settlement [65], intensity of fungal interactions [39], mycelial invasiveness [66] and degradative potential and growth [3], it seems reasonable to assume that the rates of the proposed strategies might be dependent on the traits (stoichiometry included; [67]) of the available leaf material provided by the riparian areas. This seems particularly relevant for fungal communities from salinized streams that may have lower assimilation capacity of phosphorus from the environment [21,68]; in this case, leaf litter quality will likely have a crucial role in compensating for such limitation and guaranteeing mycelial growth [67,69].
The present study shows that changes in fungal communities in streams subjected to salt contamination may, to some extent, be predicted from species-specific salt tolerances [22] and from changing interspecific competitive interactions. These changes may affect fungal succession and reduce diversity. The functional redundancy among fungal species is expected to ensure a continued high level of microbial decomposition under saline conditions. Nevertheless, nutrient transfer to the surviving salt-tolerant detritus-feeding invertebrates may be compromised by the disappearance of less tolerant aquatic hyphomycete species. While fungal colonization generally enhances palatability and nutritional value of leaf litter to shredders, these consumers often discriminate among specific fungal taxa or fungal associations [3,4]. The partial or complete loss of some fungi could therefore result in cascading effects through stream food webs in salt-contaminated streams, which may ultimately compromise vital ecosystem goods and services. More studies will be needed to clarify the importance of direct and indirect toxicity effects of salt on freshwater organisms (including physiological and behavioural tests) and under different environmental scenarios such as drought owing to its close relationship with salinization under global warming. In conclusion, the consequences of salinization in donor-controlled streams are far from clear and difficult to establish considering different contamination patterns—persistent or temporary (e.g. pulsed or chronic; [19])—and the relationships with local biotic and abiotic conditions [21,28,70]. Multiple stressors associated with agriculturalization of forested riparian areas, urbanization or global change are superimposed confounding factors that hinder evaluation but confer urgency to investigate the effects of salt contamination in streams.
Acknowledgements
Financial support by FCT, within the POCH – Human Capital Operating Programme, to A.L.G. (fellowship reference SFRH/BPD/94820/2013) is gratefully acknowledged, which was cofunded by the European Social Fund and MCTES national funds.
Data accessibility
This article has no additional data.
Authors' contributions
A.L.G., F.B. and C.C. conceived and designed the experiments. A.C. and A.L.G. carried out all the laboratory work. A.L.G. analysed data and wrote the first draft of the manuscript, and C.C. and F.B. collaborated on writing the final version.
Competing interests
We have no competing interests.
Funding
This work is also financed by the project ‘FunctionalStreams’ funded by the French National Agency (grant ANR-14-CE01-0009-01) and by FCT/MEC through national funds and the co-funding by the FEDER, within the PT2020 Partnership Agreement, and COMPETE 2020, within the project UID/BIA/04004/2013; project ReNATURE—Valorization of the Natural Endogenous Resources of the Centro Region (Centro 2020, Centro-01-0145-FEDER-000007).
References
- 1.Wipfli MS, Richardson JS, Naiman RJ. 2007. Ecological linkages between headwaters and downstream ecosystems: transport of organic matter, invertebrates, and wood down headwater channels. J. Am. Water Resour. Assoc. 43, 72–85. ( 10.1111/j.1752-1688.2007.00007.x) [DOI] [Google Scholar]
- 2.Gessner MO, Gulis V, Kuehn KA, Chauvet E, Suberkropp K. 2007. Fungal decomposers of plant litter in aquatic ecosystems. In The mycota: a comprehensive treatise on fungi as experimental systems for basic and applied research, vol. IV: environmental and microbial relationships (eds Kubicek CP, Druzhinina IS), 2nd edn, pp. 301–324. Berlin, Germany: Springer. [Google Scholar]
- 3.Canhoto C, Graça MAS. 2008. Interactions between fungi (aquatic Hyphomycetes) and invertebrates. In Novel techniques and ideas in mycology. Fungal Diversity Research Series 20 (eds Sridhar KR, Bärlocher F, Hyde KD), pp. 305–325. Chiang Rai, Thailand: Fungal Diversity Press. [Google Scholar]
- 4.Gonçalves AL, Chauvet E, Bärlocher F, Graça MAS, Canhoto C. 2014. Top-down and bottom-up control of litter decomposers in streams. Freshw. Biol. 59, 2172–2182. ( 10.1111/fwb.12420) [DOI] [Google Scholar]
- 5.Grime JP. 1977. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat. 111, 1169–1194. ( 10.1086/283244) [DOI] [Google Scholar]
- 6.Pugh GJF, 1980. Strategies in fungal ecology. Trans. Br. Mycol. Soc. 75, 1–14. ( 10.1016/S0007-1536(80)80188-4) [DOI] [Google Scholar]
- 7.Bärlocher F. 2009. Reproduction and dispersal in aquatic hyphomycetes. Mycoscience 50, 3–8. ( 10.1007/s10267-008-0449-x) [DOI] [Google Scholar]
- 8.Krauss GJ, Solé M, Krauss G, Schlosser D, Wesenberg D, Bärlocher F. 2011. Fungi in freshwaters: ecology, physiology and biochemical potential. FEMS Microbiol. Rev. 35, 620–651. ( 10.1111/j.1574-6976.2011.00266.x) [DOI] [PubMed] [Google Scholar]
- 9.Schäfer RB, Bundschuh M, Roach D, Szöcs E, von der Ohe PC, Pettigrove V, Schulz R, Nugegoda D, Kefford BJ. 2012. Relationships of selected ecosystem functions in streams with pesticide toxicity, salinity and other environmental variables and the relevance for ecosystem services. Sci. Total Environ. 415, 69–78. ( 10.1016/j.scitotenv.2011.05.056) [DOI] [PubMed] [Google Scholar]
- 10.Cañedo-Argüelles M, Kefford BJ, Piscart C, Prat N, Schäfer RB, Schulz CJ. 2013. Salinisation of rivers: an urgent ecological issue. Environ. Pollut. 173, 157–167. ( 10.1016/j.envpol.2012.10.011) [DOI] [PubMed] [Google Scholar]
- 11.Szöcs E, Coring E, Bäthe J, Schäfer RB. 2014. Effects of anthropogenic salinization on biological traits and community composition of stream macroinvertebrates. Sci. Total Environ. 468, 943–949. ( 10.1016/j.scitotenv.2013.08.058) [DOI] [PubMed] [Google Scholar]
- 12.Cañedo-Arguelles M, et al. 2016. Saving freshwater from salts. Science 351, 914–916. ( 10.1126/science.aad3488) [DOI] [PubMed] [Google Scholar]
- 13.Kaushal SS, Groffman PM, Likens GE, Belt KT, Stack WP, Kelly VR, Band LE, Fisher GT. 2005. Increased salinization of fresh water in the northeastern United States. Proc. Natl Acad. Sci. USA 102, 13 517–13 520. ( 10.1073/pnas.0506414102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tyree M, Clay N, Polaskey S, Entrekin S. 2016. Salt in our streams: even small sodium additions can have negative effects on detritivores. Hydrobiologia 775, 109–122. ( 10.1007/s10750-016-2718-6) [DOI] [Google Scholar]
- 15.Findlay SEG, Kelly VR. 2011. Emerging indirect and long-term road salt effects on ecosystems. Ann. NY Acad. Sci. 1223, 58–68. ( 10.1111/j.1749-6632.2010.05942.x) [DOI] [PubMed] [Google Scholar]
- 16.Lambert MR, Stoler AB, Smylie MS, Relyea RA, Skelly DK. 2016. Interactive effects of road salt and leaf litter on wood frog sex ratios and sexual size dimorphism. Can. J. Fish. Aquat. Sci. 6, 1–6. ( 10.1139/cjfas-2016-0324) [DOI] [Google Scholar]
- 17.Marques JM, Carvalho A, Carreira PM, Mansilha C. 2017. Impact of road de-icing on the hydrogeochemistry of groundwater from a mountain area (Serra da Estrela, Central Portugal). 15th Water–Rock Interaction International Symposium, WRI-15. Proc. Earth Planet. Sci. 17, 964–967. ( 10.1016/j.proeps.2017.01.039) [DOI] [Google Scholar]
- 18.Swan CM, DePalma CA. 2012. Elevated chloride and consumer presence independently influence processing of stream detritus. Urban Ecosyst. 15, 625–635. ( 10.1007/s11252-011-0210-7) [DOI] [Google Scholar]
- 19.Cañedo-Argüelles M, Bundschuh M, Gutiérrez-Cánovas C, Kefford BJ, Prat N, Trobajo R, Schäfer RB. 2014. Effects of repeated salt pulses on ecosystem structure and functions in a stream mesocosm. Sci. Total Environ. 476, 634–642. ( 10.1016/j.scitotenv.2013.12.067) [DOI] [PubMed] [Google Scholar]
- 20.Gómez R, Asencio AD, Picón JM, Del Campo R, Arce MI, Del Mar Sánchez-Montoya M, Suárez ML, Vidal-Abarca MR. 2016. The effect of water salinity on wood breakdown in semiarid Mediterranean streams. Sci. Total Environ. 541, 491–501. ( 10.1016/j.scitotenv.2015.09.040) [DOI] [PubMed] [Google Scholar]
- 21.Sauer FG, Bundschuh MP, Zubrod JP, Schäfer RB, Thompson K, Ben J, Kefford BJ. 2016. Effects of salinity on leaf breakdown: dryland salinity versus salinity from a coalmine. Aquat. Toxicol. 177, 425–432. ( 10.1016/j.aquatox.2016.06.014) [DOI] [PubMed] [Google Scholar]
- 22.Canhoto C, Simões S, Gonçalves AL, Guilhermino L, Bärlocher F. 2017. Stream salinization and fungal-mediated leaf decomposition: a microcosm study. Sci. Total Environ. 599–600, 1638–1645. ( 10.1016/j.scitotenv.2017.05.101) [DOI] [PubMed] [Google Scholar]
- 23.Horrigan N, Choy S, Marshall J, Recknagel F. 2005. Response of stream macroinvertebrates to changes in salinity and the development of a salinity índex. Mar. Freshw. Res. 56, 825–833. ( 10.1071/MF04237) [DOI] [Google Scholar]
- 24.Piscart C, Usseglio-Polatera P, Moreteau JC, Beisel JN. 2006. The role of salinity in the selection of biological traits of freshwater invertebrates. Arch. Hydrobiol. 166, 185–198. ( 10.1127/0003-9136/2006/0166-0185) [DOI] [Google Scholar]
- 25.Cañedo-Argüelles M, Grantham TE, Perrée I, Rieradevall M, Céspedes-Sánchez R, Prat N. 2012. Response of stream invertebrates to short-term salinization: a mesocosm approach. Environ. Pollut. 166, 144–151. ( 10.1016/j.envpol.2012.03.027) [DOI] [PubMed] [Google Scholar]
- 26.Kefford BJ, Marchant R, Schäfer RB, Metzeling L, Dunlop JE, Choy SC, Goonan P. 2011. The definition of species richness used by species sensitivity distributions approximates observed effects of salinity on stream macroinvertebrates. Environ. Pollut. 159, 302–310. ( 10.1016/j.envpol.2010.08.025) [DOI] [PubMed] [Google Scholar]
- 27.Kefford BJ, Hickey GL, Gasith A, Ben-David E, Dunlop JE, Palmer CG, Kaylene A, Choy SC, Piscart C. 2012. Global scale variation in the salinity sensitivity of riverine macroinvertebrates: Eastern Australia, France, Israel and South Africa. PLoS ONE 7, e35224 ( 10.1371/journal.pone.0035224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kefford BJ, Buchwalter D, Cañedo-Argüelles M, Davis J, Duncan R, Hoffmann A, Thompson R. 2016. Salinized rivers: degraded systems or new habitats for salt tolerant faunas? Biol. Lett. 12, 1–7. ( 10.1098/rsbl.2015.1072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Byrne PJ, Jones EB. 1975. Effect of salinity on the reproduction of terrestrial and marine fungi. Trans. Br. Mycol. Soc. 65, 185–200. ( 10.1016/S0007-1536(75)80002-7) [DOI] [Google Scholar]
- 30.Sridhar KR, Kaveriappa KM. 1988. Occurrence and survival of aquatic hyphomycetes in brackish and sea water. Arch. Hydrobiol. 113, 153–160. ( 10.1515/botm.1979.22.7.421) [DOI] [Google Scholar]
- 31.Stoler AB, Hintz WD, Jones DK, Lind L, Mattes BM, Schuler MS, Relyea RA. 2017. Leaf litter mediates the negative effect of road salt on forested wetland communities. Freshw. Sci. 36, 415–426. ( 10.1086/692139) [DOI] [Google Scholar]
- 32.Ferreira V, Gonçalves AL, Pratas J, Canhoto C. 2010. Contamination by uranium mine drainages may affect fungal growth and interactions between fungal species and strains. Mycologia 102, 1004–1011. ( 10.3852/09-248) [DOI] [PubMed] [Google Scholar]
- 33.Quainoo S, Sahadevan S, Graça MAS. 2016. Copper tolerant ecotypes of Heliscus lugdunensis differ in their ecological function and growth. Sci. Total Environ. 544, 168–174. ( 10.1016/j.scitotenv.2015.11.119) [DOI] [PubMed] [Google Scholar]
- 34.Ferreira V, Gonçalves AL, Canhoto C. 2012. Aquatic hyphomycete strains from metal contaminated and reference streams might respond differently to increases in temperature. Mycologia 104, 613–622. ( 10.3852/11-154) [DOI] [PubMed] [Google Scholar]
- 35.Dang CK, Chauvet E, Gessner MO. 2005. Magnitude and variability of process rates in fungal diversity-litter decomposition relationships. Ecol. Lett. 8, 1129–1137. ( 10.1111/j.1461-0248.2005.00815.x) [DOI] [PubMed] [Google Scholar]
- 36.Graça MAS, Bärlocher F, Gessner MO. 2005. Methods to study litter decomposition. A practical guide. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 37.Gessner MO, Chauvet E. 1993. Ergosterol-to-biomass conversion factors for aquatic hyphomycetes. Appl. Environ. Microbiol. 59, 502–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young JC. 1995. Microwave-assisted extraction of the fungal metabolite ergosterol and total fatty acids. J. Agric. Food Chem. 43, 2904–2910. ( 10.1021/jf00059a025) [DOI] [Google Scholar]
- 39.Shearer CA, Zare-Maivan H. 1988. In vitro hyphal interactions among wood- and leaf-inhabiting ascomycetes and fungi imperfect from freshwater habitats. Mycologia 80, 31–37. ( 10.2307/3807490) [DOI] [Google Scholar]
- 40.Bärlocher F. 1991. Intraspecific hyphal interactions among aquatic hyphomycetes. Mycologia 83, 82–88. ( 10.1007/BF02492836) [DOI] [Google Scholar]
- 41.Yuen TK, Hyde KD, Hodgkiss IJ. 1999. Interspecific interactions among tropical and subtropical freshwater fungi. Microb. Ecol. 37, 257–262. ( 10.1007/s002489900) [DOI] [PubMed] [Google Scholar]
- 42.Hooper DU, et al. 2012. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 486, 105–108. ( 10.1038/nature11118) [DOI] [PubMed] [Google Scholar]
- 43.Bärlocher F. 2005. Freshwater fungal communities. In The fungal community. Its organization and role in the ecosystem, 3rd edn (eds Dighton J, White JF, Oudemans P), pp. 39–59. Boca Raton, FL: Taylor & Francis. [Google Scholar]
- 44.Tilman D, Isbell F, Cowles JM. 2014. Biodiversity and ecosystem functioning. Annu. Rev. Ecol. Evol. Syst. 45, 471–493. ( 10.1146/annurev-ecolsys-120213-091917) [DOI] [Google Scholar]
- 45.Baert JM, Eisenhauer N, Janssen CR, de Laender F. 2018. Biodiversity effects on ecosystem functioning respond unimodally to environmental stress. Ecol. Lett. 21, 1191–1199. ( 10.1111/ele.13088) [DOI] [PubMed] [Google Scholar]
- 46.Herbert ER, Boon P, Burgin AJ, Neubauer SC, Franklin RB, Ardón M, Hopfensperger KN, Lamers LPM, Gell P. 2015. A global perspective on wetland salinization: ecological consequences of a growing threat to freshwater wetlands. Ecosphere 6, 1–43. ( 10.1890/ES14-00534.1) [DOI] [Google Scholar]
- 47.Bärlocher F, Brendelberger H. 2004. Clearance of aquatic hyphomycete spores by a benthic suspension feeder. Limnol. Oceanogr. 49, 2292–2296. ( 10.4319/lo.2004.49.6.2292) [DOI] [Google Scholar]
- 48.Bärlocher F, Kebede YK, Gonçalves AL, Canhoto C. 2013. Incubation temperature and substrate quality modulate sporulation by aquatic hyphomycetes. Microb. Ecol. 66, 30–39. ( 10.1007/s00248-013-0202-7) [DOI] [PubMed] [Google Scholar]
- 49.Gonçalves AL, Lírio AV, Graça MAS, Canhoto C. 2016. Fungal species diversity affects leaf decomposition after drought. Int. Rev. Hydrobiol. 100, 1–9. ( 10.1002/iroh.201501817) [DOI] [Google Scholar]
- 50.Fernandes I, Pascoal C, Cássio F. 2011. Intraspecific traits change biodiversity effects on ecosystem functioning under metal stress. Oecol. 166, 1019–1028. ( 10.1007/s00442-011-1930-3) [DOI] [PubMed] [Google Scholar]
- 51.Canhoto C, Calapez R, Gonçalves AL, Moreira-Santos M. 2013. Effects of Eucalyptus leachates and oxygen on leaf-litter processing by fungi and stream invertebrates. Freshw. Sci. 32, 411–424. ( 10.1899/12-062.1) [DOI] [Google Scholar]
- 52.Walker BH. 1992. Biodiversity and ecological redundancy. Conserv. Biol. 6, 18–23. ( 10.1046/j.1523-1739.1992.610018.x) [DOI] [Google Scholar]
- 53.Pascoal C, Cássio F, Nikolcheva L, Bärlocher F. 2010. Realized fungal diversity increases functional stability of leaf litter decomposition under zinc stress. Microb. Ecol. 59, 84–93. ( 10.1007/s00248-009-9567-z) [DOI] [PubMed] [Google Scholar]
- 54.Ferreira V, Chauvet E. 2012. Changes in dominance among species in aquatic hyphomycete assemblages do not affect litter decomposition rates. Aquat. Microb. Ecol. 66, 1–11. ( 10.3354/ame01556) [DOI] [Google Scholar]
- 55.Gonçalves AL, Graça MAS, Canhoto C. 2015. Is diversity a buffer against environmental temperature fluctuations? A decomposition experiment with aquatic fungi. Fungal Ecol. 17, 96–102. ( 10.1016/j.funeco.2015.05.013.) [DOI] [Google Scholar]
- 56.Braha B, Tintemann H, Krauss G, Ehrman J, Bärlocher F, Krauss GJ. 2007. Stress response in two strains of the aquatic hyphomycete Heliscus lugdunensis after exposure to cadmium and copper ions. Biometals 20, 93–105. ( 10.1007/s10534-006-9018-y) [DOI] [PubMed] [Google Scholar]
- 57.Asghar HN, Setia R, Marschner P. 2012. Community composition and activity of microbes from saline soils and non-saline soils respond similarly to changes in salinity. Soil Biol. Biochem. 47, 175–178. ( 10.1016/j.soilbio.2012.01.002) [DOI] [Google Scholar]
- 58.Kuehn KA, Churchill PF, Suberkropp K. 1998. Osmoregulatory responses of fungi inhabiting standing litter of the freshwater emergent macrophyte Juncus effuses. Appl. Environ. Microbiol. 64, 607–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burg MB, Ferraris JD. 2008. Intracellular organic osmolytes: function and regulation. J. Biol. Chem. 283, 7309–7313. ( 10.1074/jbc.R700042200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hellebust J. 1976. Osmoregulation. Annu. Rev. Plant. Physiol. 27, 485–505. [Google Scholar]
- 61.Hooley P, Fincham DA, Whitehead MP, Clipson NJW. 2003. Fungal osmotolerance. Adv. Appl. Microbiol. 53, 177–211. ( 10.1016/S0065-2164(03)53005-2) [DOI] [PubMed] [Google Scholar]
- 62.Overy D, et al. 2017. Does osmotic stress affect natural product expression in fungi? Mar. Drugs 15, 254 ( 10.3390/md15080254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sati SC, Arya P. 2010. Antagonism of some aquatic hyphomycetes against plant pathogenic fungi. Sci. World J. 10, 760−765. ( 10.1100/tsw.2010.80) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Allen JL, Leflaive J, Bringuier C, Ten-Hage L, Chauvet E, Cornut J, Danger M. 2017. Allelopathic inhibition of primary producer growth and photosynthesis by aquatic fungi. Fungal Ecol. 29, 133–138. ( 10.1016/j.funeco.2017.07.001) [DOI] [Google Scholar]
- 65.Dang CK, Gessner MO, Chauvet E. 2007. Influence of conidial traits and leaf structure on attachment success of aquatic hyphomycetes on leaf litter. Mycologia 99, 24–32. ( 10.3852/mycologia.99.1.24) [DOI] [PubMed] [Google Scholar]
- 66.Canhoto C, Graça MAS. 1999. Leaf barriers to fungal colonization and shredders (Tipula lateralis) consumption of decomposing Eucalyptus globulus. Microb. Ecol. 37, 163–172. [DOI] [PubMed] [Google Scholar]
- 67.Danger M, Gessner MO, Bärlocher F. 2016. Ecological stoichiometry of aquatic fungi: current knowledge and perspectives. Fungal Ecol. 19, 100–111. ( 10.1016/j.funeco.2015.09.004) [DOI] [Google Scholar]
- 68.Qualls RG, Richardson CJ. 2000. Phosphorus enrichment affects litter decomposition immobilization, and soil microbial phosphorus in wetland mesocosms. Soil Sci. Soc. Am. J. 64, 799–808. ( 10.2136/sssaj2000.642799x) [DOI] [Google Scholar]
- 69.Elser JJ, et al. et al. 2003. Growth rate–stoichiometry couplings in diverse biota. Ecol. Lett. 6, 936–943. ( 10.1046/j.1461-0248.2003.00518.x.) [DOI] [Google Scholar]
- 70.Roache P, Bailey C, Boon PI. 2006. Effects of salinity on the decay of the freshwater macrophyte, Triglochin procerum. Aquat. Bot. 84, 45–52. ( 10.1016/j.aquabot.2005.07.014) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.