Abstract
Under global change, the ion concentration of aquatic ecosystems is changing worldwide. Many freshwater ecosystems are being salinized by anthropogenic salt inputs, whereas many naturally saline ones are being diluted by agricultural drainages. This occurs concomitantly with changes in other stressors, which can result in additive, antagonistic or synergistic effects on organisms. We reviewed experimental studies that manipulated salinity and other abiotic stressors, on inland and transitional aquatic habitats, to (i) synthesize their main effects on organisms' performance, (ii) quantify the frequency of joint effect types across studies and (iii) determine the overall individual and joint effects and their variation among salinity–stressor pairs and organism groups using meta-analyses. Additive effects were slightly more frequent (54%) than non-additive ones (46%) across all the studies (n = 105 responses). However, antagonistic effects were dominant for the stressor pair salinity and toxicants (44%, n = 43), transitional habitats (48%, n = 31) and vertebrates (71%, n = 21). Meta-analyses showed detrimental additive joint effects of salinity and other stressors on organism performance and a greater individual impact of salinity than the other stressors. These results were consistent across stressor pairs and organism types. These findings suggest that strategies to mitigate multiple stressor impacts on aquatic ecosystems should prioritize restoring natural salinity concentrations.
This article is part of the theme issue ‘Salt in freshwaters: causes, ecological consequences and future prospects’.
Keywords: meta-analysis, salinization, dilution, additive effects, inland waters, transitional waters
1. Introduction
In the face of global change, understanding and predicting the effects of multiple stressors is one of the most pressing challenges in conservation and applied ecology [1,2]. In particular, aquatic organisms are exposed to a growing number of stressors [3,4], such as freshwater salinization [5–7], water acidification [8,9] or eutrophication [10,11]. Given the heterogeneous nature and different mechanisms of actions of these stressors (e.g. physical versus chemical stressors), the co-occurrence of several of them can result in additive, synergistic or antagonistic effects on organism traits (e.g. survival, fecundity, metabolic and growth rates, etc.). Additive effects occur when joint stressor effects (i.e. cumulative effects sensu Crain et al. [12]) equal the sum of individual effects. Non-additive effects are reflected by a deviation from the additive response, which can be greater (synergism) or less (antagonism) than the sum of individual effects [13] and thus exacerbate or mitigate, respectively, the effects on organism performance [14]. These changes at organism level are the primary and most sensitive responses to stress [15] but may ultimately alter community composition [16] and interfere with ecosystem processes and services, which sustain human welfare [17]. In recent years, several meta-analyses have synthesized the results of studies that have tested joint effects of multiple stressors in marine [12,18,19] and freshwater ecosystems [20] at different organizational levels, from organisms to communities, and have shown contrasting results. While an overall synergistic effect of multiple stressors has been found on marine systems, antagonistic joint effects dominate in freshwaters. However, none has specifically provided a comprehensive review of organism responses of inland aquatic species or populations to the combined effects of salinity changes with other global change stressors.
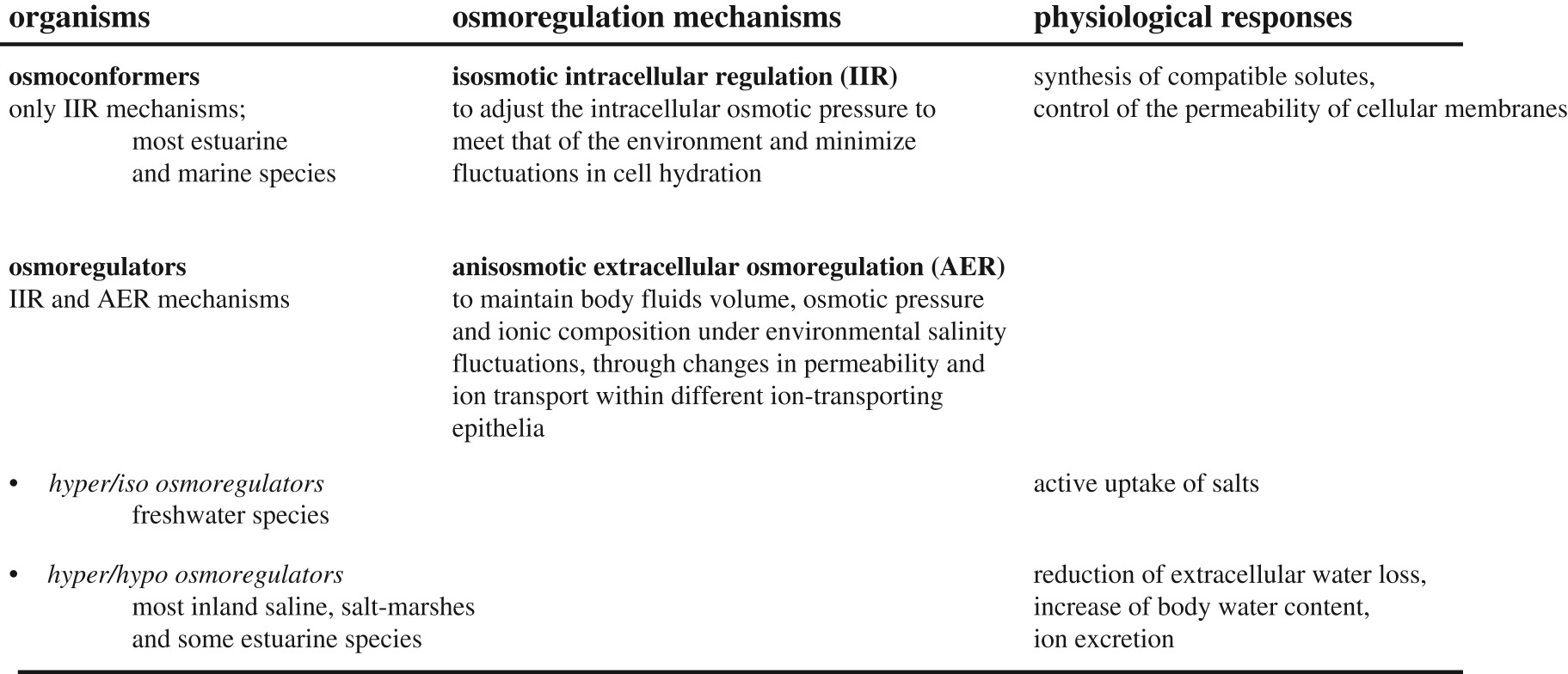
Human activities, like agriculture or salt mining, along with climatic aridification and rising sea levels, are increasing salt concentrations in inland freshwaters and coastal habitats [21], which produces severe negative economic and biological effects [6,22–24]. Contrarily, freshwater inputs, mainly caused by irrigated agriculture in arid landscapes, are diluting naturally saline rivers, estuaries and salt-marshes, with harmful effects [25]. At levels above or below the isosmotic point of organism internal fluids, salinity can disrupt metabolism and water balance [26]. Therefore, aquatic organisms have evolved different intra- and extracellular osmoregulation mechanisms to control osmotic and dehydration stress in the face of salinity changes in the external environment [27,28] (box 1). However, organism osmoregulation capacities might be insufficient to deal with anthropogenic salinization and dilution and, most importantly, it is unknown whether the derived negative effects of these salinity changes can be amplified or mitigated in the presence of additional stressors.
Box 1. Osmoregulation mechanims to deal with salinity stress based on Bradley [27] and Rivera-Ingraham & Lignot [28].
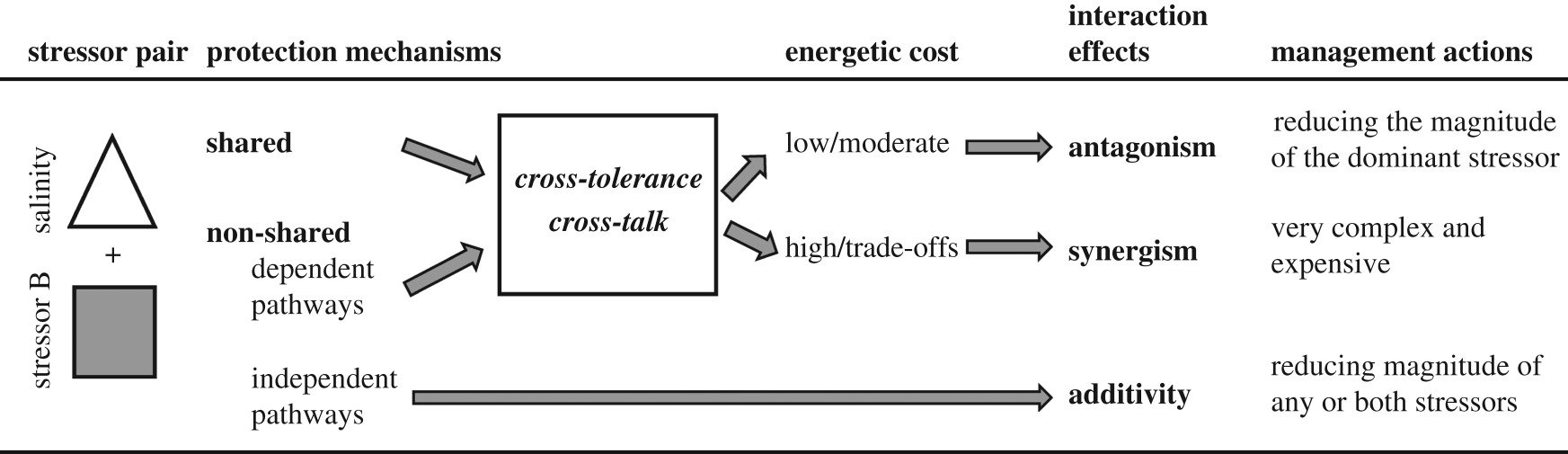
The outcomes of multiple stressor interactions are context-dependent (type of ecosystem, trophic level, response level, response metrics, specific stressor pair, stress intensity and duration, etc.) [22,23,26]. At the physiological level, the joint effect of multiple environmental stressors ultimately depends on organism sensitivity to each stressor [2] and the overlap in the underlying mechanisms and molecular pathways used to combat their effects. Exposure to one stressor can enhance resistance to another if the same protective mechanism can cope with both stressors (cross-tolerance [29–31]) (box 2). Alternatively, different stressors may activate distinct mechanisms but dependent signalling regulatory pathways (cross-talk, e.g. [32]). In these cases, the resulting interaction effect depends on the energetic cost of the upregulated mechanisms. If such cost is low, antagonistic interactions would be expected. If there are energetic trade-offs between protective mechanisms, exposure to one stressor can compromise the response to the other and the general result could be a synergistic negative effect. Finally, when independent mechanisms and pathways are activated, one stressor would have no effect on the response to the other, and an additive effect should be the most probable outcome. Some cross-tolerance and cross-talk responses involving salinity have been reported (e.g. [32–34]). Thus, this cross-tolerance/cross-talk framework, originally proposed for cold, desiccation and immune responses in overwintering insects [29], may be useful to yield broad-scale predictions of interactions among salinity and other stressors, which would require different management actions [1] (box 2).
Box 2. Cross-tolerance/cross-talk framework to predict joint effects of stressor pairs depending on the overlap between protection mechanisms (based on Sinclair et al. [29]) and recommendations for management.
Here, we review experimental studies that have explored the combined effects of changes in salinity and other key abiotic stressors associated with global change (e.g. temperature, pH, pollutants, etc.) at organism level on several physiological traits that determine the performance of aquatic organisms across inland (freshwater and saline) and transitional coastal ecosystems (estuaries and salt-marshes). Our aims were to (i) synthesize the main effects of salinity and other stressors at organism level and identify gaps in the salinity-multistressor literature, (ii) quantify the frequency of additive, synergistic and antagonistic joint effects and (iii) determine the overall individual and joint effects and their variation among salinity–stressor pairs and organism groups.
2. Methods
(a). Bibliographic search and screening
We focused on experimental studies that have assessed the effects of salinity and other stressors on organisms (autotrophs, invertebrates and vertebrates) of inland fresh and saline waters, along with estuarine and salt-marsh habitats (transitional habitats). We only considered experimental studies because they allow isolation of the effects of specific stressors from other confounding factors that cannot be controlled in the field. We searched the literature using Web of Science (last accessed in June 2018) with the following sequence of field tags and Boolean operators in advanced searches: ((((TS= ((salin* OR *osmotic* OR conductivity) AND (temperature OR heat OR thermal OR hypoxia OR nutrient* OR radiation OR humidity OR drought OR dehydration OR desiccation OR *ionic* OR pollut* OR insecticide OR pesticide OR acidity OR ‘pH’ OR metals) AND (freshwater OR aquatic NOT marine) AND (physiolog*)))))). We refined the search by stress* and no restriction was placed on publication year. From the resulting papers, we selected only those that had applied a full-factorial design, including a clearly defined control treatment (or a treatment deemed by the authors to be under non-stressful conditions for any of the stressors), treatments with one level or more of each single stressor and combined treatments of all the stressors. We also included studies from the cited literature of the selected papers that met these criteria but did not appear in the literature search. From this first filter by experimental design, we obtained two datasets.
Dataset 1 (electronic supplementary material S1) contained only those studies that statistically tested the interaction effect. These studies were used for an initial exploration of the individual and joint stressor effects reported and to identify knowledge gaps in the salinity-multistressor literature. We retrieved information for each experiment in each study to characterize stressor pairs salinity (stressor A), plus temperature, nutrients, metals, pesticides, CO2, hypoxia, sulfate or pH (stressor B), organism (autotroph, invertebrate or vertebrate), habitat (inland freshwater, inland saline or transitional) and response type (survival and tolerance limits; fitness measurements, including metabolic rates, growth and reproduction traits; molecular responses; physiological regulation, including osmotic capacity and metal uptake or accumulation measurements; and behaviour). We determined: (i) the significance of individual and joint effects by exploring the results of the statistical analyses performed in each study; (ii) the direction of such effects in individual performance terms compared with the control conditions (i.e. negative (worse performance) or positive (enhanced performance)) by looking at the post hoc tests and/or plots with errors. In multilevel experiments (i.e. those with more than two levels of each stressor), we focused on the highest level before the total mortality of individuals because the magnitude and direction of the joint effects could vary across the different levels of each stressor.
Dataset 2 (electronic supplementary material S2) was obtained by selecting exclusively those studies that report either raw data or mean, standard deviation and sample sizes for control, single and combined-stressor treatments. These data were used to quantify the frequency of joint effect types and for meta-analyses (see below). In this dataset, we simplified stressor B categories into temperature, desiccation, nutrients and toxicants (including metals, pesticides, pH and sulfates). We considered sulfates as a separate stressor from salinity because it could potentiate the negative osmotic effects of increasing salinity [35,36].
(b). Joint effect types and meta-analyses
From dataset 2 (electronic supplementary material S2), we calculated the individual, main and joint effect sizes for each experiment and study using Hedge's d according to factorial meta-analysis methods, where a significant interaction effect signifies deviation from the null model of additivity [37] (see meta-analysis details in electronic supplementary material S3). Individual effects reflect the response to one stressor alone in relation to the control. Main effects represent the individual stressor effect plus its contribution to the interaction effect, calculated in the presence and absence of the other stressor. To ensure a positive relationship of the response variables with performance, we inverted the sign of main and individual effect sizes from experiments that measured response variables negatively related with performance (e.g. mortality response was transformed into survival response by changing the effect size sign). To make a quantitative assessment of interaction type frequencies, interaction effects were classified using effect sizes according to Crain et al. [12], i.e. additive interactions were those whose 95% confidence interval (CI) include zero value. Synergistic interactions were those in which both individual effect sizes were negative, or one was negative and the other positive, and the interaction effect size was significantly lower than zero. Antagonism occurred when the interaction effect size was bigger than zero and at least one individual effect size was negative. Because studies with two positive individual effects had interaction terms opposite from the majority of studies with negative individual effects, the interaction effect sizes for these studies were inverted [12].
We used a random-effects model meta-analysis to determine the weighted mean effect sizes of the main and joint effects of stressors for the studies included in dataset 2 (electronic supplementary material S2) using the metafor R package [38]. Different meta-analyses were performed with the data subsets that allowed consistent analyses by considering a relatively balanced distribution of studies and effect sizes across moderator categories or groups (organism type, habitat). The selected categorical moderators were treated as fixed effects to assess the mean interaction effects at each level of all the categories (where n ≥ 10) (see electronic supplementary material S4 for more details). Firstly, we conducted an overall meta-analysis across all the studies that tested salinity increase effects (n = 85) and other stressors using organism type as the moderator (autotroph, n = 21; invertebrate, n = 43; vertebrate, n = 21). We also conducted meta-regressions to analyse how overall effect sizes varied with (i) publication year (n = 85), (ii) the magnitude of salinity change (treatment − control) (n = 83) and (iii) the magnitude of temperature change (n = 38). A second meta-analysis was done for autotrophs (n = 21) using habitat as the moderator (inland freshwater, n = 10, versus transitional, n = 11). Finally, for inland saline water invertebrates, three subgroup meta-analyses were run for the following stressor pairs: salinity increase × temperature increase (n = 29), salinity decrease × temperature increase (n = 16) and salinity increase×toxicant increase (n = 9). We assessed publication bias in all the meta-analyses by using funnel plots and Rosenthal's fail-safe number [39].
The codes of the functions used to run all these analyses, which were performed with R v. 3.3.2 [40], are available in electronic supplementary material S5.
3. Results and discussion
Of 2157 screened articles, only 64 studies met our experimental design criteria. Of these, 45 papers tested interaction effects and were reviewed, including a total of 208 study cases from 46 distinct species (dataset 1, electronic supplementary material S1). We obtained quantitative data from 24 papers to determine the frequency of interaction types and conduct the meta-analyses, in which the main effect sizes of stressors and their interaction were estimated for 105 study cases of 28 species (dataset 2, electronic supplementary material S2).
(a). Research contributions and gaps in the salinity-multistressor literature
Although many experimental studies have tested the effects of salinity and other stressors in combination, only a small proportion of them have employed full-factorial experimental designs and appropriate analytical approaches to identify non-additive joint effects. In dataset 1 (electronic supplementary material S1), the statistical analyses most frequently used to test interaction effects were ANOVA-type models, which assume a simple addition model as the null model [2]. Most studies tested more than two levels of each stressor, but only two of the 45 reviewed studies [41,42] used five or more treatment levels, essential to establish a reliable stressor–effect relationship from a factorial experiment [2].
Multiple stressor studies are clearly biased toward certain stressor pairs, habitats and organisms. The most frequently studied stressors in combination with salinity were temperature (34% of study cases) and metals (24%). However, other relevant stressors have received less attention (e.g. nutrients and desiccation stress). The most represented habitats were transitional ones (55%), followed by inland saline (26%) and freshwater ecosystems (18%). The number of observations made on vertebrate and invertebrate organisms was similar (36 and 34%, respectively), while autotrophs were less represented (30%). Molecular responses (e.g. activity or gene expression of ion transporter and antioxidant enzymes), and survival and tolerance limits, as well as fitness measurements (e.g. growth, reproduction and metabolic rates), were the most frequently studied traits (see electronic supplementary material S1).
The individual effect of salinity decreased organism performance in most of the observations (43%, e.g. decreased survival and growth, increased osmolyte concentration in body fluids, changed metabolic rates, etc.) and was positive in only 20% of the responses, most frequently increasing survival or tolerance to heat or cold stress. Similarly, the individual effects of the other stressors (named stressor B, see the Methods section) resulted in worse performance in most cases, but enhanced it in 30% of the cases. Approximately 50% of the studies reported significant non-additive effects of combined stressors, among which most decreased organism performance, mainly survival.
(b). Frequency of additive, antagonistic and synergistic effects
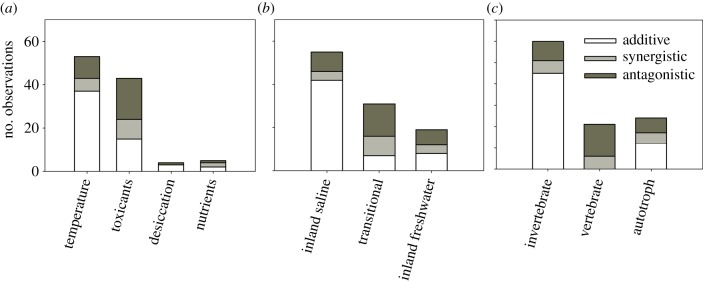
The classification of joint effect types based on effect size estimates (see electronic supplementary material S2) yielded a higher frequency of additive (54%) than antagonistic (30%) and synergistic effects (16%). These patterns varied across stressor pairs, habitat or organism categories. Additive effects were more frequent in the stressor pair salinity × temperature, inland saline habitats and invertebrates. However, antagonistic effects dominated for the combination of salinity with toxicants, and in both transitional habitats and vertebrates (figure 1).
Figure 1.
Frequency distribution of the interaction types across (a) stressor pairs, (b) habitat and (c) organism groups, estimated from effect size calculations and categorized following the classification of Crain et al. [12] (see electronic supplementary material S2). (Online version in colour.)
These results can be discussed within the cross-tolerance/cross-talk framework earlier described (box 2). In the case of high temperature and salinity, different physiological mechanisms are activated (i.e. heat shock and osmoregulatory responses, respectively), so they are more likely to interact in an additive manner, as we generally observed. For example, Garreta-Lara et al. [43] found a strong influence of salinity on the metabolomic profile of the invertebrate Daphnia magna, but no significant interaction with temperature. Though less frequent, some synergistic and antagonistic responses between salinity and temperature were also found (e.g. [44] in dataset 2, electronic supplementary material S2; see also [45] in this issue), which suggests that the mechanistic relationship between heat and osmotic stress is still not well understood.
Unlike high temperature, common homeostatic and excretory mechanisms are primarily used against the stress induced by salinity and metals, although each particular metal elicits other specific responses once it has accumulated in the organism [46]. Partially shared mechanisms and common regulatory pathways could explain the higher frequency of antagonisms found in the salinity and toxicant stressor pair (most corresponded to osmoconformer estuarine, anadromous and catadromous fish), a pattern that has also been observed in marine ecosystems [12]. For example, De Polo et al. [47] identified enzyme carbonic anhydrase (CA2) as the mechanistic link at the molecular level involved in the antagonistic effects of copper and osmotic stress on ion homeostasis in the estuarine fish Cyprinodon variegatus. In estuarine and marine invertebrates, increased salinity generally protects against the negative effects of metals [48], which can be partly explained by competitive interactions with major cations for sensitive ion transport sites [49]. These competitive interactions diminish under low salinity conditions because of lower concentrations of free ions, which facilitates metal uptake [50]. In freshwaters, poor osmoregulator species, for which these cross-protective effects of salinity likely play a smaller role, may be much more vulnerable to water pollution by metals than are saline species.
Antagonistic interactions between salinity and pesticides are also typical. In this case, neuroendocrine responses may be involved as cholinesterase inhibition is the main mode of pesticide action [51]. For example, hypersaline acclimation reduces mortality in subsequent exposure to chlorpyrifos in the euryhaline anadromous fish Salmo trutta, and it has been suggested that this protective effect could be associated with reduced neuronal signalling under hypersaline conditions [52].
A poorly explored stressor pair with shared protective physiological mechanisms is desiccation and salinity. Both stressors disrupt water and ionic balance and thus cross-tolerance might be expected (box 2). Indeed, antagonistic responses to these stressors are common in plants [53] and have been found in some aquatic insects [34,54], in which the pre-activation of osmoregulatory mechanisms during salinity exposure seems to contribute to minimize water loss during a subsequent desiccation exposure.
Interestingly, in most antagonistic interaction cases, the individual effects of stressors were both negative, which means that although the negative impact is mitigated in the presence of both stressors, they still produce a reduction in organism performance (e.g. the upper thermal limit of saline water beetles decreases after acclimation at stressful salinities and temperatures, but less than under each stress alone [55]). Opposing individual effects leading to antagonistic interactions typically occur with nutrients, whose positive effects can overcompensate for the negative effect of salinity (e.g. [56]), as happens with toxicants [12]. We also found, among the few cases of synergistic interactions, opposing individual effects (electronic supplementary material S2), mostly between salinity and toxicants. For example, in cyanobacteria, the activity of the antioxidant enzyme peroxidase increased in the presence of Cu or Cu + NaCl, but not of NaCl alone [57].
(c). Overall individual and joint stressor effects: meta-analyses
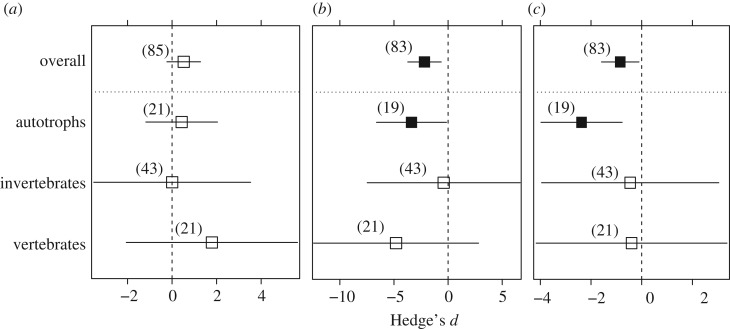
The meta-analysis conducted on all the salinity increase studies and organism groups revealed an overall additive joint effect (d = 0.527 ± 0.379, p = 0.164, n = 85; figure 2a; electronic supplementary material S4). When this dataset was moderated by organisms, joint effects were also additive for autotrophs (d = 0.464 ± 0.793, p = 0.559, n = 21), invertebrates (d = 0.005 ± 0.952, p = 0.630, n = 43) and vertebrates (d = 1.777 ± 1.117, p = 0.240, n = 21). The individual mean effect sizes of salinity increase (d = −2.223 ± 0.779, p = 0.004, n = 83) and stressor B (d = −0.907 ± 0.378, p = 0.017, n = 83) were significantly negative (figure 2b,c). Remarkably, the mean effect size of salinity increase was more than two times higher than the effect size of stressor B. In addition, overall salinity effect size became more negative with time of study publication (d = −0.326 ± 0.152, p = 0.032, n = 83). When analysed with organism taken as a moderator, we found significant negative effects of salinity (d = −3.499 ± 1.593, p = 0.028, n = 21) and stressor B (d = −2.553 ± 0.796, p = 0.001, n = 21) on autotrophs (figure 2b,c).
Figure 2.
Mean effect sizes (Hedge's d ± 95% confidence intervals), overall and by organism groups of (a) joint effect, (b) salinity individual main effect and (c) stressors B individual main effect. The number of observations (n) of each analysis is indicated in parentheses. Filled black squares indicate significant effects (p < 0.05).
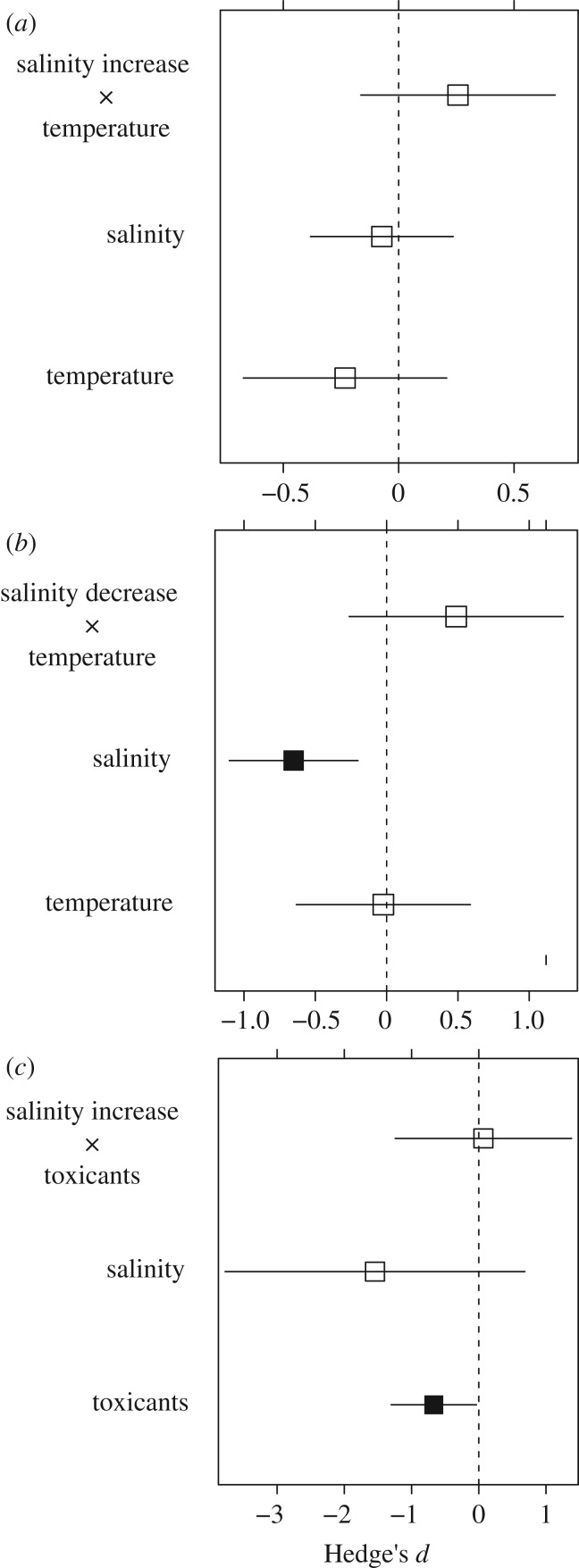
In the autotrophs subgroup meta-analysis, the joint effects of salinity and stressor B were also additive in both freshwater (d = 1.053 ± 1.402, p = 0.452, n = 10) and transitional habitats (d = 0.104 ± 1.949, p = 0.626, n = 11). In the meta-analyses performed with the subset of studies on invertebrates occurring in inland saline waters, we found additive overall joint effects for increasing salinity–temperature (d = 0.396 ± 0.324, p = 0.223, n = 19, figure 3a), decreasing salinity–temperature (d = −0.257 ± 0.215, p = 0.231, n = 29, figure 3b) and increasing salinity–toxicants stressor pairs (d = −0.068 ± 0.669, p = 0.919, n = 9, figure 3c). Salinity increase did not have a significant main individual effect but the main effect of salinity decrease was negative (d = −0.652 ± 0.23, p = 0.005). Such negative effect of salinity decrease contrasts with the general pattern of high survival of saline insects in freshwater–low salinity conditions found in a more extensive review of this topic [58]. Temperature had no significant effect, while the individual main effect of toxicants was significantly negative (d = −0.670 ± 0.323, p = 0.038). The significant results found in the meta-analyses were generally robust against publication bias according to the symmetry observed in funnel plots (see electronic supplementary material S3) and Rosenthal fail-safe numbers greater than critical thresholds (see electronic supplementary material S4).
Figure 3.
Mean effect sizes (Hedge's d ± 95% confidence intervals) on joint and individual main effects on inland saline invertebrates for (a) salinity increase and temperature, (b) salinity decrease and temperature, and (c) salinity increase and toxicants. Filled black squares indicate significant effects (p < 0.05).
The overall and relative magnitude of stressors may play a critical role in determining their interactive effects (e.g. [15]). However, our meta-regressions showed no significant relationships between the absolute salinity or temperature changes and the joint or individual effect sizes (see electronic supplementary material S4). Joint effects of multiple stressors also depend on the timing at which they act [14,31]. When stressors operate sequentially, additive effects are more likely to occur because homeostasis can be re-established in the time between exposure to the first and second stressor. By contrast, interactive effects are more frequent when the two stressors act simultaneously or very close in time. In our study, this effect was controlled because the vast majority of the experimental designs included simultaneous exposure to both stressors.
Overall, our findings revealed no interactive effects (i.e. additive effects) of salinity changes in combination with other stressors, which contrasts with the overall synergistic effects reported for marine systems [12] and the overall antagonistic effect of multiple stressor pairs found in freshwaters [20]. Nonetheless, our results should be cautiously compared with other meta-analysis studies, for several reasons. First, responses at different organizational level are highly heterogeneous [12]. Second, Crain et al. [12] and Jackson et al. [20] did not focus specifically on salinity (it was pooled with other chemical stressors in [20]) but explored instead a wide range of stressor pairs. One possible explanation for the dominance of additive effects and the higher frequency of antagonisms than synergisms in our study is that salinity may frequently act as a dominant stressor, so that the other stressors have little additional effect [2,15]. Indeed, the overall individual effect of salinity increase was generally higher than those of the other stressors analysed, such as temperature. Szöcs et al. [59] also found a greater effect of salinity than pesticides on macroinvertebrate communities, and no significant interaction effect between these stressors. These results have important implications for management of aquatic ecosystems. Mitigation strategies aimed at reducing the magnitude of salinity changes could reduce significantly the impact on organisms and substantially improve the health of populations and communities, as other authors have previously suggested [12,60]. In any case, the notable variability in the importance of the interaction types among stressor pairs in different aquatic systems suggests that responses are highly context-dependent and, therefore, a general framework for predicting interactions and guiding management could be difficult to establish [1].
Our comprehensive review included taxa with different habitats, life-history traits, stressor sensitivities and evolutionary histories (i.e. colonization from marine or terrestrial environments, transitions from fresh to saline waters, etc.), as well as a variety of strategies to cope with salinity stress. For example, while most marine and transitional water organisms are osmoconformers, the majority of organisms in saline inland waters are osmoregulators, such as aquatic insects of terrestrial origin [61–63], and can cope with wide salinity fluctuations by hyper-regulation capacity in freshwater and hypo-regulation capacity in saline waters [62] (box 1). Thus, it remains to be investigated how these different osmoregulatory strategies and their associated energetic costs determine the type of interactions with other stressors.
4. Concluding remarks and future perspectives
The multiple stressor studies reviewed herein focus primarily on the combined effects of increasing salinity and increasing temperature or toxicants (metals and pesticides), while other important stressor combinations have received very little attention (e.g. desiccation or nutrients). The number of multiple stressor experimental studies conducted in inland waters is still limited compared with those on transitional and marine systems. Thus, more research efforts are needed in freshwater and saline inland waters, which are particularly vulnerable to multiple global change pressures [1,64].
Additive effects of salinity and other stressors were prevalent, but antagonistic interactions were relatively frequent in some organism groups (vertebrates), habitats (transitional waters) or stressor pairs (salinity × toxicants). Salinity has a stronger negative individual effect on organismal performance traits than other stressors, which highlights the need to increase management efforts for this single stressor (box 2).
From this review, some considerations for future research arise. First, we need to improve our understanding of the mechanisms and pathways by which a single stressor modulates the physiological responses to other stressors. Second, to analyse multi-stressor effects, models more complex than additive ones should be applied if stressor–effect relationships and the correlation between organism's sensitivity to each stressor are known [2,65]. Third, to better understand and predict the effects of ongoing salinization and dilution processes in aquatic ecosystems, it is crucial to explore the role of the origin and evolution of the osmoregulation strategies of aquatic organisms in determining the type of interactions that arise between salinity and other stressors.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Miguel Cañedo-Argüelles for inviting us to contribute to this monographic volume, and Cristina Coccia for providing experimental data. We are also grateful to three anonymous referees who carried out constructive reviews of this work.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
J.V. conceived, designed and coordinated the study and drafted part of the manuscript; S.P. participated in the study design, carried out the literature review, data selection and analyses, created figure 1 and electronic supplementary material S1 and S2 and drafted part of the manuscript; C.G.-C. carried out the meta-analyses, created figures 2 and 3, and electronic supplementary material S3, S4 and S5, and drafted part of the manuscript; M.B.-C. carried out the literature review, data selection and analyses, and created electronic supplementary material S1 and S2, and the reference lists; D.S.-F., P.A., J.A.C. and A.M. contributed with experimental data. All the authors discussed the results and the manuscript revision and gave final approval for publication.
Competing interests
We have no competing interests.
Funding
Some physiological studies of the interaction of pair–stressors salinity–temperature, salinity–ionic composition and salinity–desiccation on the performance of aquatic saline insects were done by the Aquatic Ecology Research Group (University of Murcia, Spain) as part of the I + D + i projects CGL2010-15378 (J.V.) and CGL2013-48950-C2-2-P (J.V. and A.M.) (Spanish Ministry of Economy and Competitiveness) co-funded by FEDER funds. C.G.-C. and P.A. are supported by ‘Juan de la Cierva-Formación’ research contracts (MINECO, FJCI-2015-25785 and FJCI-2014-20581, respectively), D.S.-F. by a post-doctoral contract funded by the Universidad de Castilla-La Mancha and the European Social Fund (ESF) and M.B.-C. by a PhD grant from the Universidad de Murcia.
References
- 1.Côté IM, Darling ES, Brown CJ. 2016. Interactions among ecosystem stressors and their importance in conservation. Proc. R. Soc. B 283, 1–9. ( 10.1098/rspb.2015.2592) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schäfer RB, Piggott JJ. 2018. Advancing understanding and prediction in multiple stressor research through a mechanistic basis for null models. Glob. Chang. Biol. 24, 1817–1826. ( 10.1111/gcb.14073) [DOI] [PubMed] [Google Scholar]
- 3.Sala OE, et al. 2000. Global biodiversity scenarios for the year 2100. Science 287, 1770–1774. ( 10.1126/science.287.5459.1770) [DOI] [PubMed] [Google Scholar]
- 4.Grizzetti B, Pistocchi A, Liquete C, Udias A, Bouraoui F, Van De Bund W. 2017. Human pressures and ecological status of European rivers. Sci. Rep. 7, 1–11. ( 10.1038/s41598-017-00324-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cañedo-Argüelles M, Kefford BJ, Piscart C, Prat N, Schäfer RB, Schulz CJ. 2013. Salinisation of rivers: an urgent ecological issue. Environ. Pollut. 173, 157–167. ( 10.1016/j.envpol.2012.10.011) [DOI] [PubMed] [Google Scholar]
- 6.Cañedo-Arguelles M, et al. 2016. Saving freshwater from salts. Science 351, 914–916. ( 10.1126/science.aad3488) [DOI] [PubMed] [Google Scholar]
- 7.Abellán P, Sánchez-Fernández D, Picazo F, Millán A, Lobo JM, Ribera I. 2013. Preserving the evolutionary history of freshwater biota in Iberian National Parks. Biol. Conserv. 162, 116–126. ( 10.1016/j.biocon.2013.04.001) [DOI] [Google Scholar]
- 8.Kroeker KJ, Kordas RL, Crim R, Hendriks IE, Ramajo L, Singh GS, Duarte CM, Gattuso JP. 2013. Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob. Chang. Biol. 19, 1884–1896. ( 10.1111/gcb.12179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muniz IP. 1990. Freshwater acidification: its effects on species and communities of freshwater microbes, plants and animals. Proc. R. Soc. Edinb. B 97, 227–254. ( 10.1017/S0269727000005364) [DOI] [Google Scholar]
- 10.Smith V. 2003. Eutrophication of freshwater and coastal marine ecosystems a global problem. Environ. Sci. Pollut. Res. 10, 126–139. ( 10.1065/espr2002.12.142) [DOI] [PubMed] [Google Scholar]
- 11.Smith VH, Schindler DW. 2009. Eutrophication science: where do we go from here? Trends Ecol. Evol. 24, 201–207. ( 10.1016/j.tree.2008.11.009) [DOI] [PubMed] [Google Scholar]
- 12.Crain CM, Kroeker K, Halpern BS. 2008. Interactive and cumulative effects of multiple human stressors in marine systems. Ecol. Lett. 11, 1304–1315. ( 10.1111/j.1461-0248.2008.01253.x) [DOI] [PubMed] [Google Scholar]
- 13.Sperfeld E, Raubenheimer D, Wacker A. 2016. Bridging factorial and gradient concepts of resource co-limitation: towards a general framework applied to consumers. Ecol. Lett. 19, 201–215. ( 10.1111/ele.12554) [DOI] [PubMed] [Google Scholar]
- 14.Gunderson AR, Armstrong EJ, Stillman JH. 2016. Multiple stressors in a changing world: the need for an improved perspective on physiological responses to the dynamic marine environment. Ann. Rev. Mar. Sci. 8, 357–378. ( 10.1146/annurev-marine-122414-033953) [DOI] [PubMed] [Google Scholar]
- 15.Folt C, Chen C. 1999. Synergism and antagonism among multiple stressors. Limnol. Oceanogr. 44, 864–877. ( 10.4319/lo.1999.44.3) [DOI] [Google Scholar]
- 16.Gutiérrez-Cánovas C, et al. 2019. Do all roads lead to Rome? Exploring community trajectories in response to anthropogenic salinization and dilution of rivers. Phil. Trans. R. Soc. B 374, 20180009 ( 10.1098/rstb.2018.0009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berger E, Frör O, Schäfer RB. 2019. Salinity impacts on river ecosystem processes: a critical mini-review. Phil. Trans. R. Soc. B 374, 20180010 ( 10.1098/rstb.2018.0010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harvey BP, Gwynn-Jones D, Moore PJ. 2013. Meta-analysis reveals complex marine biological responses to the interactive effects of ocean acidification and warming. Ecol. Evol. 3, 1016–1030. ( 10.1002/ece3.516) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Przeslawski R, Byrne M, Mellin C. 2015. A review and meta-analysis of the effects of multiple abiotic stressors on marine embryos and larvae. Glob. Chang. Biol. 21, 2122–2140. ( 10.1111/gcb.12833) [DOI] [PubMed] [Google Scholar]
- 20.Jackson MC, Loewen CJG, Vinebrooke RD, Chimimba CT. 2016. Net effects of multiple stressors in freshwater ecosystems: a meta-analysis. Glob. Chang. Biol. 22, 180–189. ( 10.1111/gcb.13028) [DOI] [PubMed] [Google Scholar]
- 21.Venâncio C, Castro BB, Ribeiro R, Antunes SC, Abrantes N, Soares AMVM, Lopes I. 2019. Sensitivity of freshwater species under single and multigenerational exposure to seawater intrusion. Phil. Trans. R. Soc. B 374, 20180252 ( 10.1098/rstb.2018.0252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karim MF, Mimura N. 2008. Impacts of climate change and sea-level rise on cyclonic storm surge floods in Bangladesh. Glob. Environ. Chang. 18, 490–500. ( 10.1016/j.gloenvcha.2008.05.002) [DOI] [Google Scholar]
- 23.Bhuiyan MJAN, Dutta D. 2012. Assessing impacts of sea level rise on river salinity in the Gorai River network, Bangladesh. Estuar. Coast. Shelf Sci. 96, 219–227. ( 10.1016/j.ecss.2011.11.005) [DOI] [Google Scholar]
- 24.Nielsen DL, Brock MA. 2009. Modified water regime and salinity as a consequence of climate change: prospects for wetlands of southern Australia. Clim. Change 95, 523–533. ( 10.1007/s10584-009-9564-8) [DOI] [Google Scholar]
- 25.Millán A, Velasco J, Gutiérrez-Cánovas C, Arribas P, Picazo F, Sánchez-Fernández D, Abellán P. 2011. Mediterranean saline streams in southeast Spain: what do we know? J. Arid Environ. 75, 1352–1359. ( 10.1016/j.jaridenv.2010.12.010) [DOI] [Google Scholar]
- 26.Kefford BJ, Papas PJ, Crowther D, Nugegoda D. 2002. Are salts toxicants? Australas. J. Ecotoxicol. 8, 63–68. [Google Scholar]
- 27.Bradley TJ. 2009. Animal osmoregulation. New York: Oxford University Press. [Google Scholar]
- 28.Rivera-Ingraham GA, Lignot J-H. 2017. Osmoregulation, bioenergetics and oxidative stress in coastal marine invertebrates: raising the questions for future research. J. Exp. Biol. 220, 1749–1760. ( 10.1242/jeb.135624) [DOI] [PubMed] [Google Scholar]
- 29.Sinclair BJ, Ferguson LV, Salehipour-Shirazi G, Macmillan HA. 2013. Cross-tolerance and cross-talk in the cold: relating low temperatures to desiccation and immune stress in insects. Integr. Comp. Biol. 53, 545–556. ( 10.1093/icb/ict004) [DOI] [PubMed] [Google Scholar]
- 30.MacMillan HA, Walsh JP, Sinclair BJ. 2009. The effects of selection for cold tolerance on cross-tolerance to other environmental stressors in Drosophila melanogaster. Insect Sci. 16, 263–276. ( 10.1111/j.1744-7917.2009.01251.x) [DOI] [Google Scholar]
- 31.Todgham AE, Stillman JH. 2013. Physiological responses to shifts in multiple environmental stressors: relevance in a changing world. Integr. Comp. Biol. 53, 539–544. ( 10.1093/icb/ict086) [DOI] [PubMed] [Google Scholar]
- 32.Uyhelji HA, Cheng C, Besansky NJ. 2016. Transcriptomic differences between euryhaline and stenohaline malaria vector sibling species in response to salinity stress. Mol. Ecol. 25, 2210–2225. ( 10.1111/mec.13609) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davies SA, Overend G, Sebastian S, Cundall M, Cabrero P, Dow JAT, Terhzaz S. 2012. Immune and stress response ‘cross-talk’ in the Drosophila Malpighian tubule. J. Insect Physiol. 58, 488–497. ( 10.1016/j.jinsphys.2012.01.008) [DOI] [PubMed] [Google Scholar]
- 34.Pallarés S, Botella-Cruz M, Arribas P, Millán A, Velasco J. 2017. Aquatic insects in a multistress environment: cross-tolerance to salinity and desiccation. J. Exp. Biol. 220, 1277–1286. ( 10.1242/jeb.152108) [DOI] [PubMed] [Google Scholar]
- 35.Zalizniak L, Kefford BJ, Nugegoda D. 2006. Is all salinity the same? I. The effect of ionic compositions on the salinity tolerance of five species of freshwater invertebrates. Mar. Freshwater Res. 57, 75–82. ( 10.1071/MF05103) [DOI] [Google Scholar]
- 36.Buchwalter D, Scheibener S, Chou H, Soucek D, Elphick J. 2019. Are sulfate effects in the mayfly Neocloeon triangulifer driven by the cost of ion regulation? Phil. Trans. R. Soc. B 374, 20180013 ( 10.1098/rstb.2018.0013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gurevitch J, Morrison JA, Hedges LV. 2000. The interaction between competition and predation: a meta-analysis of field experiments. Am. Nat. 155, 435–453. ( 10.1086/303337) [DOI] [PubMed] [Google Scholar]
- 38.Viechtbauer W. 2010. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36, 1–48. ( 10.18637/jss.v036.i03) [DOI] [Google Scholar]
- 39.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. 2009. Introduction to meta-analysis. Chichester: John Wiley & Sons. [Google Scholar]
- 40.R Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.r-project.org. [Google Scholar]
- 41.Heilmayer O, Digialleonardo J, Qian L, Roesijadi G. 2008. Stress tolerance of a subtropical Crassostrea virginica population to the combined effects of temperature and salinity. Estuar. Coast. Shelf Sci. 79, 179–185. ( 10.1016/j.ecss.2008.03.022) [DOI] [Google Scholar]
- 42.Santos B, Ribeiro R, Domingues I, Pereira R, Soares AMVM, Lopes I. 2013. Salinity and copper interactive effects on Perez's frog Pelophylax perezi. Environ. Toxicol. Chem. 32, 1864–1872. ( 10.1002/etc.2257) [DOI] [PubMed] [Google Scholar]
- 43.Garreta-Lara E, Campos B, Barata C, Lacorte S, Tauler R. 2018. Combined effects of salinity, temperature and hypoxia on Daphnia magna metabolism. Sci. Total Environ. 610, 602–612. ( 10.1016/j.scitotenv.2017.05.190) [DOI] [PubMed] [Google Scholar]
- 44.Hopkins GR, French SS, Brodie ED. 2017. Interacting stressors and the potential for adaptation in a changing world : responses of populations and individuals. R. Soc. open sci. 4, 161057 ( 10.1098/rsos.161057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jackson JK, Funk DH. 2019. Temperature affects acute mayfly responses to elevated salinity: implications for toxicity of road de-icing salts. Phil. Trans. R. Soc. B 374, 20180081 ( 10.1098/rstb.2018.0081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Furness P, Rainbow S. 1990. Heavy metals in the marine environment. Boca Ratón, FL: CRC Press. [Google Scholar]
- 47.De Polo A, Margiotta-Casaluci L, Lockyer AE, Scrimshaw MD. 2014. A new role for carbonic anhydrase 2 in the response of fish to copper and osmotic stress: implications for multi-stressor studies. PLoS ONE 9, e107707 ( 10.1371/journal.pone.0107707) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heugens EHW, Hendriks AJ, Dekker T, Straalen NM, Admiraal W. 2001. A review of the effects of multiple stressors on aquatic organisms and analysis of uncertainty factors for use in risk assessment. Crit. Rev. Toxicol. 31, 247–284. ( 10.1080/20014091111695) [DOI] [PubMed] [Google Scholar]
- 49.Baysoy E, Atli G, Gürler CÖ, Dogan Z, Eroglu A, Kocalar K, Canli M. 2012. The effects of increased freshwater salinity in the biodisponibility of metals (Cr, Pb) and effects on antioxidant systems of Oreochromis niloticus. Ecotoxicol. Environ. Saf. 84, 249–253. ( 10.1016/j.ecoenv.2012.07.017) [DOI] [PubMed] [Google Scholar]
- 50.McLusky DS, Bryant V, Campbell R. 1986. The effects of temperature and salinity on the toxicity of heavy metals to marine and estuarine invertebrates. Oceanogr. Mar. Biol. Annu. Rev. 24, 481–520. [Google Scholar]
- 51.Thompson CM, Richardson RJ. 2003. Anticholinesterase insecticides. In Pesticide toxicology and international regulation (eds Mars TC, Ballantine B), pp. 89–127. Chichester, UK: John Wiley & Sons, Ltd. [Google Scholar]
- 52.Amiri BM, Xu EG, Kupsco A, Giroux M, Hoseinzadeh M, Schlenk D. 2018. The effect of chlorpyrifos on salinity acclimation of juvenile rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 195, 97–102. ( 10.1016/j.aquatox.2017.12.011) [DOI] [PubMed] [Google Scholar]
- 53.Tuteja N. 2007. Mechanisms of high salinity tolerance in plants. Methods Enzymol. 428, 419–438. ( 10.1016/S0076-6879(07)28024-3) [DOI] [PubMed] [Google Scholar]
- 54.Elnitsky MA, Benoit JB, Lopez-Martinez G, Denlinger DL, Lee RE. 2009. Osmoregulation and salinity tolerance in the Antarctic midge, Belgica antarctica: seawater exposure confers enhanced tolerance to freezing and dehydration. J. Exp. Biol. 212, 2864–2871. ( 10.1242/jeb.034173) [DOI] [PubMed] [Google Scholar]
- 55.Arribas P, Velasco J, Abellán P, Sánchez-Fernández D, Andújar C, Calosi P, Millán A, Ribera I, Bilton DT. 2012. Dispersal ability rather than ecological tolerance drives differences in range size between lentic and lotic water beetles (Coleoptera: Hydrophilidae). J. Biogeogr. 39, 984–994. ( 10.1111/j.1365-2699.2011.02641.x) [DOI] [Google Scholar]
- 56.Kamer K, Fong P. 2001. Nitrogen enrichment ameliorates the negative effects of reduced salinity on the green macroalga Enteromorpha intestinalis. Mar. Ecol. Prog. Ser. 218, 87–93. ( 10.3354/meps218087) [DOI] [PubMed] [Google Scholar]
- 57.Karimi R, Norastehnia A, Abbaspour H, Saedisar S NA. 2012. Toxicity assessment of Anabaena sp. following exposure to copper oxide nanoparticles and sodium chloride. Appl. Ecol. Environ. Res. 15, 1–15. ( 10.15666/aeer/1504_20452059) [DOI] [Google Scholar]
- 58.Arribas P, Gutiérrez-Cánovas C, Botella-Cruz M, Cañedo-Argüelles M, Antonio Carbonell J, Millán A, Pallarés S, Velasco J, Sánchez-Fernández D. 2019. Insect communities in saline waters consist of realized but not fundamental niche specialists. Phil. Trans. R. Soc. B 374, 20180008 ( 10.1098/rstb.2018.0008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Szöcs E, Kefford BJ, Schäfer RB. 2012. Is there an interaction of the effects of salinity and pesticides on the community structure of macroinvertebrates? Sci. Total Environ. 437, 121–126. ( 10.1016/j.scitotenv.2012.07.066) [DOI] [PubMed] [Google Scholar]
- 60.Kath J, Thomson JR, Thompson RM, Kefford BJ, Dyer FJ, Mac Nally R. 2018. Interactions among stressors may be weak: implications for management of freshwater macroinvertebrate communities. Divers. Distrib. 24, 939–950. ( 10.1111/ddi.12737) [DOI] [Google Scholar]
- 61.Herbst DB. 2001. Gradients of salinity stress, environmental stability and water chemistry as a templet for defining habitat types and physiological strategies in inland salt waters. Hydrobiologia 466, 209–219. ( 10.1023/A:1014508026349) [DOI] [Google Scholar]
- 62.Pallarés S, Arribas P, Bilton DT, Millán A, Velasco J. 2015. The comparative osmoregulatory ability of two water beetle genera whose species span the fresh-hypersaline gradient in inland waters (Coleoptera: Dytiscidae, Hydrophilidae). PLoS ONE 10, e0124299 ( 10.1371/journal.pone.0124299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pallarés S, Arribas P, Bilton DT, Millán A, Velasco J, Ribera I. 2017. The chicken or the egg? Adaptation to desiccation and salinity tolerance in a lineage of water beetles. Mol. Ecol. 26, 5614–5628. ( 10.1111/mec.14334) [DOI] [PubMed] [Google Scholar]
- 64.Dudgeon D, et al. 2006. Freshwater biodiversity: importance, threats, status and conservation challenges. Biol. Rev. Camb. Phil. Soc. 81, 163–182. ( 10.1017/S1464793105006950) [DOI] [PubMed] [Google Scholar]
- 65.Liess M, Foit K, Knillmann S, Schäfer RB, Liess HD. 2016. Predicting the synergy of multiple stress effects. Sci. Rep. 6, 1–8. ( 10.1038/srep32965) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.