Abstract
The salinity of many freshwaters is increasing globally as a result of human activities. Associated with this increase in salinity are losses of Ephemeroptera (mayfly) abundance and richness. The salinity concentrations at which Ephemeroptera decline in nature are lower than their internal salinity or haemolymph osmolality. Many species also suffer substantial mortality in single species laboratory toxicity tests at salinities lower than their internal salinity. These findings are problematic as conventional osmoregulation theory suggests that freshwater animals should not experience stress where external osmolality is greater than haemolymph osmolality. Here I explore three hypotheses to explain salt sensitivity in Ephemeroptera. These conceptual hypotheses are based on the observations that as the external sodium ion (Na+) concentration increases so does the Na+ turnover rate (both uptake and elimination rates increase). Sulphate (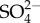 ) uptake in mayflies also increases with increasing external
) uptake in mayflies also increases with increasing external 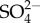 although, unlike Na+, its rate of increase decreases with increasing external
although, unlike Na+, its rate of increase decreases with increasing external 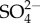 . The first hypothesis is premised on ion turnover being energetically costly. The first hypothesis proposes that individuals must devote a greater proportion of their energy to ion homeostasis at the expense of other uses including growth and development. Lethal levels of salinity presumably result from individuals not being able to devote enough energy to maintain ion homeostasis without critical loss of other vital functions. The second hypothesis is premised on the uptake of Na+ exchanged for (an outgoing) H+, leading to (localized) loss of pH regulation. The third hypothesis is premised on localized Na+ toxicity or poisoning with increased Na turnover as salinity increases. None of the proposed hypotheses is without potential problems, yet all are testable, and research effort should be focused at attempting to falsify them.
. The first hypothesis is premised on ion turnover being energetically costly. The first hypothesis proposes that individuals must devote a greater proportion of their energy to ion homeostasis at the expense of other uses including growth and development. Lethal levels of salinity presumably result from individuals not being able to devote enough energy to maintain ion homeostasis without critical loss of other vital functions. The second hypothesis is premised on the uptake of Na+ exchanged for (an outgoing) H+, leading to (localized) loss of pH regulation. The third hypothesis is premised on localized Na+ toxicity or poisoning with increased Na turnover as salinity increases. None of the proposed hypotheses is without potential problems, yet all are testable, and research effort should be focused at attempting to falsify them.
This article is part of the theme issue ‘Salt in freshwaters: causes, ecological consequences and future prospects’.
Keywords: salinity, major ions, osmoregulation, Ephemeroptera, mayfly, stream invertebrates
1. Introduction
Salinity of freshwater is increasing around the world from a range of anthropogenic factors including agricultural practices, effluents from mineral and hydrocarbon extraction and industry (the use of de-icing salts in cold regions and seawater intrusions (see review by Cañedo Argüelles et al. [1]). In addition to rises in salinity—defined as the total concentration of dissolved inorganic ions often inferred indirectly from specific conductivity of electricity [2]—there are often changes in the concentrations and relative proportions of major ions: Na+, Cl−, Ca++, 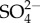 , etc. [3]. If these changes in salinity and/or ions are of sufficient magnitude, salinity has adverse effects on freshwater organisms, their populations, communities and ecosystem functions. Large rises in salinity in inland waters are well known to reduce freshwater invertebrate species richness [4], change community composition [5–7], alter the traits mix of the community [8] and reduce breakdown of leaf litter (an ecosystem function) [9,10].
, etc. [3]. If these changes in salinity and/or ions are of sufficient magnitude, salinity has adverse effects on freshwater organisms, their populations, communities and ecosystem functions. Large rises in salinity in inland waters are well known to reduce freshwater invertebrate species richness [4], change community composition [5–7], alter the traits mix of the community [8] and reduce breakdown of leaf litter (an ecosystem function) [9,10].
A picture is emerging that minor salinity increases (less than 1 mS cm−1) have detrimental effects on many Ephemeroptera (mayflies). Recently, Clements & Kotalik [11] ‘seeded’ experimental mesocosms with invertebrates from a low salinity site (0.06–0.07 mS cm−1) and then applied various experimental salinity treatments. They observed that salinity of ≈0.3 mS cm−1 caused declines in the abundance of baetid and heptageniid mayflies and total Ephemeroptera, while Ephemeroptera drift increased. The richness of the insect orders Ephemeroptera, Plecoptera (stoneflies) and Trichoptera (caddisflies), collectively abbreviated to EPT, in southeast Australia [12] and the abundance of Ephemeroptera in Kentucky, USA [13] are reduced monotonically as salinity increases above ≈0.175–0.2 mS cm−1. This is in contrast to total macroinvertebrate species richness, which peaks at intermediate salinities, ≈0.3–0.5 mS cm−1, with fewer species at lower and higher salinities [12]. In Central Appalachian Streams, USA, 5% of macroinvertebrate genera are extirpated at 0.295 mS cm−1, with Ephemeroptera genera extirpated at lower salinities than many other taxonomic groups ([14], see also [15,16]). Moreover, acute toxicity testing of 377 species from Australia, France, Israel and South Africa shows that Ephemeroptera is one of the most salinity-sensitive groups of stream macroinvertebrates [17].
The loss of species at such low salinity in nature and the results of single species toxicity testing suggest physiological harm in a significant proportion of Ephemeroptera [17,18] at salinities that cannot be explained by a widely accepted conceptual model of osmoregulation [19]. This paper conceptually summarizes this accepted conventional model and considers three conceptual hypotheses that can explain the mortality of mayflies and their loss in nature at low salinities in freshwater. These testable hypotheses expand on the conventional model and are consistent with what (little) is known of osmophysiology in Ephemeroptera [20,21]. While these hypotheses could plausibly be relevant to taxa other than Ephemeroptera [22], this paper confines itself to this taxon.
This paper deliberately takes a big picture perspective and considers ion transport mechanisms of freshwater animals only to the extent needed to explain the proposed hypotheses. This topic has been covered in more detail by recent reviews by Bradley [23], Harrison et al. [24] and Griffith [20]. Information on ionocytes (also called chloride cells and mitochondria-rich cells), which are considered to be major sites of ionic transport in freshwater insects, is available from various sources including [21,25–29].
2. The conventional model of osmoregulation in freshwater
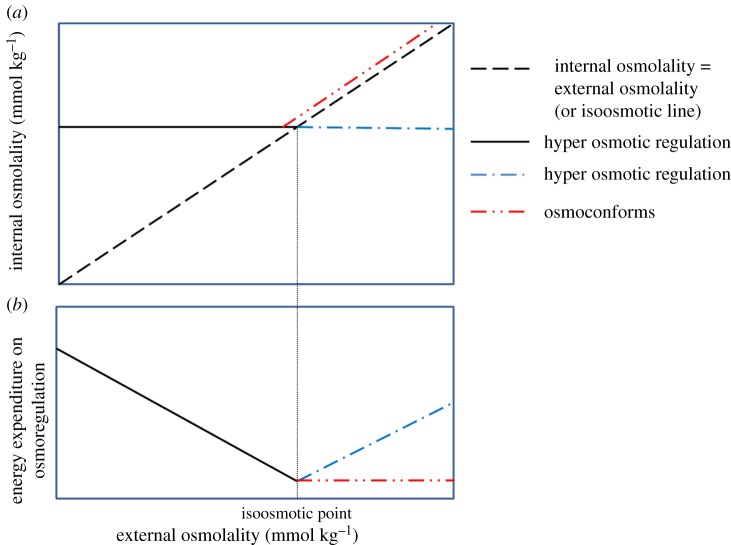
Freshwater animals must osmoregulate. Osmoconforming is not an option in freshwater because it would result in internal fluids too dilute to support normal physiological processes [23]. Thus, freshwater animals maintain their internal fluids at higher concentrations to their external water, i.e. they are hyper-osmoregulators (figure 1a), which requires energy to both excrete water and uptake ions from their food and external water. Freshwater insects are strict osmoregulators in freshwater [30]. As salinity increases, the osmolality of their internal fluids stays approximately constant despite the osmolality of their external fluids increasing (figure 1a) (e.g. [19,31,32]). If salinity increases so much that the external osmolality is equal to or surpasses the internal osmolality—the isoosmotic point—freshwater animals do one of three things: (a) regulate their haemolymph at an osmolality below their external water—referred to as hypo osmotic regulation; (b) increase their haemolymph osmolality as the osmolality of their external water increases—referred to as osmoconforming (figure 1a); or (c) die because they are not able to do (a) or (b). (Freshwater animals that start to osmoconform above the isoosmotic point do not necessarily increase their internal concentrations of inorganic ions as they may produce organic osmolytes [23,33]).
Figure 1.
The conventional model of (a) osmoregulation and its (b) energetics in freshwater animals. (Online version in colour.)
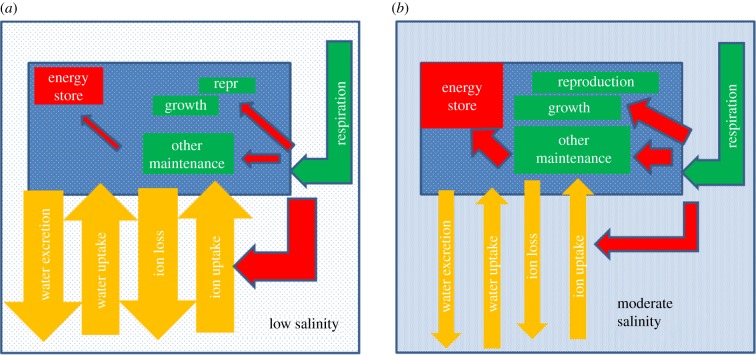
Osmoregulation in aquatic animals is thought to be one of the best understood processes in biology [19], although it is acknowledged that it has been relatively poorly studied in freshwater insects in general [20] and even more so in Ephemeroptera [21]. The introductory biology text books by Reece et al. [34], for example, state: ‘The energy cost of osmoregulation depends on how different an animal's osmolality is from its surroundings.’ They go on to say that evolution has led to freshwater animals having internal fluids with lower osmotic concentrations than related marine species, to reduce the energetic demands of osmoregulation. This conventional conceptual model of osmoregulation implies that as salinity increases from a low level towards the isoosmotic point, the uptake of ions and excretion of water will decrease, which in turn should require less energy expenditure on osmoregulation (figure 1b). Needing to spend less energy on osmoregulation would be expected to either allow freshwater animals to lower their total respiration rate and/or leave more energy available for other functions, including growth, reproduction and the accumulation of energy stores (figure 2).
Figure 2.
The conventional model of osmoregulation in freshwater animals. The individual animal is represented by a blue rectangle in either water with low salinity (a) or moderate salinity but less than the osmolality of its haemolymph (b). The level of shading in the low salinity, moderate salinity and the animal itself represent the osmolality of the internal/external fluid. The green elbow arrows represent the production of energy via respiration, red arrows the uses of this energy (with the red elbow arrow the use of energy for ion turnover), and yellow arrows the movement of substances, i.e. inorganic ions and water. The size and direction of the arrows represent the amount and direction of the substances/energy flow. The sizes of the green and red rectangles represent the size of these functions/stores. Repr, reproduction. (Online version in colour.)
Support for this conventional model is available. Some freshwater animals have higher respiration rates per unit mass of tissue than closely related marine species (e.g. [35]). The logic is that the greater difference in osmolality between the internal fluids and external water in the freshwater species, compared to their marine relatives, requires the former to spend relatively more energy on osmoregulation and thus have a higher respiration rate. Moreover, some freshwater fish [36], Gastropoda [37], Odonata [38], Chironomidae [28,39], Cladocera and Rotifera [40] have maximum growth at elevated salinities (0.1–20 mS cm−1; see electronic supplementary material, table S1), implying that individuals have more energy available for processes other than osmoregulation under these conditions [41]. A recent experiment measuring in situ growth, survival and emergence of stream invertebrates at two salinity levels found species that support this conventional model [22]. The key point for our purposes is that salinity increases below the isoosmotic point should have no negative physiological effect on freshwater animals. If increasing salinity had any effect on freshwater animals below the isoosmotic point, they should be beneficial ones, because of the need to spend less energy on osmoregulation (figure 2). Certainly, the conventional model would not expect salinity concentrations below the isoosmotic point to cause death in freshwater animals.
Organic osmolytes, such as amino acids and sugars, produced by the animal itself and not taken in from the external water [23], do not substantially change this conventional model in freshwaters. This is because these osmolytes contribute relatively little osmolality compared to Na+ and Cl− in freshwater animals [20].
3. A challenge to the conventional model of osmoregulation
The conventional model of osmoregulation does not appear to be a total explanation for salt-sensitive mayfly species. The haemolymph of aquatic insects is in the range of 250–400 mmol kg−1 [24], which corresponds to greater than 10 g l−1 or greater than 13 mS cm−1, yet a significant proportion (but not all) of Ephemeroptera species experience substantial mortality in single species experiments at lower salinities (table 1), with 72 h LC50 (concentration lethal to 50% of a test population) as low as 2.4 mS cm−1 [17] (see also Castillo et al. [18]). Indeed Austrophlebioides pusillus experienced complete mortality at 10 g l−1 (≈15 mS cm−1) but the osmolality of 10 g l−1 (256 mmol kg−1) was less than the osmolality of its haemolymph (401 mmol kg−1) [19]. Salinities that cause substantial mortality in mayfly species with chronic exposure are lower, e.g. 2.1 and 2.7 mS cm−1 [39], 1.5 mS cm−1 [42] and 2.8 mS cm−1 [43]. Reduced growth has also been reported at 0.57 mS cm−1 [43] and 0.16 mS cm−1 [44] (see also Anon [45]). Interestingly, despite several studies of sub-lethal responses of mayflies [39,43–46], none report maximum growth, development or fecundity at intermediate salinities, unlike other freshwater taxa [28,36,37,39] (see also electronic supplementary material, table S1). Ephemeroptera abundance or richness declines in the field at an external osmolality approximately an order of magnitude lower than their haemolymph osmolality [19]. These results beg the question, what is the mechanism by which salinity is adversely affecting mayflies at salinities much lower than the osmolality of the haemolymph of aquatic insects?
Table 1.
Summary of published mayfly toxicity studies to sodium chloride (NaCl)-dominated saline waters. Eph, Ephemeroptera; spp, species; SSW, synthetic seawater; h, hour; LCx, lethal concentration for x% of the test population; LOEC, lowest observed effect concentration.
| what | electrical conductivity (mS cm−1) | salt | reference |
|---|---|---|---|
| minimum 72 h LC50 of 36 Eph. spp. | 2.4 | SSW | [17] |
| 20th percentile of 72 h LC50 of 36 Eph. spp. | 6.8 | SSW | [17] |
| mean 72 h LC of 36 Eph. spp. | 12 | SSW | [17] |
| Austrophlebioides pusillus 96 h LC10 | 2.4 | SSW | [19] |
| Cloeon sp. | 2.1 | SSW | [39] |
| Centroptilum sp. | 2.7 | SSW | [39] |
| Neocloeon triangulifer LOEC (chronic) survival to pre-emergent nymph | 1.5 | NaCl | [42] |
| Neocloeon triangulifer LOEC 20 day survival | 2.8 | Mix NaCl and CaCl2 | [43] |
| Neocloeon triangulifer LOEC growth | 0.57 | [43] | |
| Neocloeon triangulifer LOEC growth | 0.16 | NaCl | [44] |
Might mortality in mayflies at salinity concentrations less than the osmolality of aquatic insects' haemolymph be owing to changes in ionic proportions and not (total) salinity [3,30]? For this to occur it would be necessary for the changes in the external proportions of total ions to cause changes in the proportions of ions inside the mayflies. These internal changes would then need to have a toxic effect or cause adverse effects via deficiencies. Owing to being the two most dominant ions in terms of maintaining hyperosmotic state in freshwater animals, including mayflies [21], external elevated Na+ and Cl− are unlikely to have adverse effects unless their concentrations in the external waters are greater than the concentration in haemolymphs [20]. Yet NaCl [18,42,44,45] and synthetic seawater [17–19,39] can cause mortality in mayflies well below the osmolality of the haemolymph of aquatic insects (table 1). It is not plausible that mortality from NaCl-dominated waters is the result of changes in the external ionic proportions.
Also contradicting the conventional model of osmoregulation is Na regulation in the mayfly Maccaffertium sp; Na is the primary cation involved in osmoregulation in animals [20]. This species increases its rate of Na uptake as its external Na+ (and salinity) increases, with no sign of a decreasing slope of Na uptake with increasing external Na+ [47]. The external Na/salinity concentration had no effect on Maccaffertium sp.'s respiration rate [47], as is the case with another mayfly species [48]. But these species should need to pull in less Na as their external Na+ (and thus salinity) concentration increases (figure 1a) and thus need less energy (figure 1b).
The conventional model of osmoregulation does not provide a full description for salt-sensitive Ephemeroptera. This model may be suitable for Ephemeroptera species that are relatively salt-tolerant. Of 36 mayflies studied, approximately 50% had 72 h LC50 values at or higher than their haemolymph osmolality [17]. Nevertheless, the global threat of freshwater salinization [3] resulting in losses to Ephemeroptera species [12–14] demands that alterative models be considered.
Next I propose three hypotheses that extend the conventional model to explain the negative effects of salinity on mayfly species below the osmolality of the haemolymph of aquatic insects. Hypothesis 1 is based on energetics of ion uptake, hypothesis 2 is based on a (localized) loss of pH regulation and hypothesis 3 is based on localized Na poisoning.
4. Hypothesis 1—energetics of ion uptake
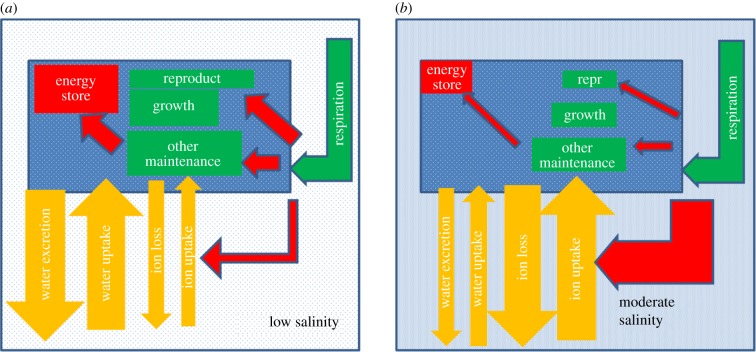
Hypothesis 1 was suggested by Scheibener et al. [47], following their observation of greater transport of Na with increasing external Na+ concentration in Maccaffertium sp., and is expanded upon here. Despite Maccaffertium sp.'s Na+ uptake increasing, its total Na body burden is invariant of the external Na+ concentration [47], so as external Na+ increases Maccaffertium sp.'s turnover of Na is increasing (figure 3).
Figure 3.
A conceptual model of hypothesis 1 in salt-sensitive Ephemeroptera (mayflies). The individual animal is represented by a blue rectangle in either water with low salinity (a) or moderate salinity but less than the osmolality of its haemolymph (b). The levels of shading in the low salinity, moderate salinity and the animal itself represent the osmolality of the internal/external fluid. The green arrows represent the production of energy via respiration, red arrows the uses of this energy and yellow arrows the movement of substances, i.e. inorganic ions and water. The size and direction of the arrows represent the amount and direction of the substances/energy flow. The sizes of the green and red rectangles represent the size of these functions/stores. Repr, reproduction. (Online version in colour.)
Potentially, the turnover of ions other than Na+ also increases as their external concentration increases in mayflies. Uptake of 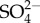 in five mayfly species (including Maccaffertium sp.) increased with increasing external
in five mayfly species (including Maccaffertium sp.) increased with increasing external 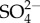 concentration, but unlike Na+, saturation of
concentration, but unlike Na+, saturation of 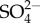 was observed ([30]; see also from this issue Buchwalter et al. [49]). That is, the rate of increase in
was observed ([30]; see also from this issue Buchwalter et al. [49]). That is, the rate of increase in 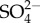 uptake decreased with increasing external
uptake decreased with increasing external 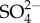 concentration. The uptake of Ca2+ was studied in seven species of ephemerellid mayflies (and five species of Hydropsychidae (Trichoptera)) but the effect of external Ca2+ on Ca's uptake rate was not measured [50]. The uptake rates of other major ions as their external concentration increases appear not to have been measured in mayflies or other freshwater insects.
concentration. The uptake of Ca2+ was studied in seven species of ephemerellid mayflies (and five species of Hydropsychidae (Trichoptera)) but the effect of external Ca2+ on Ca's uptake rate was not measured [50]. The uptake rates of other major ions as their external concentration increases appear not to have been measured in mayflies or other freshwater insects.
The uptake of ions from lower concentrations in freshwater to higher concentrations inside animals requires the expenditure of (some) energy [20,23,51], although the importance of this energy relative to the total energy expenditure is uncertain (see §7). In many incidents of anthropogenic salinization, Na+ concentration increases [43,52]. Thus, in moderate salinity (i.e. below the isoosmotic point) more energy should have to be spent on maintaining Na+ turnover (and potentially the turnover of other ions) than at lower salinities (figure 3). Maccaffertium sp. respiration does not change with salinity [47] as appears to be the case in other aquatic insects [48,53,54]. So it would appear that an increased demand for energy for osmoregulation has to occur within a fixed supply of energy. Thus, there is a zero sum game, i.e. more energy for ion homeostasis means less energy for other functions including growth, reproduction, other maintenance and the building up of stores of energy (figure 3). Individuals may even start drawing down previously deposited stores of energy. The use of more energy for ion homeostasis with increasing external salinity would be expected to result in reduced growth with increasing salinity—as has been observed [43–45]. Mortality presumably occurs when ion homeostasis demands so much energy that other vital functions are compromised.
There are three key premises made in proposing hypothesis 1. (1) The increased Na turnover observed in Maccaffertium sp. with increasing external Na+ concentration occurs in other salt-sensitive mayflies. (2) That increased Na (and 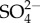 and potentially other ions) uptake requires more energy. (3) Either salt-sensitive mayflies cannot increase their total energy supply with increased energy needed or they cannot increase this supply sufficiently to meet osmoregulation demands without diverting some energy from other uses.
and potentially other ions) uptake requires more energy. (3) Either salt-sensitive mayflies cannot increase their total energy supply with increased energy needed or they cannot increase this supply sufficiently to meet osmoregulation demands without diverting some energy from other uses.
Until further studies are done on other species, it is not possible to speculate on premise (1), other than to note that physiological processes are often conserved within linages. For salinity increases that do not result in increased Na+ concentrations, hypothesis (1) would require increased turnover rates of other ions. The second premise is often said to be a well-established principle in biology (e.g. [34]), but ionic regulation is a complex phenomenon involving multiple pathways [20] and the energetic costs of osmoregulation are uncertain and dependent on various assumptions ([51]; see also §7). For the final premise not to be met, it would be necessary for individuals to produce more energy from feeding to compensate for the increased ion homeostasis demands. If it were possible for mayflies to increase feeding to compensate for increased ion transport, it begs the question: why don't they feed at this higher rate in the absence of a salinity change so as to devote more energy to increase growth, development and reproduction?
5. Hypothesis 2—(localized) loss of pH regulation
The uptake of Na+ by freshwater animals is complex. This uptake firstly involves the movement of the Na+ from the external freshwater into the ionocytes (also called chloride cells and mitochondria-rich cells) and then its movement from within the ionocytes to the organism's blood or haemolymph. Approximately nine biochemical transporters of Na have been documented in freshwater animals, of which four have been recorded in freshwater insect of the order Diptera [20].
Despite the complexity of Na+ uptake, the dominant biochemical transporter is the Na+/H+-exchanger and this transporter is used by mayflies [21]. The Na+/H+-exchanger pulls one Na+ into an ionocyte in exchange for pushing one H+ (or proton) out to the external water [20]. Another Na+ transporter in freshwater animals that is also used by mayflies [21] involves the active transport of one H+ ion out of an ionocyte into the external water via V-type H+-ATPase, which produces an electrical gradient that in turn drives one Na+ into the ionocyte via an apical Na+ channel to maintain a balance in electric charges [20,23]. Hypothesis 2 is based on the increased turnover of Na+ with increasing external Na concentration in Maccaffertium sp. [47], resulting in increased expulsion of H+ by the Na+/H+-exchanger and/or the V-type H+-ATPase. If the ionocytes are not able to regulate their pH by bringing in or creating more H+, there would be a tendency for pH to rise inside the ionocytes. Hypothesis 2 presupposes that the ability of the ionocytes to regulate their pH lags behind their pulling in of Na+ while losing H+.
While alkalization of aquatic insects has not been shown to occur as a result of Na+ uptake, the reverse, i.e. the reduction in Na+ uptake to prevent alkalization, has been suggested. Cenocorixa blaisdelli, a freshwater corixid (Hemiptera), has a lower Na+ haemolymph concentration but a higher Cl− haemolymph concentration when acclimated to alkaline (pH 9.8) conditions relative to neutral pH [55]. This change in Na+ was considered to be a response to maintain acid–base regulation by limiting Na+ uptake to reduce the loss of H+ [55]. Cl− is thought to be taken up by ionocytes from the external freshwater in exchange for a bicarbonate ion (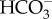 ) from the ionocyte being expelled to the external water [20,23]. So the increased haemolymph Cl− concentrations were considered to be a result of increasing Cl− uptake to increase the loss of
) from the ionocyte being expelled to the external water [20,23]. So the increased haemolymph Cl− concentrations were considered to be a result of increasing Cl− uptake to increase the loss of 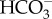 [55].
[55].
6. Hypothesis 3—localized Na poisoning
Hypothesis 3 is based on uptake of Na+ leading to localized Na toxicity or poisoning. In Maccaffertium sp., total body concentration of Na+ is invariant of external Na concentration despite an increased rate of Na+ uptake with increasing external Na concentration [47]. However, this does not rule out Na building up in specific cells, tissue(s) or organ(s) and causing localized toxicity in a small part of the mayfly. Recently, Nowghani et al. [21] showed that the mayfly Hexagenia rigida took up Na+ in its gills (which had long been hypothesized [56]) but lost this ion in regions of its alimentary canal and Malpighian tubules. If Na is travelling from the gills to the alimentary canal and Malpighian tubules at increasing rates with increasing external Na concentrations [47] there is the potential for localized build-up of Na concentrations in specific locations en route, which causes localized toxicity. In the study by Dowse et al. [19] many of the A. pusillus killed by salinity appeared to have damaged gills [57], which could be consistent with localized toxicity in the gills, the likely site of Na uptake. Low levels of localized toxicity would depress the function of the affected cells or tissue(s), resulting in sub-lethal effect. If of sufficient magnitude, localized toxicity could stop the function of the cells and tissue(s) and if these cells/tissue(s) performs vital functions, death could result.
7. Discussion
Freshwater insects, which dominate most flowing waters in terms of animal species richness and biomass, evolved from terrestrial insects on multiple occasions ([20,41] and references therein). There are critical differences in the osmoregulatory challenges between terrestrial and freshwater environments [23]. The terrestrial ancestors of freshwater insects must have evolved mechanisms to address terrestrial challenges before adapting again to meet the needs of a freshwater life. In contrast, with the exception of pulmonate gastropods [20], other freshwater animals (e.g. fish, crustaceans, other molluscs) are thought to have evolved from marine ancestors, presumably via estuaries [34]. Given differences in their evolutionary history, it should not be assumed that freshwater insects will osmoregulate in the same way as freshwater fish and crustaceans. The move to freshwater habitats occurred independently in multiple insect linages and it similarly should not be assumed that each insect linage will have evolved the same strategies for the challenges of a dilute medium. It is thus plausible that the osmoregulation of any lineage of freshwater insects, such as Ephemeroptera, might differ in important respects to other groups of freshwater animals.
Ephemeroptera (along with Plecoptera) are among the oldest flying insect orders, yet appear never to have evolved the ability to live in marine or inland saline waters [41]. This is in contrast to other major groups of freshwater insects, including: Trichoptera, Diptera, Hemiptera, Coleoptera and Odonata. There may be fundamental physiological and evolutionary constraints preventing Ephemeroptera (and Plecoptera) from inhabiting saline waters, although biotic interactions may contribute to the lack of Plecoptera in saline waters (see Bray et al. [58] from this special issue).
The mechanism responsible for Ephemeroptera increasing their Na+ uptake rate with increasing external Na+ concentration [47] is unknown, but may be a consequence of adaption to life in very dilute waters [22]. The increased rate of Na+ uptake with increasing external Na may indicate that Na+ uptake is the result of a passive process and/or driven by the exchange of other ions. Regardless, living in very dilute waters and maintaining levels of major ions, several of which are essential elements (e.g. Na, K, Ca, Mg, Cl), is challenging for osmoregulation [37]. It may be that Ephemeroptera have adopted an osmoregulatory strategy that while well suited for pulling in ions in very dilute waters, puts them at a severe disadvantage when confronted with slight increases in salinity [22].
A problem for hypotheses 2 and 3 is that they are specific to increased Na concentrations that result from salinity. Yet in the Appalachian Mountains of the USA, relatively small salinity increases from coal mining are severely detrimental to mayflies, but there can be minimal increase in Na [13,14]. There may be one mechanism accounting for mayflies' sensitivity to NaCl-dominated salinity and other explanations when increases in other ions are involved, although this explanation does not have parsimony. Hypothesis 1 in contrast could explain mayflies' sensitivity to Na+, 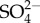 and potentially other ions.
and potentially other ions.
A problem for hypothesis 2 is that other factors should make it relatively easily to maintain pH homeostasis inside ionocytes. H+ should be relatively easily made by the hydrolysis of CO2 (that is, dissolving CO2 in water) to form H+ and 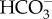 [20], and CO2 and water are unlikely to be in short supply in a freshwater animal with aerobic respiration. Furthermore if Na+ uptake is accompanied by uptake of Cl−, the pH inside the ionocytes should not tend to increase. This is because Cl− is transported into an ionocyte from the surrounding freshwater by the movement of a bicarbonate ion (
[20], and CO2 and water are unlikely to be in short supply in a freshwater animal with aerobic respiration. Furthermore if Na+ uptake is accompanied by uptake of Cl−, the pH inside the ionocytes should not tend to increase. This is because Cl− is transported into an ionocyte from the surrounding freshwater by the movement of a bicarbonate ion (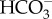 ) from inside the ionocyte to the external freshwater [20,23]. Thus with concurrent movement of both Na+ and Cl− ions, any increase in pH from the loss of H+ inside ionocytes should be neutralized by the concurrent loss of
) from inside the ionocyte to the external freshwater [20,23]. Thus with concurrent movement of both Na+ and Cl− ions, any increase in pH from the loss of H+ inside ionocytes should be neutralized by the concurrent loss of 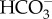 and there should be little, or no, net change in pH inside the ionocytes [23]. Furthermore Zalizniak et al. [59] observed that external pH did not affect the acute salinity tolerance of several stream invertebrates, including one Ephemeroptera (Centroptilum sp.). Nevertheless, it is difficult to exclude hypothesis 2 without empirical studies explicitly attempting to falsify it.
and there should be little, or no, net change in pH inside the ionocytes [23]. Furthermore Zalizniak et al. [59] observed that external pH did not affect the acute salinity tolerance of several stream invertebrates, including one Ephemeroptera (Centroptilum sp.). Nevertheless, it is difficult to exclude hypothesis 2 without empirical studies explicitly attempting to falsify it.
Hypothesis 1 is also not without its problems. Firstly this hypothesis is premised on ion uptake being energetically expensive. The energetic costs of osmoregulation have long been an area of controversy ([36,51,60,61]; see discussion in [37]). Measuring all the direct and indirect energetic costs of osmoregulation has proved to be complex and difficult: different methods have produced contradictory results [37] and the energetic costs of osmoregulation are unclear.
Even if the premise that ion uptake is energetically expensive holds, hypothesis 1 still has a problem. While mayflies should spend more energy on uptake of Na+, 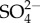 and potentially other ions with increasing salinity, they should be spending less energy on excreting water (figure 3). Osmoregulation involves both the regulation of ions and water [23]. In freshwater with increasing salinity (below the isoosmotic point), the tendency for water to rush into mayflies and the energetic cost of expelling excess water should decrease, regardless of the cost of ion regulation. For hypothesis 1 to hold, any increase in the energetic cost of ion regulation would need to be greater than the energetic saving from water regulation (figure 3).
and potentially other ions with increasing salinity, they should be spending less energy on excreting water (figure 3). Osmoregulation involves both the regulation of ions and water [23]. In freshwater with increasing salinity (below the isoosmotic point), the tendency for water to rush into mayflies and the energetic cost of expelling excess water should decrease, regardless of the cost of ion regulation. For hypothesis 1 to hold, any increase in the energetic cost of ion regulation would need to be greater than the energetic saving from water regulation (figure 3).
Another issue with hypothesis 1, at least in terms of Na+ uptake, is that freshwater animals have multiple biochemical mechanisms for the uptake of this ion [20]. The energetic costs of these various mechanisms vary [20]. So, for example, a doubling of the uptake rate of Na+ could result in less than a doubling of the energy spent on this uptake, if the animal switches to less energy expensive mechanisms for this update.
However, it is also possible that a mayfly would first use the least energetically expensive mechanism for Na+ uptake, until this mechanism is running at its maximum capacity, and only then turn to energetically more expensive mechanisms. If this were to occur, then a doubling of the uptake rate of Na+, for example, could result in more than a doubling of the energy spent on this uptake! Such an occurrence would beg the question: why does the mayfly not ‘switch off’ these expensive mechanisms so as to ‘slow down’ Na+ uptake as external Na concentration increases (figure 2) and thus avoid the problem in the first place?
The three proposed conceptual hypotheses can be tested and potentially falsified. If increased Na+ or 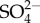 uptake with increasing external
uptake with increasing external  is not accompanied by less energy stores, reduced growth rates and/or fecundity then hypothesis 1 would not be supported. Furthermore, if hypothesis 1 is correct then the effect of salinity on mortality in mayflies should be greater when they are unfed, relative to when they are fed, because mayflies should be under greater energetic stress when not fed. If ionocytes do not have elevated pH where Na+ uptakes occurs, then hypothesis 2 would be not supported. If there are no regions within mayflies that do not have localized increases in the concentration of Na when Na+ uptake increases (with increasing external Na+ concentration) then hypothesis 3 would not be supported.
is not accompanied by less energy stores, reduced growth rates and/or fecundity then hypothesis 1 would not be supported. Furthermore, if hypothesis 1 is correct then the effect of salinity on mortality in mayflies should be greater when they are unfed, relative to when they are fed, because mayflies should be under greater energetic stress when not fed. If ionocytes do not have elevated pH where Na+ uptakes occurs, then hypothesis 2 would be not supported. If there are no regions within mayflies that do not have localized increases in the concentration of Na when Na+ uptake increases (with increasing external Na+ concentration) then hypothesis 3 would not be supported.
It is logically possible that hypothesis 2 or 3 is the mechanism that drives hypothesis 1 and that increased (localized) pH or Na occurs as described above for hypotheses 2 and 3, respectively. Yet mayflies are able to deal with the increased pH/Na but only by spending energy and taking this energy away from other processes (figure 3). If this were to occur then increased Na+ uptake should be accompanied by (1) less energy stores, reduced growth rates and/or fecundity and (2) upregulation of mechanisms to combat the localized increase in pH or Na.
Even if all of the three hypotheses proposed are ultimately shown to be particularly or totally wrong, their testing will improve the understanding of osmoregulation and ion homeostasis in mayflies. Osmoregulation and ion homeostasis in this taxon have received surprisingly little attention despite their importance for biomonitoring and their salinity sensitivity [20,21]. It is my hope that this paper will stimulate rigorous testing of the proposed hypotheses, leading to improved understanding of osmoregulation in not just Ephemeroptera but also other freshwater insects. If/where deficiencies in the proposed hypotheses are found, researchers should suggest alternative testable hypotheses.
Supplementary Material
Acknowledgements
The ideas developed here have benefited from discussions with a number of colleagues, including David Buchwalter, Charles Hawkins, Regan Ashby, Jochen Zeil and Paul Cooper. David Buchwalter, Paul Cooper, Jon Bray, Renee Dowse and Ben Moulding are thanked for comments on drafts. Comments by three anonymous reviewers also greatly improved the paper. However, I take full responsibility for the paper presented and any errors or omissions are entirely my own.
Data accessibility
Data uploaded as part of the electronic supplementary material.
Competing interests
I declare I have no competing interests.
Funding
This paper was completed while funded by an Australia Research Council Linkage Project (project no. LP130100100).
References
- 1.Cañedo-Argüelles M, Kefford BJ, Piscart C, Prat N, Schäfer RB, Schulz C-J. 2013. Salinisation of rivers: an urgent ecological issue. Environ. Pollut. 173, 157–167. ( 10.1016/j.envpol.2012.10.011) [DOI] [PubMed] [Google Scholar]
- 2.Williams WD, Sherwood JE.. 1994. Definition and measurement of salinity in salt lakes. Int. J. Salt Lake Res. 3, 53–63. ( 10.1007/BF01990642) [DOI] [Google Scholar]
- 3.Cañedo-Argüelles M, et al. 2016. Saving freshwater from salts. Science 351, 914–916. ( 10.1126/science.aad3488) [DOI] [PubMed] [Google Scholar]
- 4.Williams WD, Boulton AJ, Taaffe RG. 1990. Salinity as a determinant of salt lake fauna: a question of scale. Hydrobiologia 197, 257–266. ( 10.1007/BF00026955) [DOI] [Google Scholar]
- 5.Kefford BJ. 1998. The relationship between electrical conductivity and selected macroinvertebrate communities in four river systems of south-west Victoria, Australia. Int. J. Salt Lake Res. 7, 153–170. ( 10.1007/BF02441884) [DOI] [Google Scholar]
- 6.Short TM, Black JA, Birge WJ. 1991. Ecology of a saline stream: community responses to spatial gradients of environmental conditions. Hydrobiologia 226, 167–178. ( 10.1007/BF00006858) [DOI] [Google Scholar]
- 7.Metzeling L, Perriss S, Robinson D. 2006. Can the detection of salinity and habitat simplification gradients using rapid bioassessment of benthic invertebrates be improved through finer taxonomic resolution or alternative indices? Hydrobiologia 572, 235–252. ( 10.1007/s10750-005-9004-3) [DOI] [Google Scholar]
- 8.Kefford BJ, Schäfer RB, Metzeling L. 2012. Risk assessment of salinity and turbidity in Victoria (Australia) to stream insects’ community structure does not always protect functional traits. Sci. Total Environ. 415, 61–68. ( 10.1016/j.scitotenv.2011.05.056) [DOI] [PubMed] [Google Scholar]
- 9.Sauer FG, Bundschuh M, Zubrod JP, Schäfer RB, Thompson K, Kefford BJ. 2016. Effects of salinity on leaf breakdown: dryland salinity versus salinity from a coalmine. Aquat. Toxicol. 177, 425–432. ( 10.1016/j.aquatox.2016.06.014) [DOI] [PubMed] [Google Scholar]
- 10.Schäfer RB, et al. 2012. Relationships of selected ecosystem functions in streams with pesticide toxicity, salinity and other environmental variables and the relevance for ecosystem services. Sci. Total Environ. 415, 69–78. ( 10.1016/j.scitotenv.2011.05.063) [DOI] [PubMed] [Google Scholar]
- 11.Clements WH, Kotalik C.. 2016. Effects of major ions on natural benthic communities: an experimental assessment of the US Environmental Protection Agency aquatic life benchmark for conductivity. Freshw. Sci. 35, 126–138. ( 10.1086/685085) [DOI] [Google Scholar]
- 12.Kefford BJ, Marchant R, Schäfer RB, Metzeling L, Dunlop JE, Choy SC, Goonan P. 2011. The definition of species richness used by species sensitivity distributions approximates observed effects of salinity on stream macroinvertebrates. Environ. Pollut. 159, 302–310. ( 10.1016/j.envpol.2010.08.025) [DOI] [PubMed] [Google Scholar]
- 13.Pond GJ. 2010. Patterns of Ephemeroptera taxa loss in Appalachian headwater streams (Kentucky, USA). Hydrobiologia 641, 185–201. ( 10.1007/s10750-009-0081-6) [DOI] [Google Scholar]
- 14.USEPA. 2011. A field-based aquatic life benchmark for conductivity in Central Appalachian Streams. Washington, DC: U.S. Environmental Protection Agency. [Google Scholar]
- 15.Cormier SM, Suter GW II, Zheng L. 2013. Derivation of a benchmark for freshwater ionic strength. Environ. Toxicol. Chem. 32, 263–271. ( 10.1002/etc.2064) [DOI] [PubMed] [Google Scholar]
- 16.Cormier SM, Suter GW II, Zheng L, Pond GJ. 2013. Assessing causation of the extirpation of stream macroinvertebrates by a mixture of ions. Environ. Toxicol. Chem. 32, 277–287. ( 10.1002/etc.2059) [DOI] [PubMed] [Google Scholar]
- 17.Kefford BJ, Piscart C, Hickey HL, Gasith A, Ben-David E, Dunlop JE, Palmer CG, Allan K, Choy SC. 2012. Global scale variation in the salinity sensitivity of riverine macroinvertebrates: eastern Australia, France, Israel and South Africa. PLoS ONE 7, e35224 ( 10.1371/journal.pone.0035224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castillo AM, Sharpe DMT, Ghalambor CK, León LFD. 2018. Exploring the effects of salinization on trophic diversity in freshwater ecosystems: a quantitative review. Hydrobiologia 807, 1–17. ( 10.1007/s10750-017-3403-0) [DOI] [Google Scholar]
- 19.Dowse R, Palmer CG, Hills K, Torpy F, Kefford BJ. 2017. The mayfly nymph Austrophlebioides pusillus Harker defies common osmoregulatory assumptions. R. Soc. open sci. 4, 160520 ( 10.1098/rsos.160520) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffith MB. 2017. Toxicological perspective on the osmoregulation and ionoregulation physiology of major ions by freshwater animals: teleost fish, crustacea, aquatic insects, and Mollusca. Environ. Toxicol. Chem. 36, 576–600. ( 10.1002/etc.3676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nowghani F, Jonusaite S, Donini TW-L, Kelly SP. 2017. Strategies of ionoregulation in the freshwater nymph of the mayfly Hexagenia rigida. J. Exp. Biol. 220, 3997–4006. ( 10.1242/jeb.166132) [DOI] [PubMed] [Google Scholar]
- 22.Olson JR, Hawkins CP. 2017. Effects of total dissolved solids on growth and mortality predict distributions of stream macroinvertebrates. Freshw. Biol. 62, 779–791. ( 10.1111/fwb.12901) [DOI] [Google Scholar]
- 23.Bradley TJ. 2009. Animal osmoregulation. Oxford, UK: Oxford University Press. [Google Scholar]
- 24.Harrison JF, Woods HA, Roberts SP. 2012. Ecological and environmental physiology of insects. Oxford, UK: Oxford University Press. [Google Scholar]
- 25.Komnick H. 1977. Chloride cells and chloride epithelia of aquatic insects. Int. Rev. Cytol. 49, 285–329. ( 10.1016/S0074-7696(08)61951-8) [DOI] [Google Scholar]
- 26.Filshie BK, Campbell IC. 1984. Design of an insect cuticle associated with osmoregulation: the porous plates of chloride cells in mayfly nymph. Tissue Cell 16, 789–803. ( 10.1016/0040-8166(84)90010-7) [DOI] [PubMed] [Google Scholar]
- 27.Hirose S, Kaneko T, Naito N, Takei Y. 2003. Molecular biology components of chloride cells. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 136, 593–620. ( 10.1016/S1096-4959(03)00287-2) [DOI] [PubMed] [Google Scholar]
- 28.Kefford BJ, Reddy-Lopat K, Clay C, Hagen T, Parkanyi O, Nugegoda D. 2010. Size of anal papillae in chironomids: does it indicate their salinity stress? Limnologica 41, 96–106. ( 10.1016/j.limno.2010.09.004) [DOI] [Google Scholar]
- 29.Dymowska AK, Hwang P.-P, Goss GG. 2012. Structure and function of ionocytes in the freshwater fish gill. Respir. Physiol. Neurobiol. 184, 282–292. ( 10.1016/j.resp.2012.08.025) [DOI] [PubMed] [Google Scholar]
- 30.Scheibener S, Conley JM, Buchwalter D. 2016. Sulfate transport kinetics and toxicity are modulated by sodium in aquatic insects. Aquat. Toxicol. 190, 62–69. ( 10.1016/j.aquatox.2017.06.027) [DOI] [PubMed] [Google Scholar]
- 31.Sutcliffe DW. 1961. Studies on salt and water balance in caddis larvae (Trichoptera): I. Osmotic and ionic regulation of body fluids in Limnephilus affinis Curtis. J. Exp. Biol. 38, 501–519. [Google Scholar]
- 32.Sutcliffe DW. 1961. Studies on salt and water balance in caddis larvae (Trichoptera): II Osmotic and ionic regulation of body fluids in Limnephilus stigma Curtis and Anabolia nervosa Leach. J. Exp. Biol. 38, 521–530. [Google Scholar]
- 33.Patrick ML, Bradley TJ. 2000. The physiology of salinity tolerance in larvae of two species of Culex mosquitoes: the role of compatible solutes. J. Exp. Biol. 203, 821–830. [DOI] [PubMed] [Google Scholar]
- 34.Reece JB, Meyers N, Urry LA, Cain ML, Wasserman SA, Jackson RB, Cooke BN. 2012. Campbell biology. 9th Edition Australian Version ed, Pearson Australian Group Pty Ltd, Melbourne, Victoria, Australia.
- 35.Sutcliffe DW. 1984. Quantitative aspects of oxygen uptake by Gammarus (Crustacea, Amphipoda): a critical review. Freshw. Biol. 14, 443–489. ( 10.1111/j.1365-2427.1984.tb00168.x) [DOI] [Google Scholar]
- 36.Boeuf G, Payan P. 2001. How should salinity influence fish growth? Comp. Biochem. Physiol. C Toxicol. Pharmacol. 130, 411–423. ( 10.1016/S1532-0456(01)00268-X) [DOI] [PubMed] [Google Scholar]
- 37.Kefford BJ, Nugegoda D. 2005. No evidence for a critical salinity threshold for growth and reproduction of the freshwater snail Physa acuta. Environ. Pollut. 54, 755–765. ( 10.1016/j.envpol.2004.09.018) [DOI] [PubMed] [Google Scholar]
- 38.Kefford BJ, Zalizniak L, Nugegoda D. 2006. Growth of the damselfly Ischnura heterosticta is better in saline water than freshwater. Environ. Pollut. 141, 409–419. ( 10.1016/j.envpol.2005.08.064) [DOI] [PubMed] [Google Scholar]
- 39.Hassell KL, Kefford BJ, Nugegoda D. 2006. Sub-lethal and chronic lethal salinity tolerance of three freshwater insects: Cloeon sp. and Centroptilum sp. (Ephemeroptera: Baetidae) and Chironomus sp. (Diptera: Chironomidae). J. Exp. Biol. 209, 4024–4032. ( 10.1242/jeb.02457) [DOI] [PubMed] [Google Scholar]
- 40.Kefford BJ, Fields EJ, Nugegoda D, Clay C. 2007. The salinity tolerance of riverine microinvertebrates from the southern Murray Darling Basin. Mar. Freshw. Res. 58, 1019–1031. ( 10.1071/MF06046) [DOI] [Google Scholar]
- 41.Kefford BJ, Buchwalter D, Cañedo-Argüelles M, Davis J, Duncan RP, Hoffmann A, Thompson R. 2016. Salinized rivers: degraded systems or new habitats for salt-tolerant faunas? Biol. Lett. 12, 20151072 ( 10.1098/rsbl.2015.1072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soucek DJ, Dickinson A. 2015. Full-life chronic toxicity of sodium salts to the mayfly Neocloeon triangulifer in tests with laboratory cultured food. Environ. Toxicol. Chem. 34, 2126–2137. ( 10.1002/etc.3038) [DOI] [PubMed] [Google Scholar]
- 43.Johnson BR, Weaver PC, Nietch CT, Lazorchak JM, Struewing KA, Funk DH. 2015. Elevated major ion concentrations inhibit larval mayfly growth and development. Environ. Toxicol. Chem. 34, 167–172. ( 10.1002/etc.2777) [DOI] [PubMed] [Google Scholar]
- 44.Struewing KA, Lazorchak JM, Weaver PC, Johnson BR, Funk DH, Buchwalter DB. 2015. Part 2: Sensitivity comparisons of the mayfly Centroptilum triangulifer to Ceriodaphnia dubia and Daphnia magna using standard reference toxicants; NaCl, KCl and CuSO4. Chemosphere 139, 597–603. ( 10.1016/j.chemosphere.2014.04.096) [DOI] [PubMed] [Google Scholar]
- 45.Anon. 2015. Ecotoxological responses of stream mayflies exposed to elevated chloride in source waters that differ in hardness, p. 65 Stround, PA: Stroud Water Research Center. [Google Scholar]
- 46.MacDonald W. 2009. An examination of combination of combined stressors on macroinvertebrate salinity tolerance: the sub-lethal and lethal impacts of aluminium and salinity on freshwater macroinvertebrates. Honours Thesis, Rockhampton, QLD, Australia: Central Queensland University. [Google Scholar]
- 47.Scheibener SA, Richardi VS, Buchwalter DB. 2016. Comparative sodium transport patterns provide clues for understanding salinity and metal responses in aquatic insects. Aquat. Toxicol. 171, 20–29. ( 10.1016/j.aquatox.2015.12.006) [DOI] [PubMed] [Google Scholar]
- 48.Beaver CJOP. 1990. Respiratory rate of mayfly nymphs in water with differing oxygen and ionic concentrations. In Mayflies and Stoneflies: life histories and biology. Proceedings of the 5th International Ephemeroptera Conference and the 9th International Plecoptera Conference (ed. Campbell IC.), pp. 105–107. Dordrecht, The Netherlands: Kluwer Academic Publishers. [Google Scholar]
- 49.Buchwalter D, Scheibener S, Chou H, Soucek D, Elphick J. 2019. Are sulfate effects in the mayfly Neocloeon triangulifer driven by the cost of ion regulation? Phil. Trans. R. Soc. B 374, 20180013 ( 10.1098/rstb.2018.0013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poteat M, Buchwalter DB. 2013. Calcium uptake in aquatic insects: influences of phylogeny and metals (Cd and Zn). J. Exp. Biol. 217, 1180–1186. ( 10.1242/jeb.097261) [DOI] [PubMed] [Google Scholar]
- 51.Potts WTW. 1954. The energetics of osmotic regulation in brackish- and fresh-water animals. J. Exp. Biol. 31, 618–630. [Google Scholar]
- 52.Radke LC, Howard KWF, Gell PA. 2002. Chemical diversity in south-eastern Australian saline lakes I: geochemical causes. Mar. Freshw. Res. 53, 941–959. ( 10.1071/MF01231) [DOI] [Google Scholar]
- 53.Kapoor NN. 1979. Osmotic regulation and salinity tolerance of the stonefly nymph, Paragnetina media. J. Insect. Physiol. 25, 17–20. ( 10.1016/0022-1910(79)90031-3) [DOI] [Google Scholar]
- 54.Edwards HA. 1982. Aedes aegypti: energetics of osmoregulation. J. Exp. Biol. 101, 135–141. [Google Scholar]
- 55.Cooper PD, Scudder GGE, Quamme GA. 1987. Ion and CO2 regulation in the freshwater water boatman, Cenocorixa blaisdelli (Hung.) (Hemiptera, Corixidae). Physiol. Zool. 60, 465–471. ( 10.1086/physzool.60.4.30157908) [DOI] [Google Scholar]
- 56.Wichard W, Komnick H. 1971. Electron microscopical and histochemical evidence of chloride cells in tracheal gills of mayfly larvae. Cytobiologie 3, 215–228. [Google Scholar]
- 57.Dowse R, Kefford BJ, Palmer CG.. 2010. Salinity and river biodiversity: varying salinity and other stressors. Final Report Ref. 2006 / RD / 0027. Parramatta, NSW, Australia: The Environmental Trust.
- 58.Bray JP, Reich J, Nichols SJ, Kon Kam King G, Mac Nally R, Thompson R, O'Reilly-Nugent A, Kefford BJ. 2019. Biological interactions mediate context and species-specific sensitivities to salinity. Phil. Trans. R. Soc. B 374, 20180020 ( 10.1098/rstb.2018.0020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zalizniak L, Kefford BJ, Nugegoda D. 2009. Effects of pH on salinity tolerance of selected freshwater invertebrates. Aquat. Ecol. 43, 135–144. ( 10.1007/s10452-007-9148-5) [DOI] [Google Scholar]
- 60.Hunter WR. 1964. Physiological aspects of ecology in nonmarine molluscs. In Physiology of Mollusca, vol. 1 (eds Wilbur KM, Yonge CM), pp. 83–126. New York, NY: Academic Press. [Google Scholar]
- 61.Croghan PC. 1961. Competition and mechanisms of osmotic adaptation. Symp. Soc. Exp. Biol. 15, 156–166. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data uploaded as part of the electronic supplementary material.