Abstract
Anthropogenic activities such as mining, agriculture and industrial wastes have increased the rate of salinization of freshwater ecosystems around the world. Despite the known and probable consequences of freshwater salinization, few consequential regulatory standards and management procedures exist. Current regulations are generally inadequate because they are regionally inconsistent, lack legal consequences and have few ion-specific standards. The lack of ion-specific standards is problematic, because each anthropogenic source of freshwater salinization is associated with a distinct set of ions that can present unique social and economic costs. Additionally, the environmental and toxicological consequences of freshwater salinization are often dependent on the occurrence, concentration and ratios of specific ions. Therefore, to protect fresh waters from continued salinization, discrete, ion-specific management and regulatory strategies should be considered for each source of freshwater salinization, using data from standardized, ion-specific monitoring practices. To develop comprehensive monitoring, regulatory, and management guidelines, we recommend the use of co-adaptive, multi-stakeholder approaches that balance environmental, social, and economic costs and benefits associated with freshwater salinization.
This article is part of the theme issue ‘Salt in freshwaters: causes, ecological consequences and future prospects’.
Keywords: freshwater degradation, global change, human health, ecosystem functions, ecosystem services
1. Introduction
The global contamination of fresh water reduces biodiversity and threatens human health [1]. Toxic pollutants such as pesticides, lead and polychlorinated biphenyls (PCBs) that are deleterious to human health or biodiversity are often regulated to limit their environmental effects. Less toxic pollutants such as phosphates can also can be widespread, but the magnitude of their effects are often unknown owing to reduced research attention. This is the case for salt pollution in freshwater rivers, streams, lakes, wetlands and groundwater across the world.
Salts naturally occur in fresh waters, typically caused by rock weathering (e.g. evaporite deposits) and naturally saline groundwater. However, anthropogenic activities are further increasing concentrations of salts in fresh waters [2–4]. Over the last two decades there has been growing attention to the anthropogenic activities that lead to the salinization of fresh waters, which includes human-accelerated weathering, mining (e.g. coal and potash), shale-gas extraction, brines used for secondary oil recovery, the diversion of surface water and vegetation removal for agriculture, improper irrigation practices, industrial waste, improper storage of wastewater, and the application of fertilizers and road de-icing salts [2–12].
Common ions associated with anthropogenic salinization include chloride (Cl−), sodium (Na+), magnesium (Mg2+), calcium (Ca2+), potassium (K+), sulphate (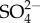 ), carbonate (
), carbonate (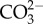 ) and bicarbonate (
) and bicarbonate (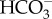 ) [2,13]. However, the dominant ions associated with each human activity are often different [13], therefore the composition of ions probably varies among freshwater environments suffering from anthropogenic salinization. Despite the global pervasiveness of freshwater salinization, and the potential for a collapse of ecosystem functions and services, few standardized, ion-specific monitoring protocols and regulations exist (e.g. the electronic supplementary material, appendix S1). Moreover, current regulatory standards are insufficient to protect freshwater ecosystems, because they lack legal consequences and often fail to provide sufficient management solutions if specific ion limits are exceeded [14–16].
) [2,13]. However, the dominant ions associated with each human activity are often different [13], therefore the composition of ions probably varies among freshwater environments suffering from anthropogenic salinization. Despite the global pervasiveness of freshwater salinization, and the potential for a collapse of ecosystem functions and services, few standardized, ion-specific monitoring protocols and regulations exist (e.g. the electronic supplementary material, appendix S1). Moreover, current regulatory standards are insufficient to protect freshwater ecosystems, because they lack legal consequences and often fail to provide sufficient management solutions if specific ion limits are exceeded [14–16].
The lack of monitoring and management standards is problematic because even if the sources of salinization were to be eliminated immediately, salts will remain in freshwater environments for years or decades [9,17,18]. Moreover, enacting regulations to protect these systems might present significant challenges, because many activities causing freshwater salinization are associated with significant sources of economic and social wellbeing [19]. Of course, human wellbeing is dependent on many ecosystem services that have social or economic implications. Hence, monitoring, regulatory, and management strategies should strive to balance the costs and benefits of ecology, society, and the economy [20] (e.g. a triple-bottom-line approach; electronic supplementary material, appendix S2). However, balancing the costs and benefits for all stakeholders can present significant challenges, even with sufficient legislative protocols (e.g. the Murray–Darling basin in Australia [21]). Therefore, multiple assessment models should be developed, to understand the range of potential outcomes following regulatory or management actions [22].
2. Consequences of salinization
Anthropogenic salinization disrupts ecosystem functions and services, degrades biological systems, and can negatively affect human health and wellbeing [9,23–26]. At high concentrations, salts are toxic to many freshwater organisms, although sensitivities vary among species [2,27]. Typically, small freshwater invertebrates are the most sensitive to increased salinity, while large vertebrates are generally the most tolerant [15]. However, ecological effects—such as a transition to salt-tolerant species—can even occur with small increases in salinity [10,28], which can have cascading effects in aquatic food webs [29,30]. Human health and wellbeing are negatively affected by the loss of ecosystem functions or services, but salinized drinking water can also cause health conditions such as hypertension [31]. Additionally, urban freshwater salinization can increase the prevalence of salt-tolerant disease vectors such as mosquitoes [32,33], and salts can mobilize toxic heavy metals into drinking water, increasing the risks to human health [23,34]. Each source of anthropogenic freshwater salinization contains a distinct set of dominant ions [13], meaning that the ion composition of freshwater environments affected by salinization are being altered in unique ways. Therefore, variable sources of ion pollution are probably associated with unique environmental, economic and social consequences.
Although many of the documented effects of ion contamination come from studies focusing on the effects of sodium or chloride [15,29], each ion associated with salinization can uniquely affect physiological processes in organisms [13,35–38]. Additionally, experimental research suggests that ions such as magnesium, potassium and sulphate, are more toxic to freshwater organisms than sodium or chloride [15,28,37,39]. Regardless of which ions have the strongest toxicological effects, the toxicity of specific ions differs among freshwater organisms, and the toxicity of specific ions often depends on the presence, ratio, and concentration of additional ions [28,40–42]. For example, when associated with chloride, magnesium and calcium have been shown to be more toxic to freshwater invertebrates than sodium [33,37,39]. However, the concentration of sodium has been demonstrated to determine the toxicity of potassium salts [40]. Additionally, the toxicity of potassium and sodium to freshwater algae was altered by their associated anion (chloride or sulphate), but both were more toxic than calcium associated with chloride [43]. Beyond the toxicological interactions among these ions, the ratio and concentration of specific ions affects the toxicity of other contaminants such as metals [13]. Also, as is the case with most metals in fresh water, increased water hardness (typically calcium carbonate plus magnesium) has been demonstrated to reduce the toxic effects of chloride and sulphate [44]. Given the variation in toxicological effects among various cations and anions, and evidence that the total concentration and ratios of ions can determine the toxicity of specific ions, it is essential to enact ion-specific monitoring programmes, regulatory actions, and management solutions [45,46].
Freshwater salinization owing to agricultural practices can have substantial economic costs [45]. For example, the annual economic cost of anthropogenic salinization in the Colorado River Basin (USA) from agricultural practices is estimated at $300 million USD [47]. This includes approximately $176 million worth of damage to crops, and $81 million worth of damage to households each year [47]. Additionally, salinization reduces agricultural revenue by $1.7–7.0 billion each year in California [48]. While these examples of economic costs associated with increased salinization are staggering, they do not often take into account social consequences. Social consequences include the displacement of hundreds of thousands to millions of people owing to loss of work, reduced access to clean drinking water, and a reduction in other ecosystem services provided by fresh water [8].
Furthermore, the social and economic costs of remediation actions are not often clearly incorporated into the cost estimates for freshwater salinization caused by agricultural practices. For any site-specific cost–benefit analysis to include remediation options, the benefits of reducing the damage through intervening actions would need to be assessed along with the social and economic costs of implementing those remediation options. The economic costs of remediation for a single watershed that has been salinized could range from millions to tens of billions of dollars (e.g. Aral Sea) [49], but estimates could be much higher if social costs are included in remediation model estimates. This might especially be true if remediation efforts are needed across multiple political boundaries. Additionally, such acts of restoration and remediation typically cannot recover all of the ecosystem services and functions required to return the ecosystem to pre-salinization state.
Salt pollution from wastewater and road salts can also have costly economic and social consequences. In cold regions of North America, 25 to 50% of applied road salts enter groundwater, leading to groundwater chloride concentrations as high as 2800 mg l−1 [50,51]. For example, in southern New York (USA), more than 50% of wells used for drinking surpassed the United States (US) Environmental Protection Agency secondary drinking water recommendation of 250 mg Cl− l−1 [52]. Furthermore, fixing road-salt contaminated water supplies in two towns in New York (USA) cost $4.7 million (20 homes) and $13.2 million (500 homes), respectively [53]. As groundwater salinization continues, the quality and availability of water used for drinking and agriculture will be reduced. Additionally, owing to chemical and physical interactions between road salts and heavy metals in soil, there is an expected increase in the concentration of heavy metals in groundwater, depending on soil composition and land-use practices [34], similar to the mechanisms causing lead contamination in Flint, Michigan, USA [23].
Beyond the economic costs of fixing water supplies that become contaminated with salt, other externalized costs include the corrosion of infrastructure, cars, industrial tools and home appliances. Vitaliano [54] estimated that the externalized costs of road salts are approximately $720 USD ($1320, 2018 adjusted) per tonne of road salt applied in the US. However, a more recent analysis of externalized costs, including additional environmental and social costs, suggests that the costs associated with road salt use could be as high as $3000 per tonne [55]. Given that transportation agencies in cold regions of the US apply an average of 15 million tonnes of road salts per year [56], a simple extrapolation would mean that the externalized costs could range from $19.8 to 45 billion per year. However, this rough estimate does not account for regional factors affecting road salting decisions, and is offered for illustration purposes only. Nonetheless, the existing research regarding the costs associated with road salt application indicates that the costs associated with this category of salinization are unlikely to be trivial. By not taking preventative measures, increased salinities will only increase the externalized costs of corrosion for infrastructure, utilities, vehicles and households. Additional research into local or regional externalized costs associated with road salt use would be beneficial for agencies seeking alternative de-icing options.
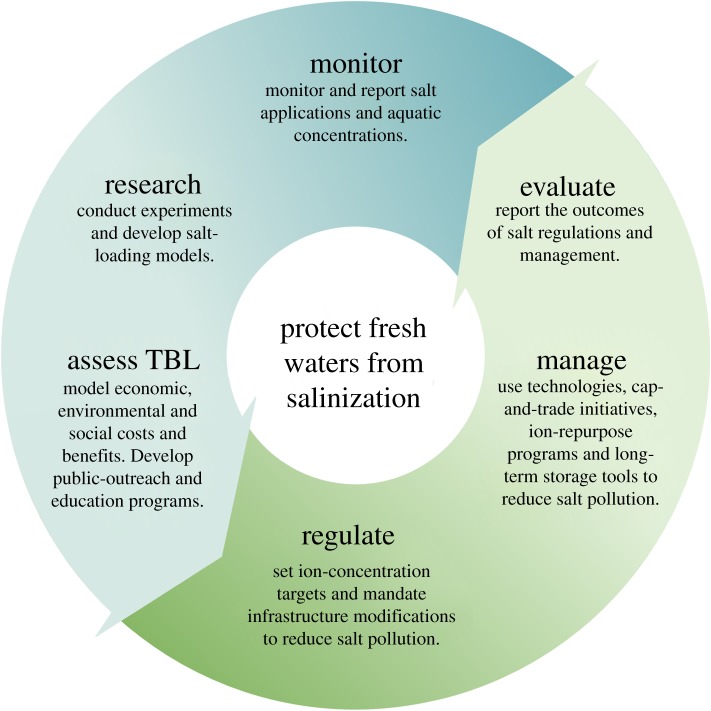
The lack of progress to protect freshwater from salinization is probably driven by limited economic, social, and ecosystem data, which is compounded by the absence of knowledge of the risks by the public and policy makers. Monitoring programmes are needed to drive scientific research investigating the causes and consequences of salinization, including experimental and modelling exercises. Data from scientific research should be used to provide information to the public and policy makers about the risks associated with salinization, driving regulatory actions and advancing management strategies to minimize the negative consequences of freshwater salinization (figure 1) [57–59]. Although management decisions and regulatory actions need to include an adequate assessment of the costs and benefits to the public and the environment, researchers must also acknowledge that any estimation of risk will be imperfect. However, an overly cautious approach might come at a cost, in terms of forgone opportunities for human wellbeing that are only discovered in retrospect [60]. Such considerations are important to consider for specific situations and locations where freshwater resources have suffered from anthropogenic salinization.
Figure 1.
Monitoring and protecting fresh waters from continued salinization using scientific evidence from experiments and models and a triple-bottom-line (TBL) approach with public outreach programmes to develop comprehensive regulations and advanced management strategies. The consequences of management strategies and regulatory actions should be evaluated and used to inform monitoring programmes and reassess models that calculate the social, environmental, and economic costs and benefits.
3. Need for ion-specific monitoring and reporting
To understand the extent of freshwater salinization and its associated impacts, it is imperative that industry, local municipalities, and state or regional governments report: (i) the quantities of each salt produced and purchased, (ii) the quantity or concentration of each salt type released into the environment, and (iii) the concentration of each salt ion in freshwater ecosystems using a standardized database (e.g. reported road salt use) [61]. For example, through the European Water Framework Directive [62], several European countries track and report ion-specific concentrations in surface waters and groundwater. Additionally, many states in the US have ion-specific monitoring programmes, especially for public water supplies. However, because the US Environmental Protection Agency and the European Water Framework Directive do not have enforceable, regulated limits for most ions (especially in surface waters), each government can select the ions that they monitor, and determine how ion concentrations are reported. An internationally recognized and standardized reporting metric for ions would help researchers investigate the potential environmental effects of ions at local, regional and continental scales.
To understand the source and potential environmental impacts of freshwater salinization, we propose that researchers report the concentration of ions measured in field or laboratory studies using standard metrics [63]. Most experimental studies report concentrations based on chloride ions (mg Cl l−1; or ppt Cl−) [29,36]. However, many field studies report salinity in various forms, such as molarity, microsiemens, per cent solution by mass, or total dissolved solids. This disorganized reporting reveals a weakness for scientists trying to communicate with managers and policy makers, and limits the potential to implement comparable quality standards. Without a consistent ion-specific monitoring and reporting protocol, it is very difficult to determine the source and potential impact of freshwater salinization, since each ion can be associated with different environmental and human-health related consequences [13,64]. Although measuring and reporting specific ion concentrations is ideal, there are significant costs associated with such measurements. Additionally, field and laboratory studies might have limited resources, such as reliable, standardized ion probes. Specific ion probes (e.g. chloride) can be cost-prohibitive, have high detection limits, need frequent calibration, and have short lifespans. Additionally, frequent ion-specific laboratory tests might be cost prohibitive. Therefore, a measure of total salt concentration (e.g. specific conductance) might be more appropriate, and would be more informative than no ionic measurements [35]. However, frequent measures of specific conductance could easily be coupled with infrequent ion-specific testing, and the relative influence of each ion can then be modelled [35,39].
We suggest four steps that will help scientists develop monitoring and reporting protocols that will be useful for (communicating with the public, managers and policy makers.
-
(i)
Characterize the baseline (current and historical) status of ions in freshwater ecosystems, focusing on their near-natural conditions and their potential sensitivity to contamination [51,64–66]. Since many freshwater systems have already experienced salinization owing to human activities, models can be used to explore long-term changes in ion concentrations. Salt loading and flow models would indicate the potential percentage of different ions that should be present in freshwater ecosystems given the extent of human activities and natural environmental changes [50,67–71].
-
(ii)
Report the distribution, fractionation, and magnitude of change of specific ions, using models and laboratory-based analyses to determine if salinization is driven by anthropogenic or natural sources [72]. To understand if sources of salinization are anthropogenic or naturally derived, the ratio of chloride to bromide (Cl−/Br−) can be explored [73], or more complex isotopic fractionation analyses (e.g. Sr : B : O : H) [74]. Further determination of ion sources could be assessed by determining long-term changes in ion fractionation [75]. The extent of mining pollution can also be assessed by tracking changes in sulfur isotopes [11].
-
(iii)
Identify thresholds that characterize effects of ion-specific salt pollution, and identify the ion-specific limits appropriate to protect human health and wellbeing, ecosystem functions, and ecosystem services [26,63,76]. It is important that multiple stakeholders are involved when identifying potential thresholds that will be proposed to determine if regulation or management actions are needed (figure 2) [15,36].
-
(iv)
Quantify the economic and social costs of past and future salt contamination using a range of approaches that are appropriate for each situation or location. These can include life-cycle-assessment models and systems-dynamic models, but must also include valuation models, which are needed to better understand the full extent of the ecosystem services associated with water quality [68,78].
Figure 2.
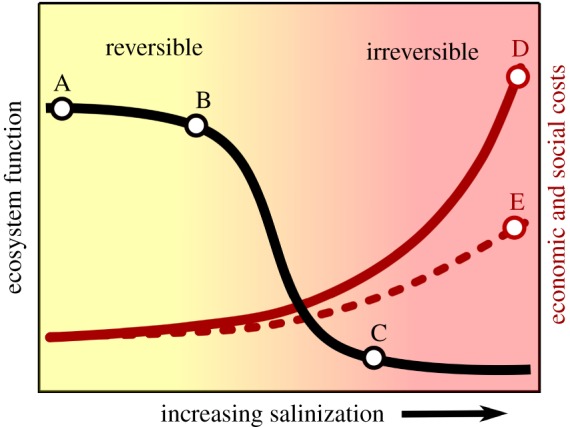
A threshold based model for monitoring, regulatory action, and management given the extent of salinization in a freshwater ecosystem, adapted from Liu et al. [77]. (A) Monitoring and modelling the economic, social, and environmental costs of salinization before environmental consequences occur. (B) Regulatory action and management can protect ecosystem function and ecosystem services while the effects of salinization are still reversible (yellow shaded area). (C) Extensive management can help mitigate some harmful ecological and economic effects. However, the overall effects are probably irreversible (red shaded area), and the economic and social costs are extensive (D), unless partnerships and cooperative agreements are reached among stakeholders to manage salinization (E).
4. Regulations to control ion-specific salinization
Current ion regulations at national or international scales typically lack judicial authority, meaning that there are no legal consequences for instances where defined ion limits are exceeded. For example, all ion-specific regulations set by the US Environmental Protection Agency are secondary recommendations, meaning that each state can set their own specific ion limits, or choose to ignore cases where limits have been exceeded. Although secondary recommendations are helpful for site-specific regulatory standards, in cases where historic ion concentrations exceed defined limits, they offer little protection for freshwater resources with historically low ion concentrations. Additionally, the lack of specified limits leads to variations in recommended ion limits among states and countries. For example, the chronic and acute chloride limits to protect freshwater organisms in the US are 230 and 860 mg l−1 respectively. Meanwhile, the chronic and acute limits for chloride in Canada are 120 and 640 mg l−1 respectively. Similarly, chloride limits for groundwater or drinking water varies among countries. The World Health Organization suggests that water used for drinking should have a chloride concentration between 200 and 300 mg l−1, because the taste of drinking water is affected above 250 mg l−1 [79]. However, the range of groundwater thresholds for chloride set by countries in the European Union varies from 24 to 12 300 mg l−1, probably owing to background concentrations. Similarly, although most countries do not set limits for sodium in drinking water, the range of limits in the European Union varies from 50 to 450 mg l−1. In the US, some states have set sodium standards between 20 and 500 mg l−1. However, other states set sodium limits based on the percentage of sodium compared to all cations, and other states set adsorption ratio limits (electronic supplementary material, appendix S1).
Effective regulations will limit contamination from anthropogenic activities by setting regional, national, or international, ion-specific contamination thresholds with legal consequences and additional regulatory or management strategies for cases where those limits are broken. These thresholds and specific limits will be established from modelled or measured baseline environmental conditions, and the assessed environmental impact of each ion [64,65]. As an example, some US states set a maximum per cent change in ion concentration, or set concentration limits based on historic categories of freshwater use and ion concentrations (electronic supplementary material, appendix S1). This recommendation represents a fundamental change, given that there are currently no national or international, judicially enforced, ion-specific, standards to protect freshwater ecosystems and human health [14,15].
To effectively regulate specific anthropogenic activities associated with freshwater salinization, governments can use existing legislation; such as the Australian and New Zealand Environmental and Conservation Council, the Agriculture and Resource Management Council of Australia and New Zealand, the Clean Water Act and Source Water Protection protocols in the US, the Canadian Environmental Protection Act in Canada, and the Water Framework Directive in Europe. Additionally, many local or regional governments have adopted similar legislation to protect freshwater resources. To successfully limit continued salinization, regulatory actions will necessarily scale from individual watersheds to continents, depending on the source and extent of freshwater salinization.
Broad-scale regulations that cross watersheds or governmental boundaries might be necessary to curb some anthropogenic activities that increase ion concentrations in fresh waters (e.g. the diversion of water for agriculture or industry). Such regulations would be similar to the Great Lakes Water Quality Agreement (GLWQA) adopted by the US and Canada in 1972 to limit phosphate pollution in the Great Lakes. Government agencies at each level must balance the social, economic and environmental impacts of any regulatory changes that can affect salinization, including the development of new technologies and remediation initiatives [20,57].
Ion-specific regulations might present legal complications in some regions, because they would counter laws regarding safety or sanitation. For example, road salts increase the ability of transportation agencies to maintain safe driving conditions; however, the continued use of road salts will increase human-health risks by contaminating water used for drinking and irrigation [52]. Additionally, the economic costs associated with some regulatory actions might be too high for local or regional municipalities. Limiting specific ion inputs from municipal wastewater treatment plants could increase the costs for taxpayers if specialized desalinization systems have to be installed. Similar complications can arise if regulations are enacted to restrict water diversions for agriculture, because this water is necessary to sustain food production and provide drinking water for residents in arid regions. In some cases, the cost of modifying infrastructure might be more costly than the future costs of desalinizing water [80], and continued contamination might increase the human-health risks [34]. Any regulatory measures taken to protect freshwater ecosystems from ion-specific contamination would need to balance the social, economic, and environmental costs and benefits.
5. Managing sources of salinization
Ion-specific regulations cannot prevent further salinization in all ecosystems, meaning that management solutions will need to be developed and paid for through stakeholder partnerships. Management practices that reduce salt input into freshwater ecosystems are vital to protect organisms, ecosystem functions and ecosystems services. Building on Cañedo-Argüelles et al.'s [45] suggestions, we have expanded management solutions to include specific ions and incorporated general engineering and stewardship practices that can be used to reduce freshwater salinization.
-
(i)
Implement comprehensive management strategies to assess options for controlling water used for agriculture that includes the evaluation of ion loading into surface and groundwater.
-
(ii)
Develop high-resolution, salt-loading models for freshwater ecosystems to target areas for management and mitigation [50]. By using flow models and dynamic models to estimate loading effects of different anthropogenic sources, managers and scientists can quantify the potential effects of different management actions on the salinization of freshwater ecosystems [67–69].
-
(iii)
Assess the costs and benefits of reducing the application rate of de-icing salts, including any externalized costs [54]. These models should also include alternative de-icing technologies that maintain safe driving conditions, but allow for reduced salt application rates. These technologies include advanced snowploughs (e.g. live-edge ploughs with GPS salt tracking), road surface modifications, targeted application zones, pre-wetting with mixed-salt brines, and alternative salts when necessary [81]. Additionally, agencies should invest in the research and development of new technologies that could further minimize economic, social and environmental costs associated with road salt use.
-
(iv)
Reduce point-source pollution by managing waste from mining (e.g. potash mining) and wastewater treatment plants [18], focusing on removing specific ions from mining waste and industrial wastewater (e.g. SO4) [82] and phytostabilization of mining wastes [83].
-
(v)
Implement ion-specific, cap-and-trade initiatives based on salt-loading model predictions, leading to long-term reductions in salinization.
-
(vi)
Develop ion-recovery programmes by managing and storing wastewater that could be used to recover salts and repurpose them.
-
(vii)
Promote desalinization technologies that could be used to recover ecosystems that would not be improved by regulatory actions, especially in arid ecosystems [80,84]. These technological advances could also be used to treat waste from mines and urban wastewater treatment plants. These technologies might initially be cost-prohibitive, and the costs and benefits of using these technologies should be assessed on a site-by-site basis. However, as more markets use these technologies, the cost of desalinization will be reduced, and the site-specific costs could be re-evaluated [84–86].
The mechanisms for achieving these management goals can come from a range of standard tools including market-based instruments and ‘command and control’ approaches [87,88]. Command and control, or non-market mechanisms, include regulations, management standards, and rules that are used to control pollution or other situations where free market activities generate socially unacceptable outcomes [87,89]. Market-based instruments are used to achieve targets by bending the free-market behaviours of businesses and individuals to maximize social wellbeing. These include emissions trading, where emission credits are used to price the externality cost imposed by pollution (e.g. carbon trades), and monetary incentives such as increased subsidies and reduced taxes. The best market-based instruments relate directly to the magnitude of pollution outputs, which in our case relates to activities that increase the concentration of specific ions in fresh waters. When non-point source pollution is the problem, practices that contribute the most to the pollution outputs can be targeted, instead of specific sources. Extensive, science-based knowledge of the pollution sources, biophysical properties of the environment, and the specific stakeholders involved are some of the necessary inputs for the proper design of market-based controls for each site and each problem. Although difficult to develop and enforce, market-based instruments are effective solutions for complex pollution problems, even in economically sensitive regions [87].
Regardless of the market-based strategies that are developed for sites and regions in the future, the management goals described in this paper can largely be achieved through adaptive co-management solutions [89]. Adaptive co-management solutions use science-based knowledge, along with interdisciplinary, multi-stakeholder collaborations to minimize the impacts of complex socio-economic, environmental problems. Importantly, adaptive co-management solutions provide key opportunities for leaders in science, industry, and policy to connect and determine solutions for complex environmental problems such as freshwater salinization [89].
6. Conclusion
Freshwater salinization caused by human activities negatively affects human health, reduces biodiversity, and disrupts ecosystem functions, leading to a loss of ecosystem services such as drinking water, agriculture, fisheries, and recreational opportunities. Controlling and remediating freshwater salinization will become more important as the demand for fresh water increases [1]. Stakeholders need to work together to ensure that salinization regulations balance complex social and economic values with ecological consequences [57].
While there is good science supporting management actions in some cases, in many cases the processes leading to a change in ecosystem functions by salinization and then to changes in ecosystem services are poorly understood, and continued research is needed [26]. Therefore, at the moment it might not be possible to accurately calculate the costs and benefits of management or regulatory actions. Unavoidably, any cost and benefit estimates would be produced with a large margin of uncertainty, suggesting that a Precautionary Principle might be a useful approach until better data are available. However, the consequences from the missed opportunities to act might supersede any costs of inaccurate value estimation [60]. Therefore, we would not recommend waiting on a full scientific accounting before action is taken.
Because monitoring is crucial for the design of effective regulatory actions and management solutions, monitoring efforts must be undertaken with the explicit goal of improving data needed for models that will inform initiatives to protect fresh water from continued salinization (figure 1). Each action adopted should be assessed within a framework that optimizes environmental, economic and social goals to minimize the negative consequences of freshwater salinization [45,57]. We have called for the establishment of an internationally standardized, scientifically-based, ion-specific monitoring programme to understand the extent, rate of change, and consequences of freshwater salinization. This is imperative because we cannot manage what is not being measured.
Along with monitoring efforts, it is crucial that policy makers draft regional, national, and international, ion-specific regulations aimed at addressing harmful salinization practices, especially in environmentally or economically sensitive regions. Uncertainties about the impacts of salinization of fresh waters should not be taken as a reason for inaction, because environmental and economic harm will continue and intensify as development and pollution progress. Finally, we recommend that multi-stakeholder, co-adaptive management strategies and technological advancements should be used to reduce and mitigate anthropogenic salt contamination when regulatory actions are inappropriate or ineffective, given the known economic, social, and environmental costs and benefits. If the necessary regulatory protections and advanced management solutions are not implemented, freshwater salinization will continue, increasing the social, economic and environmental costs, especially in developing nations.
Supplementary Material
Supplementary Material
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
Research funding was provided by The Jefferson Project at Lake George, which is a collaboration of Rensselaer Polytechnic Institute, IBM, and The FUND for Lake George.
References
- 1.Vörösmarty CJ, et al. 2010. Global threats to human water security and river biodiversity. Nature 467, 555–561. ( 10.1038/nature09440) [DOI] [PubMed] [Google Scholar]
- 2.Cañedo-Argüelles M, Kefford BJ, Piscart C, Prat N, Schäfer RB, Schulz C-J. 2013. Salinisation of rivers: an urgent ecological issue. Environ. Pollut. 173, 157–167. ( 10.1016/j.envpol.2012.10.011) [DOI] [PubMed] [Google Scholar]
- 3.Dugan HA, et al. 2017. Salting our freshwater lakes. Proc. Natl Acad. Sci. USA 114, 4453–4458. ( 10.1073/pnas.1620211114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaushal SS, Likens GE, Pace ML, Utz RM, Haq S, Gorman J, Grese M. 2018. Freshwater salinization syndrome on a continental scale. Proc. Natl Acad. Sci. USA 115, E574–E583. ( 10.1073/pnas.1711234115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mester T, Szabó G, Bessenyei É, Karancsi G, Barkóczi N, Balla D. 2017. The effects of uninsulated sewage tanks on groundwater. A case study in an eastern Hungarian settlement. J. Water Land Dev. 33, 123–129. ( 10.1515/jwld-2017-0027) [DOI] [Google Scholar]
- 6.Williams WD. 2001. Anthropogenic salinisation of inland waters. Hydrobiologia 466, 329–337. ( 10.1023/A:1014598509028) [DOI] [Google Scholar]
- 7.Kaushal SS, Groffman PM, Likens GE, Belt KT, Stack WP, Kelly VR, Band LE, Fisher GT. 2005. Increased salinization of fresh water in the northeastern United States. Proc. Natl Acad. Sci. USA 102, 13 517–13 520. ( 10.1073/pnas.0506414102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahmuduzzaman M, Ahmed ZU, Nuruzzaman AKM, Ahmed FRS. 2014. Causes of salinity intrusion in coastal belt of Bangladesh. Int. J. Plant Res. 4, 8–13. ( 10.5923/s.plant.201401.02) [DOI] [Google Scholar]
- 9.Herbert ER, Boon P, Burgin AJ, Neubauer SC, Franklin RB, Ardón M, Hopfensperger KN, Lamers LPM, Gell P. 2015. A global perspective on wetland salinization: ecological consequences of a growing threat to freshwater wetlands. Ecosphere 6, art206 ( 10.1890/ES14-00534.1) [DOI] [Google Scholar]
- 10.Cañedo-Argüelles M, Brucet S, Carrasco S, Flor-Arnau N, Ordeix M, Ponsá S, Coring E. 2017. Effects of potash mining on river ecosystems: an experimental study. Environ. Pollut. 224, 759–770. ( 10.1016/j.envpol.2016.12.072) [DOI] [PubMed] [Google Scholar]
- 11.Otero N, Soler A. 2002. Sulphur isotopes as tracers of the influence of potash mining in groundwater salinisation in the Llobregat Basin (NE Spain). Water Res. 36, 3989–4000. ( 10.1016/S0043-1354(02)00125-2) [DOI] [PubMed] [Google Scholar]
- 12.Stefan HG, Novotny E, Sander A, Mohseni O.. 2008. Study of environmental effects of de-icing salt on water quality in the twin cities metropolitan area, Minnesota. See http://hdl.handle.net/11299/132320.
- 13.Griffith MB. 2017. Toxicological perspective on the osmoregulation and ionoregulation physiology of major ions by freshwater animals: teleost fish, crustacea, aquatic insects, and Mollusca. Environ. Toxicol. Chem. 36, 576–600. ( 10.1002/etc.3676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.EPA. 2002. National Recommended Water Quality Criteria EPA-822-R-02-047. See https://nepis.epa.gov/Exe/ZyPURL.Cgi?Dockey=P1005EYQ.txt.
- 15.CCME. 2011. Canadian Water Quality Guidelines for the protection of acquatic life. See http://ceqg-rcqe.ccme.ca/download/en/337.
- 16.NSW. 2012. Aquifer Interference Policy. See http://www.water.nsw.gov.au/__data/assets/pdf_file/0004/549175/nsw_aquifer_interference_policy.pdf.
- 17.Kelly VR, Lovett GM, Weathers KC, Findlay SEG, Strayer DL, Burns DJ, Likens GE. 2008. Long-term sodium chloride retention in a rural watershed: legacy effects of road salt on streamwater concentration. Environ. Sci. Technol. 42, 410–415. ( 10.1021/es071391l) [DOI] [PubMed] [Google Scholar]
- 18.Coring E, Bäthe J. 2011. Effects of reduced salt concentrations on plant communities in the River Werra (Germany). Limnol. - Ecol. Manag. Inl. Waters 41, 134–142. ( 10.1016/j.limno.2010.08.004) [DOI] [Google Scholar]
- 19.Schwabe KA, Kan I, Knapp KC. 2006. Drainwater management for salinity mitigation in irrigated agriculture. Am. J. Agric. Econ. 88, 133–149. ( 10.1111/j.1467-8276.2006.00843.x) [DOI] [Google Scholar]
- 20.Hatton MacDonald D, Dyack BJ. 2004. Exploring the institutional impediments to conservation and water reuse: national issues. Report for the Australian Water Conservation and Reuse Research Program.
- 21.MDBA. 2016. The triple bottom line framework: a method for assessing the, economic, social and environmental outcomes of sustainable diversion limits for the northern basin. See https://www.mdba.gov.au/sites/default/files/pubs/763-NB-triple-bottom-line-report.pdf.
- 22.MDBA. 2016. Environmental outcomes of the Northern Basin Review. See https://www.mdba.gov.au/sites/default/files/pubs/Northern-basin-review-report-FINAL.pdf.
- 23.Kaushal SS. 2016. Increased salinization decreases safe drinking water. Environ. Sci. Technol. 50, 2765–2766. ( 10.1021/acs.est.6b00679) [DOI] [PubMed] [Google Scholar]
- 24.Vander Laan JJ, Hawkins CP, Olson JR, Hill RA. 2013. Linking land use, in-stream stressors, and biological condition to infer causes of regional ecological impairment in streams. Freshw. Sci. 32, 801–820. ( 10.1899/12-186.1) [DOI] [Google Scholar]
- 25.Cañedo-Argüelles M, Bundschuh M, Gutiérrez-Cánovas C, Kefford BJ, Prat N, Trobajo R, Schäfer RB. 2014. Effects of repeated salt pulses on ecosystem structure and functions in a stream mesocosm. Sci. Total Environ. 476–477, 634–642. ( 10.1016/j.scitotenv.2013.12.067) [DOI] [PubMed] [Google Scholar]
- 26.Berger E, Fro¨r O, Schäfer RB.. 2019. Salinity impacts on river ecosystem processes: a critical mini-review. Phil. Trans. R. Soc. B 374, 20180010 ( 10.1098/rstb.2018.0010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kefford BJ, Buchwalter D, Cañedo-Argüelles M, Davis J, Duncan RP, Hoffmann A, Thompson R. 2016. Salinized rivers: degraded systems or new habitats for salt-tolerant faunas? Biol. Lett. 12, 20151072 ( 10.1098/rsbl.2015.1072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mount DR, Gulley DD, Hockett JR, Garrison TD, Evans JM. 1997. Statistical models to predict the toxicity of major ions to Ceriodaphnia dubia, Daphnia magna and Pimephales promelas (fathead minnows). Environ. Toxicol. Chem. 16, 2009 ( 10.1897/1551-5028(1997)016%3C2009:SMTPTT%3E2.3.CO;2) [DOI] [Google Scholar]
- 29.Hintz WD, Mattes BM, Schuler MS, Jones DK, Stoler AB, Lind L, Relyea RA. 2017. Salinization triggers a trophic cascade in experimental freshwater communities with varying food-chain length. Ecol. Appl. 27, 833–844. ( 10.1002/eap.1487) [DOI] [PubMed] [Google Scholar]
- 30.Venâncio C, Castro BB, Ribeiro R, Antunes SC, Abrantes N, Soares AMVM, Lopes I.. 2019. Sensitivity of freshwater species under single and multigenerational exposure to seawater intrusion. Phil. Trans. R. Soc. B 374, 20180252 ( 10.1098/rstb.2018.0252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan AE, Ireson A, Kovats S, Mojumder SK, Khusru A, Rahman A, Vineis P. 2011. Drinking water salinity and maternal health in coastal Bangladesh: implications of climate change. Environ. Health Perspect. 119, 1328–1332. ( 10.1289/ehp.1002804) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petranka JW, Doyle EJ. 2010. Effects of road salts on the composition of seasonal pond communities: can the use of road salts enhance mosquito recruitment? Aquat. Ecol. 44, 155–166. ( 10.1007/s10452-009-9286-z) [DOI] [Google Scholar]
- 33.Schuler MS, Relyea RA. 2018. Road salt and organic additives affect mosquito growth and survival: an emerging problem in wetlands. Oikos 127, 866–874. ( 10.1111/oik.04837) [DOI] [Google Scholar]
- 34.Schuler MS, Relyea RA. 2018. A review of the combined threats of road salts and heavy metals to freshwater systems. Bioscience 68, 327–335. ( 10.1093/biosci/biy018) [DOI] [Google Scholar]
- 35.Clements WH, Kotalik C. 2016. Effects of major ions on natural benthic communities: an experimental assessment of the US Environmental Protection Agency aquatic life benchmark for conductivity. Freshw. Sci. 35, 126–138. ( 10.1086/685085) [DOI] [Google Scholar]
- 36.Schuler MS, Hintz WD, Jones DK, Lind LA, Mattes BM, Stoler AB, Sudol KA, Relyea RA. 2017. How common road salts and organic additives alter freshwater food webs: in search of safer alternatives. J. Appl. Ecol. 54, 1353–1361. ( 10.1111/1365-2664.12877) [DOI] [Google Scholar]
- 37.Hintz WD, Relyea RA. 2017. Impacts of road deicing salts on the early-life growth and development of a stream salmonid: salt type matters. Environ. Pollut. 223, 409–415. ( 10.1016/j.envpol.2017.01.040) [DOI] [PubMed] [Google Scholar]
- 38.Hintz WD, Jones DK, Relyea RA. 2019. Evolved tolerance to freshwater salinization in zooplankton: life-history trade-offs, cross-tolerance and reducing cascading effects. Phil. Trans. R. Soc. B 374, 20180012 ( 10.1098/rstb.2018.0012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coldsnow KD, Relyea RA. 2018. Toxicity of various road-deicing salts to Asian clams (Corbicula fluminea). Environ. Toxicol. Chem. 37, 1839–1845. ( 10.1002/etc.4126) [DOI] [PubMed] [Google Scholar]
- 40.Mount DR, et al. 2016. The acute toxicity of major ion salts to Ceriodaphnia dubia: I. Influence of background water chemistry. Environ. Toxicol. Chem. 35, 3039–3057. ( 10.1002/etc.3487) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soucek DJ, Linton TK, Tarr CD, Dickinson A, Wickramanayake N, Delos CG, Cruz LA. 2011. Influence of water hardness and sulfate on the acute toxicity of chloride to sensitive freshwater invertebrates. Environ. Toxicol. Chem. 30, 930–938. ( 10.1002/etc.454) [DOI] [PubMed] [Google Scholar]
- 42.Soucek DJ, Kennedy AJ. 2005. Effects of hardness, chloride, and acclimation on the acute toxicity of sulfate to freshwater invertebrates. Environ. Toxicol. Chem. 24, 1204 ( 10.1897/04-142.1) [DOI] [PubMed] [Google Scholar]
-
43.Simmons JA.
2012.
Toxicity of major cations and anions (Na+, K+, Ca2+, Cl−, and
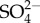 ) to a macrophyte and an alga. Environ. Toxicol. Chem.
31, 1370–1374. ( 10.1002/etc.1819) [DOI] [PubMed] [Google Scholar]
) to a macrophyte and an alga. Environ. Toxicol. Chem.
31, 1370–1374. ( 10.1002/etc.1819) [DOI] [PubMed] [Google Scholar] - 44.Elphick JR, Davies M, Gilron G, Canaria EC, Lo B, Bailey HC. 2011. An aquatic toxicological evaluation of sulfate: the case for considering hardness as a modifying factor in setting water quality guidelines. Environ. Toxicol. Chem. 30, 247–253. ( 10.1002/etc.363) [DOI] [PubMed] [Google Scholar]
- 45.Canedo-Arguelles M, et al. 2016. Saving freshwater from salts. Science 351, 914–916. ( 10.1126/science.aad3488) [DOI] [PubMed] [Google Scholar]
- 46.Bogart SJ, Azizishirazi A, Pyle GG. 2019. Challenges and future prospects for developing Ca and Mg water quality guidelines: a meta-analysis. Phil. Trans. R. Soc. B 374, 20180364 ( 10.1098/rstb.2018.0364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brady PV, Hightower MM. 2014. Desalination: water from water. Future directions, 1st edn Beverly, MA: Scrivener Publishing. [Google Scholar]
- 48.Welle PD, Mauter MS. 2017. High-resolution model for estimating the economic and policy implications of agricultural soil salinization in California. Environ. Res. Lett. 12, 094010 ( 10.1088/1748-9326/aa848e) [DOI] [Google Scholar]
- 49.Micklin P. 2007. The Aral Sea disaster. Annu. Rev. Earth Planet. Sci. 35, 47–72. ( 10.1146/annurev.earth.35.031306.140120) [DOI] [Google Scholar]
- 50.Bester ML, Frind EO, Molson JW, Rudolph DL. 2006. Numerical investigation of road salt impact on an urban wellfield. Ground Water 44, 165–175. ( 10.1111/j.1745-6584.2005.00126.x) [DOI] [PubMed] [Google Scholar]
- 51.Stoddard JL, Larsen DP, Hawkins CP, Johnson RK, Norris RH. 2006. Setting expectations for the ecological condition of streams: the concept of reference condition. Ecol. Appl. 16, 1267–1276. ( 10.1890/1051-0761(2006)016%5B1267:SEFTEC%5D2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 52.Kelly VR, Cunningham MA, Curri N, Findlay SE, Carroll SM. 2018. The distribution of road salt in private drinking water wells in a Southeastern New York suburban township. J. Environ. Qual. 47, 445 ( 10.2134/jeq2017.03.0124) [DOI] [PubMed] [Google Scholar]
- 53.French MJ. 2016. Officials in two upstate towns criticize state responses to salt contamination. See https://www.politico.com/states/new-york/albany/story/2016/12/residents-salty-over-state-response-to-contaminated-water-107851.
- 54.Vitaliano DF. 1992. An economic assessment of the social costs of highway salting and the efficiency of substituting a new deicing material. J. Policy Anal. Manage 11, 397 ( 10.2307/3325069) [DOI] [Google Scholar]
- 55.Dindorf C, Fortin C, Asleson B, Erdmann J.. 2014. Real cost salt use winter Maint. twin cities Metrop. area. See https://www.pca.state.mn.us/sites/default/files/wq-iw11-06bb.pdf.
- 56.Bolen WP. 2018. Minerals Yearbook ( 10.3133/mybvI) [DOI]
- 57.Qadir M. 2016. Policy note: reversing salt-induced land degradation requires integrated measures. Water Econ. Policy 2, 1671001 ( 10.1142/S2382624X16710016) [DOI] [Google Scholar]
- 58.Welle PD, Medellín-Azuara J, Viers JH, Mauter MS. 2017. Economic and policy drivers of agricultural water desalination in California's central valley. Agric. Water Manag. 194, 192–203. ( 10.1016/j.agwat.2017.07.024) [DOI] [Google Scholar]
- 59.Anzaldua G, et al. 2018. Getting into the water with the Ecosystem Services Approach: the DESSIN ESS evaluation framework. Ecosyst. Serv. 30, 318–326. ( 10.1016/j.ecoser.2017.12.004) [DOI] [Google Scholar]
- 60.European Environmental Agency. 2008. Late lessons from early warnings: the precautionary principle 1896–2000. See https://www.eea.europa.eu/publications/environmental_issue_report_2001_22/Issue_Report_No_22.pdf/view. [Google Scholar]
- 61.Clear Roads. 2017. Annu. Surv. state winter Maint. data. See http://clearroads.org/winter-maintenance-survey/ (accessed 20 August 2003).
- 62.EC (European Commission). 2000. Directive 2000/60/EC of the European parliament and of the council of 23 October 2000 establishing a framework for community action in the field of water policy. Off. J. Eur. Communities (doi:2004R0726-v.7of05.06.2013)
- 63.Poole GC, et al. 2004. The case for regime-based water quality standards. Bioscience 54, 155–162. ( 10.1641/0006-3568(2004)054%5B0155:TCFRWQ%5D2.0.CO;2) [DOI] [Google Scholar]
- 64.Griffith MB. 2014. Natural variation and current reference for specific conductivity and major ions in wadeable streams of the conterminous USA. Freshw. Sci. 33, 1–17. ( 10.1086/674704) [DOI] [Google Scholar]
- 65.Brucet S, Poikane S, Lyche-Solheim A, Birk S. 2013. Biological assessment of European lakes: ecological rationale and human impacts. Freshw. Biol. 58, 1106–1115. ( 10.1111/fwb.12111) [DOI] [Google Scholar]
- 66.Cormier SM, Zheng L, Flaherty CM. 2018. A field-based model of the relationship between extirpation of salt-intolerant benthic invertebrates and background conductivity. Sci. Total Environ. 633, 1629–1636. ( 10.1016/j.scitotenv.2018.02.044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zuo Q, Dou M, Chen X, Zhou K. 2006. Physically-based model for studying the salinization of Bosten Lake in China. Hydrol. Sci. J. 51, 432–449. ( 10.1623/hysj.51.3.432) [DOI] [Google Scholar]
- 68.Venkatesan AK, Ahmad S, Johnson W, Batista JR. 2011. Systems dynamic model to forecast salinity load to the Colorado River due to urbanization within the Las Vegas Valley. Sci. Total Environ. 409, 2616–2625. ( 10.1016/j.scitotenv.2011.03.018) [DOI] [PubMed] [Google Scholar]
- 69.Zhemukhov RS, Zhemukhova MM, Bechelova AR, Isakova MM, Ezaova AG. 2017. Environmental impact under development of irrigation on-site irrigation system (OS). In 2017 International Conference ‘Quality Management, Transport and Information Security, Information Technologies’ (IT&QM&IS), pp. 603–606. IEEE; ( 10.1109/ITMQIS.2017.8085896) [DOI] [Google Scholar]
- 70.Le TDH, Kattwinkel M, Schützenmeister K, Olson JR, Hawkins CP, Schäfer RB. 2019. Predicting current and future background ion concentrations in German surface water under climate change. Phil. Trans. R. Soc. B 374, 20180004 ( 10.1098/rstb.2018.0004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Olson JR. 2019. Predicting combined effects of land use and climate change on river and stream salinity. Phil. Trans. R. Soc. B 374, 20180005 ( 10.1098/rstb.2018.0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Estévez E, Rodríguez-Castillo T, González-Ferreras AM, Cañedo-Argüelles M, Barquín J.. 2019. Drivers of spatio-temporal patterns of salinity in Spanish rivers: a nationwide assessment. Phil. Trans. R. Soc. B 374, 20180022 ( 10.1098/rstb.2018.0022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Katz BG, Eberts SM, Kauffman LJ. 2011. Using Cl/Br ratios and other indicators to assess potential impacts on groundwater quality from septic systems: a review and examples from principal aquifers in the United States. J. Hydrol. 397, 151–166. ( 10.1016/j.jhydrol.2010.11.017) [DOI] [Google Scholar]
- 74.Cary L, et al. 2015. Origins and processes of groundwater salinization in the urban coastal aquifers of Recife (Pernambuco, Brazil): a multi-isotope approach. Sci. Total Environ. 530–531, 411–429. ( 10.1016/j.scitotenv.2015.05.015) [DOI] [PubMed] [Google Scholar]
- 75.Sinha R, Raymahashay BC. 2004. Evaporite mineralogy and geochemical evolution of the Sambhar Salt Lake, Rajasthan, India. Sediment. Geol. 166, 59–71. ( 10.1016/j.sedgeo.2003.11.021) [DOI] [Google Scholar]
- 76.Muschal M. 2006. Assessment of risk to aquatic biota from elevated salinity: a case study from the Hunter River, Australia. J. Environ. Manage. 79, 266–278. ( 10.1016/j.jenvman.2005.08.002) [DOI] [PubMed] [Google Scholar]
- 77.Liu J, Kattel G, Arp HPH, Yang H. 2015. Towards threshold-based management of freshwater ecosystems in the context of climate change. Ecol. Model. 318, 265–274. [Google Scholar]
- 78.Keeler BL, Polasky S, Brauman KA, Johnson KA, Finlay JC, O'Neill A, Kovacs K, Dalzell B. 2012. Linking water quality and well-being for improved assessment and valuation of ecosystem services. Proc. Natl Acad. Sci. USA 109, 18 619–18 624. ( 10.1073/pnas.1215991109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.World Health Organisation. 2017. Guidelines for drinking-water quality: fourth edition incorporating the first addendum. WHO Libr. Cat. Data ( 10.1016/S1462-0758(00)00006-6) [DOI]
- 80.Kang M, Jackson RB. 2016. Salinity of deep groundwater in California: water quantity, quality, and protection. Proc. Natl Acad. Sci. USA 113, 7768–7773. ( 10.1073/pnas.1600400113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kelly VR, Findlay SEG, Schlesinger WH, Menking K, Chatrchyan AM.. 2010. Road salt Mov. Towar. Solut. See http://www.caryinstitute.org/sites/default/files/public/downloads/report_road_salt.pdf (accessed 20 August 2005).
- 82.Pinto PX, Al-Abed SR, Balz DA, Butler BA, Landy RB, Smith SJ. 2016. Bench-scale and pilot-scale treatment technologies for the removal of total dissolved solids from coal mine water: a review. Mine Water Environ. 35, 94–112. ( 10.1007/s10230-015-0351-7) [DOI] [Google Scholar]
- 83.Mendez MO, Maier RM. 2007. Phytostabilization of mine tailings in arid and semiarid environments: an emerging remediation technology. Environ. Health Perspect. 116, 278–283. ( 10.1289/ehp.10608) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gude VG. 2018. Desalination of deep groundwater aquifers for freshwater supplies: challenges and strategies. Groundw. Sustain. Dev. 6, 87–92. ( 10.1016/j.gsd.2017.11.002) [DOI] [Google Scholar]
- 85.Kokabian B, Ghimire U, Gude VG. 2018. Water deionization with renewable energy production in microalgae—microbial desalination process. Renew. Energy 122, 354–361. ( 10.1016/j.renene.2018.01.061) [DOI] [Google Scholar]
- 86.Ackerman F, Gallagher K. 2001. Getting the prices wrong: the limits of market-based environmental policy. Tufts University: Global Development and Environment Institute working paper 00-05 (available at http://ase.tufts.edu/gdae/publications.html).
- 87.Blackman A, Li Z, Liu AA. 2018. Efficacy of command-and-control and market-based environmental regulation in developing countries. Annu. Rev. Resour. Econ. 10, 381–404. ( 10.1146/annurev-resource-100517-023144) [DOI] [Google Scholar]
- 88.Grafton RQ, Adamowicz W, Dupont D, Nelson H, Hill RJ, Renzetti S. 2004. The economics of the environment and natural resources. Malden, MA: Blackwell Publishing Ltd. [Google Scholar]
- 89.Armitage DR, et al. 2009. Adaptive co-management for social–ecological complexity. Front. Ecol. Environ. 7, 95–102. ( 10.1890/070089) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.