Abstract
In many dry parts of the world, salinization of water resources threatens freshwater biodiversity and the livelihood of people. However, ecological impact studies remain scarce. Here, we review field-observations of salinity impacts on ecosystem processes such as leaf decomposition, metabolism, biomass production and nutrient cycling, with a special emphasis on dryland ecosystems. In addition, we discuss the potential linkages of these processes to ecosystem service delivery—the benefits that humans derive from ecosystems—as additional nature conservation arguments and the challenges associated with this endeavour.
This article is part of the theme issue ‘Salt in freshwaters: causes, ecological consequences and future prospects'.
Keywords: desert rivers, salinization, ecosystem functions, freshwater management
1. Background
All life depends on water. Since water is scarce in drylands, rivers are the most dominant factor shaping the ecology of these environments including people [1]. Drylands occupy around 40% of the world's land mass and host over one-third of the world's population [2,3]. However, little is known on the ecology of dryland rivers compared with their temperate counterparts [4–6]. Intermittency is often found in these rivers, meaning that they cease to flow or dry out for variable periods of time at differing locations [7]. Only recently several publications have called for increased research efforts on such intermittent rivers and ephemeral streams (IRES), partially driven by the fact that their occurrence is expected to increase as many parts of the world have become drier, a trend that is predicted to continue owing to climate change [4–6]. As their temperate counterparts, dryland rivers, both perennial and intermittent, are under extreme pressure from artificial flow modifications (e.g. dams for irrigation, water retention or power generation, water abstraction, and channelization for shipping), exotic species introductions, waste-disposal effluents from households and industry, changes in catchment land-use (e.g. agriculture and mining) and climate change [8–10]. These factors contribute variously to changes in water physico-chemistry including salinization—the focus of this special issue. In semiarid and arid regions, rivers and streams are often naturally saline owing to underlying geology and high evaporation; however, irrigation agriculture is one of the main causes of secondary, i.e. anthropogenic, salinization [11,12], the mechanisms of which have been summarized and management options suggested [13]. In addition, flow modifications through dams, mining activities, and industrial and household wastewater input can be important contributors. Although salinization is not a recent phenomenon [14], studies on the ecological impacts are scarce [12,15]. The impacts of stressors on river ecosystems are predominantly investigated through the lens of structural descriptors of river biota, such as species richness, abundance diversity and community composition [16]. Salinization of freshwater habitats has been correlated with reduced biodiversity indices such as species or functional richness and has been related to changes in aquatic community composition (see references within reviews, [12,17,18]). It is known that freshwater organisms have varying sensitivities towards salinity stress [19–22] and thus increasing salinity can safely be expected to alter community compositions either directly (species loss due to toxicity) or indirectly through altered species interactions [23,24], irrespective of whether acting as a single stressor or in concert with multiple stressors [25,26]. However, in the past 20 years, nature conservation has departed from protecting species or habitats for their intrinsic value towards preserving ecosystem services—the benefits that humans derive from ecosystem functions—as a stronger conservation argument [27–29]. For this reason, the interest in measuring ecosystem functions in relation to environmental change directly has been increasing, providing a potentially more suitable and mechanistic link to ecosystem service delivery than species compositions [30–34]. Unfortunately, the term ecosystem functions is rather ill-defined and various meanings can be identified in the literature [35,36]. It is used to refer variously to the habitat, biological or system properties or processes of ecosystems [28]. Here, we understand ecosystem functions as a set of processes that regulate the fluxes of energy and matter in ecosystems as a consequence of the joint activity of organisms [33,37].
The aim of this review is to summarize the knowledge on changes in ecosystem processes such as organic matter breakdown, metabolism and nutrient cycling in relation to salinity. Our main interest was to advance understanding of anthropogenic dryland salinization; however, anthropogenic as well as natural salinity gradients in inland rivers from dry as well as humid climates are considered, owing to the scarcity of studies. Moreover, potential links to ecosystem service delivery are discussed and research gaps identified.
2. Material and methods
We reviewed changes in ecosystem processes related to salinization and natural salinity gradients in river ecosystems, focusing on field studies in arid and semiarid regions. Salinity can alter purely physical and chemical processes, as well as coupled biogeochemical cycles that mobilize, for example carbon, nitrogen, phosphorus and sulfur [38]. However, we focused on studies including aquatic organisms such periphyton, invertebrates or fish. A recent review [20] has a stronger focus on salinization impacts on chemical and biogeochemical cycles in aquatic environments, though not focused on dryland rivers (see also [38]). Ecosystem processes such as organic matter decomposition as well as primary and secondary production are at the base of many potential ecosystem services [36] and were the main target of our search in the Web of Science. The key words were: ‘salinization’ (or ‘salinit*’, ‘conductivi*’, ‘ion*’, ‘hardness*’) and ‘ecosystem process*’ (or ecosystem function*, ‘purification’, ‘metabolism’, ‘productivity’, ‘production’, ‘biomass’, ‘decomposition’, ‘regulation’, ‘uptake’, ‘removal’) and ‘river’ (or ‘aquatic’, ‘freshwater’, ‘lotic’, ‘stream’, ‘wetland’) and ‘dryland’ (or ‘arid’, ‘semiarid’, ‘dry’, ‘oasis’, ‘desert’) and ‘impact’ (or ‘affect’, ‘effect’, ‘correlat*’, ‘relationship*’, ‘gradient*’, ‘experiment*’). Approximately 3000 publications were scanned by title, resulting in approximately 150 studies that appeared to deal with salinity impacts (excluding salinity gradients studied in estuaries) on an ecosystem process. These were analysed in greater detail and their reference lists as well as citing papers were screened for relevant literature sources (footnote chasing). Studies from estuaries or coastal wetlands were excluded, because our focus was on inland rivers. Each study was classified into a climatic category (hyper-arid, arid, semiarid or dry subhumid or humid) based on reported location and the associated aridity index [3]. Owing to a scarcity of studies in real drylands, studies from humid climates were also included for comparison, if all other selection criteria were met (investigation of a biologically mediated in-stream ecosystem process in relation to salinity that is not influenced by seawater intrusion). Manipulative mesocosm studies were included, because of their power to differentiate effects from correlated variables, although the literature search was not specifically targeting mesocosm studies and their coverage may not be exhaustive. Single species studies investigating growth, nutrient uptake or processing were not considered, nor studies investigating the composition of species without at least one process-oriented measure.
(a). Data analysis
The ranges of response variables were extracted from tables or using Plot Digitizer v. 2.6.8 (http://plotdigitizer.sourceforge.net) from figures as necessary. Reported salt concentrations were converted to mS cm−1; if reported in weights a factor of 0.7 was used to convert to electric conductivity. Response patterns were classified based on the reported outcome in the individual studies and were summarized into four broad categories: increase, decrease, no response or an inverted U-shaped response pattern (initial increase followed by a decrease) based on statistical significance in the related studies. For joint analysis of decomposition rates, results from four case studies published in three papers were combined that were most similar in terms of the methods used (deployment of leaf bags (3×) and wood sticks (1×) that allowed decomposition by microorganisms and invertebrates). Breakdown rates per degree day (dday−1) were standardized relative to the breakdown rate determined at a conductivity of approximately 1700 µS cm−1, which was a level occurring across all studies. Linear regression was used to analyse the relationship across studies using the statistical software R, v. 3.4.1 [39]. The effect of increased salinity on decomposition in different mesocosm studies was compared by calculating the mean % difference in leaf mass loss relative to the respective control level.
3. Results
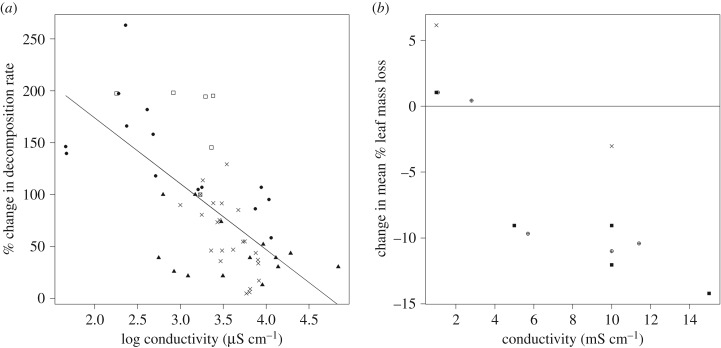
The search criteria were met by 10 studies from semiarid climates in Australia, the USA and Spain, and a further eight studies from humid climates in Australia, Canada, Japan and Poland. Six studies investigated terrestrial leaf litter decomposition [40–45], 11 studies looked at metabolism, biomass production or dietary carbon source use [41,43,46–54], of which two considered the dilution of naturally saline streams as a stressor instead of salinization [46,47]. Nutrient cycling (nitrate uptake and denitrification) was addressed in two studies [55,56]. One study investigated pollution attenuation [57]. A further study investigating primary and secondary production in an agriculturally influenced prairie river was omitted, because siltation confounded the results and the sample size was too low [14]. The origin of salinity gradients in the field studies varied from coal mining, through groundwater treatment effluents and land-use change, to natural geology, and the salinity ranges varied broadly (table 1). These differences as well as the variety of applied methods render quantitative comparisons between studies difficult. The methods, salinity ranges, sources, response ranges and response directions from the 13 field investigations and six mesocosm or microcosm experiments are summarized in tables 1 and 2. For example, the maximum considered salinity varied between 2 mS cm−1 and more than 70 mS cm−1, and some studies focused on the spatial variability, investigating up to 24 sites; others focused on temporal variability of a single site. The most frequently and uniformly assessed ecosystem process was organic matter decomposition, for which the joint analysis displayed a reduced decomposition (R2 = 0.43, p < 0.001) with increasing salinity (figure 1a,b). Including the factor ‘study’ as an explanatory variable in addition to ‘salinity’ in the linear regression models increased model prediction (R2 = 0.61, p < 0.001), where an approximately equal amount of variability in decomposition rates was explained by the two factors (‘study’ = 52% and ‘salinity’ = 48%) in hierarchical partitioning [58].
Table 1.
Summary of field studies investigating ecosystem processes in relation to salinity changes. DE, decomposition; ME, metabolism; BP, biomass production; DC, dietary carbon source use; NC, nutrient cycling; PA, pollutant attenuation; RD, response direction of measured variables with increasing salinity (↓ negative association, ↑ positive association, ∩ inverted U shape, — no clear response pattern). ref., reference; AFDM, ash-free dry mass; FW, fresh weight; Chl a, chlorophyll a; dday, degree day; sal, naturally saline site; fw, chronically freshwater disturbed site.
| process | country, climate | salinity origin | no. of sites | salinity range (mS cm−1) | method | response variable | result range | RD | ref. |
|---|---|---|---|---|---|---|---|---|---|
| DE | Australia, semiarid | anthropogenic (agriculture, coal mining) | 22 | 0.05–2.4 | fine and coarse litter bags (Eucalyptus) ergosterol measurement |
leaf breakdown rates leaf associated fungal biomass |
0.3−1.2 × 10−3 dday−1 0–54 mg kg−1 |
↓ ↓ |
[39] |
| DE | Australia, humid | anthropogenic | 24 | 0.05–3.5 | fine and coarse litter bags (Eucalyptus) cotton strips ergosterol measurement different carbon sources offered to microbes |
leaf breakdown rates microbial cotton strip breakdown rates leaf associated fungal biomass functional microbial richness |
1–2 × 10−3 dday−1 0.07–0.3 × 10−3 dday−1 0–123 mg kg−1 13–26 carbon sources used |
↓ ↓ ↓ ↓ |
[41] |
| DE | Spain, semiarid | natural | 7 | not reported | coarse litter bags (Alnus glutinosa) ergosterol measurement length–mass equations |
leaf breakdown rates leaf associated fungal biomass invertebrate biomass in litter bags |
0.7–5.8 × 10−3 dday−1 6–304 mg kg−1 0–245 mg AFDM bag–1 |
— — — |
[42] |
| DE | Spain, semiarid | natural | 14 | 0.5–69.3 | birch wood sticks in streams ergosterol measurement hydrolysis of fluorescein diacetate |
breakdown rate of wood sticks associated fungal biomass microbial activity |
0.1–0.23 × 10−3 dday−1 0–162.3 ± 7.1 mg kg−1 0.9–3.3 µmol g−1 AFDM h−1 |
↓ ↓ ↓ |
[45] |
| ME | Australia, humid | anthropogenic | 24 | 0.05–3.5 | continuous oxygen measurements | gross primary production ecosystem respiration |
0.001–0.044 g O2 l−1 d−1 0.003–0.101 g O2 l−1 d−1 |
— — |
[41] |
| ME | Spain, semiarid | natural | 1 (2 years) | 6.4–99 | continuous oxygen measurements chlorophyll a concentrations length–mass relations and AFDM |
gross primary production ecosystem respiration biomass of all primary producers total consumer biomass |
0.07–21.05 g O2 m−2 d−1 0.19–17.39 g O2 m−2 d−1 7–461 g Chl a m−2 0–13.7 g AFDM m−2 |
— — — — |
[46] |
| ME | Spain, semiarid | natural | 2 (2 years) | 1–77.6 | continuous oxygen measurements chlorophyll a concentration and AFDM length–mass relations and AFDM stable isotope analysis |
gross primary production ecosystem respiration total biomass of primary producers invertebrate biomass dietary carbon source |
1–170 g O2 d−1 m−2 1–90 g O2 d−1 m−2 sal: 96.5 ± 65.4 g Chl a m−2, fw: 5.1 ± 1.6 g Chl a m−2 sal: 1000–14 000 mg AFDM m−2 fw: 10–500 mg AFDM m−2 based on autochthonous primary sources at both sites |
↑ — ↑ ↑ — |
[47] |
| ME | Canada, humid | simulated agricultural effluent | 1 (120 experimental chambers) | 1.7, 3.4, 6.4 and 11.7 | closed-system O2 production closed-system O2 consumption by invertebrates |
net primary productivity community respiration |
0.90–2.07 µg O2 Chl a µg−1 h−1 0.0032–0.0123 mg O2 FW mg−1 h−1 |
∩ ∩ |
[48] |
| BP | Japan, humid | anthropogenic (ground-water treatment effluent) | 9 | 0.1–5.5 | colonization of Plexiglass plates in stream for 4 weeks | primary production (epilithic algal growth) | 1.82–154.7 mg m−2 | ∩ | [49] |
| BP | Spain, semiarid | natural | 6 | 7.00–17.50 | chlorophyll a and AFDM | primary production | values not reported | ↑ | [54] |
| DC | USA, semiarid | anthropogenic | 12 | 0.1–39.4 | stable isotope analysis | dietary carbon source use | increased relevance of in-stream carbon sources at salinized sites | [50] | |
| NC | Spain, semiarid | natural and anthropogenic | 9 | 9 0.5–11.7 | pulse NO3− additions (tracer addition for spiralling curve characterization) acetylene block technique |
nitrate uptake sediment denitrification |
uptake at 5 sites; maximum values 0.3–60.5 mg NO3− m−2 min−1 68 ± 0.37–40.97 ± 6.06 µg N g−1 AFDM h−1 |
— — |
[55] |
| NC | Spain, semiarid | natural and anthropogenic | 12 | 2.7–39 | acetylene block technique | sediment denitrification | 0.2 ± 0.01−30.0 ± 8.8 µg N g−1 AFDM h−1 | —↓ | [56] |
| PA | Poland, humid | anthropogenic | 2 sites, 40 samples | 0.7–7 | measuring pharmaceuticals added to river water after 28 days | half biodegradation rates of pharmaceuticals | approx. 0−0.9 mg l−1 d−1 | ↓ | [57] |
Table 2.
Summary of mesocosm and microcosm studies investigating ecosystem processes in relation to salinity changes. DE, decomposition; ME, metabolism; BP, biomass production; RD, response direction of measured variables with increasing salinity (↓ negative association, ↑ positive association, ∩ inverted U-shaped, — no clear response pattern); ref., reference; DO, dissolved oxygen.
| process | country and climate | experimental unit | treatments | response variable (method) | RD | ref. |
|---|---|---|---|---|---|---|
| DE | Australia, semiarid | mesocosms colonized by microorganism | three salt types (sea salt, NaCl and NaHCO3), two salinity levels (1, 10 mS cm−1) | % leaf mass loss (fine leaf bags) fungal biomass (ergosterol measurement) |
↓ — |
[39] |
| DE | Australia, semiarid | mesocosms colonized by microorganism | six salt (NaCl) concentrations: (0, 0.05, 0.1, 0.2, 0.5, 1 mS cm−1) | % leaf mass loss (fine leaf bags) | ∩— | [39] |
| DE | Spain, semiarid | artificial flow-through stream systems colonized with invertebrates and microorganisms | three salt concentrations (sea salt): 5, 10 and 15 mS cm−1, as repeated pulses of 3 h duration | leaf mass loss (coarse litter bags, Populus nigra) fungal biomass (as ergosterol) biofilm biomass (chlorophyll a) |
↓ ∩ —↓ |
[43] |
| DE | Portugal, humid | microcosms conditioned with 5000 fungal conidia | four salinity (NaCl) concentrations: 0, 2.8, 5.7, 11.4 mS cm−1 | leaf mass loss (discs of Quercus robur L.) fungal respiration, growth and sporulation rate (oxygen consumption, counting of conidia) |
↓ ↓ |
[44] |
| ME/BP | Argentina, humid | recirculating microcosms with stream colonized glass tiles | salinity (NaCl) pulse (30 min d−1) and press (72 h) exposure with 1.5 mS cm−1, control with no salt addition | biofilm biomass (chlorophyll a concentration) bacterial density (counting) community respiration (oxygen consumption) |
— ↓ ↓ |
[51] |
| BP | Germany, humid | microcosm with stream conditioned periphyton | salinity (Na2SO4 and CaCl2) at 2 and 5 mS cm−1, control 0.6 mS cm−1 | biofilm biomass (minimal fluorescence of photosynthesis pigments) | ↑ | [52] |
| ME | USA, humid | mesocosms with invertebrate community | three salt types (NaHCO3, MgSO4, NaCl) with six concentrations between 0.07 and 4.4 mS cm−1 | difference between day and night-time DO concentrations (open-system, single-station approach for continuous DO measurement) | ↓ | [53] |
Figure 1.
(a) Relationship between conductivity and organic matter breakdown rates (R2 = 0.43, p < 0.001). Data are combined from four case studies (different symbols) published in three papers [39,41,45], n = 57. Breakdown rates per degree day were standardized relative to the breakdown rate determined at a conductivity of approximately 1700 µS cm−1, which was a level occurring across all studies. (b) Difference in the mean % leaf mass loss relative to a low salinity control level from controlled mesocsom experiments [39,43,44]. Different symbols denote different salt types used to achieve experimental conductivity levels; circle with plus sign, NaCl; filled square, sea salt; times, NaHCO3.
4. Discussion
(a). Context-dependent impact: rivers in dry versus humid climates
The effect of salinity on ecosystem processes depends on many factors, such as the salt concentration, salt type, exposure duration (acute or chronic) and most importantly the environmental context (e.g. the natural background salinity, climate and biotic community). All these factors varied between studies and the overall scarcity of studies dealing with salinity impacts on ecosystem processes limited the derivation of generalizable associations or mechanisms as well as direct comparisons between different climatic regions. Generally rivers in dry and humid regions share many similarities, but a very distinctive difference is the much more variable nature of dryland rivers. Their extreme hydrology, where flow gradually decreases, leaving behind isolated water pools, before drying up completely, as well as the altered lateral, vertical and longitudinal connectivity (or fragmentation, respectively) between stream subsystems and the terrestrial surrounding, is known to influence all ecological and biogeochemical processes [59,60]. Their mean coefficient of variation in annual flows is twice that of rivers from humid regions and changes in long periods of drought and extreme floods shape the variable stream bed morphology and sediment transport [61]. Ideally anthropogenic salinization needs to be investigated against this naturally variable background. For example, drying is naturally associated with increases in salinity, and thus river discharge is often negatively correlated with salinity [46,56]. Thus, depending on the cause of salinization, salinity may occur as a single stressor (e.g. point source effluent) or as a stressor complex together with increased habitat fragmentation and flow reduction (e.g. water abstraction). However, reviewing the impact of intermittency on ecosystem processes, which is an important field of research, is beyond the scope of this review (see [4] for an overview). Notwithstanding, we discovered trends of effects and research gaps that may inform future research and management and discuss potential differences between climatic regions.
(b). Leaf litter decomposition
Leaf organic matter may be respired or assimilated by fungi, bacteria, invertebrates or even fish. Both field and mesocosm studies display a rather consistent trend of reduced decomposition rates with increasing salinity in humid as well as semiarid climates (figure 1a, tables 1 and 2; [62,63]). Unclear or nonlinear response patterns were found exclusively at low salinity ranges (less than 3 mS cm−1) [39,64,65]. Interestingly, the effect of conductivity on decomposition rates was related to the salt type in two studies (figure 1b; [39,66]), which confirms studies suggesting that ion-specific thresholds are required in management [15,67]. Mostly, leaf associated fungal biomass or microbial activity is measured together with decomposition rates, because microorganisms are important decomposers. Negative associations (see also [66]) as well as no apparent relation between these potential decomposers with salinity have been observed (table 1). Thus, the underlying mechanisms for reduced decomposition remain controversial. Whereas a recent study [68] showed that fungal decomposers obtained from both salinized and undisturbed sites largely maintained their function at experimental high salinity concentrations, a previous study [39] revealed lower leaf decomposition rates for microorganism communities from a salinized reach compared with those from an undisturbed site, which may hint towards a salinity-driven selection of less efficient bacterial decomposers. Despite the rather consistent reduction in decomposition with increased salinity, many other factors, such as nutrient availability, litter type and temperature, affect decomposition rates [46,65]. These may explain why the highest decomposition rate was observed at the most saline site in a study comparing different Mediterranean stream types [42].
Leaf litter is considered the key carbon source sustaining the biomass of higher trophic levels in temperate forest streams, although the importance of algae is disputed [69], and is therefore connected to ecosystem services such as fish production. Owing to generally more sparse vegetation, and consequently less litter input in arid than in temperate regions [70], less shading and the intense photosynthetically active radiation in drylands, it is almost universally agreed that autotrophic organisms such as algae are the dominant carbon source for animal production in these rivers [47,69,71,72], but see [50]. If other carbon sources can sustain biomass production, reduced decomposition will have little direct impact on secondary production. However, litter could accumulate, leading to habitat modifications and increased downstream transport of organic matter, with indirect negative effects on production as well as other processes [45,47]. Moreover, litter decomposition may be considered as proxy for organic matter processing, a reduction of which may indicate slower carbon turnover and production. Reduced decomposition could also lead to lower CO2 emissions and to carbon sequestration, which may be considered a positive effect for the ecosystem service of climate regulation.
(c). Metabolism and biomass production
Whole river ecosystem metabolism refers to ‘the production and destruction of organic matter, and the associated fluxes of nutrients, through the gross photosynthetic and respiratory activity of organisms' [73, p. S101]. It is estimated from the variation in oxygen production and consumption using simple [73,74] or increasingly sophisticated models [75].
Key factors influencing metabolism are light, which is controlled by latitude, season, shading and turbidity, as well as temperature, disturbances and nutrient availability. Disturbances such as high floods and desiccation are thought to control large parts of the variability in stream metabolism by removing photosynthetically active stream bed biota and habitat available for production, respectively [73,76,77], which may apply particularly to the highly dynamic dryland IRES. However, overall our understanding of factors controlling river metabolism across regions and seasons is still limited compared with lake and forest metabolism and is based on few studies only [73]. Some desert rivers have among the highest rates of primary and secondary production that have been recorded in lotic systems [71,78]. Since they are naturally more saline than temperate rivers, there may not be a universally negative association between salinity and metabolism. For example, biomass depletion and a reduction in the production to respiration ratio to values below 1 were observed for a saline stream chronically disturbed by freshwater input compared with an undisturbed saline stream [47]. Additional short-term dilution stress at both sites induced, however, different responses, with biofilm mass decreasing at the saline reference, but increasing at the chronically disturbed site. This study highlights the complexity and context-dependency of responses as well as the need to differentiate between short-term and chronic disturbances that can have very different impacts [47]. Unfortunately, we found no field study that investigated stream metabolism in relation to increasing salinity from a semiarid or arid region. From humid regions, no response [41] and an initial increase in metabolism followed by a reduction at higher salt concentrations were observed [48], whereas a mesocosm study with similar salinity concentration ranges reported a negative correlation [53] (see tables 1 and 2 for concentrations). Measures of biomass production are often restricted to specific components. Here, biomass from biofilms increased rather consistently, also in different environments [79,80] with some exceptions [43,51], and may be attributed to high salt tolerance of cyanobacteria and diatoms [47]. Total primary and secondary biomass production are, however, poorly investigated.
Overall, dryland rivers are more likely to be net carbon exporters fuelling adjacent low productivity terrestrial dryland ecosystems than to rely on the subsidies of energy and nutrients [71,73]. For example, riparian spiders from Sycamore Creek, USA obtained 68–100% of their carbon intake from in-stream sources [81]. Thus, although generalizable relations between salinity and metabolism are lacking, changes in river metabolism and primary production may strongly impact terrestrial biota such as birds, which are often highly valued by society, constituting an ecosystem service.
(d). Nutrient uptake and retention
Human activities—mainly fertilizer production—convert around 120 million tonnes of N2 into bioavailable nitrogen species, globally, which is considered to exceed ‘the safe operating space for humanity’ [82, p. 472]. Therefore, the capacity of rivers to assimilate NO3− into biomass and to remove N via denitrification (transformation of NO3− to gaseous N2) is generally considered an ecosystem service per se. It is strongly associated with the self-purification capacity of water and it prevents downstream nutrient transport and associated eutrophication of coastal zones [33]. Thus, although nutrient cycling refers to all nutrients, the main interest lies in the limiting nutrients nitrogen and phosphorus. However, only two studies from the same authors investigated denitrification or whole ecosystem NO3− uptake through nutrient addition experiments in relation to salinity gradients in streams (table 1). Salinity explained 5% of the variability in denitrification rates in one study [56]; however, no association between denitrification or NO3− uptake and salinity was found in a follow-up study [55]. It was concluded that salinity does apparently not constrain these processes. By contrast, wetland salinization is generally associated with decreasing nitrogen removal [20]. Thus, further research is required, including improvement of concept and methods for application in nutrient saturated streams, where added nutrients cannot be taken up and uptake rates can hardly be quantified [83,84]. Moreover, current studies strongly focus on the bottom-up regulation of nutrient cycling. However, it may also be worth targeting the effect of altered top-down regulation in future. Charismatic megafauna are most prone to extinction, because of their large size, sparseness and rarity, late maturity and low fecundity, as well as high market value [85]. Their loss may substantially alter ecosystem processes, which is currently hardly considered, but may serve as strong impetus for conservation action [86].
(e). Associations with ecosystem services
Ecosystem services represent the benefits humans/society receive from ecosystems and their processes and functions; therefore, a link between ecosystem processes and the human sphere must be established and must be demonstrated [87,88] and, ideally, it should be quantified how and to what extent the well-being of humans is influenced by these functions. Research in this field has thrived in recent years as the need to develop sound methods to identify, quantify and value ecosystem services has become apparent and such information has been demanded more and more by policy makers [89]. Currently, the identification, quantification and valuation of ecosystem services of rivers and river catchments, in general, is an on-going research process that is still in its infancy [31]. To achieve these objectives, sound and comprehensive knowledge about water-related ecosystem processes and functions and their interconnectedness with human activities is required and should be coupled in system-like models. Such integrating endeavours are, for example, the research and modelling frameworks InVEST and ARIES [90]. While these are large projects or even well-established scientific institutions with significant personnel, the respective models still have gaps and the valuation of services is still rather coarse. We see that for the specific context of intermittent streams, dryland areas and especially the problem of freshwater salinization, the existing approaches are by far not suited to adequately capture the challenges laid out above. Therefore, as a first step, we approach this lack of a coherent knowledge and modelling framework by identifying those specific ecosystem services under focus in dryland areas and their intermittent streams and laying out the specific challenges for future research. Following [91], we suggest six most important ecosystem services of dryland rivers, of which four belong to provisioning services (crop irrigation, drinking water for livestock, drinking water for humans and fishing), one to regulating services (controlling desertification) and one to cultural services (supporting vegetation for cultural identity with the landscape). There may be more specific types of ecosystem services to be identified for specific kinds of landscapes (e.g. the dry phase of intermittent rivers, [92]) and areas depending on the settlement and economic structure of those areas.
Focusing on the effects of in-channel salinization in dryland rivers, it is clear that an assessment of the associated changes in ecosystem services requires understanding and quantitative assessment of the effects on the adjacent natural or managed systems because ecosystem services materialize mostly not only in the streams themselves, but also in those adjacent systems in connection to human use, for example, reduced crop growth related to irrigation with salinized water [93] or stream energy transfer to birds. In this context, desert rivers may represent a model system for investigating aquatic–terrestrial linkages, because of their rather well-defined boundary between river-associated oasis and desert landscapes. We, therefore, argue that the assessment of ecosystem service changes due to salinization of intermittent streams in dryland areas is still in its infancy and requires (i) deeper and broader understanding of the ecosystem processes in the rivers and especially their links to adjacent systems (see [94]), (ii) integrative modelling of processes in connection to human use of those systems and (iii) valuation approaches adapted to diverse and heterogeneous socio-economic contexts.
(f). Conclusions and outlook
We have reviewed salinity-driven changes in ecosystem processes of rivers, with special interest in dryland intermittent rivers and ephemeral streams (IRES) and discussed potential links to ecosystem service delivery. Most uncertainties remain for the effects on the processes of metabolism and nutrient cycling, where studies are scarce, but may be particularly important for ecosystem services of dryland rivers. More information regarding natural background variability is also needed and increasingly affordable sensors for continuous monitoring of, for example, dissolved oxygen may revolutionize our understanding of river ecosystem energetics in future [73]. Moreover, a recent review [33] on various methods for the quantification of different river ecosystem processes may aid in the design of further salinity impact studies. The establishment of close links between salinization and ecosystem service delivery mediated through changes in ecosystem processes is currently a major research gap. To address this gap it will be necessary to transcend the natural sciences and to integrate social sciences in inter- and transdisciplinary research processes, where rivers are conceptualized and modelled as socio-ecological systems [95,96].
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
The authors thank the German Ministry of Education and Research (BMBF) for project funding (SALIDRAA-01DH17002).
References
- 1.Kingsford RT, Thompson JR. 2006. Desert or dryland rivers of the word: an introduction. In Ecology of desert rivers (ed. Kingsford R.), pp. 3–10. New York, NY: Cambridge University Press. [Google Scholar]
- 2.Maestre FT, Salguero-Gómez R, Quero JL. 2012. It is getting hotter in here: determining and projecting the impacts of global environmental change on drylands. Phil. Trans. R Soc. B 367, 3062–3075. ( 10.1098/rstb.2011.0323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cherlet M, Hutchinson C, Reynolds J, Hill J, Sommer S, Maltitz G. 2018. World atlas of desertification. Luxembourg: Publication Office of the European Union. [Google Scholar]
- 4.Datry T, Bonada N, Boulton A. 2017. General introduction. In Intermittent rivers and ephemeral streams (eds Datry T, Bonada N, Boulton A), ch. 1. San Diego, CA: Academic Press. [Google Scholar]
- 5.Döll P, Schmied HM. 2012. How is the impact of climate change on river flow regimes related to the impact on mean annual runoff? A global-scale analysis. Environ. Res. Lett. 7, 014037 ( 10.1088/1748-9326/7/1/014037) [DOI] [Google Scholar]
- 6.Acuña V, Hunter M, Ruhí A. 2017. Managing temporary streams and rivers as unique rather than second-class ecosystems. Biol. Conserv. 211, 12–19. ( 10.1016/j.biocon.2016.12.025) [DOI] [Google Scholar]
- 7.Datry T, Larned ST, Tockner K. 2014. Intermittent rivers: a challenge for freshwater ecology. BioScience 64, 229–235. ( 10.1093/biosci/bit027) [DOI] [Google Scholar]
- 8.Malmqvist B, Rundle S. 2002. Threats to the running water ecosystems of the world. Environ. Conserv. 29, 134–153. ( 10.1017/S0376892902000097) [DOI] [Google Scholar]
- 9.Nel JL, Roux DJ, Maree G, Kleynhans CJ, Moolman J, Reyers B, Rouget M, Cowling RM. 2007. Rivers in peril inside and outside protected areas: a systematic approach to conservation assessment of river ecosystems. Divers. Distrib. 13, 341–352. ( 10.1111/j.1472-4642.2007.00308.x) [DOI] [Google Scholar]
- 10.Vörösmarty CJ, et al. 2010. Global threats to human water security and river biodiversity. Nature 467, 555–561. ( 10.1038/nature09440) [DOI] [PubMed] [Google Scholar]
- 11.Bailey PCE, Boon PI, Blinn DW, Williams WD. 2006. Salinisation as an ecological perturbation to rivers, streams and wetlands of arid and semi-arid regions. In Ecology of desert rivers (ed. Kingsford R.), pp. 280–314. New York, NY: Cambridge University Press. [Google Scholar]
- 12.Cañedo-Argüelles M, Kefford BJ, Piscart C, Prat N, Schäfer RB, Schulz C-J. 2013. Salinisation of rivers: an urgent ecological issue. Environ. Pollut. 173, 157–167. ( 10.1016/j.envpol.2012.10.011) [DOI] [PubMed] [Google Scholar]
- 13.Smedema LK, Shiati K. 2002. Irrigation and salinity: a perspective review of the salinity hazards of irrigation development in the arid zone. Irrig. Drain. Syst. 16, 161–174. ( 10.1023/A:1016008417327) [DOI] [Google Scholar]
- 14.Silva EIL, Davies R. 1997. The effects of irrigation effluent on a western Canadian prairie river. Hydrobiologia 344, 103–109. ( 10.1023/A:1002906428167) [DOI] [Google Scholar]
- 15.Cañedo-Argüelles M, et al. 2016. Saving freshwater from salts. Science 351, 914–916. ( 10.1126/science.aad3488) [DOI] [PubMed] [Google Scholar]
- 16.Birk S, et al. 2012. Three hundred ways to assess Europe's surface waters: an almost complete overview of biological methods to implement the Water Framework Directive. Ecol. Indic. 18, 31–41. ( 10.1016/j.ecolind.2011.10.009) [DOI] [Google Scholar]
- 17.Hart BT, Bailey P, Edwards R, Hortle K, James K, McMahon A, Meredith C, Swadling K. 1991. A review of the salt sensitivity of the Australian freshwater biota. Hydrobiologia 210, 105–144. ( 10.1007/BF00014327) [DOI] [Google Scholar]
- 18.Nielsen DL, Brock MA, Rees GN, Baldwin DS. 2003. Effects of increasing salinity on freshwater ecosystems in Australia. Aust. J. Bot. 51, 655–665. ( 10.1071/bt02115) [DOI] [Google Scholar]
- 19.Castillo AM, Sharpe DMT, Ghalambor CK, León LFD. 2018. Exploring the effects of salinization on trophic diversity in freshwater ecosystems: a quantitative review. Hydrobiologia 807, 1–17. ( 10.1007/s10750-017-3403-0) [DOI] [Google Scholar]
- 20.Herbert ER, Boon P, Burgin AJ, Neubauer SC, Franklin RB, Ardón M, Hopfensperger KN, Lamers LPM, Gell P. 2015. A global perspective on wetland salinization: ecological consequences of a growing threat to freshwater wetlands. Ecosphere 6, art206 ( 10.1890/ES14-00534.1) [DOI] [Google Scholar]
- 21.Hills KA, Hyne RV, Kefford BJ. 2019. Species of freshwater invertebrates that are sensitive to one saline water are mostly sensitive to another saline water but an exception exists. Phil. Trans. R. Soc. B 374, 20180003 ( 10.1098/rstb.2018.0003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venâncio C, Castro BB, Ribeiro R, Antunes SC, Abrantes N, Soares AMVM, Lopes I. 2019. Sensitivity of freshwater species under single and multigenerational exposure to seawater intrusion. Phil. Trans. R. Soc. B 374, 20180252 ( 10.1098/rstb.2018.0252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hintz WD, Mattes BM, Schuler MS, Jones DK, Stoler AB, Lind L, Relyea RA. 2017. Salinization triggers a trophic cascade in experimental freshwater communities with varying food-chain length. Ecol. Appl. Publ. Ecol. Soc. Am. 27, 833–844. ( 10.1002/eap.1487) [DOI] [PubMed] [Google Scholar]
- 24.Hintz WD, Relyea RA. 2017. A salty landscape of fear: responses of fish and zooplankton to freshwater salinization and predatory stress. Oecologia 185, 147–156. ( 10.1007/s00442-017-3925-1) [DOI] [PubMed] [Google Scholar]
- 25.Beermann AJ, Elbrecht V, Karnatz S, Ma L, Matthaei CD, Piggott JJ, Leese F. 2018. Multiple-stressor effects on stream macroinvertebrate communities: a mesocosm experiment manipulating salinity, fine sediment and flow velocity. Sci. Total Environ. 610–611, 961–971. ( 10.1016/j.scitotenv.2017.08.084) [DOI] [PubMed] [Google Scholar]
- 26.Velasco J, Gutiérrez-Cánovas C, Botella-Cruz M, Sánchez-Fernández D, Arribas P, Carbonell JA, Millán A, Pallarés S. 2019. Effects of salinity changes on aquatic organisms in a multiple stressor context. Phil. Trans. R. Soc. B 374, 20180011 ( 10.1098/rstb.2018.0011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costanza R, de Groot R, Sutton P, van der Ploeg S, Anderson SJ, Kubiszewski I, Farber S, Turner RK. 2014. Changes in the global value of ecosystem services. Glob. Environ. Change 26, 152–158. ( 10.1016/j.gloenvcha.2014.04.002) [DOI] [Google Scholar]
- 28.Costanza R, et al. 1997. The value of the world's ecosystem services and natural capital. Nature 387, 253–260. ( 10.1038/387253a0) [DOI] [Google Scholar]
- 29.Mace GM. 2014. Whose conservation? Science 345, 1558–1560. ( 10.1126/science.1254704) [DOI] [PubMed] [Google Scholar]
- 30.Duncan C, Thompson JR, Pettorelli N. 2015. The quest for a mechanistic understanding of biodiversity–ecosystem services relationships. Proc. R. Soc. B 282, 20151348 ( 10.1098/rspb.2015.1348) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grizzetti B, Lanzanova D, Liquete C, Reynaud A, Cardoso AC. 2016. Assessing water ecosystem services for water resource management. Environ. Sci. Policy 61, 194–203. ( 10.1016/j.envsci.2016.04.008) [DOI] [Google Scholar]
- 32.O'Higgins TG. 2017. You can't eat biodiversity: agency and irrational norms in European aquatic environmental law. Chall. Sustain. 5, 43–51. ( 10.12924/cis2017.05010043) [DOI] [Google Scholar]
- 33.von Schiller D, et al. 2017. River ecosystem processes: a synthesis of approaches, criteria of use and sensitivity to environmental stressors. Sci. Total Environ. 596, 465–480. ( 10.1016/j.scitotenv.2017.04.081) [DOI] [PubMed] [Google Scholar]
- 34.Woodward G. 2009. Biodiversity, ecosystem functioning and food webs in fresh waters: assembling the jigsaw puzzle. Freshw. Biol. 54, 2171–2187. ( 10.1111/j.1365-2427.2008.02081.x) [DOI] [Google Scholar]
- 35.Jax K. 2005. Function and ‘functioning’ in ecology: what does it mean? Oikos 111, 641–648. ( 10.1111/j.1600-0706.2005.13851.x) [DOI] [Google Scholar]
- 36.Pettorelli N, et al. 2017. Satellite remote sensing of ecosystem functions: opportunities, challenges and way forward. Remote Sens. Ecol. Conserv. 4, 71–93. ( 10.1002/rse2.59) [DOI] [Google Scholar]
- 37.Tilman D, Isbell F, Cowles JM. 2014. Biodiversity and ecosystem functioning. Annu. Rev. Ecol. Evol. Syst. 45, 471–493. ( 10.1146/annurev-ecolsys-120213-091917) [DOI] [Google Scholar]
- 38.Duan S, Kaushal SS. 2015. Salinization alters fluxes of bioreactive elements from stream ecosystems across land use. Biogeosciences 12, 7331–7347. ( 10.5194/bg-12-7331-2015) [DOI] [Google Scholar]
- 39.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/. [Google Scholar]
- 40.Sauer FG, Bundschuh M, Zubrod JP, Schäfer RB, Thompson K, Kefford BJ. 2016. Effects of salinity on leaf breakdown: dryland salinity versus salinity from a coalmine. Aquat. Toxicol. 177, 425–432. ( 10.1016/j.aquatox.2016.06.014) [DOI] [PubMed] [Google Scholar]
- 41.Schäfer RB, et al. 2012. Effects of pesticide toxicity, salinity and other environmental variables on selected ecosystem functions in streams and the relevance for ecosystem services. Sci. Total Environ. 415, 69–78. ( 10.1016/j.scitotenv.2011.05.063) [DOI] [PubMed] [Google Scholar]
- 42.Casas JJ, Gessner MO, López D, Descals E. 2011. Leaf-litter colonisation and breakdown in relation to stream typology: insights from Mediterranean low-order streams. Freshw. Biol. 56, 2594–2608. ( 10.1111/j.1365-2427.2011.02686.x) [DOI] [Google Scholar]
- 43.Cañedo-Argüelles M, Bundschuh M, Gutiérrez-Cánovas C, Kefford BJ, Prat N, Trobajo R, Schäfer RB. 2014. Effects of repeated salt pulses on ecosystem structure and functions in a stream mesocosm. Sci. Total Environ. 476–477, 634–642. ( 10.1016/j.scitotenv.2013.12.067) [DOI] [PubMed] [Google Scholar]
- 44.Canhoto C, Simões S, Gonçalves AL, Guilhermino L, Bärlocher F. 2017. Stream salinization and fungal-mediated leaf decomposition: a microcosm study. Sci. Total Environ. 599–600, 1638–1645. ( 10.1016/j.scitotenv.2017.05.101) [DOI] [PubMed] [Google Scholar]
- 45.Gómez R, Asencio AD, Picón JM, Del Campo R, Arce MI, del Mar Sánchez-Montoya M, Suárez ML, Vidal-Abarca MR. 2016. The effect of water salinity on wood breakdown in semiarid Mediterranean streams. Sci. Total Environ. 541, 491–501. ( 10.1016/j.scitotenv.2015.09.040) [DOI] [PubMed] [Google Scholar]
- 46.Gutiérrez-Cánovas C, Velasco J, Millán A. 2009. Effects of dilution stress on the functioning of a saline Mediterranean stream. Hydrobiologia 619, 119–132. ( 10.1007/s10750-008-9604-9) [DOI] [Google Scholar]
- 47.Gutiérrez-Cánovas C, Hernández J, Millán A, Velasco J. 2012. Impact of chronic and pulse dilution disturbances on metabolism and trophic structure in a saline Mediterranean stream. Hydrobiologia 686, 225–239. ( 10.1007/s10750-012-1004-5) [DOI] [Google Scholar]
- 48.Silva EIL, Davies RW. 1999. The effects of simulated irrigation induced changes in salinity on metabolism of lotic biota. Hydrobiologia 416, 193–202. ( 10.1023/A:1003827807547) [DOI] [Google Scholar]
- 49.Silva EIL, Shimizu A, Matsunami H. 2000. Salt pollution in a Japanese stream and its effects on water chemistry and epilithic algal chlorophyll-a. Hydrobiologia 437, 139–148. ( 10.1023/A:1026598723329) [DOI] [Google Scholar]
- 50.East JL, Wilcut C, Pease AA. 2017. Aquatic food-web structure along a salinized dryland river. Freshw. Biol. 62, 681–694. ( 10.1111/fwb.12893) [DOI] [Google Scholar]
- 51.Cochero J, Licursi M, Gómez N. 2017. Effects of pulse and press additions of salt on biofilms of nutrient-rich streams. Sci. Total Environ. 579, 1496–1503. ( 10.1016/j.scitotenv.2016.11.152) [DOI] [PubMed] [Google Scholar]
- 52.Rotter S, Heilmeier H, Altenburger R, Schmitt-Jansen M. 2013. Multiple stressors in periphyton—comparison of observed and predicted tolerance responses to high ionic loads and herbicide exposure. J. Appl. Ecol. 50, 1459–1468. ( 10.1111/1365-2664.12146) [DOI] [Google Scholar]
- 53.Clements WH, Kotalik C. 2016. Effects of major ions on natural benthic communities: an experimental assessment of the US Environmental Protection Agency aquatic life benchmark for conductivity. Freshw. Sci. 35, 126–138. ( 10.1086/685085) [DOI] [Google Scholar]
- 54.Ros MD, Marín-Murcia JP, Aboal M. 2009. Biodiversity of diatom assemblages in a Mediterranean semiarid stream: implications for conservation. Mar. Freshw. Res. 60, 14–24. ( 10.1071/MF07231) [DOI] [Google Scholar]
- 55.Arce MI, von Schiller D, Gómez R. 2014. Variation in nitrate uptake and denitrification rates across a salinity gradient in Mediterranean semiarid streams. Aquat. Sci. 176, 295–311. ( 10.1007/s00027-014-0336-9) [DOI] [Google Scholar]
- 56.Arce MI, Gómez R, Suárez ML, Vidal-Abarca MR. 2013. Denitrification rates and controlling factors in two agriculturally influenced temporary Mediterranean saline streams. Hydrobiologia 700, 169–185. ( 10.1007/s10750-012-1228-4) [DOI] [Google Scholar]
- 57.Adamek E, Baran W, Sobczak A. 2016. Assessment of the biodegradability of selected sulfa drugs in two polluted rivers in Poland: effects of seasonal variations, accidental contamination, turbidity and salinity. J. Hazard. Mater. 313, 147–158. ( 10.1016/j.jhazmat.2016.03.064) [DOI] [PubMed] [Google Scholar]
- 58.Chevan A, Sutherland M. 1991. Hierarchical partitioning. Am. Statist. 45, 90–96. [Google Scholar]
- 59.Schiller DV, Bernal S, Dahm CN, Marti E. 2017. Nutrient and organic matter dynamics in intermittent rivers and ephemeral streams. In Intermittent rivers and ephemeral streams (eds Datry T, Bonada N, Boulton A), pp. 135–160. San Diego, CA: Academic Press. [Google Scholar]
- 60.Romani AM, Chauvet E, Febria C, Mora-Gómez J, Risse-Buhl U, Timoner X, Weitere M, Zeglin L. 2017. The biota of intermittent rivers and ephemeral streams: prokaryotes, fungi, and protozoans. In Intermittent rivers and ephemeral streams (eds Datry T, Bonada N, Boulton A), pp. 161–188. San Diego, CA: Academic Press. [Google Scholar]
- 61.Thoms MC, Beyer PJ, Rogers KH. 2006. Variability, complexity and diversity: the geomorphology of river ecosystems in dryland regions. In Ecology of desert rivers (ed. Kingsford R.), pp. 47–75. New York, NY: Cambridge University Press. [Google Scholar]
- 62.Swan CM, DePalma CA. 2012. Elevated chloride and consumer presence independently influence processing of stream detritus. Urban Ecosyst. 15, 625–635. ( 10.1007/s11252-011-0210-7) [DOI] [Google Scholar]
- 63.Van Meter RJ, Swan CM, Trossen CA. 2012. Effects of road deicer (NaCl) and amphibian grazers on detritus processing in pond mesocosms. Environ. Toxicol. Chem. 31, 2306–2310. ( 10.1002/etc.1949) [DOI] [PubMed] [Google Scholar]
- 64.Tyree M, Clay N, Polaskey S, Entrekin S. 2016. Salt in our streams: even small sodium additions can have negative effects on detritivores. Hydrobiologia 775, 109–122. ( 10.1007/s10750-016-2718-6) [DOI] [Google Scholar]
- 65.Stoler AB, Hintz WD, Jones DK, Lind L, Mattes BM, Schuler MS, Relyea RA. 2017. Leaf litter mediates the negative effect of road salt on forested wetland communities. Freshw. Sci. 36, 415–426. ( 10.1086/692139) [DOI] [Google Scholar]
- 66.Roache MC, Bailey PC, Boon PI. 2006. Effects of salinity on the decay of the freshwater macrophyte, Triglochin procerum. Aquat. Bot. 84, 45–52. ( 10.1016/j.aquabot.2005.07.014) [DOI] [Google Scholar]
- 67.Bogart SJ, Azizishirazi A, Pyle GG. 2019. Challenges and future prospects for developing Ca and Mg water quality guidelines: a meta-analysis. Phil. Trans. R. Soc. B 374, 20180364 ( 10.1098/rstb.2018.0364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gonçalves AL, Carvalho A, Bärlocher F, Canhoto C. 2019. Are fungal strains from salinized streams adapted to salt-rich conditions? Phil. Trans. R. Soc. B 374, 20180018 ( 10.1098/rstb.2018.0018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brett MT, et al. 2017. How important are terrestrial organic carbon inputs for secondary production in freshwater ecosystems? Freshw. Biol. 62, 833–853. ( 10.1111/fwb.12909) [DOI] [Google Scholar]
- 70.Datry T, et al. 2018. A global analysis of terrestrial plant litter dynamics in non-perennial waterways. Nat. Geosci. 11, 497–503. ( 10.1038/s41561-018-0134-4) [DOI] [Google Scholar]
- 71.Bunn SE, Balcombe SR, Davies PM, Fellows CS, Mckenzie-smith FJ. 2006. Aquatic productivity and food webs of desert river ecosystems. In Ecology of desert rivers (ed. Kingsford R.), pp. 76–99. New York, NY: Cambridge University Press. [Google Scholar]
- 72.Fellows CS, Bunn SE, Sheldon F, Beard NJ. 2009. Benthic metabolism in two turbid dryland rivers. Freshw. Biol. 54, 236–253. ( 10.1111/j.1365-2427.2008.02104.x) [DOI] [Google Scholar]
- 73.Bernhardt ES, et al. 2018. The metabolic regimes of flowing waters. Limnol. Oceanogr. 63, S99–S118. ( 10.1002/lno.10726) [DOI] [Google Scholar]
- 74.Odum HT. 1965. Primary production in flowing waters. Limnol. Oceanogr. 1, 102–117. ( 10.4319/lo.1956.1.2.0102) [DOI] [Google Scholar]
- 75.Demars BOL, Thompson J, Manson JR. 2015. Stream metabolism and the open diel oxygen method: principles, practice, and perspectives. Limnol. Oceanogr. Methods 13, 356–374. ( 10.1002/lom3.10030) [DOI] [Google Scholar]
- 76.Acuña V, Muñoz I, Giorgi A, Omella M, Sabater F, Sabater S. 2005. Drought and postdrought recovery cycles in an intermittent Mediterranean stream: structural and functional aspects. J. North Am. Benthol. Soc. 24, 919–933. ( 10.1899/04-078.1) [DOI] [Google Scholar]
- 77.Beaulieu JJ, Arango CP, Balz DA, Shuster WD. 2013. Continuous monitoring reveals multiple controls on ecosystem metabolism in a suburban stream. Freshw. Biol. 58, 918–937. ( 10.1111/fwb.12097) [DOI] [Google Scholar]
- 78.Grimm NB. 1987. Nitrogen dynamics during succession in a desert stream. Ecology 68, 1157–1170. ( 10.2307/1939200) [DOI] [Google Scholar]
- 79.Cant B, Nally RM, Thomson JR, Beardall J. 2010. Relative effects of local and landscape factors on wetland algal biomass over a salinity gradient. Aquat. Sci. 72, 191–202. ( 10.1007/s00027-009-0119-x) [DOI] [Google Scholar]
- 80.Asencio AD. 2013. Permanent salt evaporation ponds in a semi-arid Mediterranean region as model systems to study primary production processes under hypersaline conditions. Estuar. Coast Shelf Sci. 124, 24–33. ( 10.1016/j.ecss.2013.03.006) [DOI] [Google Scholar]
- 81.Sanzone DM, Meyer JL, Marti E, Gardiner EP, Tank JL, Grimm NB. 2003. Carbon and nitrogen transfer from a desert stream to riparian predators. Oecologia 134, 238–250. ( 10.1007/s00442-002-1113-3) [DOI] [PubMed] [Google Scholar]
- 82.Rockström J, et al. 2009. A safe operating space for humanity. Nature 461, 472–475. ( 10.1038/461472a) [DOI] [PubMed] [Google Scholar]
- 83.Covino TP, Bernhardt ES, Heffernan JB. 2018. Measuring and interpreting relationships between nutrient supply, demand, and limitation. Freshw. Sci. 37, 448–455. ( 10.1086/699202) [DOI] [Google Scholar]
- 84.Mulholland PJ, et al. 2008. Stream denitrification across biomes and its response to anthropogenic nitrate loading. Nature 452, 202–205. ( 10.1038/nature06686) [DOI] [PubMed] [Google Scholar]
- 85.He F, Zarfl C, Bremerich V, Henshaw A, Darwall W, Tockner K, Jähnig SC. 2017. Disappearing giants: a review of threats to freshwater megafauna. WIREs Water 4, e1208 ( 10.1002/wat2.1208) [DOI] [Google Scholar]
- 86.Lindsay MK, Zhang Y, Forstner MRJ, Hahn D. 2013. Effects of the freshwater turtle Trachemys scripta elegans on ecosystem functioning: an approach in experimental ponds. Amphib. Reptil. 34, 75–84. ( 10.1163/15685381-00002871) [DOI] [Google Scholar]
- 87.Jardine A, Corkeron M, Weinstein P. 2011. Dryland salinity and vector-borne disease emergence in southwestern Australia. Environ. Geochem. Health 33, 363–370. ( 10.1007/s10653-011-9387-1) [DOI] [PubMed] [Google Scholar]
- 88.Petranka JW, Doyle EJ. 2010. Effects of road salts on the composition of seasonal pond communities: can the use of road salts enhance mosquito recruitment? Aquat. Ecol. 44, 155–166. ( 10.1007/s10452-009-9286-z) [DOI] [Google Scholar]
- 89.Daily GC, Polasky S, Goldstein J, Kareiva PM, Mooney HA, Pejchar L, Ricketts TH, Salzman J, Shallenberger R. 2009. Ecosystem services in decision making: time to deliver. Front. Ecol. Environ. 7, 21–28. ( 10.1890/080025) [DOI] [Google Scholar]
- 90.Bagstad KJ, Semmens DJ, Waage S, Winthrop R. 2013. A comparative assessment of decision-support tools for ecosystem services quantification and valuation. Ecosyst. Serv. 5, 27–39. ( 10.1016/j.ecoser.2013.07.004) [DOI] [Google Scholar]
- 91.Dudley N, MacKinnon K, Stolton S. 2014. The role of protected areas in supplying ten critical ecosystem services in drylands: a review. Biodiversity 15, 178–184. ( 10.1080/14888386.2014.928790) [DOI] [Google Scholar]
- 92.Datry T, Boulton AJ, Bonada N, Fritz K, Leigh C, Sauquet E et al. 2017. Flow intermittence and ecosystem services in rivers of the Anthropocene. J. Appl. Ecol. 55, 353–364. ( 10.1111/1365-2664.12941) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.de Lange WJ, Mahumani BK, Steyn M, Oelofse SHH. 2012. Monetary valuation of salinity impacts and microbial pollution in the Olifants Water Management Area, South Africa. Water SA 38, 241–248. ( 10.4314/wsa.v38i2.9) [DOI] [Google Scholar]
- 94.Schulz R, Bundschuh M, Gergs R, Brühl CA, Diehl D, Entling MH et al. 2015. Review on environmental alterations propagating from aquatic to terrestrial ecosystems. Sci. Total Environ. 538, 246–261. ( 10.1016/j.scitotenv.2015.08.038) [DOI] [PubMed] [Google Scholar]
- 95.Dunham JB, Angermeier PL, Crausbay SD, Cravens AE, Gosnell H, McEvoy J, Moritz MA, Raheem N, Sanford T. 2018. Rivers are social–ecological systems: time to integrate human dimensions into riverscape ecology and management. Wiley Interdiscip. Rev. Water 5, e1291 ( 10.1002/wat2.1291) [DOI] [Google Scholar]
- 96.Gorostiza S, Saurí D. 2019. Naturalizing pollution: a critical social science view on the link between potash mining and salinization in the Llobregat river basin, northeast Spain. Phil. Trans. R. Soc. B 374, 20180006 ( 10.1098/rstb.2018.0006) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.