Abstract
Widespread changes in water temperatures, salinity, alkalinity and pH have been documented in inland waters in North America, which influence ion exchange, weathering rates, chemical solubility and contaminant toxicity. Increasing major ion concentrations from pollution, human-accelerated weathering and saltwater intrusion contribute to multiple ecological stressors such as changing ionic strength and pH and mobilization of chemical mixtures resulting in the freshwater salinization syndrome (FSS). Here, we explore novel combinations of elements, which are transported together as chemical mixtures containing salts, nutrients and metals as a consequence of FSS. First, we show that base cation concentrations have increased in regions primarily in North America and Europe over 100 years. Second, we show interactions between specific conductance, pH, nitrate and metals using data from greater than 20 streams located in different regions of the USA. Finally, salinization experiments and routine monitoring demonstrate mobilization of chemical mixtures of cations, metals and nutrients in 10 streams draining the Washington, DC–Baltimore, MD metropolitan regions. Freshwater salinization mobilizes diverse chemical mixtures influencing drinking water quality, infrastructure corrosion, freshwater CO2 concentrations and biodiversity. Most regulations currently target individual contaminants, but FSS requires managing mobilization of multiple chemical mixtures and interacting ecological stressors as consequences of freshwater salinization.
This article is part of the theme issue ‘Salt in freshwaters: causes, ecological consequences and future prospects’.
Keywords: non-point source water pollution, salinization, inland waters, water quality
1. Introduction
The chemistry of fresh water is rapidly changing in some regions because of human activities resulting in major changes in land use, climate, atmospheric deposition and natural resource extraction [1–3]. These regional changes are evident in the widespread increases in temperature, salinity, hardness, alkalinity and pH documented in fresh waters draining North America (e.g. [4–7]). These synchronous trends in freshwater quality are also recognized as emerging environmental issues in some other regions of the world [8–10]. Changes in concentrations and relative compositions of major ions trigger cascading ecosystem impacts on solute ionic strengths, acid–base properties and contaminant mobilization. We previously proposed these collective impacts of salinization on water quality as the freshwater salinization syndrome (FSS) [7]. A consequence of FSS is novel and variable combinations of elements and compounds, which are anthropogenically enhanced and transported together. These elements and compounds can be characterized as ‘chemical cocktails’ or chemical mixtures [11,12]. Throughout this article, we will refer to them as chemical mixtures, but we recognize these mixtures are novel because of their elevated concentrations relative to natural baseline conditions and anthropogenically enhanced transport, formation and transformation in the environment [11]. Environmental impacts of chemical mixtures associated with FSS should be considered within existing frameworks such as those for multiple stressors [13,14].
Concentrations and compositions of chemical mixtures of major ions vary across gradients of atmospheric deposition, geology, land use and water management. For example, Na+ and Cl− from road salts can mobilize nutrients ( ,
, 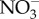 and
and  ), cations (Ca2+, Mg2+, K+) and metals (Cu, Cd, Zn, etc.) [15–18]. Chemical mixtures can become enriched in different proportions of: (i) Ca2+, Mg2+,
), cations (Ca2+, Mg2+, K+) and metals (Cu, Cd, Zn, etc.) [15–18]. Chemical mixtures can become enriched in different proportions of: (i) Ca2+, Mg2+,  and
and  from human-accelerated weathering of sedimentary and metamorphic rocks and/or impervious surfaces in urban watersheds [6,19,20]; (ii) K+ and
from human-accelerated weathering of sedimentary and metamorphic rocks and/or impervious surfaces in urban watersheds [6,19,20]; (ii) K+ and  from potash and lime in agricultural watersheds [7,21,22]; (iii) Na+, Cl−,
from potash and lime in agricultural watersheds [7,21,22]; (iii) Na+, Cl−,  and other marine salts from saltwater intrusion [23]; and (iv) multiple major ions owing to increased evaporation from global warming and freshwater losses owing to diversions and damming [24–26]. Ultimately, concentrations and compositions of different chemical mixtures of major ions impact solubility, bioavailability and toxicity of inorganic and organic contaminants and the release of CO2 and other greenhouse gases from inland waters to the atmosphere [11].
and other marine salts from saltwater intrusion [23]; and (iv) multiple major ions owing to increased evaporation from global warming and freshwater losses owing to diversions and damming [24–26]. Ultimately, concentrations and compositions of different chemical mixtures of major ions impact solubility, bioavailability and toxicity of inorganic and organic contaminants and the release of CO2 and other greenhouse gases from inland waters to the atmosphere [11].
While environmental causes and consequences of FSS are diverse (e.g. [3,27–29]), we focus primarily on the effects of chemical mixtures resulting from nonpoint sources and in situ modes of formation in the environment. Our analysis, interpretations and implications in this paper have a strong geographical bias toward North America and Europe, where nonpoint sources are important. There can be many patterns of FSS that appear in other freshwater ecosystems experiencing ion enrichment, and many can be traced back to the type of ion enrichment occurring (e.g. road salting versus increased mineral weathering versus increased evaporative loss) and the underlying biogeochemistry of the system (i.e. FSS is a polymorphic syndrome). For example, work in Australia has focused on salinity with the ionic proportions found in seawater, as these proportions are common in salinization as a result of dryland salinity [30]. While field studies that associate biota to total salinity (expressed as electrical conductivity) do encompass the interactive effects of complex mixtures, the chemical concentrations, compositions and consequences of mixtures are not always characterized or understood (but see [30,31]). In North America and Europe, road salt, acid rain, mine drainage, rising temperatures and widespread exploitation of mineral resources (gravel, limestone, halite, gypsum, potash, etc.) can enhance weathering rates and ion exchange. As a consequence, concentrations and compositions of different ion mixtures in fresh waters are altered such as Ca2+, Mg2+, K+,  , acetate and
, acetate and  (sensu [4,7,18,20,32–36]).
(sensu [4,7,18,20,32–36]).
Interactions between FSS and eutrophication, acid–base status, organic contaminants and metals toxicity are poorly understood and represent a research frontier in ecosystem science. Sodium mobilizes hydrogen ions and induces temporary episodic acidification or long-term alkalinization as hydrogen ions are depleted from exchange sites [37]. In addition, there can be either net alkalinization or acidification of fresh waters based on the substrates available for chemical weathering and potential for neutralization reactions along hydrologic flowpaths [6,20,22,35,38]. Changes in pH based on ionic compositions influence the solubility of dissolved organic carbon, organic contaminants and metals (e.g. Cu, Pb, Cd, Zn) [13,39]. Metal solubility can be enhanced owing to increased complexation with dissolved Cl−,  , OH− and/or
, OH− and/or  (e.g. [16]). Organic matter solubility is altered based on molecular size and polarity and proteins 'salt in' (become more soluble in water) based on base cation concentrations in the following order: Ca2+ > Mg2+ > K+ > Na+ and anions in the following order: Cl− >
(e.g. [16]). Organic matter solubility is altered based on molecular size and polarity and proteins 'salt in' (become more soluble in water) based on base cation concentrations in the following order: Ca2+ > Mg2+ > K+ > Na+ and anions in the following order: Cl− >  >
>  [39,40]. Alkaline conditions favour the release of phosphorus from oxyhydroxides in sediments and soils which contribute to freshwater eutrophication [17,18]. Microbial nitrification and denitrification are both stimulated at slightly alkaline pH in soils and sediments affected by road salts, which contributes to either nitrogen transformations and/or coastal eutrophication (e.g. [40]). Alkalinity also results from microbial reduction reactions (e.g. in anaerobic sediments) and shifts source versus sink dynamics of CO2 and solubility of greenhouse gases [41]. Clearly, diagnosing water quality symptoms of FSS requires moving beyond focusing on single contaminants, while considering mobilization of toxic metals, shifts in quantity and quality of organic matter, liberation of CO2 and other gases and release of N and P buried in sediments.
[39,40]. Alkaline conditions favour the release of phosphorus from oxyhydroxides in sediments and soils which contribute to freshwater eutrophication [17,18]. Microbial nitrification and denitrification are both stimulated at slightly alkaline pH in soils and sediments affected by road salts, which contributes to either nitrogen transformations and/or coastal eutrophication (e.g. [40]). Alkalinity also results from microbial reduction reactions (e.g. in anaerobic sediments) and shifts source versus sink dynamics of CO2 and solubility of greenhouse gases [41]. Clearly, diagnosing water quality symptoms of FSS requires moving beyond focusing on single contaminants, while considering mobilization of toxic metals, shifts in quantity and quality of organic matter, liberation of CO2 and other gases and release of N and P buried in sediments.
In this paper, we show how distinct chemical mixtures can form and vary in response to FSS across varying spatial and temporal scales. First, we document increases in formation, concentration and variable composition of base cation mixtures in fresh waters primarily in North America and Europe over timeframes as long as 100 years. Second, we investigate how major ion mixtures represented by specific conductance are linked to freshwater alkalinization and nutrient mobilization over shorter timescales using high-frequency sensor data from streams located in different regions of the USA. Finally, we show how episodic salinization is linked to the mobilization of chemical mixtures of different cations, nutrients and metals in streams across the Washington, DC and Baltimore, MD metropolitan regions using a combination of monitoring and salinization experiments in the laboratory and field. Salinization leads to a set of interactive environmental effects that significantly alter chemical mixtures in fresh waters and are tied together as a complex syndrome (FSS). Further, we expand the FSS to include additional chemical interactions and multiple stressors, which have been less considered. In some cases, we acknowledge that the causes and consequences of FSS are inseparable. We also acknowledge that our interpretations and implications may be geographically biased towards regions in North America and Europe, where we have conducted most of our research, and there can be variations in causes and consequences in FSS across other regions in Australia and Africa [42]. Ultimately, we suggest that freshwater salinization needs to be seen as a more complex process than only increases in concentrations of primary geochemical ions, however, which is critical for managing non-point source pollution of inland waters.
2. Material and methods
(a). Trends in major ions in fresh waters
We used data from previous literature studies to illustrate trends in the formation of chemical mixtures of multiple ions potentially as a consequence of FSS in well-studied freshwater systems (which were located primarily in North America and Europe with a few sites from Russia, China and elsewhere). We acknowledge that there are some significant regions of the world which are not represented at all in our illustration (e.g. the Southern Hemisphere, Africa and the tropics) or are poorly represented (e.g. Asia). We chose these examples from the literature because the patterns, processes and methods have been discussed extensively (electronic supplementary material, table S1). Although there are currently not enough examples from the literature across all the continents to make generalizations regarding FSS globally, our illustration attempts to stimulate questions regarding the spatial and temporal extent of FSS and regional variability in causes and consequences. We focus primarily on North America and Europe, which share a broadly similar climate and level of industrial/economic development in the Northern Hemisphere, but these regions may not be representative of other parts of the world. Our illustration of some well-studied trends includes the Laurentian Great Lakes in the USA, which contain approximately 20% of the world's fresh water supply. Major rivers examined from the USA include the Potomac River, which is the drinking water supply for Washington, DC. Our illustration also includes lakes and rivers across different regions in Europe and a few examples from China, Russia and Iran. Select examples include Lago Maggiore and Lago Como in Italy, which have experienced land use change, industrial pollution and ecosystem recovery over time [43]. Another notable example is the Vantaanjoki River [44], which primarily drains forests and fields and has been a drinking water source to Helsinki, Finland. Trends in base cation concentrations were estimated at all sites after digitizing and interpolating published datasets. Further details on methods, sites, data quality assurance, sample size, etc. can be found in the electronic supplementary material, table S1.
(b). High-frequency relationships between specific conductance, pH and nitrate
We analysed high-frequency sensor data from the United States (US) Geological Survey (USGS) stations in the midwestern USA and eastern USA. High-frequency sensors measure water quality variables continuously at finer temporal scales (e.g. every 15 min). USGS sites have sensors for estimating continuous nitrate, specific conductance, pH, turbidity and discharge. We performed regression analyses of all parameters with specific conductance at more than 20 sites (electronic supplementary material, table S2). These relationships between specific conductance and other parameters are not intended to represent global relationships and interpretations are restricted only to the sites and regions of the data analyses in the USA. Further details on methods, sites, data quality assurance and study periods can be found in the electronic supplementary material.
(c). Effects of freshwater salinization syndrome on mixtures of metals: monitoring, laboratory and ecosystem experiments
We addressed the following questions: (i) what is the potential release of base cations and/or metals during winter months when road salts are applied and in response to experimental salinization experiments? (ii) do the relationships between specific conductance and metals change during winter months? and (iii) how far downstream does a salt pulse travel before being attenuated? Road salt obtained from the Maryland Department of State Highways was used in stream salinization experiments. Road salt may have contained trace metals, and we compared results from manipulations in streams using road salt to experiments in the laboratory using 100% pure NaCl to isolate the effects of salt on the mobilization of metals and nutrients (detailed methods in [18]). We also monitored metals concentrations in streams during snow storms in the Baltimore, MD–Washington, DC metropolitan region and analysed metals concentrations at USGS sites. Descriptions of monitoring frequency, experimental manipulations and analytical methods can be found in the electronic supplementary material.
3. Results
(a). Trends in base cation mixtures in fresh waters
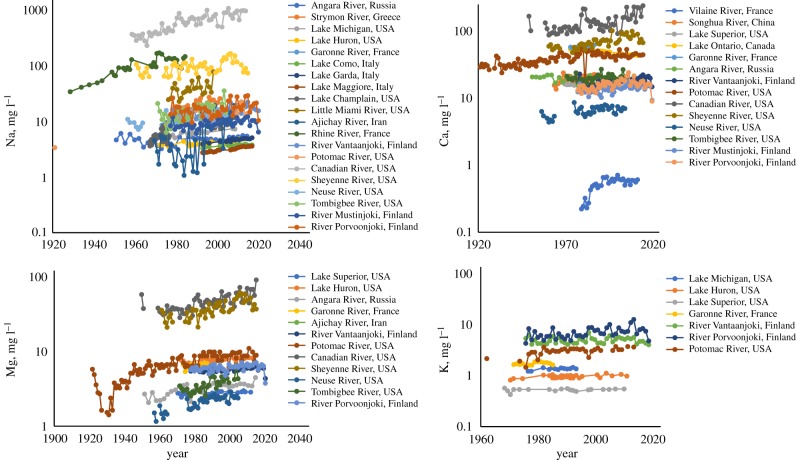
Many well-studied inland waters, primarily in North America and Europe, have shown increasing trends in dissolved salts (particularly Na+) over the past 100 years (figure 1). Statistical analyses of these trends can be found in previous literature studies pertaining to these sites (electronic supplementary material, table S1). In general, concentrations of base cations in fresh waters followed the sequence Na+ > Ca2+ > Mg2+ > K+, which is similar to the relative mobility for these ions in terms of dissolution [45]. The exception is K+, which is a limiting nutrient and in biological demand in terrestrial and aquatic ecosystems (e.g. [38,46]). Ionic enrichment from a variety of mineral sources has affected a significant fraction of the Great Lakes [47]. Also, other large water bodies such as Lago Maggiore and Lago Como have been affected by multiple salts [43]. It is important to note that we are reporting on inland waters where long-term changes and mechanisms have been discussed extensively in the literature (electronic supplementary material, table S1), but there are others that do not show a trend or show decreasing trends, such as those documented in our previous work [6,7].
Figure 1.
Trends in mean annual concentrations of base cations in rivers and lakes. Examples include rivers/lakes from USA, Canada, Europe, Russia and Iran. Citations of data sources are in the electronic supplementary material.
For example, while we highlight the many cases that are increasing, we also note that some are not. While our illustration showed increasing trends in Na+ concentrations, there have been decreasing trends in Ca2+ and Mg2+ in some cases (e.g. [47]). This change can be owing to a variety of factors such as increased calcite precipitation and decreased human-accelerated weathering of sedimentary rocks and ion exchange in response to recovery from acid rain [6,25,47]. However, these explanations may not be applicable everywhere because it is important to note that other regions have not experienced significant levels of acid rain. Similarly, our previous work and other work has shown declining trends in total dissolved solids and major ions in the southwestern USA compared to increasing trends in the eastern USA [7,24]. These differences are probably related to water diversions, damming, irrigation, wastewater treatment, urban wastewater return flows and increasing pH and mineral precipitation in rivers and reservoirs in the southwestern USA [7,24]. Ultimately, our illustration of long-term patterns is intended to provide context for FSS in well-studied freshwater ecosystems in North America and Europe and stimulate further discussion and research. For example, results suggest further research is needed on factors that might cause some sites and regions to have increased concentrations, while other sites show decreased or not significantly changed concentrations, and/or what might explain some of the variability in the rates of increase across different sites and regions.
(b). High-frequency relationships between specific conductance, pH and nutrients
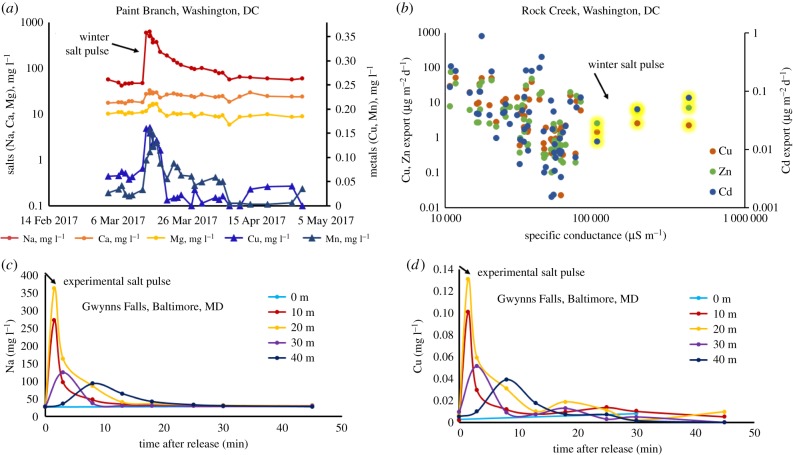
We analysed sensor data to investigate associated changes in chemical properties over short timescales. In general, there were positive relationships between specific conductance and pH and negative relationships between specific conductance and turbidity across more than 20 sites (electronic supplementary material, table S2). There were examples of significant positive relationships between nitrate concentrations and specific conductance in the eastern and midwestern USA (figure 2), but there were also examples of other more complex relationships (electronic supplementary material, figures S2–5). Concentrations of nitrate were sometimes inversely related to specific conductance in the agricultural midwestern USA (probably owing to the relative effects of different ions on specific conductance) (electronic supplementary material, table S2). In an urban stream in the Baltimore, MD–Washington, DC metropolitan area and also the nearby Potomac River (during winter months of January–March, when specific conductance is at its highest levels), there were cyclical patterns between nitrate concentrations and specific conductance during snowstorms, which suggested concurrent flushing of road salts and nitrate and/or ion exchange (figure 2 and electronic supplementary material, figure S5).
Figure 2.
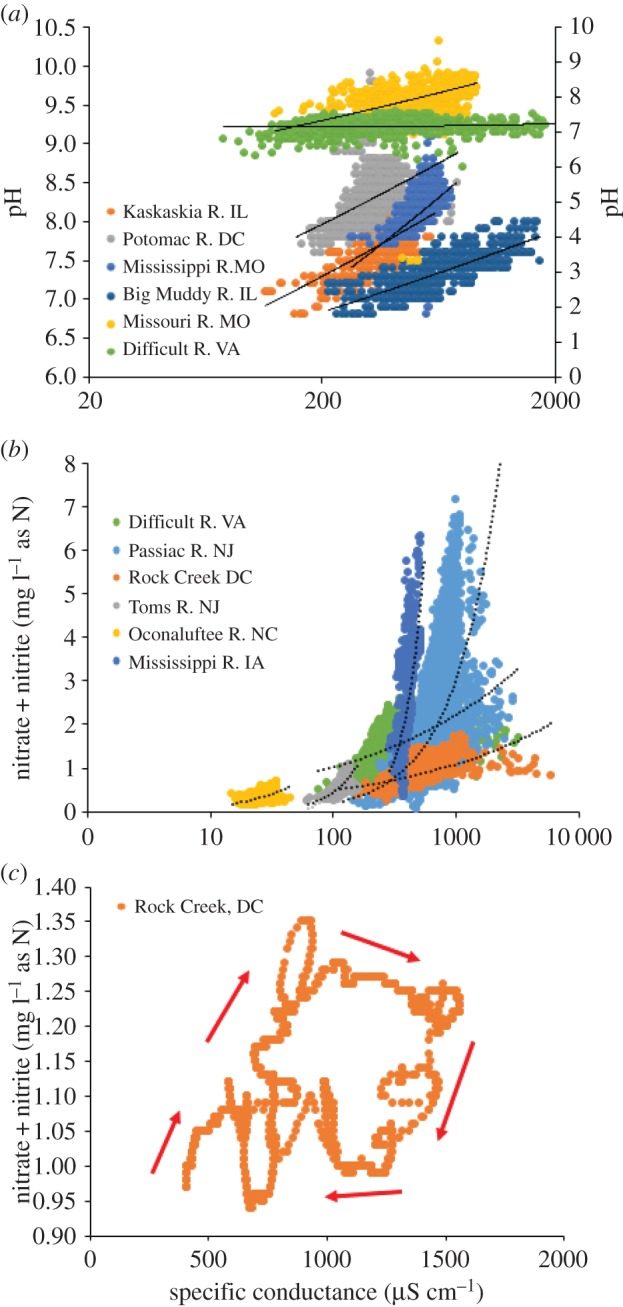
High-frequency daily sensor data illustrating examples of significant relationships over months and years between (a) specific conductance and pH for rivers in the midwestern and eastern USA (Potomac, Mississippi, Kaskaskia, Big Muddy rivers are plotted on the left axis, while Missouri River and Difficult Run are plotted on the right axis), and (b) significant positive relationships between specific conductance and nitrate for some rivers, while other rivers had more complex patterns (electronic supplementary material, figures S2–S4). In Rock Creek, Maryland (c), there was a cyclical relationship between nitrate concentrations and specific conductance (measured every 15 min) over 14 days directly before, during and after a winter snow event.
(c). Freshwater salinization syndrome mobilizes mixtures of metals: monitoring, laboratory and ecosystem experiments
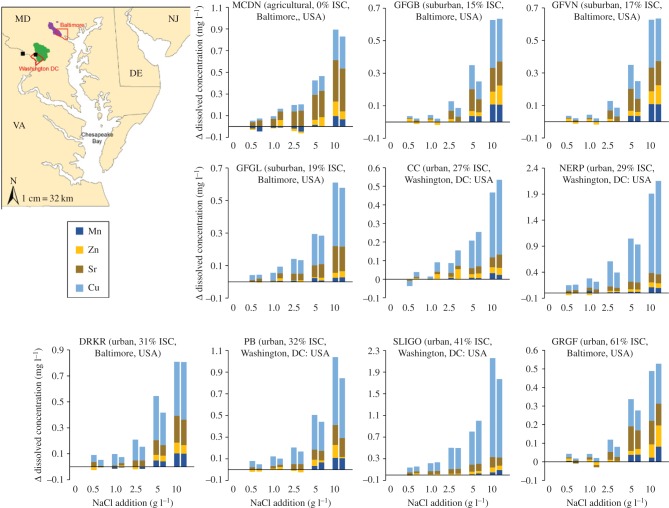
There were sharp increases in concentrations of base cations and Cu, Mn, Zn and Cd in streams during winter months in the Baltimore, MD–Washington, DC metropolitan area (figure 3a,b). The immediate runoff from the melting of snow associated with road salt applications led to these sharp peaks in concentrations. Concentrations of Cu, Mn, Na, Ca and Mg decayed slowly towards lower baseline values but rates of return varied element-by-element (figure 3a). Concentrations of Na remained elevated for weeks following just one isolated snowstorm and didn't return to pre-storm concentrations for months (figure 3a). Watershed exports of Cu, Cd and Zn (mass fluxes transported in streams per unit area of watershed) decreased as specific conductance increased until reaching a threshold value in winter, when there were the highest levels of specific conductance and an abrupt increase in watershed Cu, Cd and Zn exports (figure 3b and electronic supplementary material, figure S6). However, it may be important to note that the exports of Cd, Cu and Zn do not ever surpass the range of exports at lower conductivities and more data is needed during winter months. There were also pulses in metal concentrations in streams during experimental road salt additions (figure 3c,d). Pulses in Na and Cu concentrations coincided simultaneously as road salt migrated downstream over the span of a few minutes. There were also significant increases in the mobilization of metals that were measured (Cu, Mn, Zn, Sr) from stream sediments across 10 sites from the Baltimore, MD–Washington, DC metropolitan area in controlled laboratory experiments with 100% pure NaCl (figure 4).
Figure 3.
Increases in: (a) major and trace metals concentrations before, during, after snowmelt (spanning two months) in urban watersheds of the eastern USA demonstrated by routine monitoring of ambient concentrations in stream water, and (b) peaks in ambient Cu, Cd and Zn watershed exports (mass flux in stream per unit area of watershed) in Rock Creek in Washington, DC highlighted in yellow during winter months in response to road salting (examples from other nearby streams can be found in the electronic supplementary material, figure S6). (c) Experimental salinization of an urban stream with real road salt caused an experimental salt pulse to migrate quickly downstream; during the experiment, Na+ concentrations were elevated above baseline pre-disturbance conditions. (d) During the experiment, Cu concentrations peaked with Na+ concentrations as the experimental road salt pulse migrated downstream.
Figure 4.
Increases in concentrations of metals and nutrients in stream sediments exposed to varying levels of NaCl. There are negative dissolved metal concentrations reported in a few cases because we are comparing the values to incubations without salinization treatments as a control. Streams from 10 watersheds were located across the Baltimore, MD and Washington, DC metropolitan region [15] (electronic supplementary material, table S3). ISC denotes impervious surface cover in the watershed.
4. Discussion
A growing body of work demonstrates that the chemistry of fresh waters has been rapidly changing in the Anthropocene with a wide geographical scope and novel interactions between solutes forming novel chemical mixtures [11]. Interactions between major ions in salts and other chemical cycles in ecosystems are important but are sometimes poorly recognized. For example, concentrations and compositions of salts influence pH, conductivity, solubility, mobility and chemical and biological reactivity of metals, nutrients, organics and greenhouse gases in fresh waters [16,18,41,48]. Managing freshwater salinization requires considering chemical mixtures and potential interactive effects as a syndrome of multiple stressors instead of single contaminants. Managing multiple stressors simultaneously instead of managing single contaminants is a critical frontier for dealing with FSS in the future. Below, we discuss some factors influencing formation, concentrations and variations in chemical mixtures across varying spatial and temporal scales and environmental implications.
(a). Freshwater salinization syndrome mobilizes mixtures of major ions in fresh waters
Our analysis illustrates increasing trends in salt ions in diverse freshwater ecosystems such as rivers and lakes primarily in North America and Europe, which varied in size and land use of the drainage basin (figure 1). Our analysis consistently showed Na+ concentrations increasing for many sites including the Great Lakes [47]. Increasing Na+ concentrations are caused by the combination of road salts, water softeners, irrigation waters, fracking brines and sewage inputs (e.g. [27,32,34]). Many countries outside of North America and certain regions of Europe have little snow and do not rely on road salts. Studies on the effects of road salts may not be applicable to these fresh waters. However, chemical mixtures enriched in Na+ may be a surrogate for other anthropogenic activities related to sewage inputs, water softeners or irrigation (e.g. [27,34]). Tracing and quantifying relative contributions of Na+ ions from different non-point sources in watersheds warrants further study.
Although K+ concentrations are under stronger biotic demand, they can increase in runoff draining agricultural land and mines [7,49]. We also observed significantly increasing trends in mean Ca2+ and Mg2+ concentrations across many sites, but there were some cases where there were decreasing trends [47]. Increasing or decreasing trends in Ca2+ and Mg2+ are strongly dependent on chemical weathering and the underlying geology of watersheds, historical changes in acidic deposition and mineral precipitation influenced by ion concentrations, pH, direct anthropogenic inputs and warming temperatures [24,25]. Ca2+ and Mg2+ concentrations have decreased in atmospheric deposition owing to clean air regulations, which contributes to decreasing concentrations in watersheds underlain by crystalline lithology, where Ca2+ and Mg2+ pools have been depleted for decades by acid rain in some regions (but not other regions less affected by acid rain) [35,38]. In watersheds draining sedimentary or metamorphic lithology, increased erosion rates and human-accelerated weathering of rocks and soils (which contain ample Ca2+ and Mg2+) can contribute to increasing trends [6]. Anthropogenic inputs of Ca2+ and Mg2+ from pollution sources can also influence concentrations in fresh waters. Urban and agricultural watersheds experience human-accelerated weathering of the built environment and sewage and fertilizer inputs, which contribute to increasing trends in concentrations of Na+, Ca2+, Mg2+ and K+ ions [20,48]. Thus, differences in trends across sites may be explained by variations in underlying geology, atmospheric deposition, land use, anthropogenic inputs and geochemical transformations in fresh waters.
Many sites in the illustration we show are primarily in North America and Europe and many of these sites experienced glaciation during the last ice age and have predominately young soils (less than 15 000 years) and recently exposed unweathered rock [50]. In other regions (such as most of Australia and Africa and significant regions of South America and Asia), soils have been developing and rocks have weathered over longer geological timescales such as millions of years. Older soils have had greater time for pools of major ions to accumulate in the subsurface and ground water [9]. These subsurface stores of major ions can then be mobilized following the clearing of native vegetation and certain agricultural practices in Australia, Africa and the Middle East (e.g. [42]). The age of exposed rock may be relevant in explaining some potential differences in mechanisms of FSS, as greater exposure leads to greater weathering and dissolution of ions from minerals; however, more work is necessary to investigate and compare different patterns and processes related to FSS across regions (B. Kefford 2018, personal communication). Interestingly, our previous work has shown increasing trends in concentrations of base cations, specific conductance and alkalinity in areas of the mid-Atlantic and southern USA, which did not experience glaciation during the last ice age [6,7,20]. Nonetheless, the causes and consequences of FSS in North America and Europe can be quite different when compared to other regions. Thus, we emphasize that FSS is actually quite polymorphic because of the underlying causes, and biogeochemical relationships operating in different watersheds. Based on complicated interactions of the FSS, there is not a single uniform response to salinization, but that there can be a suite of interactions, which affect chemical mixtures, water quality and/or multiple stressors such as changing pH, alkalinity and mobilization of organics, nutrients and metals.
Differing concentrations and compositions of mixtures of major ions have differing environmental consequences across watersheds. For example, Na+-rich mixtures weaken soil aggregate structure, increase soil erosion, decrease water infiltration capacity, decrease plant root penetration and can mobilize contaminants bound to solid soil particles and colloids [8,9]. Because Na+ has a weaker charge and smaller radius than Ca2+ and Mg2+ ions, which stabilize soil aggregates, it allows negatively charged soil particles to repel each other [51,52]. In addition, Na+ has a larger hydration shell, which can physically force clay particles away from one another [53]. Previous work has shown that salinization from NaCl alters the density of water in lakes and bays, which intensifies stratification and influences mixing dynamics [54]. The K+ and  mixtures formed in agricultural regions have the potential to fertilize freshwater and marine ecosystems and increase primary productivity [55]. We hypothesize that salinization of lakes with alkaline ion mixtures may also influence internal loading of phosphorus owing to ion exchange with sediments or desorption of phosphorus bound to sesquioxides in response to elevated pH, decreased oxygen and reducing conditions in the hypolimnion of thermally stratified lakes. More research is needed to investigate the effects of salinization and alkalinization on internal loading of phosphorus in smaller lakes where salt concentration increases can be greater.
mixtures formed in agricultural regions have the potential to fertilize freshwater and marine ecosystems and increase primary productivity [55]. We hypothesize that salinization of lakes with alkaline ion mixtures may also influence internal loading of phosphorus owing to ion exchange with sediments or desorption of phosphorus bound to sesquioxides in response to elevated pH, decreased oxygen and reducing conditions in the hypolimnion of thermally stratified lakes. More research is needed to investigate the effects of salinization and alkalinization on internal loading of phosphorus in smaller lakes where salt concentration increases can be greater.
(b). Freshwater salinization syndrome mobilizes mixtures of alkaline salts and nutrients: evidence from high-frequency sensors
High-frequency sensor data showed a positive relationship between specific conductance and pH at 26 out of the 26 sites with available data (electronic supplementary material, table S2) and between nitrate and specific conductance at some select sites. The positive relationship between specific conductance and pH suggests that the pH change is embodied by an increase in alkaline salts. This relationship may result from mobile anions, such as chloride and nitrate, transported together as chemical mixtures and ion exchange reactions in soils [17,18]. There was a cyclical relationship between nitrate concentrations and specific conductance during a discrete snowstorm demonstrating a flushing effect in an urban stream and the nearby Potomac River (electronic supplementary material, figure S5). Winter flushing of urban contaminants indicates potential water quality impacts from snowstorms which have been less studied than urban rainstorms [17,18]. Inverse and more complex relationships between specific conductance and nitrate at other sites were probably related to shifts in nitrate and other ion sources across streamflow and season (e.g. differences in groundwater versus surface sources or deicer versus fertilizer sources). Alternatively, these relationships may have been owing to high density road salting in the east and intensive agriculture in the midwest and not be significantly influenced by the relative contributions of different ions to conductivity (but mostly attributed to different land use practices).
(c). Freshwater salinization syndrome mobilizes chemical mixtures of metals: monitoring and laboratory and ecosystem experiments
Our results from routine monitoring and field and laboratory salinization experiments demonstrated elevated concentrations of metals in solution, particularly for Cu, following episodic salinization. High concentrations of solutes such as Na+ mobilize metals into solution in soils and waters, although higher valance cations such as Ca2+ and Mg2+ can compete even more effectively for soil cation exchange sites. For example, Ca2+ competes more effectively than Pb, Zn and Cd for soil exchange sites, promoting dissolution of these metals. The Mg2+ competes more effectively than Cu and brings it into solution perhaps owing to the nearly identical ionic radius and charge of the two cations and also the typically higher relative concentrations of Mg2+ than Cu [56]. The Cu concentrations are highest near the soil surface but also show notable migration throughout the soil profile [57]. Cd, a highly mobile heavy metal, is readily brought into solution by higher ionic strength solutions associated with salinization, whereas Pb and Zn are also mobilized but at a much lower rate. Different metals and anions also have different affinities for one another. For example, Cl− plays a major role in the solubility of Cd and Pb whereas  plays a major role in Cu and Zn solubility [56,58]. Some metals preferentially come into solution with NaCl through chloro-complexation such as Cd and Pb. The affinity for Cl− increases bioavailability and toxicity of Cd and other metals [59].
plays a major role in Cu and Zn solubility [56,58]. Some metals preferentially come into solution with NaCl through chloro-complexation such as Cd and Pb. The affinity for Cl− increases bioavailability and toxicity of Cd and other metals [59].
Our salinization experiments in the laboratory showed potential increases in Cu concentrations in stream water that were higher than concentrations contributing to acute and chronic toxicity for four species of Daphnia in a previous study [60]; this previous study showed changes in survival and instantaneous population growth at concentrations greater than 0.040–0.060 mg l−1 [60]. However, many other factors influence the toxicity of Cu and other metals such as water hardness and relative concentrations and compositions of dissolved organic matter [61]. In fresh waters, there is competition between toxic metal ions and other metal cations in solution (such as Ca2+), and there is also competition between biotic ligands and organic matter for metal ions [61]. Both mixtures of major ions and concentrations and compositions of organic matter can change as a consequence of FSS [17,18], which can make evaluating toxicity more complex under environmental conditions in streams. However, it is important to note that there are tools like the Biotic Ligand Model for Cu that can provide a means of quantifying relative toxicity based on different or changing concentrations of major ions, pH, organic matter, etc. [61]. Environmental and health impacts of mixtures of metals and ecotoxicological effects of mixtures of major ions warrant further investigation (sensu [30,62,63]).
(d). Freshwater salinization syndrome: implications for ecosystems, infrastructure and management
Currently, most water quality regulations are driven by individual elements or compounds. Yet, nature consists of different chemical mixtures. Major ions in freshwater ecosystems and drinking water are typically not regulated by most water-related legislation [10,64]. The FSS raises a need to consider regulating chemical mixtures because they produce broader environmental effects than individual elements or compounds on aquatic life, safe drinking water and infrastructure [10,64,65]. A growing body of work demonstrates that toxicity of ion mixtures to aquatic life is greater than individual ions or pairs [29,66,67], but there needs to be bioavailability measurements and toxicity testing in the case of metals. However, more research is necessary to determine which ions have synergistic or antagonistic effects on toxicity based on their relative concentrations in chemical mixtures and which ions have neutral or subtractive effects on toxicity chemistry [31]. Some work has shown toxicity of major ions depends on the influence of background water chemistry and additive toxicity of major ions to species like Ceriodaphnia dubia [31]. However, other field studies have concluded that the effect of salinity and other stressors on freshwater biota is not interactive [68,69]. While the interaction between the multiple stressors in these studies may not always be relevant to the FSS, it is important to note that the combined effects of multiple stressors and salinity can be complex in general [13,70,71].
From an infrastructure and drinking water perspective, non-point source ion inputs from human-accelerated weathering need to be further considered. Initially, just NaCl was thought to be reaching levels of concern in major drinking water supplies in the USA [4,64], but we now know that multiple ions such as Na+, Cl−, Ca2+, Mg2+, K+,  , etc., have been increasing in concentration over the past century [6,7,10,20] (figure 1). Factors such as conductivity, total dissolved solids, pH and chloride affect corrosion potential and mobilization of metals from pipes to drinking water [64,65]. In industrial settings, damage to pipes by chemical mixtures rich in Ca2+, Mg2+ and carbonates can make energy production more expensive. Coal, nuclear, oil and natural gas power plants rely on fresh water for generation of steam. Salts concentrate during evaporation of steam and deposit on pipes as ‘scales' depending upon Ca2+, Mg2+, pH, hardness, alkalinity and conductivity [72]. Scaling from 'hard water' chemical mixtures increases corrosion and diminishes efficiency in power generation. Controlling scaling is complex, costly and detrimental to the environment owing to the release of wastewaters high in salt [72]. As another example, desalination plants, which are common in many warm and dry regions typically generate a hypersaline discharge, which is greater than the salinity of seawater and may have impacts when released into marine waters. Overall, broader impacts of different chemical mixtures on safe drinking water and infrastructure scaling and/or corrosion warrants further consideration in research and management.
, etc., have been increasing in concentration over the past century [6,7,10,20] (figure 1). Factors such as conductivity, total dissolved solids, pH and chloride affect corrosion potential and mobilization of metals from pipes to drinking water [64,65]. In industrial settings, damage to pipes by chemical mixtures rich in Ca2+, Mg2+ and carbonates can make energy production more expensive. Coal, nuclear, oil and natural gas power plants rely on fresh water for generation of steam. Salts concentrate during evaporation of steam and deposit on pipes as ‘scales' depending upon Ca2+, Mg2+, pH, hardness, alkalinity and conductivity [72]. Scaling from 'hard water' chemical mixtures increases corrosion and diminishes efficiency in power generation. Controlling scaling is complex, costly and detrimental to the environment owing to the release of wastewaters high in salt [72]. As another example, desalination plants, which are common in many warm and dry regions typically generate a hypersaline discharge, which is greater than the salinity of seawater and may have impacts when released into marine waters. Overall, broader impacts of different chemical mixtures on safe drinking water and infrastructure scaling and/or corrosion warrants further consideration in research and management.
Another emerging question is related to effects of chemical mixtures resulting from FSS on aquatic foodwebs and eutrophication. Changes in chemical concentrations and compositions associated with FSS have the capacity to cause losses of sensitive species to different chemical mixtures and enhancement of relatively tolerant species. These species shifts may or may not lead to changes in ecosystems similar to the types observed owing to nutrient enrichment or cascading impacts from fairly subtle shifts in species compositions? For example, increased salinization may sometimes trigger trophic cascades by stimulating phytoplankton growth and toxicity to grazers [73], although trophic cascades have not been detected in other studies [74]. Further,  and Na+ have been shown to stimulate algal growth [8]. In addition, FSS alters solubility and distribution of organic matter as dissolved versus particulate fractions [37,39], which may have implications for energy and contaminant flow through aquatic foodwebs. Strongly hydrated cations (e.g. Mg2+, Ca2+ and H+) and weakly hydrated anions (e.g.
and Na+ have been shown to stimulate algal growth [8]. In addition, FSS alters solubility and distribution of organic matter as dissolved versus particulate fractions [37,39], which may have implications for energy and contaminant flow through aquatic foodwebs. Strongly hydrated cations (e.g. Mg2+, Ca2+ and H+) and weakly hydrated anions (e.g.  and Cl−) denature proteins and increase hydrophobic solubility, which may increase the bioavailability of protein-rich organic matter and dissolved organic nitrogen to heterotrophic microbes. Conversely, non-polar or weakly polar organic compounds such as polycyclic aromatic hydrocarbons may 'salt out' (become less soluble in water) and sorb onto particulate matter, which increases their potential for sedimentation or ingestion by higher trophic levels and transfers up the food chain [75]. Mixtures of major ions can also increase decomposition rates of organic compounds, ammonification and nitrification [40]. The FSS may influence trophic cascades, eutrophication and regime shifts in some cases and not others [23,73,74], but these types of effects need to be further evaluated and compared across diverse environments and regions (sensu [76]).
and Cl−) denature proteins and increase hydrophobic solubility, which may increase the bioavailability of protein-rich organic matter and dissolved organic nitrogen to heterotrophic microbes. Conversely, non-polar or weakly polar organic compounds such as polycyclic aromatic hydrocarbons may 'salt out' (become less soluble in water) and sorb onto particulate matter, which increases their potential for sedimentation or ingestion by higher trophic levels and transfers up the food chain [75]. Mixtures of major ions can also increase decomposition rates of organic compounds, ammonification and nitrification [40]. The FSS may influence trophic cascades, eutrophication and regime shifts in some cases and not others [23,73,74], but these types of effects need to be further evaluated and compared across diverse environments and regions (sensu [76]).
Overall, our analysis shows that baseline concentrations of major ions are increasing in well-studied freshwater ecosystems located primarily in North America and Europe, and that episodic salinization pulses have significant effects on water quality. Previous bio-assessments in Maryland, USA, where most of our urban research was conducted, has linked elevated baseline ion concentrations with degrading biological conditions in stream surveys [77], but more work is necessary across other regions. These results stimulate an overall question regarding what is the full suite of chemical, biological and physical changes that can happen in response to episodic versus gradual increases in baseline concentrations over time? This study also raises other questions regarding the long-term effects of FSS on shifting ecosystem resistance and resilience to episodic salinization. High-frequency sensors are now being increasingly deployed and data obtained from them will be critical in investigating if/how fresh waters exceed secondary contaminant levels (unregulated levels that are recommended by the US Environmental Protection Agency) and how much stream length is impaired. Ecosystem scale experiments in streams and lakes (similar to those described in this paper and elsewhere) are useful for studying how effects of chemical mixtures persist in streams and what biological effects these mixtures of major ions and metals have close to non-point sources or further downstream. There are also questions regarding consequences of varying salt mixtures on the mobilization of chemical mixtures across pollutant levels, underlying geology, temperature and land use.
In conclusion, we found that many drainage waters show a temporal trend toward increasing ion concentrations over time. The biogeochemical consequences of ion enrichment go well beyond the effects of ions adding to ‘salinity’ and extend to nutrients, metals and hydrogen ions (pH). The relationships among biogeochemical variables were not consistent across systems and can vary according to the type of ion enrichment and the underlying geochemistry. We also suggest that the ecological consequences of the multiple biogeochemical changes that can accompany ion enrichment require further study. Management of ion enrichment as an environmental stressor should incorporate all of the points above and insure that the management focus is not inappropriately narrow. Current approaches used in watershed management, particularly in North America and Europe, can be applied to managing FSS. For example, non-point source pollution strategies target reducing inputs of nutrients and metals and there are also strategies for enhancing riparian buffers and wetlands in agricultural and urban watersheds, which can attenuate major ions, nutrients and metals. Avoiding or minimizing the use of road salts in the environment and minimizing sewer overflows and high nutrient loads can also reduce the interactive effects of FSS on water quality. Our results show that there are direct environmental impacts of chemical mixtures of major ions themselves, and these mixtures enhance the mobilization of other contaminants. Therefore, recognition of the interactive effects of chemical mixtures and multiple stressors associated with freshwater salinization on enhancing contaminant mobilization suggests we need to be even more aggressive with addressing our current non-point source pollution issues.
Supplementary Material
Acknowledgements
M. P. Dubbin assisted with analyses. We thank B. J. Kefford for insights regarding regional variations in causes and ecological consequences of salinization. We also thank four anonymous reviewers for constructive comments and suggestions.
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
This work was primarily supported by NSF grant nos. EAR 1521224, DEB 1027188 and CBET 105850.
References
- 1.Jackson RB, Carpenter SR, Dahm CN, Mcknight DM, Naiman RJ, Postel SL, Running SW. 2001. Water in a changing world. Ecol. Appl. 11, 1027–1045. ( 10.1890/1051-0761(2001)011%5B1027:WIACW%5D2.0.CO;2) [DOI] [Google Scholar]
- 2.Oki T, Kanae S. 2006. Global hydrological cycles and world water resources. Science 313, 1068–1072. ( 10.1126/science.1128845) [DOI] [PubMed] [Google Scholar]
- 3.Cañedo-Argüelles M, Kefford BJ, Piscart C, Prat N, Schäfer RB, Schulz C-J. 2013. Salinization of rivers: an urgent ecological issue. Environ. Pollut. 173, 157–167. ( 10.1016/j.envpol.2012.10.011) [DOI] [PubMed] [Google Scholar]
- 4.Kaushal SS, Groffman PM, Likens GE, Belt KT, Stack WP, Kelly VR, Band LE, Fisher GT. 2005. Increased salinization of fresh water in the northeastern United States. Proc. Natl Acad. Sci. USA 102, 13 517–13 520. ( 10.1073/pnas.0506414102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaushal SS, Likens GE, Jaworski N, Pace ML, Sides AM, Seekell D, Belt KT, Secor DH, Wingate RL. 2010. Rising stream and river temperatures in the United States. Front. Ecol. Environ. 8, 461–466. ( 10.1890/090037) [DOI] [Google Scholar]
- 6.Kaushal SS, Likens GE, Utz R, Pace ML, Grese M, Yepsen M. 2013. Increased river alkalinization in the eastern US. Environ. Sci. Technol. 47, 10 302–10 311. [DOI] [PubMed] [Google Scholar]
- 7.Kaushal SS, Likens GE, Pace ML, Utz RM, Haq S, Gorman J, Grese M. 2018. Freshwater salinization syndrome on a continental scale. Proc. Natl Acad. Sci. USA 8, E574–E583 ( 10.1073/pnas.1711234115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams WD. 2001. Anthropogenic salinization of inland waters. Hydrobiologia 466, 329–337. ( 10.1023/A:1014598509028) [DOI] [Google Scholar]
- 9.Rengasamy P. 2006. World salinization with emphasis on Australia. J. Exp. Bot. 57, 1017–1023. ( 10.1093/jxb/erj108) [DOI] [PubMed] [Google Scholar]
- 10.Cañedo-Argüelles M, et al. 2016. Saving freshwater from salts. Science 351, 914–916. ( 10.1126/science.aad3488) [DOI] [PubMed] [Google Scholar]
- 11.Kaushal SS, et al. In press Watershed ‘chemical cocktails’: forming novel elemental combinations in Anthropocene fresh waters. Biogeochemistry ( 10.1007/s10533-018-0502-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Relyea RA. 2009. A cocktail of contaminants: how pesticide mixtures at low concentrations affect aquatic communities. Oecologia 159, 363–376. ( 10.1007/s00442-008-1213-9) [DOI] [PubMed] [Google Scholar]
- 13.Piggot JJ, Townsend CR, Matthaei CD. 2015. Reconceptualizing synergism and antagonism among multiple stressors. Ecol. Evol. 5, 1538–1547. ( 10.1002/ece3.1465) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schafer RB, Piggot JJ. 2018. Advancing understanding and prediction in multiple stressor research through a mechanistic basis for null models. Global Change Biol. 24, 1817–1826. ( 10.1111/gcb.14073) [DOI] [PubMed] [Google Scholar]
- 15.Shanley JB. 1994. Effects of ion-exchange on stream solute fluxes in a basin receiving highway deicing salts. J. Environ. Qual. 23, 977–986. ( 10.2134/jeq1994.00472425002300050019x) [DOI] [PubMed] [Google Scholar]
- 16.Löfgren S. 2001. The chemical effects of deicing salt on soil and stream water of five catchments in southeast Sweden. Water Air Soil Pollut. 130, 863–868. ( 10.1023/A:1013895215558) [DOI] [Google Scholar]
- 17.Duan S, Kaushal SS. 2015. Salinization alters fluxes of bioreactive elements from stream ecosystems across land use. Biogeosciences 12, 7331–7347. ( 10.5194/bg-12-7331-2015) [DOI] [Google Scholar]
- 18.Haq S, Kaushal SS, Duan S. In press Episodic salinization and freshwater salinization syndrome mobilize base cations, carbon, and nutrients to streams across urban regions. Biogeochemistry. ( 10.1007/s10533-018-0514-2) [DOI] [Google Scholar]
- 19.Zampella RA, Procopio NA, Lathrop RG, Dow CL. 2007. Relationship of land-use/land-cover patterns and surface-water quality in the Mullica River Basin. JAWRA 43, 594–604. ( 10.1111/j.1752-1688.2007.00045.x) [DOI] [Google Scholar]
- 20.Kaushal SS, et al. 2017. Human-accelerated weathering increases salinization, major ions, and alkalinization in fresh water across land use. Appl. Geochem. 83, 121–135. ( 10.1016/j.apgeochem.2017.02.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raymond PA, Oh N-H, Turner RE, Broussard W. 2008. Anthropogenically enhanced fluxes of water and carbon from the Mississippi River. Nature 451, 449–452. ( 10.1038/nature06505) [DOI] [PubMed] [Google Scholar]
- 22.Raymond PA, Hamilton SK. 2018. Anthropogenic influences on riverine fluxes of dissolved inorganic carbon to the oceans. Limnol. Oceanogr. 3, 143–155. ( 10.1002/lol2.10069) [DOI] [Google Scholar]
- 23.Helton AM, Bernhardt ES, Fedders A. 2014. Biogeochemical regime shifts in coastal landscapes: the contrasting effects of saltwater incursion and agricultural pollution on greenhouse gas emissions from a freshwater wetland. Biogeochemistry 120, 133–147. ( 10.1007/s10533-014-9986-x) [DOI] [Google Scholar]
- 24.Anning DW, Flynn ME. 2014. Dissolved-solids sources, loads, yields, and concentrations in streams of the conterminous United States. U.S. Geological Survey Scientific Investigations Report 2014–5012. See https://pubs.usgs.gov/sir/2014/5012/.
- 25.Stets EG, Kelly VJ, Crawford CG. 2014. Long-term trends in alkalinity in large rivers of the conterminous US in relation to acidification, agriculture, and hydrologic modification. Sci. Total Environ. 488–489, 280–289. ( 10.1016/j.scitotenv.2014.04.054) [DOI] [PubMed] [Google Scholar]
- 26.Jeppesen E, et al. 2015. Ecological impacts of global warming and water abstraction on lakes and reservoirs due to changes in water level and related changes in salinity. Hydrobiologia 750, 201–227. ( 10.1007/s10750-014-2169-x) [DOI] [Google Scholar]
- 27.Steele MK, Aitkenhead-Peterson JA. 2011. Long-term sodium and chloride surface water exports from the Dallas/Fort Worth region. Sci. Total Environ. 409, 3021–3032. ( 10.1016/j.scitotenv.2011.04.015) [DOI] [PubMed] [Google Scholar]
- 28.Mahmuduzzaman M, Ahmed ZU, Nuruzzaman AKM, Ahmed FRS. 2014. Causes of salinity intrusion in coastal belt of Bangladesh. Int. J. Plant Res. 4, 8–13. [Google Scholar]
- 29.Hintz WD, Relyea RA. 2017. Impacts of road deicing salts on the early-life growth and development of a stream salmonid: salt type matters. Environ. Pollut. 223, 409–415. ( 10.1016/j.envpol.2017.01.040) [DOI] [PubMed] [Google Scholar]
- 30.Zalizniak L, Kefford BJ, Dayanthi N. 2006. Is all salinity the same? I. The effect of ionic compositions on the salinity tolerance of five species of freshwater invertebrates. Mar. Freshwater Res. 57, 75–82. ( 10.1071/MF05103) [DOI] [Google Scholar]
- 31.Mount DR, et al. 2016. The acute toxicity of major ion salts to Ceriodaphnia dubia: 1. Influence of background water chemistry. Environ. Toxicol. Chem. 35, 3039–3057. ( 10.1002/etc.3487) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corsi SR, Graczyk DJ, Geis SW, Booth NL, Richards KD. 2010. A fresh look at road salt: aquatic toxicity and water-quality impacts on local, regional, and national scales. Environ. Sci. Technol. 44, 7376–7382. ( 10.1021/es101333u) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dugan HA, et al. 2017. Salting our freshwater lakes. Proc. Natl Acad. Sci. USA 114, 4453–4458. ( 10.1073/pnas.1620211114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelly VR, Cunnigham MA, Curri N, Findlay SE, Carroll SM. 2018. The distribution of road salt in private drinking water wells in a southeastern New York suburban township. J. Environ. Qual. 47, 445–451. ( 10.2134/jeq2017.03.0124) [DOI] [PubMed] [Google Scholar]
- 35.Likens GE, Driscoll CT, Buso DC. 1996. Long-term effects of acid rain: response and recovery of a forest ecosystem. Science 272, 244–246. ( 10.1126/science.272.5259.244) [DOI] [Google Scholar]
- 36.Rosenberry DO, Bukaveckas PA, Buso DC, Likens GE, Shapiro AM, Winter TC. 1999. Movement of road salt to a small New Hampshire lake. Water Air Soil Pollut. 109, 179–206. ( 10.1023/A:1005041632056) [DOI] [Google Scholar]
- 37.Green SM, Machin R, Cresser MS. 2009. Does road salting induce or ameliorate DOC mobilisation from roadside soils to surface waters in the long term? Environ. Monit. Assess. 153, 435–448. ( 10.1007/s10661-008-0369-4) [DOI] [PubMed] [Google Scholar]
- 38.Likens GE. 2013. Biogeochemistry of a forested ecosystem, 3rd edn New York, NY: Springer. [Google Scholar]
- 39.Amrhein C, Strong JE, Mosher PA. 1992. Effect of deicing salts on metal and organic matter mobilization in roadside soils. Environ. Sci. Technol. 26, 703–709. ( 10.1021/es00028a006) [DOI] [Google Scholar]
- 40.Green SM, Machin R, Cresser MS. 2009. Effect of long-term changes in soil chemistry induced by road salt applications on N-transformations in roadside soils. Environ. Pollut. 152, 20–31. ( 10.1016/j.envpol.2007.06.005) [DOI] [PubMed] [Google Scholar]
- 41.Guo JH, Wang FS, Vogt RD, Zhang YH, Liu C. 2015. Anthropogenically enhanced chemical weathering and carbon evasion in the Yangtze Basin. Sci. Rep. 5, 11941 ( 10.1038/srep11941) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kefford BJ, Palmer CG, Dayanthi N. 2005. Relative salinity tolerance of freshwater macroinvertebrates from the south-east Eastern Cape, South Africa compared with the Barwon Catchment, Victoria, Australia. Mar. Freshwater Res. 56, 163–171. ( 10.1071/MF04098) [DOI] [Google Scholar]
- 43.Rogora M, et al. 2015. Recent trends in chloride and sodium concentrations in the deep subalpine lakes (Northern Italy). Environ. Sci. Pollut. R. 22, 19 013–19 026. ( 10.1007/s11356-015-5090-6) [DOI] [PubMed] [Google Scholar]
- 44.Räike A, Kortelainen P, Mattsson T, Thomas DN. 2012. 36 year trends in dissolved organic carbon export from Finnish rivers to the Baltic Sea. Sci. Total Environ. 435–436, 188–201. ( 10.1016/j.scitotenv.2012.06.111) [DOI] [PubMed] [Google Scholar]
- 45.Stumm W, Morgan JJ. 1996. Aquatic chemistry: chemical equilibria and rates in natural waters, 3rd edn Hoboken, NJ: Wiley. [Google Scholar]
- 46.Tripler CE, Kaushal SS, Likens GE, Walter MT. 2006. Patterns in potassium dynamics in forest ecosystems. Ecol. Lett. 9, 451–466. ( 10.1111/j.1461-0248.2006.00891.x) [DOI] [PubMed] [Google Scholar]
- 47.Chapra SC, Dove A, Warren GJ. 2012. Long-term trends of Great Lakes major ion chemistry. J. Great Lakes Res. 38, 550–560. ( 10.1016/j.jglr.2012.06.010) [DOI] [Google Scholar]
- 48.Aquilina L, Poszwa A, Walter C, Vergnaud V, Pierson-Wickmann A-C, Ruiz L. 2012. Long-term effects of high nitrogen loads on cation and carbon riverine export in agricultural catchments. Environ. Sci. Technol. 46, 9447–9455. ( 10.1021/es301715t) [DOI] [PubMed] [Google Scholar]
- 49.Cañedo-Argüelles M, Brucet S, Carrasco S, Flor-Arnau N, Ordeix M, Ponsá S, Coring E. 2017. Effects of potash mining on river ecosystems: an experimental study. Environ. Pollut. 224, 759–770. ( 10.1016/j.envpol.2016.12.072) [DOI] [PubMed] [Google Scholar]
- 50.Davis MB. 1983. Quaternary history of deciduous forests of Eastern North America and Europe. Ann. Missouri Bot. Gard. 70, 550–563. ( 10.2307/2992086) [DOI] [Google Scholar]
- 51.Miller WP, Frenkel H, Newman KD. 1990. Flocculation concentration and sodium/calcium exchange of kaolinitic soil clays. Soil Sci. Soc. Am. J. 54, 346–351. ( 10.2136/sssaj1990.03615995005400020008x) [DOI] [Google Scholar]
- 52.Brady NC, Weil RR. 2010. Elements of the nature and properties of soils, 3rd edn Upper Saddle River, NJ: Pearson Prentice Hall. [Google Scholar]
- 53.Sumner ME. 1992. The electrical double layer and clay dispersion. In Soil crusting: chemical and physical processes (eds Sumner ME, Stewart BA), pp. 1–31. Boca Raton, FL: CRC Press. [Google Scholar]
- 54.Bubeck RC, Diment WH, Deck BL, Baldwin AL, Lipton SD. 1971. Runoff of deicing salt: effect on Irondequoit Bay, Rochester, New York. Science 172, 1128–1132. ( 10.1126/science.172.3988.1128) [DOI] [PubMed] [Google Scholar]
- 55.White DA, Pagarette A, Rooks P, Ali ST. 2013. The effect of sodium bicarbonate supplementation on growth and biochemical composition of marine microalgae cultures. J. Appl. Phycol. 25, 153–165. ( 10.1007/s10811-012-9849-6) [DOI] [Google Scholar]
- 56.Acosta JA, Jansen B, Kalbitz K, Faz A, Martinez-Martinez S. 2011. Salinity increases mobility of heavy metals in soils. Chemosphere 85, 1318–1324. ( 10.1016/j.chemosphere.2011.07.046) [DOI] [PubMed] [Google Scholar]
- 57.Jones PS, Davis AP. 2013. Spatial accumulation and strength of affiliation of heavy metals in bioretention media. J. Environ. Eng. ASCE 139, 479–487. ( 10.1061/(ASCE)EE.1943-7870.0000624) [DOI] [Google Scholar]
- 58.Zhao S, Feng CH, Wang DX, Liu YZ, Shen ZY. 2013. Salinity increases the mobility of Cd, Cu, Mn, and Pb in the sediments of Yangtze Estuary: relative role of sediments' properties and metal speciation. Chemosphere 91, 977–984. ( 10.1016/j.chemosphere.2013.02.001) [DOI] [PubMed] [Google Scholar]
- 59.Lopez-Chuken UJ, Young SD, Sanchez-Gonzalez MN. 2010. The use of chloro-complexation to enhance cadmium uptake by Zea mays and Brassica juncea: testing a free ion activity model and implications for phytoremediation. Int. J. Phytoremediat. 12, 680–696. ( 10.1080/15226510903353161) [DOI] [PubMed] [Google Scholar]
- 60.Winner RW, Farrell MP. 1976. Acute and chronic toxicity of copper to four species of Daphnia. J. Fish. Res. Board Can. 33, 1685–1691. ( 10.1139/f76-215) [DOI] [Google Scholar]
- 61.Toro D, Allen HE, Bergman HL, Meyer JS, Paquin PR, Santore RC. 2009. Biotic ligand model of the acute toxicity of metals. I. Technical basis. Environ. Toxicol. Chem. 20, 2383–2396. ( 10.1002/etc.5620201034) [DOI] [PubMed] [Google Scholar]
- 62.Mount DR, Barth AK, Garrison TD, Barten KA, Hockett JR. 1994. Dietary and waterborne exposure of rainbow trout (Onorhynchus mykiss) to copper, cadmium, lead and zinc using a live diet. Environ. Toxicol. Chem. 13, 2031–2041. ( 10.1897/1552-8618(1994)13%5B2031:DAWEOR%5D2.0.CO;2) [DOI] [Google Scholar]
- 63.Mount DR, Gulley DD, Hockett JR, Garrison TD, Evans JM. 2009. Statistical models to predict the toxicity of major ions to Ceriodaphnia dubia, Daphnia magna and Pimephales promelas (fathead minnows). Environ. Toxicol. Chem. 16, 2009–2019. ( 10.1897/1551-5028(1997)016%3C2009:SMTPTT%3E2.3.CO;2) [DOI] [Google Scholar]
- 64.Kaushal SS. 2016. Increased salinization decreases safe drinking water. Environ. Sci. Technol. 50, 2765–2766. ( 10.1021/acs.est.6b00679) [DOI] [PubMed] [Google Scholar]
- 65.Stets EG, Lee CJ, Lytle DA, Schock MR. 2017. Increasing chloride in rivers of the conterminous U.S. and linkages to potential corrosivity and lead action level exceedances in drinking water. Sci. Total Environ. 613–614, 1498–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cormier SM, Suter GW, Zheng L, Pond GL. 2013. Assessing causation of the extirpation of stream macroinvertebrates by a mixture of ions. Environ. Toxicol. Chem. 32, 277–287. ( 10.1002/etc.2059) [DOI] [PubMed] [Google Scholar]
- 67.Kunz JL, Conley JM, Buchwalter DB, Norberg-King TJ, Kemble NE, Wang N, Ingersoll CG. 2013. Use of reconstituted waters to evaluate effects of elevated major ions associated with mountaintop coal mining on freshwater invertebrates. Environ. Toxicol. Chem. 32, 2826–2835. ( 10.1002/etc.2391) [DOI] [PubMed] [Google Scholar]
- 68.Kath J, Thomson JR, Thompson RM, Kefford BJ, Dyer FJ, Mac Nally R. 2018. Interactions among stressors may be weak: implications for management of freshwater macroinvertebrate communities. Divers. Distrib. 24, 939–950. ( 10.1111/ddi.12737) [DOI] [Google Scholar]
- 69.Szocs E, Kefford BJ, Schafer RB. 2012. Is there an interaction of the effects of salinity and pesticides on the community structure of macroinvertebrates? Sci. Tot. Environ. 437, 121–126. ( 10.1016/j.scitotenv.2012.07.066) [DOI] [PubMed] [Google Scholar]
- 70.Hall LW, Anderson RD. 1995. The influence of salinity on the toxicity of classes of chemicals to aquatic biota. Crit. Rev. Toxicol. 25, 281–346. ( 10.3109/10408449509021613) [DOI] [PubMed] [Google Scholar]
- 71.Holmstrup M, et al. 2010. Interactions between effects of environmental chemicals and matural stressors: a review. Sci. Total. Environ. 408, 3746–3762. ( 10.1016/j.scitotenv.2009.10.067) [DOI] [PubMed] [Google Scholar]
- 72.Li H, Hsieh MK, Chien SH, Monnell JD, Dzombak DA, Vidic RD. 2011. Control of mineral scale deposition in cooling systems using secondary-treated municipal wastewater. Water Res. 45, 748–760. ( 10.1016/j.watres.2010.08.052) [DOI] [PubMed] [Google Scholar]
- 73.Hintz WD, Mattes BM, Schuler MS, Jones DK, Stoler AB, Lind L, Relyea RA. 2017. Salinization triggers a trophic cascade in experimental freshwater communities with varying food-chain length. Ecol. Appl. 27, 833–844. ( 10.1002/eap.1487) [DOI] [PubMed] [Google Scholar]
- 74.Canedo-Arguelles M, Sala M, Peixoto G, Prat NÃ, Faria M, Soares AMVM, Barata C, Kefford B. 2016. Can salinity trigger cascade effects on streams? A mesocosm approach. Sci. Total Environ. 540, 3–10. ( 10.1016/j.scitotenv.2015.03.039) [DOI] [PubMed] [Google Scholar]
- 75.Brunk BK, Jirka GH, Lion LW. 1997. Effects of salinity changes and the formation of dissolved organic matter coatings on the sorption of phenanthrene: implications for pollutant trapping in estuaries. Environ. Sci. Technol. 31, 119–125. ( 10.1021/es9602051) [DOI] [Google Scholar]
- 76.Pace ML, Cole JJ, Carpenter SR, Kitchell JF. 1999. Trophic cascades revealed in diverse ecosystems. Trends Ecol. Evol. 14, 483–488. ( 10.1016/S0169-5347(99)01723-1) [DOI] [PubMed] [Google Scholar]
- 77.Morgan RP, Kline KM, Cushman SF. 2007. Relationships among nutrients, chloride and biological indices in urban Maryland streams. Urban Ecosyst. 10, 153–166. ( 10.1007/s11252-006-0016-1) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.