Abstract
Objective
Little was known about the role of socioeconomic status as a risk factor for epistaxis in adult population. The objective of this study was to determine whether socioeconomic status influences the presentation to emergency department for anterior epistaxis in an adult population.
Methods
Retrospective review of emergency department visits from January 2012 to May 2014. The setting is in an emergency department of a Canadian tertiary care centre. Adult patients with primary diagnosis of anterior epistaxis in the emergency department were included in this study. The main outcome was emergency department visits for anterior epistaxis visits.
Results
A total of 351 cases of anterior epistaxis were included. The mean age was 70 years and 51% of patients were male. The patients were stratified into two groups based on whether their age was equal to and above, or below 75 years. Our analysis indicated that those 75 years or older in higher income quintiles have an increased risk of anterior epistaxis compared to the subjects in the lower income quintiles (P < 0.05). This association did not hold true for those younger than 75 years or for all age groups combined.
Conclusion
There is an association between higher socioeconomic status and the presentation to the emergency department with anterior epistaxis in the population older than 75 years but not in younger patients.
Keywords: Epistaxis, Anterior, Socioeconomicstatus, Income, Social determinants of health
Introduction
Epistaxis is one of the most common otolaryngology health problems, affecting 60% of the population at least once in their lifetime.1 Even though most cases are self-limiting, it accounts for 0.46% of all emergency department (ED) visits in the United States.2 The age distribution is bimodal,2, 3 with peak incidences in those under 10 years and over 40 years of age. In adults, more than 90% of bleeds are anterior arising from the Kiesselbach's plexus on the nasal septum.1, 4 The most common causes of epistaxis have been characterised as idiopathic, traumatic, iatrogenic, and neoplastic.5
Several factors influence the risk of epistaxis including age, sex, seasonal variation, smoking, nasal steroid spray use, and the presence of a coagulopathy.2, 5, 6, 7, 8, 9 Less is known about the extent to which any social determinants of health affect the risk of epistaxis. Up to this time, there are no studies investigating the relationship between socioeconomic status (SES) and the incidence of epistaxis. It has been shown the lower SES is associated with higher risk of upper gastrointestinal bleed,10 abnormal uterine bleed11 and hemorrhage during warfarin therapy.12 Our purpose was to determine if there is a significant correlation between SES and the occurrence of anterior epistaxis observed in the ED.
Material and methods
Study design and setting
This retrospective study was approved by the Research Ethics Board at the Ottawa Hospital Research Institute. All the patient visits to the ED at the Ottawa Hospital (TOH), a Canadian tertiary care centre, with the primary diagnosis of anterior epistaxis during the period of January 2012 to May 2014 were reviewed.
Selection of participants
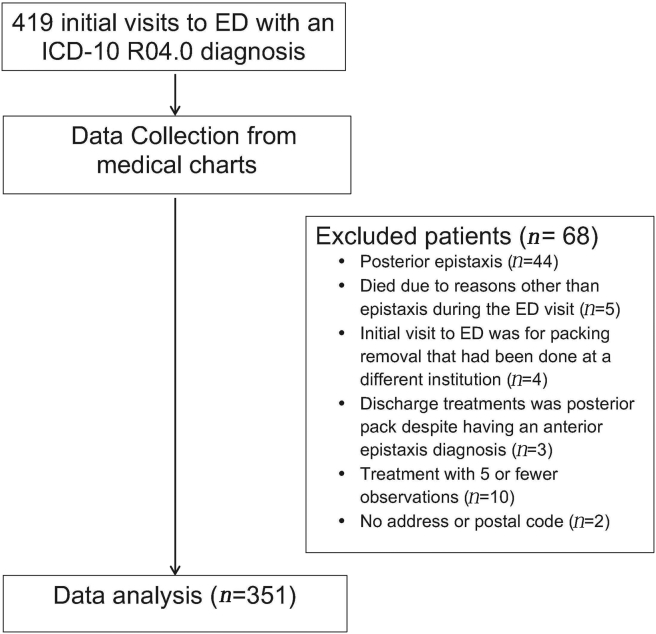
Adult patients with primary diagnosis of epistaxis in the ED were included in the study. The International Classification of Disease, version 10 (ICD-10), code for epistaxis (R04-0) was used to identify the patients and obtain the health records. Since the epistaxis code does not differentiate between anterior and posterior epistaxis, all the records were hand searched to exclude patients with the diagnosis of posterior epistaxis or concurrent anterior and posterior epistaxis. Patients who presented with epistaxis as a complication of a secondary condition, such as end-stage malignancies, or those who died during the ED visit were excluded. Patients with an initial visit to the ED to remove the packing that had been placed at a different institute and those who were treated as posterior epistaxis despite having an anterior epistaxis were excluded as well. Patients who received a treatment modality that was used in less than five cases were also excluded from analysis. Refer to Fig. 1 for the study flow chart.
Fig. 1.
Study flowchart.
Methods and measurements
The data on patient demographics, including age, sex, and postal code, comorbidities, the treatment modalities used, the course in emergency department, admission, concurrent medical disorders, medications and recurrence or ED follow-up information were extracted from the identified charts. Treatment modalities identified for data abstraction included conservative (no treatment), nasal clip, petroleum gauze packing, polyvinyl alcohol (PVA) sponge packing, Gelatin-Thrombin Matrix, Surgicel® Absorbable hemostat, Epistat, silver nitrate cautery, electrocautery, endoscopic surgery, arterial embolization and other treatments not otherwise specified (NOS). These data were used in a previous study13 to assess the current practices for anterior epistaxis management and evaluate the outcomes.
The individual postal codes at the time of presentation were matched to Statistics Canada Census using Statistics Canada Postal Code Conversion File (PCCF + Version 5F) in order to determine the neighbourhood income quintile at the level of dissemination area. The dissemination areas are the smallest standard geographical units in Canada, which provide a more accurate estimate of socioeconomic status compared to other larger reporting areas.14, 15 This method of determining socioeconomic status has been used in several other studies in Ontario.12, 16, 17, 18
Outcome
The primary outcome of this study was the occurrence of anterior epistaxis based on ED visits. We determined the number of outcomes for each of the income quintiles and stratified the data into two groups based on whether the patients were older or younger than the median age (75 years) which created two groups roughly equal in number.
Statistical analysis
All statistical calculations were done using SAS Version 9.3 (SAS Institute, Cary, NC). The categorical variables were summarized using frequency counts and percentages, while continuous variables were summarized by mean (SD) or median (IQR), as appropriate. Chi-square test was used for statistical analysis. A 5% (P < 0.05) level of statistical significant was chosen. Post-hoc power calculation was performed.
Results
Characteristics of study subjects
During the period of January 2012 to May 2014, there were 419 visits to the ED with a diagnosis of anterior epistaxis. After excluding 68 patients due to reasons listed in Fig. 1, a total of 351 anterior epistaxis cases were included in the study. The characteristics recorded co-morbidities of the included patients are summarized in Table 1. The mean age of patients was 70 years, the median was 75 years and 179 (51%) were male. There were 217 (62%) patients on some type of antiplatelet or anticoagulant medication at the time of ED presentation. Overall, the patients were evenly distributed amongst the quintiles with 63 (18%), 74 (21%), 66 (19%), 71 (20%) and 77 (22%) patients in each income quintile from lowest to highest quintiles respectively.
Table 1.
Patient demographics.
| Characteristic | Value |
|---|---|
| Age [mean y (range)] | 70 (14–97) |
| Sex [No. (%)] | |
| Male | 179 (51) |
| Female | 172 (49) |
| Comorbidities [No. (%)] | |
| Hypertension | 198 (56) |
| Diabetes | 67 (19) |
| CADa | 97 (28) |
| Afibb | 94 (27) |
| HHTc | 3 (1) |
| Other Blood disorders | 12 (3) |
| AC/APd Medication Use | 217 (62) |
Coronary artery disease.
Atrial fibrillation.
Hereditary hemorrhagic telangiectasia.
Anticoagulation or antiplatelet.
Main results
Table 2 summarizes the number of patients in each income quintile with diagnosis of anterior epistaxis who is above or under age 75. There were 177 patients equal to or older than 75 years of age, and 174 patients younger than 75 years. For those at or over the age of 75 years, the percentage of patients with anterior epistaxis in each income quintile from lowest to highest was 14%, 19%, 15%, 27% and 24% respectively. There was a statistically significant correlation between the presentation to ED for anterior epistaxis and income quintiles (P < 0.05) for this age group with higher income quintiles being associated with higher number of ED visits for anterior epistaxis. The post-hoc power calculation was completed for this age group to be 0.77 with an alpha of 5%, detecting no change in effect size. However, this correlation was not significant for those under the age of 75 or for the pooled data of patients of all ages.
Table 2.
Presentation to emergency department for anterior epistaxis according to the age and income quintile [No. (%)].
| Years | n | Lowest quintile | Second quintile | Third quintile | Fourth quintile | Highest quintile |
|---|---|---|---|---|---|---|
| ≥75a | 177 | 25 (14) | 34 (19) | 27 (15) | 48 (27) | 43 (24) |
| <75 | 174 | 38 (22) | 40 (23) | 39 (22) | 23 (13) | 34 (19) |
aStatistically significant (P < 0.05).
Discussion
This study suggests that the number of ED visits for anterior epistaxis is significantly higher in those with higher SES and older than 75 years. In addition, utilization of the ED was more common among elderly as the mean and median age of patients were 70 and 75 respectively. This is in accordance to a previous study by Pallin et al2 which demonstrated that the age group of 70–79 had the highest presentation of epistaxis in the adult population.
There are many variables that could contribute to higher utilization of ED for epistaxis in the elderly population. Studies have found that co-morbidities, such as hypertension, diabetes and congestive heart failure, are potential risk factors for epistaxis.6, 19 Hypertension and diabetes are hypothesized to contribute to atherosclerosis of vessels and congestive heart failure has been shown to increase the venous pressure in the nasal vessels, causing damage to the nasal blood vessels.20 Also, there is a higher use of anticoagulant and antiplatelet treatment for various chronic diseases in the elderly which contributes to a higher incidence of epistaxis.
There are possible reasons for which higher SES is associated with a higher presentation to ED for anterior epistaxis. Although many studies conducted in Ontario have concluded that those individuals with lower SES have more frequent emergency department visits compared with higher SES,21, 22, 23 there are several studies which imply that individuals with higher SES seek out and receive treatment sooner and more often than those of lower SES. For example, many studies suggest that people with moderate to higher income and education levels have higher specialist utilization.24, 25, 26 This could have several potential implications. First, it might result in a higher incidence of diagnosis and self-reported severity of diseases, including epistaxis, among a higher SES population; second, it could lead to a higher reported prevalence of chronic disease, such as hypertension, diabetes, and coagulopathies. Third, it could lead to higher medication use, such as anticoagulation therapy, and the inappropriate use of topical nasal steroids, both of which are associated with anterior epistaxis. In support, an Ontario study indicates that individuals with complementary insurance coverage have significantly increased medication use.27 In addition, presentation to ED could be influenced by distance from the hospital, means of transportation and threshold to be present to the hospital, as the first two could be associated with lower socioeconomic status and the latter could be associated with older age.
Furthermore, many environmental factors tend to be geographical or dependent on the living condition and therefore affect people with certain postal codes more than others. Temperature, humidity and pollution levels were found to be associated with higher ED visits for epistaxis.7, 8, 28 Alcohol use is an established risk factor for epistaxis29, 30 and those with higher SES may consume similar or higher amounts of alcohol compared with the lower SES population.31
Study limitations
A limitation of this study is the small sample size, which limited the power of the study to find a statistically significant difference between different SES groups in patients younger than 75. The retrospective design of the study and reliance on hospital medical records of patients visiting ED is another limitation. Furthermore, the data does not represent incidence of anterior epistaxis as it captures only those individuals with a severe enough condition (as determined by the patient) to seek medical attention in the ED. Since the study was conducted at TOH, and socioeconomic status was based on postal codes, there is a potential for recruitment bias. The external applicability of the study also becomes limited, as this population may not be representative of the distribution of SES in Canada or North America. There are other confounding variables that were not captured in this study, seasonal variation, pollution levels, smoking status, and whether the co-morbidities, such as hypertension and diabetes, were controlled. These variables were not adjusted for in the analysis.
It is possible that the findings regarding a higher occurrence of epistaxis in higher SES individuals in the elderly subgroup is an artifact of survival bias of this population. Studies have shown that in patients with co-morbidities associated with epistaxis a higher SES is associated with a lower mortality rate.32, 33 This implies that a greater number of patients in the high SES group in comparison to the low SES group survive over the age of 75 years resulting in variation in baseline characteristics in terms of co-morbidities among different SES groups which is thereby reflected in a higher rate of epistaxis. However, this effect is less pronounced in the younger subgroup as they have not lived long enough in order for the effects of these chronic diseases to affect mortality, and create this bias. This idea of survival bias has been identified and described previously in the medical literature.34, 35, 36, 37
Despite these limitations, this is the first study that looks at the role of SES and occurrence of epistaxis in the adult population. Whether this is a true occurrence rate or simply higher rate of presentation to the hospital in patients of higher SES, the result of this study could be used by physicians for risk recognition and patient education, and by public health planners for assessing the population risk of this Otolaryngological emergency. Primary care providers may be better able to focus their education efforts for anterior epistaxis towards the elderly at risk population in particular when prescribing medications that could increase the occurrence of this emergency. On the other hand, this information will allow Emergency Medicine physicians and Otolaryngologists to be more aware of these risk factors to better understand the presentation of patients who present to a hospital emergency department with anterior epistaxis.
Conclusion
In conclusion, there appears to be an association between higher SES and the occurrence of anterior epistaxis in the population older than 75. Such association does not exist for the population under age of 75. Future studies with prospective design and larger sample size must be conducted in order to re-evaluate whether this association is applicable to other age groups.
Edited by Yu-Xin Fang
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Viehweg T.L., Roberson J.B., Hudson J.W. Epistaxis: diagnosis and treatment. J Oral Maxillofac Surg. 2006;64:511–518. doi: 10.1016/j.joms.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 2.Pallin D.J., Chng Y.M., McKay M.P., Emond J.A., Pelletier A.J., Camargo C.A., Jr. Epidemiology of epistaxis in US emergency departments, 1992 to 2001. Ann Emerg Med. 2005;46:77–81. doi: 10.1016/j.annemergmed.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 3.Walker T.W., Macfarlane T.V., McGarry G.W. The epidemiology and chronobiology of epistaxis: an investigation of Scottish hospital admissions 1995–2004. Clin Otolaryngol. 2007;32:361–365. doi: 10.1111/j.1749-4486.2007.01530.x. [DOI] [PubMed] [Google Scholar]
- 4.Douglas R., Wormald P.J. Update on epistaxis. Curr Opin Otolaryngol Head Neck Surg. 2007;15:180–183. doi: 10.1097/MOO.0b013e32814b06ed. [DOI] [PubMed] [Google Scholar]
- 5.Simmen D.B., Jones N.S. Epistaxis. In: Flint P.W., Haughey B.H., Niparko J.K., editors. Cummings Otolaryngology-Head and Neck Surgery Head and Neck Surgery, 3-Volume Set. Elsevier Health Sciences; London: 2010. pp. 678–690. [Google Scholar]
- 6.Abrich V., Brozek A., Boyle T.R., Chyou P.H., Yale S.H. Risk factors for recurrent spontaneous epistaxis. Mayo Clin Proc. 2014;89:1636–1643. doi: 10.1016/j.mayocp.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Purkey M.R., Seeskin Z., Chandra R. Seasonal variation and predictors of epistaxis. Laryngoscope. 2014;124:2028–2033. doi: 10.1002/lary.24679. [DOI] [PubMed] [Google Scholar]
- 8.Sowerby L.J., DeSerres J.J., Rudmik L., Wright E.D. Role of season, temperature and humidity on the incidence of epistaxis in Alberta, Canada. J Otolaryngol Head Neck Surg. 2014;43:10. doi: 10.1186/1916-0216-43-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waddell A.N., Patel S.K., Toma A.G., Maw A.R. Intranasal steroid sprays in the treatment of rhinitis: is one better than another. J Laryngol Otol. 2003;117:843–845. doi: 10.1258/002221503322542818. [DOI] [PubMed] [Google Scholar]
- 10.Crooks C.J., West J., Card T.R. Upper gastrointestinal haemorrhage and deprivation: a nationwide cohort study of health inequality in hospital admissions. Gut. 2012;61:514–520. doi: 10.1136/gutjnl-2011-300186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matteson K.A., Raker C.A., Clark M.A., Frick K.D. Abnormal uterine bleeding, health status, and usual source of medical care: analyses using the medical expenditures panel survey. J Womens Health (Larchmt) 2013;22:959–965. doi: 10.1089/jwh.2013.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cressman A.M., Macdonald E.M., Yao Z. Socioeconomic status and risk of hemorrhage during warfarin therapy for atrial fibrillation: a population-based study. Am Heart J. 2015;170:133–140. doi: 10.1016/j.ahj.2015.03.014. 140.e1-3. [DOI] [PubMed] [Google Scholar]
- 13.Newton E., Lasso A., Petrcich W., Kilty S.J. An outcomes analysis of anterior epistaxis management in the emergency department. J Otolaryngol Head Neck Surg. 2016;45:24. doi: 10.1186/s40463-016-0138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Southern D.A., Ghali W.A., Faris P.D. Misclassification of income quintiles derived from area-based measures. A comparison of enumeration area and forward sortation area. Can J Public Health. 2002;93:465–469. doi: 10.1007/BF03405041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peller P. University of Calgary; Calgary: 2011. An Analysis of the Postal Code Conversion File's Use in Research; pp. 1–24. [Google Scholar]
- 16.Gershon A.S., Warner L., Cascagnette P., Victor J.C., To T. Lifetime risk of developing chronic obstructive pulmonary disease: a longitudinal population study. Lancet. 2011;378:991–996. doi: 10.1016/S0140-6736(11)60990-2. [DOI] [PubMed] [Google Scholar]
- 17.Kapral M.K., Wang H., Mamdani M., Tu J.V. Effect of socioeconomic status on treatment and mortality after stroke. Stroke. 2002;33:268–273. doi: 10.1161/hs0102.101169. [DOI] [PubMed] [Google Scholar]
- 18.Alter D.A., Naylor C.D., Austin P., Tu J.V. Effects of socioeconomic status on access to invasive cardiac procedures and on mortality after acute myocardial infarction. N Engl J Med. 1999;341:1359–1367. doi: 10.1056/NEJM199910283411806. [DOI] [PubMed] [Google Scholar]
- 19.Ibrashi F., Sabri N., Eldawi M., Belal A. Effect of atherosclerosis and hypertension on arterial epistaxis. J Laryngol Otol. 1978;92:877–881. doi: 10.1017/s0022215100086254. [DOI] [PubMed] [Google Scholar]
- 20.Kanowitz S.J., Citardi M.J., Batra P.S. Contemporary management strategies for epistaxis. In: Stucker F.J., de Souza C., Kenyon G.S., Lian T.S., Draf W., Schick B., editors. Rhinology and Facial Plastic Surgery. Springer; Berlin Heidelberg: 2009. pp. 139–149.http://link.springer.com/chapter/10.1007/978-3-540-74380-4_12 [cited 2016 Apr 20] Available from: [Google Scholar]
- 21.Tozer A.P., Belanger P., Moore K., Caudle J. Socioeconomic status of emergency department users in Ontario, 2003 to 2009. CJEM. 2014;16:220–225. doi: 10.2310/8000.2013.131048. [DOI] [PubMed] [Google Scholar]
- 22.Vanstone N.A., Belanger P., Moore K., Caudle J.M. Socioeconomic composition of low-acuity emergency department users in Ontario. Can Fam Physician. 2014;60:355–362. [PMC free article] [PubMed] [Google Scholar]
- 23.Khan Y., Glazier R.H., Moineddin R., Schull M.J. A population-based study of the association between socioeconomic status and emergency department utilization in Ontario, Canada. Acad Emerg Med. 2011;18:836–843. doi: 10.1111/j.1553-2712.2011.01127.x. [DOI] [PubMed] [Google Scholar]
- 24.Glazier R.H., Agha M.M., Moineddin R., Sibley L.M. Universal health insurance and equity in primary care and specialist office visits: a population-based study. Ann Fam Med. 2009;7:396–405. doi: 10.1370/afm.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunlop S., Coyte P.C., McIsaac W. Socio-economic status and the utilisation of physicians' services: results from the Canadian national population health survey. Soc Sci Med. 2000;51:123–133. doi: 10.1016/s0277-9536(99)00424-4. [DOI] [PubMed] [Google Scholar]
- 26.Haider A., Mamdani M., Shaw J.C., Alter D.A., Shear N.H. Socioeconomic status influences care of patients with acne in Ontario, Canada. J Am Acad Dermatol. 2006;54:331–335. doi: 10.1016/j.jaad.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 27.Allin S., Law M.R., Laporte A. How does complementary private prescription drug insurance coverage affect seniors' use of publicly funded medications. Health Policy. 2013;110:147–155. doi: 10.1016/j.healthpol.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Szyszkowicz M., Shutt R., Kousha T., Rowe B.H. Air pollution and emergency department visits for epistaxis. Clin Otolaryngol. 2014;39:345–351. doi: 10.1111/coa.12296. [DOI] [PubMed] [Google Scholar]
- 29.McGarry G.W., Gatehouse S., Vernham G. Idiopathic epistaxis, haemostasis and alcohol. Clin Otolaryngol Allied Sci. 1995;20:174–177. doi: 10.1111/j.1365-2273.1995.tb00039.x. [DOI] [PubMed] [Google Scholar]
- 30.McGarry G.W., Gatehouse S., Hinnie J. Relation between alcohol and nose bleeds. BMJ. 1994;309:640. doi: 10.1136/bmj.309.6955.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones L., Bates G., McCoy E., Bellis M.A. Relationship between alcohol-attributable disease and socioeconomic status, and the role of alcohol consumption in this relationship: a systematic review and meta-analysis. BMC Public Health. 2015;15:400. doi: 10.1186/s12889-015-1720-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saydah S.H., Imperatore G., Beckles G.L. Socioeconomic status and mortality: contribution of health care access and psychological distress among U.S. adults with diagnosed diabetes. Diabetes Care. 2013;36:49–55. doi: 10.2337/dc11-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bassuk S.S., Berkman L.F., Amick B.C. Socioeconomic status and mortality among the elderly: findings from four US communities. Am J Epidemiol. 2002;155:520–533. doi: 10.1093/aje/155.6.520. [DOI] [PubMed] [Google Scholar]
- 34.Weuve J., Proust-Lima C., Power M.C. Guidelines for reporting methodological challenges and evaluating potential bias in dementia research. Alzheimers Dement. 2015;11:1098–1109. doi: 10.1016/j.jalz.2015.06.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hernán M.A., Alonso A., Logroscino G. Cigarette smoking and dementia: potential selection bias in the elderly. Epidemiology. 2008;19:448–450. doi: 10.1097/EDE.0b013e31816bbe14. [DOI] [PubMed] [Google Scholar]
- 36.van Rein N., Cannegieter S.C., Rosendaal F.R., Reitsma P.H., Lijfering W.M. Suspected survivor bias in case-control studies: stratify on survival time and use a negative control. J Clin Epidemiol. 2014;67:232–235. doi: 10.1016/j.jclinepi.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 37.Naimi A.I., Cole S.R., Hudgens M.G., Brookhart M.A., Richardson D.B. Assessing the component associations of the healthy worker survivor bias: occupational asbestos exposure and lung cancer mortality. Ann Epidemiol. 2013;23:334–341. doi: 10.1016/j.annepidem.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]