Abstract
Micronized particles released from car tires have been found to contribute substantially to microplastic pollution, triggering the need to evaluate their effects on biota. In the present study, four freshwater benthic macroinvertebrates were exposed for 28 days to tread particles (TP; 10–586 μm) made from used car tires at concentrations of 0, 0.1, 0.3, 1, 3, and 10% sediment dry weight. No adverse effects were found on the survival, growth, and feeding rate of Gammarus pulex and Asellus aquaticus, the survival and growth of Tubifex spp., and the number of worms and growth of Lumbriculus variegatus. A method to quantify TP numbers inside biota was developed and here applied to G. pulex. In bodies and faces of G. pulex exposed to 10% car tire TP, averages of 2.5 and 4 tread particles per organism were found, respectively. Chemical analysis showed that, although car tire TP had a high intrinsic zinc content, only small fractions of the heavy metals present were bioavailable. PAHs in the TP-sediment mixtures also remained below existing toxicity thresholds. This combination of results suggests that real in situ effects of TP and TP-associated contaminants when dispersed in sediments are probably lower than those reported after forced leaching of contaminants from car tire particles.
Introduction
During the past decade, extensive research has been conducted to evaluate the emissions and environmental concentrations of microplastics worldwide. A few studies considered micronized particles released from car tires as part of microplastic pollution and concluded that they constitute a significant global source of microplastics.1−3 This fact, together with concerns raised by governmental institutions and the general public about potential adverse effects of particles released from car tires, brings out the need to quantify the amount of car tire particles in the environment and to evaluate their bioavailability and effects on biota.2,4−6
Depending on the generation process and their composition, different car tire particle types are formed. Tread particles (TP) originate from the grinding or abrasion of a tire tread,7 which include the finely crushed rubber particles made from old car tires that are commonly used in synthetic turf fields.4 Tire wear particles (TWP) can be released into the environment as a result of the mechanical abrasion of car tires with the road surface.8 Although rubber is the main constituent of car tires, sulfur and zinc oxide are added during the vulcanization process, black carbon or silica are added as fillers, and oil is added to increase the wet grip performance.2 Besides these general additives, tires can contain other additives depending on their specific properties defined by the application.2,5 In aquatic systems, chemicals may leach out into the aqueous phase at different rates depending on the environmental conditions (temperature, pH, and salinity) and the composition and size of the particles.5,9,10 For instance, total zinc content has been found to be three times higher in TP than in TWP.7 Different release rates can lead to differences in chemical bioavailability and to complex mixture effects.
TWP released from car tires and old tire TP used as infill in artificial turfs are the most important sources for micronized rubber particles in the environment.2 Whereas microplastic detection methods have evolved considerably over the past decade,11 the methodology used to quantify the amount of car tire particles in the environment is still limited. Car tire particle concentrations in water and sediments are estimated based on chemical markers, such as benzothiazoles or zinc, or the rubber type,9 markers that are unable to distinguish between TP and TWP, and may include chemicals from other environmental sources.5 Concentrations in biota have never been measured, probably because their levels are below the detection limits. No laboratory studies on the ingestion of TWP or TP are available either. Car tire particle concentrations have been measured in surface waters (0.09–6.3 mg/L) and sediments (0.3–155 g/kg dry weight).12−17 This indicates that part of the car tire particles entering the surface water may sink to the sediment compartment due to their higher density.18
To date, effect studies done on aquatic biota have mostly focused on the evaluation of the effects of car tire leachates, which were often extracted under conditions forcing chemical release, such as high temperatures and low pH values.9 Some of the leachates were prepared using whole tires,19,20 whereas others were extracted from TP or TWP,10,21−23 which seems to be more toxic, probably due to a faster release of chemicals from smaller particles.9 Although studying the effects of elutriates extracted from car tire particles may be useful for screening level assessments, evaluating the effects of car tire particles in water or sediment under natural conditions is more environmentally realistic.24 For instance, earlier studies showed negative effects of car tire TP on the development of Rana sylvatica larvae at a concentration of 83.8 g/kg of sediment dry weight.25 In contrast, no effects of 10 g of TRWP per kg of sediment dry weight were found for the amphipod Hyalella azteca and the larvae midge Chironomus dilutus.24
Knowing that sediments accumulate settling car tire particles, their bioavailability and effects on benthic macroinvertebrates should be evaluated. Moreover, for a proper assessment of the risks of car tire TP, not only environmentally realistic conditions and concentrations should be used, but also a systematic setup should be followed in order to ensure the comparability among species. In the present study, chronic effects of car tire TP were evaluated for four freshwater benthic macroinvertebrates: the amphipod Gammarus pulex, the isopod Asellus aquaticus, and the worms Tubifex spp. and Lumbriculus variegatus. We used a standardized setup, previously used to evaluate the effects of polystyrene microplastics on the same species.26 Effects were assessed using a wide range of environmentally relevant concentrations of car tire TP, under environmentally realistic conditions. Additionally, for the first time tread particle ingestion and egestion were investigated. This was done for G. pulex by quantifying the amount of particles in their body and in faces at the end of the experiment. Here, G. pulex was used as a model invertebrate species for ingestion, as it was demonstrated to be sensitive to ingested microplastic exposure in an earlier work.26 We developed a method in order to be able to assess TP particles inside organisms, which included testing the resistance of TP rubber materials to animal tissue digestion fluids and developing an image analysis approach for quantifying ingested TP particles.
Materials and Methods
Preparation of Car Tire Tread Particles
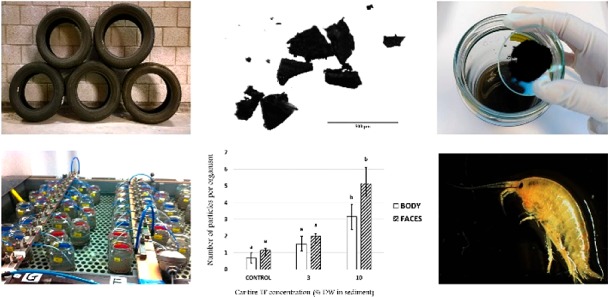
With the purpose of mimicking an environmentally relevant scenario of car tire TP exposure, five second hand tires of various brands were bought in Supervelg (Drunen, The Netherlands) (Supporting Information (SI) Table S1. Using a metal grater, milimiter sized particles were scapped from the first 2 cm of each tire. After freezing the particles with liquid nitrogen to prevent them from burning, they were grinded and sieved over a 500 μm sieve in an Ultra Centrifugal Mill ZM 1000 (Retsch, Germany). A mixture was made by the combination of the five car tires particles at equal weight proportions and this mixture was sieved again over a 500 μm sieve to guarantee any bigger particles being removed.
Characterization of the Car Tire Tread Particles
Particle size distribution (PSD) of the car tire TP mixture was measured by laser diffraction using a Mastersizer 3000 (Malvern Instruments), which is capable to measure particle sizes between 0.01 and 3500 μm. Particle shape was examined under an Olympus SZX10 stereomicroscope. Main constituents of the car tire TP were quantified using thermogravimetric analysis (TGA/DSC 3+, Mettler Toledo). Upon heating the sample, the mass loss was determined allowing to distinguish between (i) volatile substances (e.g., plasticizers, that vaporize between 30- 300 °C), (ii) the actual polymer (300–600 °C), (iii) carbon black (600–850 °C), and the residue, which is composed of (iv) inorganic fillers (e.g., zinc oxide).27,28 For the combustion of carbon black the gas was switched from nitrogen to air (50 mL min–1, see SI Table S2 for instrumental settings). While heating from 300 to 600 °C, the evolved gases were trapped to further characterize comprised polymers. This was done by coupling a cartridge filled with a hydrophilic- lipophilic balanced polymer (HLB, Oasis Water Corporation, Milford, MA) directly to the TGA. Trapped decomposition products were extracted by flushing with 2 mL of Dichlormethane (DCM, Honeywell Research Chemicals, U.S.) of which 2 μL were injected manually in a gas chromatograph coupled to a mass spectrometer (GC–MS, Agilent Technologies, SI Table S2). Characteristic mass spectra of the decomposition products (pyrolysates) of polymers typically used in tire production were taken from literature (SI Table S3).29,30 Their presence was used to identify polymers incorporated in the car tire TP. Finally, the total amount of zinc was quantified. The inorganic residues (120 mg) were exposed to microwave acid extraction using 13% nitric acid (Merck, Suprapur), heated under pressure and kept at temperatures between 133 and 163 °C for 30 min. Subsequently, the sample was filtered and the total amount of zinc was determined using ICP–MS (X Series 2, Thermo Fisher Scientific) (SI Table S2).
Sediments
Freshwater sediments were collected with a standard dip net at Veenkampen (Wageningen, The Netherlands) in December 2016. Previous studies have shown that PAH background concentrations at this location are below toxicity thresholds.26,31−33 Sediments were sieved, homogenized and placed in a freezer at −20 °C. Prior to the experiments, sediments were thawed and thoroughly homogenized again, and four representative subsamples were taken to determine the percentage of Total Organic Matter (TOM) through loss on ignition (3 h, 550 °C), which was 40 ± 0.8% (n = 4). All data are depicted with mean ± standard deviation, unless otherwise stated.
Test Organisms
Following previous procedures,26,31G. pulex and A. aquaticus were collected from a relatively unpolluted brook (Heelsum, The Netherlands) and ditch (Heteren, The Netherlands). L. variegatus were obtained from Wageningen Environmental Research (Wageningen, The Netherlands) and Tubifex spp. were purchased at a local pet shop. Prior to the experiments, organisms were acclimatized for 1 week in aerated buckets with copper-free Dutch Standard Water (DSW) inside a water bath at 16 ± 1 °C while maintaining a 12:12 light:dark cycle. During the acclimatization, G. pulex and A. aquaticus were fed with dry poplar leaves that were collected in the field and Tubifex spp. and L. variegatus were fed with TetraMin fish food pellets.
Experimental Design
Sediments were spiked to achieve the concentrations: 0, 0.1, 0.3, 1, 3, and 10% of car tire TP dry weight in the total sediment mixture. These concentrations correspond to 0, 1.0, 3.0, 10, 30, and 100 g/kg respectively, which are within the range of measured car tire wear and tear particle concentrations in sediments.2 Each experimental unit consisted of a 750 mL glass beaker filled with 211 g of the corresponding car tire TP – sediment mixture. For each concentration, three replicas were made. Two weeks prior to the start of the experiment, beakers were placed in a water bath at a constant temperature of 16 ± 1 °C and aerated. Then, 11 randomly selected individuals from the corresponding species were placed in the experimental units. The starting length of 33 randomly selected individuals from the initial population was assessed as body lengh for A. aquaticus, and head capsule (HD in mm) for G. pulex, from which total length (TL) was calculated as TL = −2.07 + 9.82 HD.34 The average size of G. pulex and A. aquaticus was 4.6 ± 0.8 mm (n = 33) and 4.5 ± 0.5 mm (n = 33), respectively. The starting dry weight of 33 active adult worms from the initial population of Tubifex spp. and L. variegatus was determined. The average dry weight per worm was 0.42 ± 0.05 mg (n = 33) for Tubifex spp. and 1 ± 0.08 mg (n = 33) for L. variegatus. During the experiments, two poplar leaves discs with a diameter of 3 cm were supplied to the beakers of G. pulex and A. aquaticus at day 0 and 14. Poplar leaves discs were previously conditioned with DSW for 3 days. No additional food was needed for Tubifex spp. and L. variegatus due to the high organic matter content of the sediment. Temperature, dissolved oxygen (DO), pH, and NH3 were measured in all beakers once a week, whereas conductivity (EC) was measured only at the start and at the end of the experiment. To keep water levels constant, DSW was added weekly until the end of the experiment. Water quality variables remained constant in all beakers along the experiment (SI Table S4). Unionised levels of ammonia decreased along the experiment for all species, reaching an average of 0.03 ± 0.01 mg NH3/L (n = 12) at the end of the experiment. All un-ionized ammonia levels were always below the LC50 values available for these species.35−37
Analysis of Heavy Metals and PAHs in Sediments Mixed with Car Tire Tread Particles
Especially heavy metals and polycyclic aromatic hydrocarbons (PAH) are relevant in explaining potential chemical effects from sediments polluted with TP.9,38,39 Therefore, sediments with TP mixed from all treatments were analyzed for heavy metals and PAHs at t = 0. Two extra beakers were prepared the same way and at the same time as the experimental units. After the 2 week acclimatization period, sediments from the two duplicates were mixed and freeze-dried. The total amount of zinc (Zn), sulfur (S), cadmium (Cd), chromium (Cr), copper (Cu), nickel (Ni), and lead (Pb) were analyzed using microwave acid extraction with ICP-AES and ICP-MS after destruction with HNO3–HCl.40 Additionally, the sediment-TP mixtures were extracted with 0.01 M CaCl2 to determine the mildly extractable concentrations as a proxy for bioavailable metal concentrations.41 Following earlier procedures,31 PAHs were extracted from the sediment-TP mixtures using accelerated solvent extraction (ASE) and analyzed by HPLC after sample cleanup. Clean-up recoveries for 14 PAH were 91 ± 6%, and ranged from 78 to 98%. Concentrations were corrected for blanks and recoveries. For further details on PAH analysis, the reader is referred to the SI.
Effects on Survival, Growth, and Feeding Rate
After 28 days, the content of each experimental unit was sieved over a 0.35 mm sieve. Surviving organisms were collected, counted and transferred to clean DSW to depurate their gut for 24 h, following procedures from previous microplastic ingestion studies.42−44 The number of worms per replicate was used as end point for L. variegatus instead of survival, as they reproduced by fragmentation during the experiments.45G. pulex and A. aquaticus were preserved in 70% ethanol until their length was measured, which was done in the same way as for the starting population. Growth was determined as the difference in mean length (in mm) of the animals in each replicate at the end minus the mean length from 33 animals at the start of the experiment.The growth of Tubifex spp. and L. variegatus was determined as biomass increase per replicate by subtracting the average dry weight of the starting population from the average dry weight of the populations at the end of the exposure test. Feeding rate (mg dry weight leaf/organism/d) of G. pulex and A. aquaticus was calculated from the loss of the added poplar leaves using the equation from Maltby et al., 2002, described in the SI.46
Resistance of Car Tire TP to H2O2 and Ingestion by G. pulex
Surviving individuals of G. pulex from controls (TP concentration of 0% sediment dry weight) and the two highest exposure concentrations (3 and 10% car tire TP in sediment dry weight), as well as the faeces excreted by these organisms during the 24 h defeacation period, were analyzed for the presence of car tire TP using 30% H2O2 to purify the biota samples.
Resistance of Car Tire TP to H2O2
Prior to the purification of the samples, the resistance of car tire TP to 30% H2O2 was tested. For this, 80 car tire TP cut from the scrapped sample were distributed in eight porcelain cups and dried in an oven at 40 °C for 72h. The mean dry weight of the particles from each cup (n = 10) was measured with a Cubis Micro balance (Sartorius, Germany). Pictures of each particle were taken with a CMEX camera (Euromex, The Netherlands) under an Olympus SZX10 stereomicroscope and the mean particle area (n = 10) was measured using ImageJ Software. Four groups of 10 particles were added to glass beakers containing 30% H2O2 and the other four were added to glass beakers containing Milli-Q water. All glass beakers were placed in a New Brunswick Scientific G25 shaking incubator at 45 °C and 80 rpm for 24 h. After this period, all particles were flushed with water and dried in an oven at 40 °C for 72h. Finally, the mean weight of the particles was measured again and new pictures were taken to calculate the mean particle area.
Ingestion of TP by G. pulex
Following the protocol by Löder et al., 2017 with modifications,47 bodies and faeces of G. pulex were added to 10 mL of 30% H2O2 and placed in a New Brunswick Scientific G25 shaking incubator at 45 °C and 80 rpm for 24 h. The presence of car tire TP in bodies and faeces was studied separatelly, whereas bodies and faeces from each individual replica were pooled and treated together. A subsequent Chitinase step was needed for the body samples to remove all chitine leftovers. For this, to remove the 30% H2O2, each sample was filtered through a stainless steel filter with a mesh size of 10 μm. The residues on the filter were rinsed with 15 mL of phosphate-buffered saline (PBS) solution (pH 5) into a beaker in which 1 mL of Chitinase (EC 3.2.1.14, ASA Spezialenzyme GmbH, Wolfenbüttel, Germany) was added. Samples were placed in a New Brunswick Scientific G25 shaking incubator at 37 °C and 80 rpm for 5 days. Finally, samples were filtered through 25 mm aluminum oxide filters (Anodisc, Whatman, UK) with a pore size of 0.2 μm, which were dried in an oven at 45 °C for at least 48 h. Note that FTIR or Raman microscopy used to identify microplastics in biota, such as in Redondo-Hasselerharm et al., 2018, are not applicable to black particles due to high IR absorption.48,49 Therefore, all black particles found on each of the filters were photographed with a CMEX camera (Euromex, The Netherlands) under an Olympus SZX10 stereomicroscope. Particle size was measured using image analysis software (ImageJ). Only particles within the size range of the original TP mixture were accounted. Finally, the number of black particles within the size range of 10–586 μm found in each filter was divided by the number of surviving individuals in the corresponding replicate at the end of the experiment to obtain the number of particles per organism. Blanks were included (n = 3) to correct for contamination by particles within the targeted size range. Size frequency of the particles found in bodies and faeces of G. pulex at concentrations 3 and 10% were analyzed after measuring their length (in μm) in ImageJ. Following our previously published approach,26 the number of car tire TP per gram of sediment was calculated from the mass of car tire TP per dose, TP density and the measured particle volume distribution.26
Statistical Analysis
Data analysis was done in SPSS 23 (IBM Corp., NY). Generalized Linear Models (GLMs) were applied to study the effects of car tire TP on all end points using the log-transformed concentration as covariate. GLMs were selected based on the data distribution of each end point. One-way ANOVA (p < 0.05) were conducted to determine the effects of car tire TP on the number of worms of L. variegatus, the growth of G. pulex, A. aquaticus, Tubifex spp., and L. variegatus, and the feeding rate of G. pulex and A. aquaticus. One-way ANOVA was also used to study differences in the number of car tire TP found in bodies and faeces of G. pulex at zero concentration (i.e., the blanks) and the two highest concentrations. Residuals were tested for normality using Shapiro-Wilk test (p > 0.05) and visualized with a Q-Q plot. Variances were tested for homogeneity using Levene’s test (p > 0.05). Post hoc multiple comparisons were done using Tukey’s and Bonferroni tests. If the assumption of homogeneity of variances was violated, one-way Welch ANOVA (p < 0.05) was conducted. Independent t test was applied to compare the average dry weight (mg) and the average area (mm2) of the particles before and after the H2O2 and the H2O treatments. The difference in dry weight and area between the particles before and after each treatment was compared between treatments as well.
Results and Discussion
Characterization of Car Tire TP
Particle size distribution of the car tire TP mixture showed an unimodal distribution spanning from approximately 10–586 μm with a modus centered at 239 μm by volume (SI Figure S1A) and 10.5 μm by number of particles (SI Figure S1B). This size distribution included previously reported size ranges for TWP and TP.7,50 Particle shape was found to be generally angulated (SI Figure S2), as described by Kreider et al., 2010 for TP.7
TGA analysis revealed that the tire mixture comprised of volatile substances (7%), polymeric substances (52.4%), carbon black (6.5%) and inorganic fillers (34.1%) (SI Figure S3). Car tire TP were also analyzed individually, revealing a similar composition for all tires used (SI Figure S3). Thus, further analyses were only conducted for the car tire TP mixture. The MS data were screened for the presence of decomposition products of polymers typically used during tire production29,51 after which methyl-butadiene and dipentene, butadiene and styrene were confirmed (SI Figure S4). That implies that the car tire TP consisted of blends of polyisoprene and styrene butadiene rubber (SBR) (SI Table S3). Benzothiazole (indicator m/z 135), used as a vulcanizator during the tire production, was identified too (SI Figure S4). The total amount of zinc was determined for the inorganic tire residues (16.58 g kg–1) which corresponds to 5.65 g zinc kg–1 tire TP mixture.
Analysis of Heavy Metals and PAHs in Sediments Mixed with Car Tire Tread Particles
Metal Analysis
Concentrations of Zn, Cd, Cr, Cu, Ni, Pb, and S in the sediment-TP mixtures did not vary among treatments, except for Zn (SI Table S5). This implies that the added TP did not contain sufficient quantities of these elements to cause a measurable change in overall concentrations, except for Zn. The total concentration of Zn in sediments was linearly correlated (R2 = 0.99) with the nominal concentration of car tire TP in sediment (SI Figure S5). The slope of this line represents the Zn added with every extra 1% of TP, which translates into a tire TP mixture Zn content of 6.54 ± 0.37 g/kg. This is only slightly different from the value of 5.65 mentioned above, which is explained from the different digestion and analytical method used. The linearity illustrates the accuracy of the dosing and the mixing. The data show that by adding TP up to 10% dry weight, the sediment background Zn concentration of 75 mg/kg was increased almost 10-fold to 735 mg/kg. The CaCl2 extractable (bioavailable) concentrations of Zn, however, were a factor of 1000 times lower than the total amount (SI Table S5), and in fact were below the detection limit and remained at least a factor 30 below the LC50 values for Tubifex spp. and L. variegatus (990.1 μg/kg and 2954 μg/kg, respectively).52,53 As for the other CaCl2 extractable elements, only S, Cr, and Ni were detected. They also did not increase with increasing car tire TP concentrations in sediment and also remained at nontoxic concentrations. These chemical data already show that TP elutriate tests are not likely to represent ecologically relevant results as they do not account for the limited bioavailability of metals in the sediment mixture.10,21
PAH Analysis
PAH concentrations did not increase with increasing car tire TP concentrations in sediment (SI Table S6), and PAH concentrations in controls (TP concentration of 0% sediment dry weight) were similar to previously reported PAH concentrations for the same sediment.31 Therefore, we conclude that PAHs did not leach from the car tire TP to the sediment and did not contribute to the PAH concentration in the systems. This is in agreement with previous studies that also reported a low contribution of TP and TWP to PAH concentrations to the environment.7,54 PAHs are not easily extracted even under extreme environmental conditions and its bioavailability is expected to be low.5,7 The Sum of PAHs (∑PAH) for all sediment-TP mixtures was at least 2 times lower than the probable effect concentration (PEC) reported by MacDonald et al., 2000, which is 22.8 mg/kg dry weight.55 Note that outlying PAH concentrations were observed for the lowest TP treatment (0.1%) with factor 10 higher numbers than those for all other treatments and those previously reported for the same sediment.31 We have no conclusive explanation for the outlier but an incidental contamination may have played a role.
Effects on Survival, Growth, and Feeding Rate
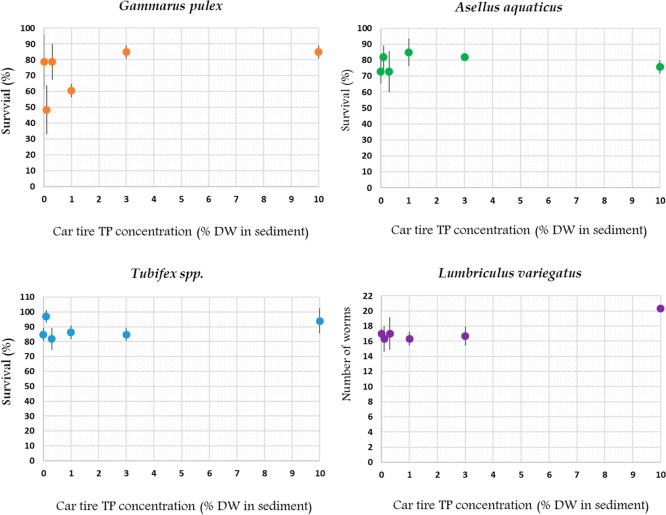
Survival in controls (TP concentration of 0% sediment dry weight) was on average 79%, 73%, and 84% for G. pulex, A. aquaticus, and Tubifex spp., respectively. Data analysis with GLM revealed no significant relationship between the survival of G. pulex, A. aquaticus, and Tubifex spp. and increasing car tire TP concentrations in sediment (GLM, pG. pulex = 0.063; pA. aquaticus = 0.654; pTubifex spp. = 0.692) (Figure 1). For L. variegatus, there was no significant relationship between the number of worms and increasing car tire TP concentrations (GLM, pL. variegatus = 0.380) and no significant differences were found between car tire TP concentration in sediment and the number of worms at the end of the experiment (ANOVA, pL. variegatus = 0.084) (Figure 1). No significant relationship between the growth of G. pulex, A. aquaticus, Tubifex spp., and L. variegatus and increasing car tire TP concentrations in sediment was found (GLM, pG. pulex = 0.554; pA. aquaticus = 0.470; pTubifex spp. = 0.160; pL.variegatus = 0.262). No significant differences between car tire TP concentration in sediment and growth were found for G. pulex, A. aquaticus, Tubifex spp., and L. variegatus (Welch, pG. pulex = 0.334; ANOVA pA. aquaticus = 0.143; pTubifex spp. = 0.054; pL.variegatus = 0.441) (Figure 2). Mean feeding rates were 0.098 ± 0.023 (n = 3) mg d.w./org/d and 0.089 ± 0.029 (n = 3) mg d.w./org/d for G. pulex and A. aquaticus, respectively (sI Figure S6). There was no significant relationship between the feeding rate of G. pulex and A. aquaticus and increasing car tire TP concentrations in sediment (GLM, p G. pulex = 0.520; p A. aquaticus = 0.336) and no significant differences between car tire TP concentration and feeding rate were found for G. pulex and A. aquaticus (ANOVA, pG. pulex= 0.26; pA. aquaticus = 0.595). No adverse effects were found on the survival and growth of G. pulex, A. aquaticus, and Tubifex spp., and the number of worms and growth of L. variegatus. This means that neither the particles themselves nor any of the associated chemicals were toxic at TP concentrations up to 10% sediment dry weight, which complies to the low chemical bioavailability discussed in the previous section. Interestingly, there seems to be a positive trend (GLM p = 0.063) between the survival of G. pulex and car tire TP concentrations in sediment. However, this could have been caused by the incidental high PAH level at 0.1% and lower survival observed for this treatment.
Figure 1.
Survival of G. pulex, A. aquaticus, and Tubifex spp. and number of worms of L. variegatus after 28 days of exposure to car tire TP at increasing concentrations in sediment. Error bars are mean ±1 SD n = 3, except for treatment 1% of Tubifex spp., where n = 2.
Figure 2.
Growth of G. pulex, A. aquaticus, Tubifex spp., and L. variegatus after 28 days of exposure to car tire TP at increasing concentrations in sediment. Error bars are mean ±1 SD n = 3, except for treatment 1% of Tubifex spp., where n = 2.
Our results are in accordance with the findings of Panko et al., 2013, who found no significant adverse effects for benthic invertebrates H. azteca and C. dilutus after a chronic exposure to 10 g TRWP per kg of sediment dry weight.24 In contrast, Camponelli et al. 2009 showed that car tire TP with size <590 μm at 83.8 g/kg of sediment dry weight slowed down the metamorphosis of R. sylvatica larvae and zinc accumulated in their tissues.25 Zinc content in the car tire TP mixture was roughly 0.6% in our study, in comparison to the 1.26% reported by Camponelli et al., 2009. Metal analysis showed that, although zinc content in our sediment was 735 mg/kg at the highest car tire TP concentration, only a small fraction of zinc embedded in the rubber polymer was bioavailable. Several studies are in conformity with this observation.10,56,57 We have two explanations for the differences between our results and those from Camponelli et al., 2009.25 First, their much longer sediment aging time may have increased desorption and thus chemical bioavailability, leading to more pronounced effects. Second, our OM content was much higher than in the study by Camponelli et al., 2009 (40% vs 1.57%). OM is one of the dominant binding phases for hydrophobic organic chemicals as well as for many heavy metals.58−60 Therefore, the desorption of chemicals from our TP and subsequent uptake by OM can be assumed to be higher, leading to a lower bioavailability in our experiments.
Quantifcation of Car Tire TP in Body and Faeces of G. pulex
Resistance of Car Tire TP to H2O2
Mean dry weight (in mg) and area (in mm2) of the car tire tread particles before and after their addition to H2O2 and H2O for 24 h are shown in SI Table S7. No significant differences were found between the mean dry weight (mg) from all particles before and after the H2O2 and H2O treatments (Independent t test, p H2O2 = 0.995; p H2O = 0.955). No significant differences were found between the mean area (mm2) from all particles before and after the H2O2 and H2O treatments (Independent t test, p H2O2 = 0.968; p H2O = 0.974). Furthermore, the difference in area and weight before and after each treatment was not statistically different between H2O2 and H2O treatments (Independent t test, pweight = 0.168; parea = 0.385). These results indicate that the treatment with H2O2 did not affect mass and area of the TP, and thus is not expected to affect the number of car tire TP found in the body and faces of G. pulex.
Ingestion of TP by G. pulex
One-way ANOVA revealed significant differences in the number of black particles with a size range of 10–586 μm found per organism in the body and faces of G. pulex exposed to the control treatment (TP concentration of 0% sediment dry weight) and 10% car tire TP in sediment (ANOVA, pBody = 0.008; pFaces = 0.001) (Figure 3). Significant differences in the number of particles with the same characteristics were also found between organisms of G. pulex exposed to 3 and 10% car tire TP in sediment dry weight (ANOVA, pBody = 0.037; pFaces = 0.003) (Figure 3). After correcting for the number of black particles with a size range of 10–586 μm in controls (TP concentration of 0% sediment dry weight), considering them as contamination of the samples, an average of 2.5 and 4 car tire TP per organism were found in bodies and faces of G. pulex exposed to 10% car tire TP in sediment, respectively. Size frequency of the particles found in bodies of G. pulex ranged from 14 to 272 μm, with an average size of 66 μm (SI Figure S7). Size frequency of the particles found in faces of G. pulex ranged from 14 to 555 μm and had a mean size of 65 μm (SI Figure S8). Although the average particle size found in bodies and faces of G. pulex were similar to the ones reported for PS microplastics, the upper size range was higher for car tire TP than for PS microplastics.26
Figure 3.
Number of black particles with a size between 10 and 500 μm found per organism in the body (white column) and faces (stripped column) of G. pulex after the exposure to 0, 3, and 10% car tire TP in sediment dry weight (DW) for 28 days. Error bars are mean ±1 SD n = 3.
When calculating the number of car tire TP at 10% sediment dry weight, using the average density given by Verschoor et al., 2017 for TWP (1.20 g/cm3), a value of 5.28 × 108 is obtained.61 If we calculate the trophic transfer factor (TTF)62 for car tire TP at 10% sediment dry weight as the number concentration in G. pulex divided by the number of car tire TP in the sediment, we get a TTF of 4.7 × 10–9. In an earlier study,26 we reported a TTF of (4.47 ± 0.35) × 10–11 for PS microplastics retained by G. pulex. This indicates that the TTF for car tire TP is approximately 100 times higher than the TTF of PS microplastics. A total of 1.4 and 5.9 PS microplastics per organism were retained and egested, respectively, at 10% PS microplastics in sediment dry weight.26 This indicates that, although the number of car tire TP retained by G. pulex was higher than the number of retained PS microplastics, the total number of PS microplastics ingested was similar (1.4 + 5.9 = 7.3) as the total number of car tire TP ingested (2.5 + 4 = 6.5). When comparing this value with the number of PS microplastics found in the sediment at the same dose of 10%, which was 3.15 × 1010 PS microplastics, we realize that at the same %, a lower number of car tire TP are found in the sediment. This is due to the higher density of the car tire TP, as well as the presence of a higher number of smaller particles (10–20 μm) in the car tire TP mixture in comparison to the PS microplastics used before.26
General Discussion and Implications
Our study showed that car tire TP, including chemicals associated with this material, did not negatively affect four freshwater benthic invertebrates, even at concentrations of 10% sediment dry weight. This implies that car tire TP effects can be more mild or even absent under ecologically relevant conditions than suggested in elutriate tests.10,19,22 As the maximum Predicted Environmental Concentrations (PEC) in sediments range from 0.3 to 155 g/kg dry weight,9 we can conclude that car tire TP pose a low risk to freshwater benthic invertebrates. This is in agreement with previous studies evaluating the effects of TRWP mixed with sediments on aquatic organisms.21,24 However, potential long-term effects caused by the slow release and gradual environmental increase of bioavailable zinc and other substances caused by aging of rubber particles are not expressed by these experiments. For G. pulex, the ingestion of car tire TP was demonstrated after a 28 day exposure to 10% car tire TP in sediment. An average of 2.5 and 4 car tire TP were found in bodies and faces of G. pulex at this concentration, respectively. This ingestion did not led to negative effects on its survival, growth, or feeding rate. In contrast, in an earlier work, the ingestion of another particle type (PS microplastic) was found to cause a reduction in the growth of the same species, using the same methodology.26 In both cases, particles ingested by G. pulex were found to have a similar average size (57–66 μm).26 This demonstrates that implications of particles probably may be case-specific and that the probably multicausal mechanisms underlying such effects need further attention.
Acknowledgments
This study was funded by the Dutch Technology Foundation TTW, project no. 13940. We acknowledge additional support from KWR; IMARES; NVWA; RIKILT; The Dutch Ministry of Infrastructure and the Environment; The Dutch Ministry of Health, Welfare and Sport; Wageningen Food & Biobased Research; STOWA; RIWA; and water boards Hoogheemraadschap van Delfland, Zuiderzeeland, Rijn en IJssel, Vechtstromen, Scheldestromen, Aa en Maas, de Dommel, and Rivierenland. We gratefully acknowledge Frits Gillissen, John Beijer, Marlies Vollebregt and Guus Frissen for their technical assistance; Patrick Bäuerlein and Claudia Kooijman (KWR Watercycle Research Institute) for their contribution to the characterization of the car tire TP; and Edwin Peeters for his advice on the statistical analysis.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.est.8b05035.
Car tires used for the manufacturing of TP (Table S1); instrumental settings to analyze car tire TP (Table S2); polymer pyrolysates detected in the used car tire TP (Table S3); mean temperature, pH, DO and EC (Table S4); Zn, S, Cd, Cr, Cu, Ni, and Pb concentrations (Table S5); concentration of PAHs (Table S6); mean dry weight and area of car tire TP before and after addition to H2O2 and H2O for 24 h (Table S7); particle size distribution of the car tire TP (Figure S1); pictures of the car tire TP (Figure S2); weight loss of individual car tire TP and their mixture (Figure S3); pyrogram of the analyzed car tire TP (Figure S4); nominal against measured Zn concentration in TP-sediment mixtures (Figure S5); feeding rate of G. pulex and A. aquaticus (Figure S6); size frequency the car tire TP measured in the body (Figure S7) and faces (Figure S8) of G. pulex (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Sundt P.; Schulze P.-E.; Syversen F.. Sources of Microplastic- Pollution to the Marine Environment Project Report, 2014. [Google Scholar]
- Kole P. J.; Löhr A. J.; Van Belleghem F.; Ragas A. Wear and Tear of Tyres: A Stealthy Source of Microplastics in the Environment. Int. J. Environ. Res. Public Health 2017, 14, 1265. 10.3390/ijerph14101265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher J.; Friot D.. Primary Microplastics in the Oceans: A Global Evaluation of Sources; IUCN International Union for Conservation of Nature, 2017. [Google Scholar]
- Oomen A.; de Groot G.. Evaluation of Health Risks of Playing Sports on Synthetic Turf Pitches with Rubber Granulate, 2017. [Google Scholar]
- Wagner S.; Hüffer T.; Klöckner P.; Wehrhahn M.; Hofmann T.; Reemtsma T. Tire wear particles in the aquatic environment - a review on generation, analysis, occurrence, fate and effects. Water Res. 2018, 139, 83–100. 10.1016/j.watres.2018.03.051. [DOI] [PubMed] [Google Scholar]
- Cheng H.; Hu Y.; Reinhard M. Environmental and Health Impacts of Artificial Turf: A Review. Environ. Sci. Technol. 2014, 48, 2114–2129. 10.1021/es4044193. [DOI] [PubMed] [Google Scholar]
- Kreider M. L.; Panko J. M.; McAtee B. L.; Sweet L. I.; Finley B. L. Physical and chemical characterization of tire-related particles: Comparison of particles generated using different methodologies. Sci. Total Environ. 2010, 408, 652–659. 10.1016/j.scitotenv.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Rogge W. F.; Hildemann L. M.; Mazurek M. A.; Cass G. R.; Simoneit B. R. T. Sources of fine organic aerosol. 3. Road dust, tire debris, and organometallic brake lining dust: roads as sources and sinks. Environ. Sci. Technol. 1993, 27, 1892–1904. 10.1021/es00046a019. [DOI] [Google Scholar]
- Wik A.; Dave G. Occurrence and effects of tire wear particles in the environment – A critical review and an initial risk assessment. Environ. Pollut. 2009, 157, 1–11. 10.1016/j.envpol.2008.09.028. [DOI] [PubMed] [Google Scholar]
- Gualtieri M.; Andrioletti M.; Vismara C.; Milani M.; Camatini M. Environ. Int. 2005, 31, 723–730. 10.1016/j.envint.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Mintenig S. M.; Bäuerlein P. S.; Koelmans A. A.; Dekker S. C.; van Wezel A. P. Closing the gap between small and smaller: towards a framework to analyse nano- and microplastics in aqueous environmental samples. Environ. Sci.: Nano 2018, 5, 1640–1649. 10.1039/C8EN00186C. [DOI] [Google Scholar]
- Kumata H.; Sanada; Takada H.; Takashi Historical Trends of N-Cyclohexyl-2-benzothiazolamine, 2-(4-Morpholinyl)benzothiazole, and Other Anthropogenic Contaminants in the Urban Reservoir Sediment Core. Environ. Sci. Technol. 2000, 34, 246–253. 10.1021/es990738k. [DOI] [Google Scholar]
- Spies R. B.; Andresen B. D.; Rice D. W. Jr Benzthiazoles in estuarine sediments as indicators of street runoff. Nature 1987, 327, 697–699. 10.1038/327697a0. [DOI] [Google Scholar]
- Reddy C. M.; Quinn J. G. Environmental Chemistry of Benzothiazoles Derived from Rubber. Environ. Sci. Technol. 1997, 31, 2847–2853. 10.1021/es970078o. [DOI] [Google Scholar]
- Wik A.; Lycken J.; Dave G. Sediment Quality Assessment of Road Runoff Detention Systems in Sweden and the Potential Contribution of Tire Wear. Water, Air, Soil Pollut. 2008, 194, 301–314. 10.1007/s11270-008-9718-8. [DOI] [Google Scholar]
- Ni H.-G.; Lu F.-H.; Luo X.-L.; Tian H.-Y.; Zeng E. Y. Occurrence, Phase Distribution, and Mass Loadings of Benzothiazoles in Riverine Runoff of the Pearl River Delta, China. Environ. Sci. Technol. 2008, 42, 1892–1897. 10.1021/es071871c. [DOI] [PubMed] [Google Scholar]
- Schuchardt B.; Beilfuß S.; Reincke T.; Hofmann O.; Ziebarth N.; Liebezeit G.; Dubaish F.. Müll in der Nordsee – Pilotprojekt zur Relevanz des Eintragspfades Ästuar am Beispiel der Unterweser Studie vor dem Hintergrund der Meeresstrategie-Rahmenrichtlinie (MSRL); Bremen, Germany, 2013. [Google Scholar]
- Besseling E.; Quik J. T. K.; Sun M.; Koelmans A. A. Fate of nano- and microplastic in freshwater systems: A modeling study. Environ. Pollut. 2017, 220, 540–548. 10.1016/j.envpol.2016.10.001. [DOI] [PubMed] [Google Scholar]
- Day K. E.; Holtze K. E.; Metcalfe-Smith J. L.; Bishop C. T.; Dutka B. J. Toxicity of leachate from automobile tires to aquatic biota. Chemosphere 1993, 27, 665–675. 10.1016/0045-6535(93)90100-J. [DOI] [Google Scholar]
- Stephensen E.; Adolfsson-Erici M.; Celander M.; Hulander M.; Parkkonen J.; Hegelund T.; Sturve J.; Hasselberg L.; Bengtsson M.; Förlin L. Biomarker responses and chemical analyses in fish indicate leakage of polycyclic aromatic hydrocarbons and other compounds from car tire rubber. Environ. Toxicol. Chem. 2003, 22, 2926. 10.1897/02-444. [DOI] [PubMed] [Google Scholar]
- Marwood C.; McAtee B.; Kreider M.; Ogle R. S.; Finley B.; Sweet L.; Panko J. Acute aquatic toxicity of tire and road wear particles to alga, daphnid, and fish. Ecotoxicology 2011, 20, 2079–2089. 10.1007/s10646-011-0750-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villena O. C.; Terry I.; Iwata K.; Landa E. R.; LaDeau S. L.; Leisnham P. T. Effects of tire leachate on the invasive mosquito Aedes albopictus and the native congener Aedes triseriatus. PeerJ 2017, 5, e3756 10.7717/peerj.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A.; Rice L. Toxicity of tire wear particle leachate to the marine macroalga, Ulva lactuca. Environ. Pollut. 2010, 158, 3650–3654. 10.1016/j.envpol.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Panko J. M.; Kreider M. L.; Mcatee B. L.; Marwood C. Chronic toxicity of tire and road wear particles to water-and sediment-dwelling organisms. Ecotoxicology 2013, 22, 13–21. 10.1007/s10646-012-0998-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camponelli K. M.; Casey R. E.; Snodgrass J. W.; Lev S. M.; Landa E. R. Impacts of weathered tire debris on the development of Rana sylvatica larvae. Chemosphere 2009, 74, 717–722. 10.1016/j.chemosphere.2008.09.056. [DOI] [PubMed] [Google Scholar]
- Redondo-Hasselerharm P. E.; Falahudin D.; Peeters E. T. H. M.; Koelmans A. A. Microplastic Effect Thresholds for Freshwater Benthic Macroinvertebrates. Environ. Sci. Technol. 2018, 52 (4), 2278–2286. 10.1021/acs.est.7b05367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Januszewicz K.; Klein M.; Klugmann-Radziemska E.; Kardas D. Physicochemical Problems of Mineral Processing Thermogravimetric analysis/pyrolysis of used tyres and waste rubber. Physicochem. Probl. Miner. Process 2017, 53, 802–811. [Google Scholar]
- Wagner M.Thermal Analysis in Practice. Fundamental Aspects; Hanser: München, 2009. [Google Scholar]
- Gueissaz L.; Massonnet G. Study on the discrimination of tires using chemical profiles obtained by Py-GC/MS. J. Anal. Appl. Pyrolysis 2017, 124, 704–718. 10.1016/j.jaap.2016.11.024. [DOI] [Google Scholar]
- Tsuge S.; Ohtani H.; Watanabe C.. Pyrolysis - GC/MS Data Book of Synthetic Polymers : Pyrograms, Thermograms and MS of Pyrolyzates; Elsevier Science, 2011. [Google Scholar]
- Kupryianchyk D.; Reichman E. P.; Rakowska M. I.; Peeters E. T. H. M.; Grotenhuis J. T. C.; Koelmans A. A.; C Grotenhuis J. T.; Koelmans A. A. Ecotoxicological effects of activated carbon amendments on macroinvertebrates in nonpolluted and polluted sediments. Environ. Sci. Technol. 2011, 45, 8567–8574. 10.1021/es2014538. [DOI] [PubMed] [Google Scholar]
- Velzeboer I.; Kupryianchyk D.; Peeters E. T. H. M.; Koelmans A. A. Community effects of carbon nanotubes in aquatic sediments. Environ. Int. 2011, 37 (6), 1126–1130. 10.1016/j.envint.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Kupryianchyk D.; H M Peeters E. T.; Rakowska M. I.; Reichman E. P.; C Grotenhuis J. T.; Koelmans A. A. Long-Term Recovery of Benthic Communities in Sediments Amended with Activated Carbon. Environ. Sci. Technol. 2012, 46, 10735–10742. 10.1021/es302285h. [DOI] [PubMed] [Google Scholar]
- Wilhelm F. M.; Lasenby D. C. Seasonal Trends in the Head Capsule Length and Body Length/Weight Relationships of Two Amphipod Species. Crustaceana 1998, 71, 399–410. 10.1163/156854098X00518. [DOI] [Google Scholar]
- Maltby L. Sensitivity of the crustaceans Gammarus pulex (L.) and Asellus aquaticus (L.) to short-term exposure to hypoxia and unionized ammonia: observations and possible mechanisms. Water Res. 1995, 29, 781–787. 10.1016/0043-1354(94)00231-U. [DOI] [Google Scholar]
- Huang X.; Liang P.; Qian Y. Excess sludge reduction induced by Tubifex tubifex in a recycled sludge reactor. J. Biotechnol. 2007, 127 (3), 443–451. 10.1016/j.jbiotec.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Schubaur-Berigan M. K.; Monson P. D.; West C. W.; Ankley G. T. Influence of pH on the toxicity of ammonia to Chironomus tentans and Lumbriculus variegatus. Environ. Toxicol. Chem. 1995, 14, 713–717. 10.1897/1552-8618(1995)14[713:IOPOTT]2.0.CO;2. [DOI] [Google Scholar]
- Sadiktsis I.; Bergvall C.; Johansson C.; Westerholm R. Automobile tires-A potential source of highly carcinogenic dibenzopyrenes to the environment. Environ. Sci. Technol. 2012, 46 (6), 3326–3334. 10.1021/es204257d. [DOI] [PubMed] [Google Scholar]
- Horner J. M. Environmental health implications of heavy metal pollution from car tires. Rev. Environ. Health 1996, 11, 175–178. 10.1515/REVEH.1996.11.4.175. [DOI] [PubMed] [Google Scholar]
- Van Griethuysen C.; Van Baren J.; Peeters E. T. H. M.; Koelmans A. A. Trace metal availability and effects on benthic community structure in floodplain lakes. Environ. Toxicol. Chem. 2004, 23, 668. 10.1897/02-583. [DOI] [PubMed] [Google Scholar]
- Katayama A.; Bhula R.; Burns G. R.; Carazo E.; Felsot A.; Hamilton D.; Harris C.; Kim Y.-H.; Kleter G.; Koedel W.; et al. Bioavailability of Xenobiotics in the Soil Environment. In Reviews of Environmental Contamination and Toxicology, Vol. 203, pp 1–86. [DOI] [PubMed] [Google Scholar]
- Hurley R. R.; Woodward J. C.; Rothwell J. J. Ingestion of Microplastics by Freshwater Tubifex Worms. Environ. Sci. Technol. 2017, 51 (21), 12844–12851. 10.1021/acs.est.7b03567. [DOI] [PubMed] [Google Scholar]
- Cole M.; Lindeque P.; Fileman E.; Halsband C.; Goodhead R.; Moger J.; Galloway T. S. Microplastic ingestion by zooplankton. Environ. Sci. Technol. 2013, 47 (12), 6646–6655. 10.1021/es400663f. [DOI] [PubMed] [Google Scholar]
- Besseling E.; Wegner A.; Foekema E. M.; Van Den Heuvel-Greve M. J.; Koelmans A. A. Effects of microplastic on fitness and PCB bioaccumulation by the lugworm Arenicola marina (L.). Environ. Sci. Technol. 2013, 47 (1), 593–600. 10.1021/es302763x. [DOI] [PubMed] [Google Scholar]
- Organization for Economic Co-operation and Development . OECD 225 Guidelines for the testing of chemicals. Sediment-Water Lumbriculus Toxicity Test Using Spiked Sediment. Test 225 2007, No. October, 1–31. [Google Scholar]
- Maltby L.; Clayton S. A.; Wood R. M.; McLoughlin N. Evaluation of the Gammarus pulex in situ feeding assay as a biomonitor of water quality: Robustness, responsiveness, and relevance. Environ. Toxicol. Chem. 2002, 21, 361–368. 10.1002/etc.5620210219. [DOI] [PubMed] [Google Scholar]
- Löder M. G. J.; Imhof H. K.; Ladehoff M.; Lö L. A.; Lorenz C.; Mintenig S.; Piehl S.; Primpke S.; Schrank I.; Laforsch C.; et al. Enzymatic Purification of Microplastics in Environmental Samples. Environ. Sci. Technol. 2017, 51, 14283–14292. 10.1021/acs.est.7b03055. [DOI] [PubMed] [Google Scholar]
- Käppler A.; Fischer D.; Oberbeckmann S.; Schernewski G.; Labrenz M.; Eichhorn K.-J.; Voit B. Analysis of environmental microplastics by vibrational microspectroscopy: FTIR, Raman or both?. Anal. Bioanal. Chem. 2016, 408 (29), 8377–8391. 10.1007/s00216-016-9956-3. [DOI] [PubMed] [Google Scholar]
- Löder M. G. J.; Gerdts G.. Methodology used for the detection and identification of microplastics - a critical appraisal. In Marine Anthropogenic Litter; Bergmann M., Gutow L., Klages M., Eds.; Springer International Publishing, 2015; pp 201–227. [Google Scholar]
- Persson B. N. J. Theory of powdery rubber wear. J. Phys.: Condens. Matter 2009, 21, 8. 10.1088/0953-8984/21/48/485001. [DOI] [PubMed] [Google Scholar]
- Milani M.; Pucillo F. P.; Ballerini M.; Camatini M.; Gualtieri M.; Martino S. First evidence of tyre debris characterization at the nanoscale by focused ion beam. Mater. Charact. 2004, 52, 283–288. 10.1016/j.matchar.2004.06.001. [DOI] [Google Scholar]
- Back H. Epidermal uptake of Pb, Cd, and Zn in tubificid worms. Oecologia 1990, 85, 226–232. 10.1007/BF00319405. [DOI] [PubMed] [Google Scholar]
- Phipps G. L.; Mattson V. R.; Ankley G. T. Relative sensitivity of three freshwater benthic macroinvertebrates to ten contaminants. Arch. Environ. Contam. Toxicol. 1995, 28, 281–286. 10.1007/BF00213103. [DOI] [Google Scholar]
- Zakaria M. P. 200. Distribution of polycyclic aromatic hydrocarbons (PAHs) in rivers and estuaries in Malaysia. A Widespread Input Petrogenic PAHs 2002, 36, 1907–1918. [DOI] [PubMed] [Google Scholar]
- MacDonald D. D.; Ingersoll C. G.; Berger T. A. Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch. Environ. Contam. Toxicol. 2000, 39, 20–31. 10.1007/s002440010075. [DOI] [PubMed] [Google Scholar]
- Wik A.; Dave G. Acute toxicity of leachates of tire wear material to Daphnia magna—Variability and toxic components. Chemosphere 2006, 64, 1777–1784. 10.1016/j.chemosphere.2005.12.045. [DOI] [PubMed] [Google Scholar]
- Wik A.; Nilsson E.; Källqvist T.; Tobiesen A.; Dave G. Toxicity assessment of sequential leachates of tire powder using a battery of toxicity tests and toxicity identification evaluations. Chemosphere 2009, 77, 922–927. 10.1016/j.chemosphere.2009.08.034. [DOI] [PubMed] [Google Scholar]
- Schwarzenbach R. P.; Gschwend P. M.; Imboden D. M.. Environmental Organic Chemistry, 3rd ed.; 2016. [Google Scholar]
- Lin J.-G.; Chen S.-Y. The relationship between adsorption of heavy metal and organic matter in river sediments. Environ. Int. 1998, 24 (3), 345–352. 10.1016/S0160-4120(98)00012-9. [DOI] [Google Scholar]
- Karickhoff S. W.; Brown D. S.; Scott T. A. Sorption of hydrophobic pollutants on natural sediments. Water Res. 1979, 13 (3), 241–248. 10.1016/0043-1354(79)90201-X. [DOI] [Google Scholar]
- Verschoor A.; de Poorter L.; Dröge R.; Kuenen J.; de Valk E.. Emission of Microplastics and Potential Mitigation Measures, 2016. [Google Scholar]
- DeForest D. K.; Brix K. V.; Adams W. J. Assessing metal bioaccumulation in aquatic environments: The inverse relationship between bioaccumulation factors, trophic transfer factors and exposure concentration. Aquat. Toxicol. 2007, 84, 236–246. 10.1016/j.aquatox.2007.02.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.