Abstract
Semi-intensive free-range farm systems are common in Australia, and these systems frequently practise on-range feeding. The objective of this study was to investigate the benefit of on-range choice feeding on flock performance, egg quality, and range use of free-range laying hens using black soldier fly larvae (Hermetia illucens, BSF). A total of 160 mature ISA brown laying hens, previously determined to range daily, were allocated to a control group (control) or a treatment group (BSF) with various replicates depending on the parameter investigated. All hens were fed ad libitum indoors with a wheat-soy based diet formulated according to breed requirements. Black soldier fly hens were offered dried BSF larvae ad libitum on the range. Body weight, feed intake, BSF intake, egg production, feed conversion ratio, internal and external egg quality parameters, and individual range use using radio-frequency identification (RFID) technology was evaluated. Black soldier fly hens consumed on average 15 ± 1.7 g BSF larvae/hen per day. There were no differences between BSF and control hens for any of the performance parameters obtained (P > 0.05). Egg weight, shell weight, and shell thickness of eggs from BSF hens were significantly lower (P = 0.003, P = 0.001, and P = 0.004, respectively) than those of eggs from control hens. Egg yolk colour was significantly paler in eggs from BSF hens (P < 0.001). No significant ranging differences between the BSF and control hens were observed (P > 0.05) except for BSF hens showing longer total maximum time for a single visit to the range (P = 0.011). In conclusion, the average intake of BSF larvae indicated a good level of acceptance. Feed formulation should be adjusted for the intake of the choice fed source. The impact of choice-feeding on range use was minor.
Keywords: Behaviour, Chicken, Insect protein, Nutrition, Poultry, Radio-frequency identification
1. Introduction
Following the trend in Europe and the USA, free-range egg production in Australia is a rapidly growing sector, with an ongoing volume growth of 10.2% annually (Australian Eggs, 2017). On-range feeding of free-range laying hens frequently occurs in Australia, and is especially common in farms using mobile housing sheds (Ruhnke et al., 2015a, Singh et al., 2017). Feed supplements offered on-range to free-range hens have been reported to include shell grit (42.9%), limestone (40.0%), hay (28.6%), silage (8.6%), and others such as vegetables, pasture, insects, and harvested grass (37.1%) (Ruhnke et al., 2015a). Beneficial effects of diet supplementation on gizzard barrier function and a positive modification of intestinal microbiota including reduced intestinal Escherichia coli counts and increased lactic acid bacteria concentrations have been observed in laying hens fed with maize silage, barley-pea silage, or carrots (Shrestha et al., 2013, Steenfeldt et al., 2007). However, access to feed supplements can dilute the formulated nutrient intake significantly with severe consequences; for example, pasture on the range has been associated with gut impaction, malnourishment, and increased flock mortality (Ruhnke et al., 2015b, Iqbal et al., 2017). Therefore, only choice feeding of high nutritional value feeds should be considered.
Black soldier fly (Hermetia illucens, BSF) larvae contain up to 35% ether extract and 47% crude protein on a dry matter basis (Newton et al., 1977, St-Hilaire et al., 2007, De Marco et al., 2015, Payne et al., 2015). Black soldier fly larvae also contain around 5% calcium, much higher than many insect species (Khusro et al., 2012). A recent report indicated that there was no effect on meat colour, pH, meat composition, and sensory traits in broiler quails when 15% of BSF was used in the diet (Cullere et al., 2016, Cullere et al., 2017). There is very limited information available on the impact of BSF larvae on layer performance and egg quality.
Previous research indicates indoor feeding schedules may be a factor in limiting range use as birds may prefer to stay inside or close to the main barn to ensure feed access at specific times of day (Bubier and Bradshaw, 1998). While there are biosecurity risks linked to on-range feeding, the potential impact of on-range feeding on range usage has not yet been examined. Good use of the outdoor area is of interest to egg producers since the Federal Court of Australia determined that in order to declare eggs “free range”, “hens have meaningful and regular access to the outdoors” (CAF, 2014). The possibility that feed on the range encourages hens to increase their range use may be one of the main motivations for farmers to conduct this practice. The objective of the present study was to investigate the impact of on-range choice feeding of BSF larvae on (objective part A) flock performance (objective part B) egg quality, and (objective part C) range use of free-range laying hens.
2. Materials and methods
The study was approved by the Animal Ethics Committee of the University of New England (AEC15-120). Animals were housed and treated in accordance with the Australian Model Code of Practice for domestic poultry (Primary Industries Standing Committee, 2002).
2.1. Diet and BSF larvae
Feed was formulated using commercial software (Concept 5, version 12.07.01, CFC Tech Services Inc, Pierz, MN, USA) according to the breeders' standard recommendations and mixed at the University of New England, Australia (ISA, 2016). Details of the diet composition are outlined in Table 1. Hens were fed ad libitum indoors with this complete wheat-soy based diet in mash form. Black soldier fly larvae were obtained from a commercial producer (Entofood SDN BHD, Rawang, Malaysia) and were reared on brewery spent grain before being dried at 90 °C for 60 min until the dry matter content of 90% was reached. Black soldier fly larvae were analysed by a commercial company (APAF, Australian Proteome Analysis Facility, Maquarie University, NSW, Australia; Table 2) and fed whole (Fig. 1). Table 3 provides an overview of the experimental design and outlines the timeline of data collection.
Table 1.
Ingredients and nutrient concentration of the complete diet offered ad libitum to all hens subject to this study.
| Item | Content |
|---|---|
| Ingredients, kg/100 kg | |
| Wheat | 64.5 |
| Soybean meal | 21.8 |
| Meat meal | 1.00 |
| Canola oil | 2.02 |
| Limestone | 6.06 |
| Limestone fine grit | 4.03 |
| Salt | 0.23 |
| UNE layer premix1 | 0.10 |
| Choline Cl 60% | 0.039 |
| DL-methionine | 0.160 |
| Xylanase powder | 0.006 |
| Phytase (5,000 U/g) | 0.012 |
| Jabiru red 10% | 0.004 |
| Jabiru yellow | 0.003 |
| Nutrient concentration (as calculated)2, g/kg | |
| Crude protein | 184.2 |
| Ether extract | 37.4 |
| Crude fibre | 22.7 |
| Calcium | 42.0 |
| Available phosphorus | 3.45 |
| Dig. lysine | 8.15 |
| Dig. methionine | 4.00 |
| Dig. methionine & cysteine | 7.09 |
| Dig. threonine | 5.63 |
| Dig. isoleucine | 7.87 |
| Dig. valine | 8.75 |
| Dig. arginine | 11.0 |
| Linoleic acid | 17.9 |
| Metabolisable energy, kcal/kg | 2.797 |
Provided g/kg of diet: DL-α-tocopheryl acetate 10; menadione 1.5; thiamine 1.2; riboflavin 3; pyridoxine hydrochloride 2; niacin 15; pantothenic acid 6; folic acid 0.35; ferrous iron 30; zinc 50; manganese 50; copper 6.5; selenium 0.1; molybdenum 1; cobalt 0.2; cyanocobalamin 0.01 biotin 0.065; retinol 6.5; cholecalciferol 2.0.
The nutritional value of the feed was calculated using a commercial feed formulation software (Concept 5, version 12.07.01, CFC Tech Services Inc, Pierz, MN, USA).
Table 2.
Composition of the black soldier fly (BSF) larvae offered to hens of the treatment group (% in dry matter).
| Nutrient concentration | Content |
|---|---|
| Crude protein | 46.7 |
| Ether extract | 42.2 |
| Crude fibre | 1.56 |
| Ash | 5.56 |
| Calcium | 2.94 |
| Total phosphorus | 0.91 |
| Avail. phosphorus | 0.80 |
| Dig. lysine | 5.44 |
| Dig. methionine | 0.73 |
| Dig. methionine & cysteine | 1.24 |
| Dig. cysteine | 0.52 |
| Dig. threonine | 2.91 |
| Dig. isoleucine | 4.04 |
| Dig. tryptophane | 1.28 |
| Dig. valine | 4.92 |
| Dig. arginine | 4.82 |
| Dig. histamine | 3.19 |
| Dig. leucine | 6.00 |
| Dig. phenylalanine | 3.54 |
| Dig. tyrosine | 7.73 |
| Linoleic acid | 0.17 |
| Apparent metabolisable energy, kcal/kg | 4.156 |
Fig. 1.
Black soldier fly (BSF) larvae were offered to hens subject to the treatment group as whole. Courtesy: David Waugh.
Table 3.
This table provides an overview outlining the various objectives evaluated at various time points and hen age.
| Experimental week | Week 1 | Week 6 | Week 12 | ||
|---|---|---|---|---|---|
| Age of the hens, weeks | 50 | 56 | 62 | ||
| Parameters evaluated | performance | range use | performance | range use | egg quality |
| Assessment of objective | part A | part C | part A | part C | part B |
| Numbers of hens used | 160 | 120 | 160 | 120 | 70 |
2.2. Investigation of choice feeding with BSF larvae on flock performance – objective part A
2.2.1. Animals and housing
A total of 160 ISA brown laying hens, 47 weeks of age, were used from a previous experiment conducted at the same facility as the current experiment that assessed the impacts of 3 different outdoor stocking densities on ranging behaviour and welfare (Campbell et al., 2017a, Campbell et al., 2017b). In the previous experiment, a total of 450 hens from a flock of 900 (housed within 6 pens, of the total 9 pens available at the facility) had been evaluated for their individual range use using radio-frequency identification (RFID) tracking across 15 weeks (starting at 21 weeks of age). The birds for the current experiment were selected based on showing daily range access during the tracking periods but with individual variation in total time spent outdoors each day. These exclusively outdoor ranging birds compared to birds that preferred to stay indoors, or sporadically accessed the outdoors (Campbell et al., 2017a) were selected to maximise the opportunities for BSF hens to consume the insects.
The majority of birds in the previous experiment did show daily range access (Campbell et al., 2017a), and thus 160 hens were randomly selected from a list of outdoor-preferring birds within each pen, equally (within one bird) across the 3 different stocking densities. Birds from all stocking densities were balanced across 8 pens with 20 birds per pen. Birds for the current experiment remained within the same facility and were housed in the 8 pens at 41 weeks of age with the current experiment beginning at 47 weeks of age. Between 37 and 39 weeks of age, selected birds remained within their different stocking densities while a separate set of birds underwent behavioural testing (Campbell et al., 2016). From 40 to 41 weeks of age, the birds selected for the current experiment were housed together in 2 pens while the litter from the previous experiment was replaced; pen resources were reorganised; and all other birds were rehomed. Birds used in the current experiment still had opportunities for daily range access during the transition period between experiments but their range usage was not tracked.
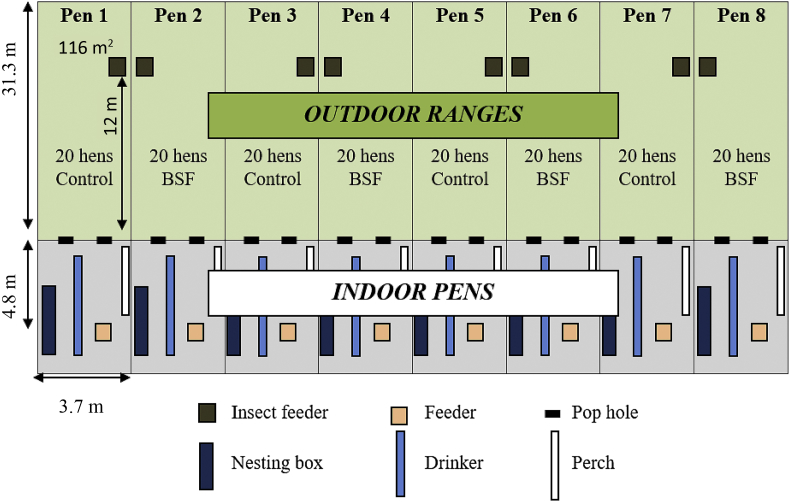
Each of the pens was of identical configuration and equipped with perches, nestboxes, a water nipple line, and a floor area of 38.9 m2 covered with rice hulls (Fig. 2). Hens were assigned to a treatment group (BSF) or a control group (control), allowing 4 replicate pens of each treatment (20 birds per pen). Each pen replicate had access to a separate range area of 115.8 m2 between 09:00 and 19:00 daily via pop holes (Fig. 2). This range area was reconfigured from the previous experiment on outdoor stocking densities (Campbell et al., 2017a). There was minimal vegetation on the range as it had been consumed by birds from the previous experiment. The range area was devoid of any additional trees or structures and birds had visual contact between the range areas. Birds were kept inside at all other times. The health of all birds (general activity, feather cover, visible signs of disease/injury) and presence of adequate food and water was visually checked twice daily.
Fig. 2.
The arrangement of the pens housing hens of the black soldier fly (BSF) group and the control groups subject to objective part A: Investigating the impact of BSF choice feeding on flock performance. The outlined set up resulted in a stocking density of 0.88 m2/hen indoors, and 5.79 m2/hen on the range.
2.2.2. Data collection
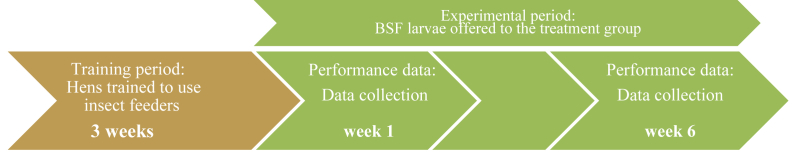
All hens were trained for the duration of 3 weeks to use biosecure insect feeders next to their feeder in the pen, filled with the complete diet (training period). Dried BSF were subsequently made available using the same biosecure feeders and offered ad libitum on the range for the 4 BSF pen replicates (experimental period), while the hens of the control group had empty feeders present. This experimental feeding period lasted 6 weeks. The timeline of the experiment is summarised in Fig. 3. Eggs were collected and individually weighed daily during the 6 weeks of the experiment. The average daily egg weight and egg production per pen were calculated. Individual body weights and feeder weights were obtained weekly using a 3-digit scale (BAT1, Veit Electronics, Moavany, Czech Republic). Feeders were refilled on the same occasion. Body weight gain, feed intake per pen Eq. (1) and BSF intake per pen Eq. (2) were recorded weekly. Egg mass and FCR were calculated as follows Eq. (3) and Eq. (4).
| (1) |
| (2) |
where B is the beginning of the week (Monday), E is the end of the week (Monday), and n is the number of hens in the pen.
| (3) |
| (4) |
Fig. 3.
Schematic overview of the timeline to evaluate the impact of choice feeding with black soldier fly (BSF) larvae on flock performance.
2.3. Investigation of choice feeding with BSF larvae on egg quality – objective part B
2.3.1. Animals and housing
A total of 70 ISA brown laying hens randomly selected hens from the cohort used for part A of the objective were randomly assigned to either a control group (no BSF offered, n = 35), or a treatment group (BSF offered, n = 35). All 70 hens (selected from the 160 hens used in part A of the study) were treated and fed as described above. The biosecure feeders on the range were available to these hens for 6 additional weeks, allowing for a complete experimental period of 12 weeks (Fig. 4). All 70 hens used for this study were individually numbered and had been randomly allocated to groups of 5 within the BSF and control groups. Thus, the experimental unit was defined as 5 pooled hens per treatment and a total of 7 replicates per group were used for statistical analyses. The 70 hens were consolidated into 2 separate pens for the data collection.
Fig. 4.
Schematic overview of the timeline to evaluate the impact of choice feeding with black soldier fly (BSF) larvae on egg quality.
2.3.2. Data collection
Eggs from individual hens were collected after the 12 week experimental period on 2 consecutive days. Internal and external egg quality parameters were evaluated on the day of collection by a single person blinded to the treatment and then averaged for the individual hen across the 2 days: egg weight, shell colour by reflectivity, egg shell breaking strength by quasi-static compression, shell deformation to breaking point, albumen height, Haugh Unit, and yolk colour (all egg quality equipment: Technical Services and Supplies, Dunnington, York, UK) (Haugh, 1937, Bhale et al., 2003). Egg yolk colour was measured within a range from 1 to 15, palest to the darkest. Each shell was weighed and its thickness measured (Mitutoyo Dial Comparator gauge Model 2109-10, Kawasaki, Japan) (Roberts et al., 2013).
2.4. Investigation of choice feeding with BSF larvae on hen range use – objective part C
2.4.1. Animals and housing
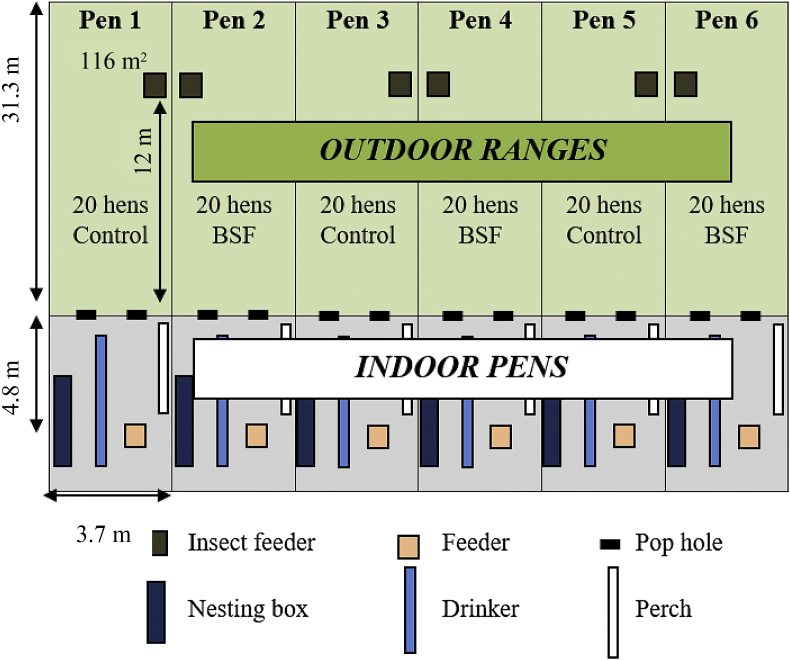
Six of the 8 pens used for the performance evaluation (objective part A) were also used to monitor range use at the same time using RFID technology. Only 6 pens were used due to availability of the RFID monitoring equipment. Three RFID antenna systems were obtained from Microchips Australia Pty Ltd (Keysborough, VIC, Australia), using software developed and built by Dorset Identification B.V. (Aalten, Netherlands) based on Trovan technology and were situated within the pens prior to bird placement. Hens were individually banded with a numbered leg band (Roxan Developments, Datamars Agri UK Ltd, Selkirk, UK) containing a RFID microchip (Trovan Unique ID 100 FDX-A, Trovan Ltd, East Yorkshire, UK) as described by Campbell et al. (2017a). Hens of 3 pens were assigned to the BSF group (BSF larvae access), and hens of the control group (3 pens) were exposed to an empty feeder on the range (control; Fig. 5). Two pop holes leading to the same range were available for each pen (20 hens/pen; Fig. 2). For more details on the set-up and validation of the RFID system see Campbell et al. (2017a).
Fig. 5.
The pens used for objective part C: Investigating the impact of black soldier fly (BSF) choice feeding on range use.
2.4.2. Data collection
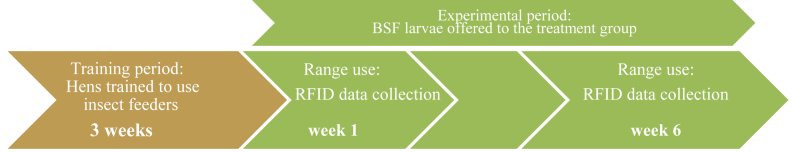
Range use was recorded during the first and last 7 consecutive days of the 6 week experimental period (Fig. 6). Each pop hole antenna registered the date, time, and microchip of the individual bird moving through the pop hole onto the range, and passing back through the pop hole into the indoor pen. All RFID data were then run through a custom-built software program (Bryce Little; CSIRO, Agriculture and Food, St Lucia QLD, Australia) to filter out unpaired ‘false’ readings if a bird sits in the pop hole for example. The program then summarised the daily data per hen from week 1 to week 6 to provide the average daily hours spent outside, total hours outside, the average number of daily visits, total visits outside, average minimum and maximum time per visit, and the total minimum and maximum length of a single visit.
Fig. 6.
Schematic overview of the timeline to evaluate the impact of choice feeding with black soldier fly (BSF) larvae on range use. RFID = radio-frequency identification.
2.5. Statistical analysis
To analyse data obtained for objective part A, mean values per replicate (pen) were used. Data were tested for normality using the Komolgov-Smirnoff, and analysed using a 2 × 2 factorial arrangement (treatment × time including their interaction) in a general linear model.
Data obtained for objective part B were tested for normality of distribution using the Shapiro–Wilk normality test and then compared to each other using the t-test or Wilcoxon–Mann–Whitney test when appropriate.
The ranging data/hen obtained for objective part C were summarised each day and averaged per week and pen, as the pen was considered the statistical unit. All data were arranged 2 × 2 factorial (time point × treatment group and their interaction) tested for normality and analysed using a general linear model.
All statistical analyses were processed in SPSS statistics 24 (SPSS Inc., Chicago, IL, USA) with significance set at P = 0.05.
3. Results
3.1. Flock performance
Hens allocated to the BSF treatment consumed on average 15 ± 1.7 g BSF/hen per day, which equals 16% of their diet. Flock performances of control vs. BSF group at the end of the experiment were respectively: egg production 82.8% vs. 91.6%, egg weight 66.9 vs. 67.5 g, feed intake (including BSF intake) 94.6 vs. 94.3 g/day, feed conversion ratio (FCR) 1.71 vs. 1.73, body weight 2.00 vs. 2.04 kg. The large variation in some of the data was the major reason why no statistically significant differences were obtained (Table 4). There were no significant differences (all P > 0.05) between control and BSF treatment groups or between week 1 and week 6 of the evaluation period for these parameters (Table 4). All birds were in visibly good condition throughout the trial.
Table 4.
The effect of choice feeding with black soldier fly (BSF) dry larvae (Hermetia illucens) for the duration of 6 weeks on the flock performances of 160 ISA Brown free range laying hens.
| Item | Treatment | Time1 |
P-value |
|||
|---|---|---|---|---|---|---|
| Week 1 | Week 6 | Treatment | Time | Treatment × Time | ||
| Egg production, % | Control group | 95.2 ± 0.62 | 82.8 ± 4.24 | 0.482 | 0.165 | 0.681 |
| BSF group | 91.7 ± 2.47 | 91.6 ± 2.11 | ||||
| Egg weight, g | Control group | 67.9 ± 0.62 | 66.9 ± 0.75 | 0.548 | 0.219 | 0.637 |
| BSF group | 68.1 ± 0.65 | 67.5 ± 0.34 | ||||
| Egg mass, g | Control group | 64.7 ± 2.07 | 55.5 ± 3.47 | 0.415 | 0.063 | 0.093 |
| BSF group | 62.4 ± 2.19 | 61.9 ± 1.36 | ||||
| Feed intake, g/day per hen | Control group | 108.6 ± 4.01 | 94.6 ± 6.98 | 0.674 | 0.074 | 0.958 |
| BSF group | 107.7 ± 4.06 | 94.3 ± 9.59 | ||||
| Feed conversion ratio, kg egg/kg feed | Control group | 1.68 ± 0.03 | 1.71 ± 0.07 | 0.916 | 0.834 | 0.190 |
| BSF group | 1.73 ± 0.04 | 1.73 ± 0.08 | ||||
| Body weight, kg/hen | Control group | 2.03 ± 0.03 | 2.00 ± 0.03 | 0.412 | 0.558 | 0.650 |
| BSF group | 2.04 ± 0.02 | 2.04 ± 0.02 | ||||
Mean values ± standard error of the mean of 80 hens, 4 replicates per treatment.
3.2. Egg quality
Eggs produced from hens with access to the BSF larvae for the duration of 12 weeks were significantly lower in weight (P = 0.003), shell weight (P = 0.001), and shell thickness (P = 0.004) than the eggs from hens in the control group (Table 5). Egg yolk colour was significantly paler (P = 0.001), with a lower yolk colour number, in eggs from the treatment group. There was no significant effect of BSF choice feeding on the egg quality for the following parameters: shell breaking strength, shell deformation, shell thickness, shell reflectivity, albumen height, and Haugh unit (all P > 0.05).
Table 5.
Effect of choice feeding with black soldier fly (BSF) dry larvae (Hermetia illucens) for the duration of 12 weeks on the internal and external egg quality.
| Item | Control group1 | BSF group1 | P-value |
|---|---|---|---|
| Egg weight, g | 71.7 ± 0.97a | 67.3 ± 0.57b | 0.0032 |
| Breaking strength, N | 46.0 ± 1.18 | 47.3 ± 1.42 | 0.506 |
| Shell deformation, mm | 0.290 ± 0.00 | 0.283 ± 0.00 | 0.467 |
| Albumen height, mm | 9.18 ± 0.28 | 8.91 ± 0.21 | 0.466 |
| Haugh unit | 92.7 ± 4.44 | 92.1 ± 4.01 | 0.798 |
| Yolk colour score | 11.7 ± 0.35a | 10.3 ± 0.69b | <0.0012 |
| Shell weight, g | 6.99 ± 0.17a | 6.55 ± 0.28b | 0.0012 |
| Shell thickness, mm | 0.457 ± 0.00a | 0.446 ± 0.00b | 0.0042 |
| Shell reflectivity | 24.9 ± 2.22 | 24.4 ± 1.77 | 0.643 |
a, b Different superscripts in each row for each factor differ significantly (P < 0.05).
Mean values ± standard error of the mean of 35 hens, 7 replicates per treatment.
Statistically highly significant (P < 0.005).
3.3. Range use
There were no significant differences in range usage between the control and BSF group at either time point except for the BSF hens showing a longer total maximum time for a single visit outside across the evaluation period (P = 0.011). However, the average time on the range, the total time on the range, the average number of visits per day and the total number of visits significantly decreased over time (P < 0.001; Table 6).
Table 6.
Range usage of control hens compared to hens with on-range choice feeding of black soldier fly (BSF).
| Item | Treatments | Time1 |
P-value |
|||
|---|---|---|---|---|---|---|
| Week 1 | Week 6 | Treatment | Time | Treatment × Time | ||
| Average time on range per day, h | Control group | 5.82 ± 0.12a | 5.12 ± 0.15b | 0.675 | 0.0002 | 0.299 |
| BSF group | 6.05 ± 0.13a | 5.03 ± 0.20b | ||||
| Total time on range, h | Control group | 40.8 ± 0.83a | 35.9 ± 1.07b | 0.675 | 0.0002 | 0.299 |
| BSF group | 42.4 ± 0.94a | 35.2 ± 1.40b | ||||
| Average visits on range per day | Control group | 25.0 ± 1.13a | 18.0 ± 0.80b | 0.056 | 0.0002 | 0.0273 |
| BSF group | 21.1 ± 0.87a | 18.3 ± 0.93b | ||||
| Total visits | Control group | 175.1 ± 7.92a | 125.9 ± 5.61b | 0.056 | 0.0002 | 0.0273 |
| BSF group | 147.7 ± 6.07a | 127.9 ± 6.47b | ||||
| Average minimum time outside per visit, h | Control group | 0.016 ± 0.00 | 0.032 ± 0.00 | 0.123 | 0.589 | 0.978 |
| BSF group | 0.062 ± 0.04 | 0.079 ± 0.05 | ||||
| Average maximum time outside per visit, h | Control group | 0.975 ± 0.03 | 1.13 ± 0.08 | 0.058 | 0.369 | 0.164 |
| BSF group | 1.19 ± 0.06 | 1.16 ± 0.08 | ||||
| Minimum time outside, h | Control group | 0.004 ± 0.001 | 0.006 ± 0.001 | 0.061 | 0.587 | 0.0323 |
| BSF group | 0.006 ± 0.001 | 0.005 ± 0.001 | ||||
| Maximum time outside, h | Control group | 1.46 ± 0.06b | 1.72 ± 0.14b | 0.0113 | 0.303 | 0.451 |
| BSF group | 1.94 ± 0.15a | 1.98 ± 0.19a | ||||
a,b Different superscripts in each row for each factor differ significantly (P < 0.05).
Mean values ± standard error of the mean of 60 hens within 3 replicates per treatment, total n = 120 hens.
Statistically highly significant (P < 0.005).
Statistically significant (P < 0.05).
4. Discussion
The laying hens consumed an average of 15 g/hen per day of BSF which equals 16% of their diet and indicates a good level of acceptance. In broiler chickens and broiler quails, H. illucens was included at 15% and 25% in the formulated diet, which did not affect overall feed intake, nor product quality (De Marco et al., 2015, Cullere et al., 2016). The fact that choice feeding with BSF did not have any effect on flock performance for the duration of 6 weeks of feeding indicates the high nutritional value of this ingredient. The total feed intake (control diet and larvae) was much lower in both groups at the end of the experimental period, compared to the beginning (108 vs. 94 g/hen per day control; 107 vs. 94 g/hen per day BSF; Table 4), but failed to be statistically significant due to the high variation. With hens aged 50 to 60 weeks, feed intake is expected to remain constant (ISA, 2016). In addition, the total feed intake during the experiment was lower than the breeder standard (122 g/hen per day; ISA, 2016). While the standards are generated on hens kept in optimal housing conditions with controlled environmental temperature and humidity, hens in this experiment were exposed to fluctuating summer temperatures up to 38 °C. Therefore, a reduced feed intake of the experimental animals could be expected (Talukder et al., 2010).
The observed egg production corresponds to the value found in the literature for 50-week-old hens and decreased as expected with the age of the hens (from 95% to 83% in the control group) but remained constant for hens of the BSF group (92%; Table 4) (ISA, 2016). Potential factors affecting the production rate of the control group only, such as plumage damage and/or wounds indicative of severe feather pecking and/or aggression, were not observed and do not provide a possible explanation for the obtained results. Overall, the average egg weights obtained of >67 g can be classified as large, extra large, or jumbo depending on the country of evaluation. These relatively large eggs are most likely a consequence of the relatively heavy hen body weight and a possibly positive influence of the more than satisfactory linoleic acid content of the feed (Leeson and Summers, 2005). However, egg weight decreased in both groups between the beginning and the end of the trial (from 67.9 to 66.9 g in the control group; from 68.1 to 67.5 g in the treatment group), during a period when it would be expected to increase slightly (from 63.5 to 64 g; ISA, 2016). This could be due to the (non-significant) loss of body weight, the reduction of the feed intake, or an early indicator of the fact that BSF larvae intake dilutes some of the nutrients of the formulated diet. Table 7 compares the composition of the treatment diet taking the dilution factor of the BSF larvae into account (84% of control diet + 16% of BSF larvae). While the dietary requirements of the main components are met, the impact of feed dilution on microminerals can only be speculated. The yolk colour was significantly paler in the BSF hens (10.3 in the treatment group vs. 11.7 in the control group), suggesting that BSF larvae are less pigmented than the control diet. Artificial colourings (red and yellow) were used in the control diets. The amount of colouring received for BSF hens was lower compared to the control hens due to the feed dilution and hence the results obtained on the egg yolk colour support the possibility that micronutrients may be lacking in hens of the BSF group. However, when hens were 61 weeks of age at the end of the experiment, the observed egg weight for the control and BSF groups was 66.9 and 67.5 g, which is within the range of the expected 60 to 70 g (Roberts and Ball, 2003, ISA, 2016).
Table 7.
Comparison of the control and treatment diets with the nutritional requirements for >50-week-old laying hens (% as fed).1
| Item | 100% control diet | 84% control diet + 16% BSF larvae | Requirements >50 weeks of age |
|---|---|---|---|
| Crude protein | 18.4 | 22.2 | 17.5 |
| Calcium | 4.20 | 4.97 | 4.5 |
| Available phosphorus | 0.35 | 0.41 | 3.8 |
| Dig. lysine | 0.82 | 1.47 | 0.85 |
| Dig. methionine | 0.40 | 0.44 | 0.46 |
| Dig. methionine & cysteine | 0.71 | 0.77 | 0.73 |
| Dig. threonine | 0.56 | 0.89 | 0.59 |
| Dig. isoleucine | 0.79 | 1.24 | 0.76 |
| Dig. valine | 0.88 | 1.44 | 0.90 |
| Apparent metabolisable energy, kcal/kg | 2.794 | 2.938 | 2.842 |
BSF = black soldier fly.
Reference: ISA, 2016, Leeson and Summers, 2005.
The nutritional profile of the BSF larvae used in this experiment is well within the expected profile (Tran et al., 2015, Makkar et al., 2014). However, exposing the BSF larvae to an intense drying process of 90 °C for 60 min may have resulted in a reduced availability of heat labile amino acids (lysine, arginine, threonine) due to the Maillard reaction between the former and the aldehyde group of reducing sugars (Newkirk et al., 2003). It is known that increasing feed processing temperatures between 60 and 95 °C significantly impairs nutrient availability and ileal digestibility in broilers diets (Amezcua and Parsons, 2007, Lundblad et al., 2011, Liu et al., 2013). The fact that choice feeding hens with BSF for a duration of 12 weeks affected some egg quality parameters suggests that digestibility studies are needed to evaluate the best method for BSF processing. Feed formulation should be adjusted and feed intake should be accounted for if BSF are to be offered free choice as an alternative protein source.
A decrease in egg mass combined with a decrease of the feed intake led to a constant FCR during the experiment. While the FCR remained constant, the observed values were much lower than expected (1.7 vs. 2.2 kg egg/kg feed), as a consequence of the low feed intake (ISA, 2016). The only research related to the use of insect feeding on FCR was performed in broilers using Musca domestica and Tenebrio molitor (Ocio and Viñaras, 1979, Awoniyi et al., 2003, Dordevic et al., 2008). In those studies, no significant effect of using these alternative protein sources on FCR was observed (Ocio and Viñaras, 1979, Awoniyi et al., 2003, Dordevic et al., 2008). Further studies on the use of BSF on long term performance and maximum inclusion levels in the feed are warranted. Shell weight was significantly lower in the treatment group, due to a lower egg weight compared to the control group. The observed values for the egg shell quality correspond to the values found in the literature for 60-week-old hens (Roberts and Ball, 2003).
In order to provide all hens with the same opportunity to access the insect feeder on the range, hens that were known to access the outdoors daily were specifically selected for this study. We also provided hens with a housing and range space that resulted in a stocking density of 0.88 m2/hen indoors and 5.79 m2/hen on the range. This is much lower than the current Australian industry standard of 30 kg (∼20 hens) per m2 indoors and 1 m2/hen on the range (Primary Industries Standing Committee, 2002, CAF, 2016), and also lower than the current European standard of 9 hens/m2 indoors, and at least 4 m2/hen outdoors (Directive EU, 1999, Regulation, 1999). Using such a low stocking density allowed us to evaluate hen movement without competition and constraints on resources (e.g., pop hole access, feeder space). Despite these extremely low densities, range usage was not enhanced by the presence of an additional feed source on the range. The additional attraction of the BSF reward did not encourage hens to range more compared to hens of the control group in birds with established range use patterns. This finding contradicts the general perception that feed on the range is a suitable tool to encourage range usage. However, the incentive for hens to access the range for a food reward might be higher in hens that generally prefer to stay indoors and needs further research.
In general, range usage depends on factors such as flock size, number of pop holes, shelter on the range, age and experience of the flock, and weather conditions (Bubier and Bradshaw, 1998, Nicol et al., 2003, Hegelund et al., 2005, Glatz et al., 2010). As the flock and housing parameters were the same for all hens in this study, the weather conditions and the age are the most likely major factors contributing to the change in range use over time. The impact of hen age on range use has been equivocal in various studies. While some researchers found range usage to be positively correlated with age and explained this by an increase in familiarity, others researchers found a decrease with age and suspected it was due to a loss of interest based on novelty (Hegelund et al., 2005, Pettersson et al., 2016). In the present study, the biosecure insect feeders may have been the novelty factor leading to initial curiosity and ranging motivation. Since hens were rewarded with feed being present in the feeders during the training period, the association with the feeder would have been positive. However, hens of both treatment groups decreased their ranging activity over time regardless the content of the feeder (BSF or no BSF larvae).
5. Conclusion
In conclusion, laying hens consumed an average of 15 g BSF larvae/hen per day, indicating a good level of acceptance. Choice feeding with BSF larvae for the duration of 6 weeks had no effect on hen performance, indicating its high nutritional value. Choice feeding hens with BSF larvae for the duration of 12 weeks did affect egg quality, indicating that feed formulation needs to be adjusted in choice feeding situations. However, further studies on the long term performance and maximum inclusion level of BSF larvae in feed are warranted. The additional on-range feeding with BSF larvae did not affect the range usage of outdoor preferring hens with established range use patterns.
Acknowledgments
This study was funded by the Poultry CRC, established and supported under the Australian Government's Cooperative Research Centres Program. Poultry CRC, PO Box U242, University of New England, Armidale, NSW 2351, Australia (project number 1.5.6). We thank the following persons for their help in field and laboratory work: C. Keerqin, N. Morgan, N. Sharma and N.K. Sharma. We are also grateful to the staff from the University of New England for the support they provided, particularly: S. Song, R. Woodgate, L. Lisle, G. Cluley, M. Porter, G. Chaffey and L. Roan.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Amezcua C.M., Parsons C.M. Effect of increased heat processing and particle size on phosphorus bioavailability in corn distillers dried grains with solubles. Poult Sci. 2007;86:331–337. doi: 10.1093/ps/86.2.331. [DOI] [PubMed] [Google Scholar]
- Australian Eggs . 2017. Annual report.https://www.australianeggs.org.au/who-we-are/annual-reports/#item-754 [Google Scholar]
- Awoniyi T.A.M., Aletor V.A., Aina J.M. Performance of broiler – chickens fed on maggot meal in place of fishmeal. Intern J Poult Sci. 2003;2:71–274. [Google Scholar]
- Bhale S., No H.K., Prinyawiwatkul W., Farr A.J., Nadarajah K., Meyers S.P. Chitosan coating improves shelf life of eggs. J Food Sci. 2003;68:2378–2383. [Google Scholar]
- Bubier N.E., Bradshaw R.H. Movement of flocks of laying hens in and out of the hen house in four free range systems. Br Poult Sci. 1998;39:5–6. doi: 10.1080/00071669888025. [DOI] [PubMed] [Google Scholar]
- CAF . Meeting of ministers for consumer affairs. Thursday 31 march 2016 canberra. 2016. Ministers for consumer affairs. http://consumerlaw.gov.au/files/2016/03/CAF_Communique_March_2016.pdf. [Google Scholar]
- CAF. 2014. http://www.judgments.fedcourt.gov.au/judgments/Judgments/fca/single/2014/2014fca1028.
- Campbell D.L.M., Hinch G.N., Dyall T.R., Warin L., Little B.A., Lee C. Outdoor stocking density in free-range laying hens: radio-frequency identification of impacts on range use. Animal. 2017;11:121–130. doi: 10.1017/S1751731116001154. [DOI] [PubMed] [Google Scholar]
- Campbell D.L.M., Hinch G.N., Downing J.A., Lee C. Outdoor stocking density in free-range laying hens: effects on behaviour and welfare. Animal. 2017;11:1036–1045. doi: 10.1017/S1751731116002342. [DOI] [PubMed] [Google Scholar]
- Campbell D.L.M., Hinch G.N., Downing J.A., Lee C. Fear and coping styles of outdoor-preferring, moderate-outdoor and indoor-preferring free-range laying hens. Appl Anim Behav Sci. 2016;185:73–77. [Google Scholar]
- Cullere M., Tasoniero G., Giaccone V., Mitto-Scapin R., Claeys E., DeSmet S., Dalle Zotte A. Black soldier fly as dietary protein source for broiler quails: apparent digestibility, excreta microbial load, feed choice, performance, carcass and meat traits. Animal. 2016;10:1923–1930. doi: 10.1017/S1751731116001270. [DOI] [PubMed] [Google Scholar]
- Cullere M., Tasoniero G., Giaccone V., Acuti G., Marangon A., Dalle Zotte A. Black soldier fly as dietary protein source for broiler quails: meat proximate composition, fatty acid and amino acid profile, oxidative status and sensory traits. Animal. 2017:1–8. doi: 10.1017/S1751731117001860. [DOI] [PubMed] [Google Scholar]
- Primary Industries Standing Committee . 4th ed. CSIRO Publishing; Collingwood, Victoria, Australia: 2002. Model code of practice for the welfare of animals, domestic poultry. [Google Scholar]
- De Marco M., Martínez S., Hernandez F., Madrid J., Gai F., Rotolo L., Belforti M., Bergero D., Katz H., Dabbou S., Kovitvadhi A., Zoccarato I., Gasco L., Schiavone A. Nutritional value of two insect larval meals (Tenebrio molitorand Hermetia illucens) for broiler chickens: apparent nutrient digestibility, apparent ileal amino acid digestibility and apparent metabolizable energy. Anim Feed Sci Technol. 2015;20:209–218. [Google Scholar]
- Directive EU Council Directive 99/74/EC of 19 July 1999 laying down minimum standards for the protection of laying hens. Off J Eur Commun. 1999:53–57. [Google Scholar]
- Dordevic M., Radenkovic-Damnjanovic B., Vucinic M., Baltic M., Teodorovic R., Jankovic L., Vukasinovic M., Rajkovic M. Effect of the substitution of fish meal with fresh and dehydrated larvae of the house fly (Musca domestica L.) on productive performance and health of broilers. Acta Vet. 2008;58:357–368. [Google Scholar]
- Glatz P.C., Rodda B.K., Rimmington H., Wyatt C., Miao Z.H. Attracting laying hens into range areas using shade and forage. APSS. 2010;21:134. [Abstract] [Google Scholar]
- Haugh R.R. The Haugh unit for measuring egg quality. US Egg Poult Mag. 1937;43 522–555 and 572–573. [Google Scholar]
- Hegelund L., Sørensen J.T., Kjær J.B., Kristensen I.S. Use of the range area in organic egg production systems: effect of climatic factors, flock size, age and artificial cover. Br Poult Sci. 2005;46(1):1–8. doi: 10.1080/00071660400023813. [DOI] [PubMed] [Google Scholar]
- Iqbal Z., Roberts J., Perez-Maldonado R.A., Goodarzi Boroojeni F., Swick R.A., Ruhnke I. Pasture, multi-enzymes, benzoic acid and essential oils positively influence performance, intestinal organ weight and egg quality in free-range laying hens. Br Poult Sci. 2017 doi: 10.1080/00071668.2017.1403566. [DOI] [PubMed] [Google Scholar]
- ISA . 2016. Isa Brown production guide alternative production systems; p. 56. [Google Scholar]
- Khusro M., Andrew N.R., Nicholas A. Insects as poultry feed: a scoping study for poultry production systems in Australia. World’s Poult Sci J. 2012;68:435–446. [Google Scholar]
- Leeson S., Summers J.D. Nottingham University Press; Nottingham, UK: 2005. Commercial poultry nutrition 3rd ed. [Google Scholar]
- Liu S.Y., Selle P.H., Cowieson A.J. Influence of white- and red-sorghum varieties and hydrothermal component of steam-pelleting on digestibility coefficients of amino acids and kinetics of amino acids, nitrogen and starch digestion in diets for broiler chickens. Anim Feed Sci Technol. 2013;186:53–63. [Google Scholar]
- Lundblad K.K., Issa S., Hancock J.D., Behnke K.C., McKinney L.J., Alavi S., Prestlokken E., Sorensen M. Effects of steam conditioning at low and high temperature, expander conditioning and extruder processing prior to pelleting on growth performance and nutrient digestibility in nursery pigs and broiler chickens. Anim Feed Sci Technol. 2011;169:208–217. [Google Scholar]
- Makkar H.P.S., Tran G., Heuze V., Ankers P. State-of-the-art on use of insects as animal feed. Anim Feed Sci Technol. 2014;197:1–33. [Google Scholar]
- Nicol C.J., Potzsch C., Lewis K., Green L.E. Matched concurrent case control study of risk factors for feather pecking hens on free range commercial farms in the UK. Br Poult Sci. 2003;44:515–523. doi: 10.1080/00071660310001616255. [DOI] [PubMed] [Google Scholar]
- Newkirk R.W., Classen H.L., Scott T.A., Edney M.J. The digestibility and content of amino acids in toasted and non-toasted canola meals. Can J Anim Sci. 2003;83:131–139. [Google Scholar]
- Newton G.L., Booram C.V., Barker R.W., Hale O.M. Dried Hermetia illucenslarvae meal as a supplement for swine. J Anim Sci. 1977;44:395–400. [Google Scholar]
- Ocio E., Viñaras R. House fly larvae meal grown on municipal organic waste as a source of protein in poultry diets. Anim Feed Sci Technol. 1979;4:227–231. [Google Scholar]
- Payne C.L.R., Scarborough P., Rayner M., Nonaka K. A systematic review of nutrient composition data available for twelve commercially available edible insects, and comparison with reference values. Trends Food Sci Technol. 2015;47:69–77. [Google Scholar]
- Pettersson I.C., Freire R., Nicol C.J. Factors affecting ranging behaviour in commercial free-range hens. World’s Poult Sci J. 2016;72:137–150. [Google Scholar]
- Regulation, Council Council Regulation (EC) No 1804/1999 of 19 July 1999 supplementing Regulation (EEC) No 2092/91 on organic production of agricultural products and indications referring thereto on agricultural products and foodstuffs to include livestock production. Off J. 1999;24:08. [Google Scholar]
- Roberts J.R., Ball W. Egg and egg shell quality guidelines for the Australian egg industry. APSS. 2003;15:91–94. [Google Scholar]
- Roberts J.R., Chousalkar K., Samiullah K. Egg quality and age of laying hens: implications for product safety. CSIRO publishing. Anim Prod Sci. 2013;53:1291–1297. [Google Scholar]
- Ruhnke I., De Koning C., Drake K., Choct M., Singh M. Feed practices in Australian free-range egg production. ESPN. 2015;20:250. [Abstract] [Google Scholar]
- Ruhnke I., Cowling G., Sommerlad M., Swick R.A., Choct M. Gut impaction in free range hens. APSS. 2015;26:242–246. [Google Scholar]
- Shrestha A., Norup L.R., Juul-Madsen H.R., Steenfeldt S., Afrose S., Engberg R.M. The influence of maize silage supplementation on selected intestinal bacteria and the course of Ascaridia galli infection in organic layers. ESPN. 2013:160. [Abstract] [Google Scholar]
- Singh M., Ruhnke I., DeKoning C., Drake K., Skerman A., Hinch G.N., Glatz P.C. Free-range poultry survey: demographics and practices of semi-intensive free-range farming systems in Australia with an outdoor stocking density of ≤1500 hens/hectare. PLoS One. 2017;12:10. doi: 10.1371/journal.pone.0187057. e0187057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Hilaire S., Sheppard C., Tomberlin J.K., Irving S., Newton L., McGuire M.A., Mosley E.E., Hardy R.W., Sealey W. Fly prepupae as a feedstuff forrainbow trout, Oncorhynchus mykiss. J World Aquacult Soc. 2007;38:59–67. [Google Scholar]
- Steenfeldt S., Kjaer J.B., Engberg R.M. Effect of feeding silages or carrots as supplements to laying hens on production performance, nutrient digestibility, gut structure, gut microflora and feather pecking behaviour. Br Poultry Sci. 2007;48:454–468. doi: 10.1080/00071660701473857. [DOI] [PubMed] [Google Scholar]
- Talukder S., Islam T., Sarker S., Islam M.M. Effects of environment on layer performance. J Bangladesh Agric Univ. 2010;8:253–258. [Google Scholar]
- Tran G., Heuze V., Makkar H.P.S. Insects in fish diets. Anim Front. 2015;5:37–44. [Google Scholar]