Abstract
The gut microbiota plays important roles in animal overall health and productiveness. Balancing host gut microbiota by probiotics has been documented. Our previous study showed that Saccharomyces boulardii (Sb) and Bacillus subtilis B10 (Bs) significantly improve growth performance and modulate the intestinal histomorphology in broilers. To increase the knowledge regarding Sb and Bs, this study investigated the effects of these 2 probiotic strains on the gut microbiota in broilers. Three hundred 1-day-old Sanhuang broilers (Chinese cross breed) were randomly divided into 3 groups, each group with 5 replications (n = 20). The control group (CK) was fed a basal diet containing an antibiotic (virginiamycin, 20 mg/kg) and the other 2 groups received Sb and Bs (1 × 108 cfu/kg of feed) in addition to the basal diet. After 72 d of treatment, pyrosequencing revealed that the bacterial communities varied along the section of intestinal tract in the control and Bs groups, but not in the Sb group. No difference in microbial diversity was observed among 3 groups. The major phyla observed along the GI tract of broilers (particularly in the duodenum and cecum) were Firmicutes, Bacteroidetes, Proteobacteria, and Verrucomicrobia, which were considered potentially growth performance-related. Bacteroidetes, Proteobacteria, and Verrucomicrobia were observed at a much higher abundance in the jejunums and ileums of the Sb group (P < 0.05). In addition, the jejunal microbial communities formed 3 different clusters at either the genus level or the category of metabolism among the groups, based on the principal component analyses. These data indicated that Sb and Bs can modulate the microbial ecosystem, and subsequently enhance the health status of broilers.
Keywords: Saccharomyces boulardii, Bacillus subtilis B10, Gastrointestinal tract, Microbiota, Broiler
1. Introduction
The gastrointestinal (GI) tract is home to a complex and dynamic microbial ecosystem — a so-called ‘microbiota superorganism’, which contains hundreds of microbe species (Apajalahti et al., 2004, Wei et al., 2013, Zoetendal et al., 1998). The genomes of these intestinal microbes form a microbiome that by far outnumbers the host's genome (Cisek and Binek, 2014). Consequently, these gut microbes play a key role in host energy metabolism and immune functions and greatly contribute to a wide range of processes involved in gastrointestinal development, including regulation of intestinal epithelial proliferation (Forder et al., 2007), vitamin synthesis and ion absorption, carbohydrate and protein fermentation (Hamer et al., 2012), bile acid biotransformation (Degirolamo et al., 2014, Ridlon et al., 2006), protection against pathogens and immune system modulation (Guarner, 2006, Guarner and Malagelada, 2003, Noverr and Huffnagel, 2004, Round and Mazmanian, 2009).
Bacterial colonization of the animal gut by environmental microbes begins immediately at birth or after hatching. The composition of the gut microbiota is modulated by numerous extrinsic factors such as age, diet, medication, and stress (Claesson et al., 2011, De La Cochetiere et al., 2008, Louis et al., 2007, O'Toole and Claesson, 2010). Additionally, balance among the gastrointestinal microbial communities is crucial for host health maintenance. Disturbances of the gut microbiota, also known as dysbiosis, have often been associated with several diseases including inflammatory bowel diseases (Frank et al., 2011), diabetes (Cani et al., 2008), obesity (Ley et al., 2006), fatty liver (Dumas et al., 2006), and anxiety (Neufeld et al., 2011). The gut microbiota composition is readily changeable (Jia et al., 2008); consequently, this plasticity favors the development of gut microbiota-targeted therapies such as antibiotics, prebiotics, and probiotics. Antibiotics in feed have been successfully utilized since the 1950s for growth promotion during food-animal production (Dibner and Richards, 2005, Gaskins et al., 2002). However, antibiotic resistance among bacterial pathogens and antibiotic residues in animal products has garnered global interest in limiting antibiotic use in animal agriculture (Seal et al., 2013). Probiotics are live microorganisms that provide beneficial effects to the host when adequately administered. Researchers have shown that probiotic bacteria have a variety of beneficial effects, including counteraction of dysbiosis, promotion of gut health and homeostasis, promotion of growth enhancement of immune defenses and protection of the host from infection by pathogens (Aureli et al., 2011, Cisek and Binek, 2014).
Poultry has become one of the most prominent sources of animal protein worldwide; therefore, the gut microbiome of chicken is a major interest of investigators attempting to improve the growth, health and food safety of poultry (Kohl, 2012, Oakley et al., 2013, Wise and Siragusa, 2007). Our previous study showed that Saccharomyces boulardii (Sb) and Bacillus subtilis B10 (Bs) significantly improve the growth performance and modulate the morphology of the intestine (the intestinal villus height, width, and goblet cell number are increased in the Sb and Bs groups) in broilers (Rajput et al., 2013a). Accordingly, we speculated that the administration of these 2 probiotic strains might yield a common or similar gut microbial community to that induced by virginiamycin treatment or even a more optimal gut microbial structure than virginiamycin in broilers. In addition, there is little information available concerning the effect of these 2 probiotic strains on the gut microbiota in broilers. Therefore, this study is aimed to interpret the beneficial effects of the 2 probiotic strains in the gut microbiota perspective.
2. Materials and methods
Procedures involving animals were performed in according to the guideline of the declaration of Zhejiang Animal Center at the Institute of Medical Science, and approved by the Ministry of Livestock, Zhejiang University, Hangzhou, China.
2.1. Bacteria and yeast
Bacterial strains Sb and Bs used here were isolated and identified by the Institute of Feed Sciences, Zhejiang University, China. S. boulardii was cultured in yeast peptone dextrose broth (Oxoid, Basingstoke, UK) in aerobic conditions at 30 °C for 24 h, and B. subtilis in Luria-Bertani broth (Oxoid) for 12 h. After cultivation, the yeast and bacterial were collected by centrifugation (6,000 × g for 5 min at 4 °C). The pellets were washed twice with PBS (pH 7.4) and resuspended in sterile water at a final concentration of 1.0 × 109 cfu/mL. The prepared mixture was added into the basal diet (Table 1) and maintained at 1 × 108 cfu/kg.
Table 1.
Ingredients (as-fed basis) and calculated composition of the basal diet (%).
| Item | 1 to 35 d | 36 to 72 d |
|---|---|---|
| Ingredients | ||
| Corn | 55.90 | 61.60 |
| Soybean meal | 31.00 | 27.00 |
| Wheat shorts | 3.00 | 4.00 |
| Imported fish meal | 5.00 | 2.00 |
| Rapeseed oil | 1.50 | 2.00 |
| Salt | 0.30 | 0.30 |
| Dicalcium phosphate | 1.20 | 1.00 |
| Limestone | 1.00 | 1.00 |
| DL-Met | 0.10 | |
| Lysine | 0.10 | |
| Premix1 | 1.00 | 1.00 |
| Total | 100.00 | 100.00 |
| Calculated composition | ||
| ME, MJ/kg | 12.78 | 13.05 |
| Crude protein | 22.86 | 19.14 |
| Lys | 1.07 | 0.98 |
| Met + Cys | 0.86 | 0.72 |
| Ash | 7.38 | 6.41 |
| Ca | 0.93 | 0.91 |
| Total phosphorus | 0.64 | 0.56 |
Each kilogram of premix compound contained: vitamin A, 7,000 IU; vitamin D3, 2,500 IU; vitamin E, 30 mg; vitamin K3, 1 mg; vitamin B1, 1.5 mg; vitamin B2, 4 mg; vitamin B6, 2 mg; vitamin B12, 0.02 mg; niacin, 30 mg; folic acid, 0.55 mg; pantothenic acid, 10 mg; biotin, 0.16 mg; choline chloride, 400 mg; Cu, 20 mg; Fe, 70 mg; Mn, 100 mg; Zn, 70 mg; I, 0.4 mg and Se, 0.5 mg.
2.2. Animals
Three hundred 1-day-old Sanhuang broilers (a Chinese cross breed) were randomly divided into 3 groups, each group with 5 replications (n = 20). The control group (CK) was fed a basal diet (Table 1) supplemented with an antibiotic (virginiamycin, 20 mg/kg), whereas broilers in Sb and Bs groups were fed the basal diet supplemented with Sb and Bs (1 × 108 cfu/kg of feed) for 72 d.
2.3. Sample collection
After the 72-d feeding treatment, 10 broilers from each replicate of each treatment group were killed by administering lethobarb (0.5 mL/bird) intravenously and then weighed before sample collection. Duodenum, jejunum, ileum and cecum were collected aseptically. The contents of each section of the gut (duodenum, jejunum, ileum, and cecum) were isolated. And, the contents of each section of the gut of each replicate from different treatment groups were pooled and stored at −70 °C for microbiota analysis.
2.4. DNA extraction and pyrosequencing
For each treatment group, 3 pool samples of each section of the gut were selected randomly to use for microbiota analysis. Total bacteria DNA from each sample (200 mg) was extracted using a DNA Isolation Kit (Tiangen, Beijing, China). Sequencing was performed at Tongji-SCBIT Biotechnology Co. Ltd. Shanghai, China. Briefly, DNA was amplified using the conserved primers 341F (5′-XXXXXXXCCTACGGGAGGCAGCAG-3′) and 534R (5′- ATGAGCTGATTACCGCGGCTGCT-3′), which targets the V3 region of the 16S rRNA, with the forward primer containing a 7-bp barcode unique to each sample. The PCR was performed with the following condition: 94 °C for 5 min (94 °C for 40 s, 55 °C for 30 s, and 72 °C for 30 s) × 30 cycles. The PCR products were purified using a gel extraction kit (Axygen Scientific Inc., USA). The concentration was measured with a UV–vis spectrophotometer (NanoDrop ND1000, USA) and then adjusted to 50 ng/μL for each sample. Finally, equal amounts of DNA from each sample were mixed together and sequenced by Tongji-SCBIT Biotechnology Co., Ltd. (Shanghai, China) using the 454 Life Sciences/Roche GS-FLX sequencing system (Roche Applied Science, Penzburg, Germany).
Sequences obtained through 454 pyrosequencing were then filtered by QIIME software (QIIME version 1.9.0) with default parameters (Caporaso et al., 2010, Zhang et al., 2014). Low-quality or ambiguous reads were discarded, and primer sequences and barcodes were trimmed from the 5′ region. The operational taxonomic unit (OTU) clustering pipeline UPARSE was used to select OTU at 97% similarity. Diversity between the samples (Shannon index) was also analyzed by QIIME. The final taxonomic assignment was based on the consensus identification for each OTU. A multivariate data analysis was performed using METAGENassist (http://www.metagenassist.ca/METAGENassist/faces/Home.jsp), a web server tool that assigns probable microbial functions based on taxonomy (16 S ribosomal subunit) as described by Arndt et al. (2012) and Badri et al. (2013). Principal component analyses (PCA) and the identification of significant features were performed for all treatments combined.
2.5. Statistical analyses
Data were represented as means ± SEM and analyzed with SPSS 16.0. The intergroup variation was tested with paired-samples t-test, followed by Fisher's least significant difference test among the groups. P-value ≤ 0.05 is considered significant.
3. Results
3.1. Microbial diversity along the broiler intestinal tract
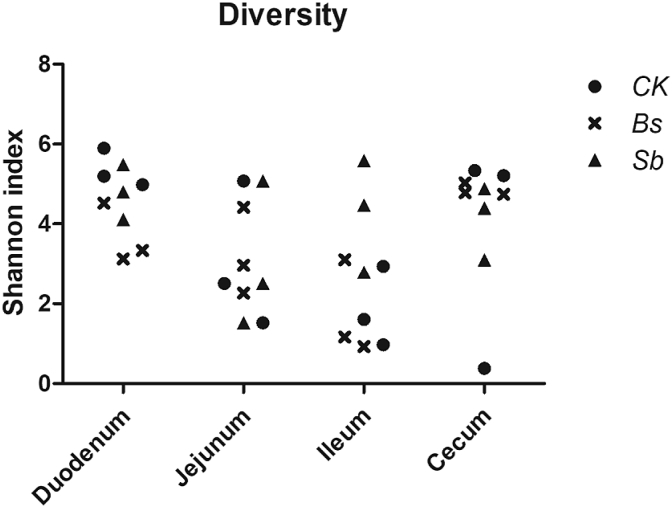
Shannon index was used to evaluate the microbial ecological diversity of each sample (Fig. 1). The results revealed that the ileal and jejunal samples had much lower diversity, whereas the cecal and duodenal samples had much higher Shannon index values (Fig. 1). B. subtilis B10 and Sb administrations did not affect the microbiota diversity along the GI tract compared with the control, except in the duodenums of the Bs group, the microbiota diversity was lower than that of the control (Fig. 1, P < 0.05).
Fig. 1.
Shannon diversity of each sample from different treatment groups. CK: birds fed the basal diet supplemented with virginiamycin; Bs: birds fed the basal diet supplemented with Bacillus subtilis B10; Sb: birds fed the basal diet supplemented with Saccharomyces boulardii.
3.2. Changes in bacterial community structure along the broiler intestinal tract
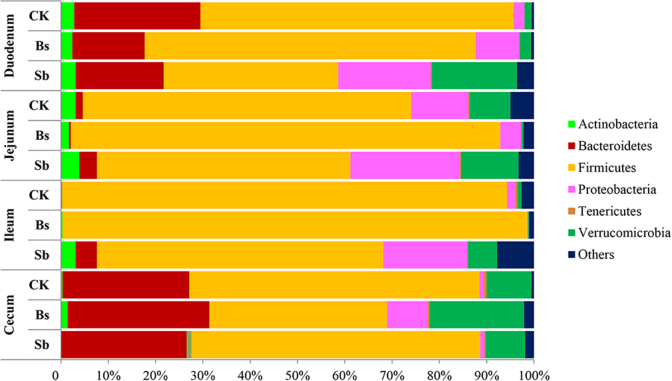
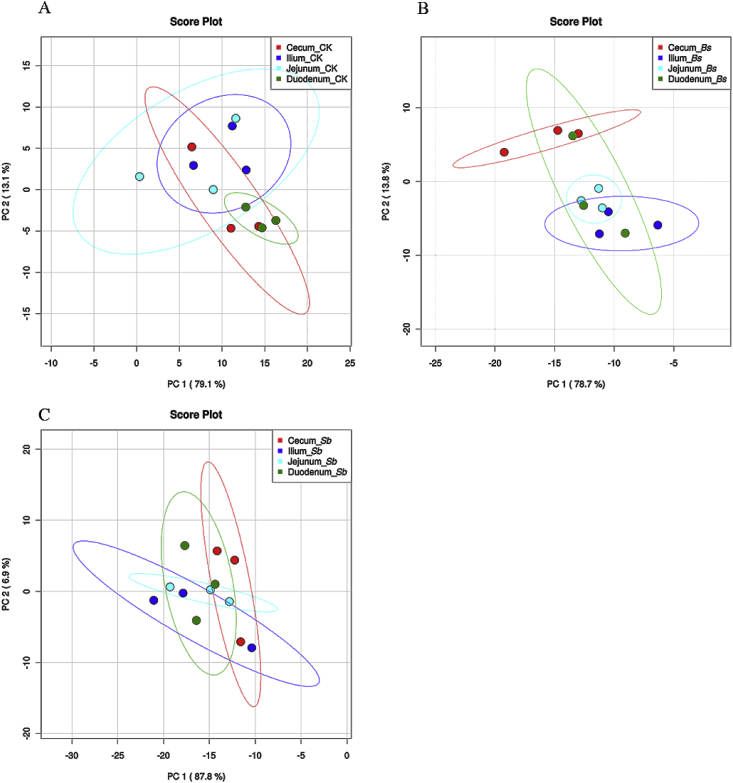
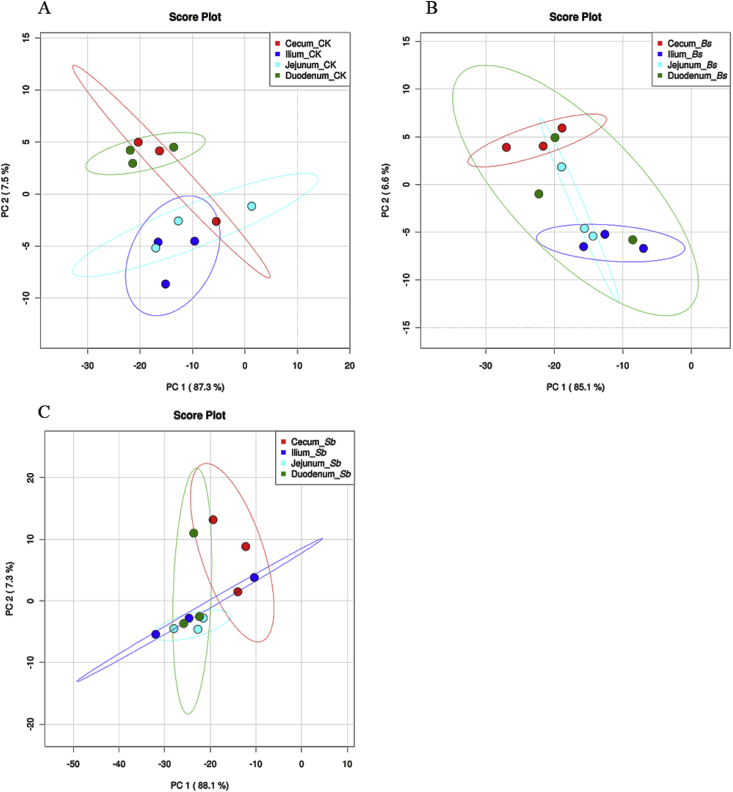
The bacterial community structure varied among different anatomical regions along the chicken intestinal tract (Fig. 2, Fig. 3). Taxonomically, 19 different bacterial phyla were identified in the broiler intestinal tract (Fig. 2). The major bacterial phyla identified were Bacteroidetes, Firmicutes, Proteobacteria, and Verrucomicrobia. Only Firmicutes, Proteobacteria, and Verrucomicrobia were found in all sections of the intestine in all treatment groups (Fig. 2). Firmicutes was dominant along the broiler intestinal tract and with much higher abundance in the jejunum and ileum than in the duodenum and cecum (Fig. 2). However, Actinobacteria were much enriched in the duodenum and jejunum, and the relative abundance of Bacteroidetes was much higher in the duodenum and cecum than in the jejunum and ileum (Fig. 2, P < 0.01). Based on the PCA, we observed that the bacterial community among the duodenum, ileum, and jejunum formed into 2 different clusters in control (Fig. 4A), whereas in the Bs group, the bacterial community of the cecal was separated from those of the ileum and jejunum (Fig. 4B). Interestingly, no significant difference was observed in the bacterial communities along the intestinal tract in the Sb group (Fig. 4C).
Fig. 2.
Relative abundance of bacterial phyla present in the intestinal tract in different treatment groups revealed by pyrosequencing. CK: birds fed the basal diet supplemented with virginiamycin; Bs: birds fed the basal diet supplemented with Bacillus subtilis B10; Sb: birds fed the basal diet supplemented with Saccharomyces boulardii.
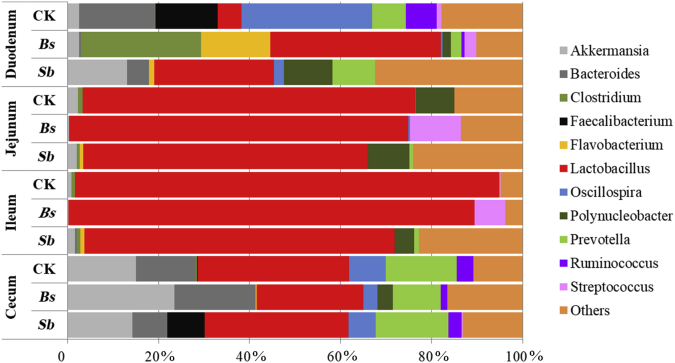
Fig. 3.
Relative abundance of bacterial genus present in the intestinal tract in different treatment groups revealed by pyrosequencing. CK: birds fed the basal diet supplemented with virginiamycin; Bs: birds fed the basal diet supplemented with Bacillus subtilis B10; Sb: birds fed the basal diet supplemented with Saccharomyces boulardii.
Fig. 4.
Microbial community analyzed by principal component (PC) analyses at phyla levels. (A) CK: birds fed the basal diet supplemented with virginiamycin; (B) Bs: birds fed the basal diet supplemented with Bacillus subtilis B10; (C) Sb: birds fed the basal diet supplemented with Saccharomyces boulardii.
At the genus level, Akkermansia, Bacteroides, Oscillospira, Prevotella, and Ruminococcus were all enriched in the duodenum and cecum (Fig. 3). However, Lactobacillus was enriched in the jejunum and ileum, and the abundance of Lactobacillus was much higher in the jejunum and ileum than in the duodenum and cecum (Fig. 3, P < 0.05).
3.3. Effect of probiotics on the microbial structure along the intestinal tract
The effects of probiotics on the microbial community structure in the chicken intestinal tract were identified at the phylum (Fig. 2) and genus (Fig. 3) by pyrosequencing analyses. The microbial community structure after the probiotic treatments were mainly changed at the genus levels in the duodenum and jejunum compared with the control group (Fig. 3). However, the microbial community structure in the ileum and cecum was relative stable among the 3 groups (Fig. 3).
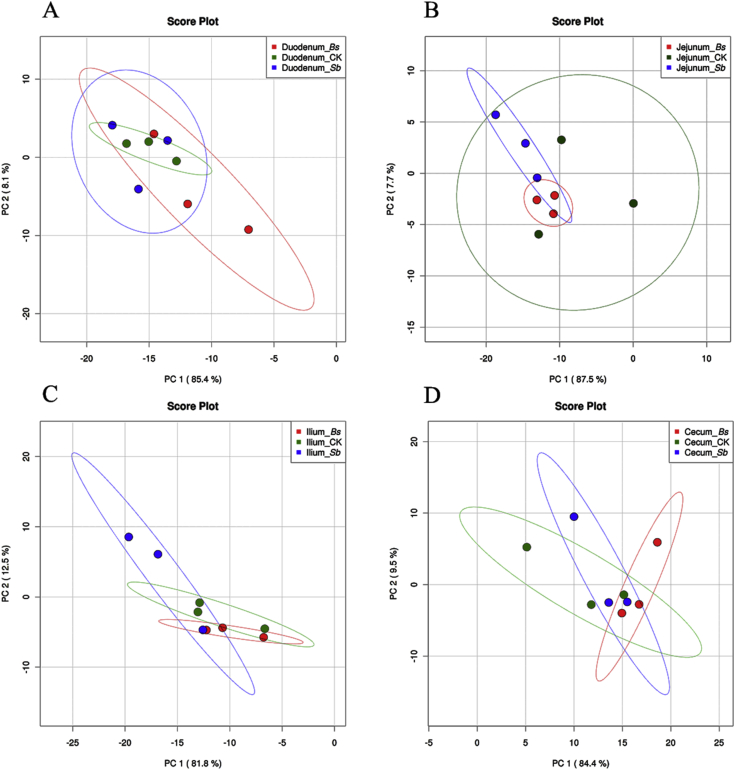
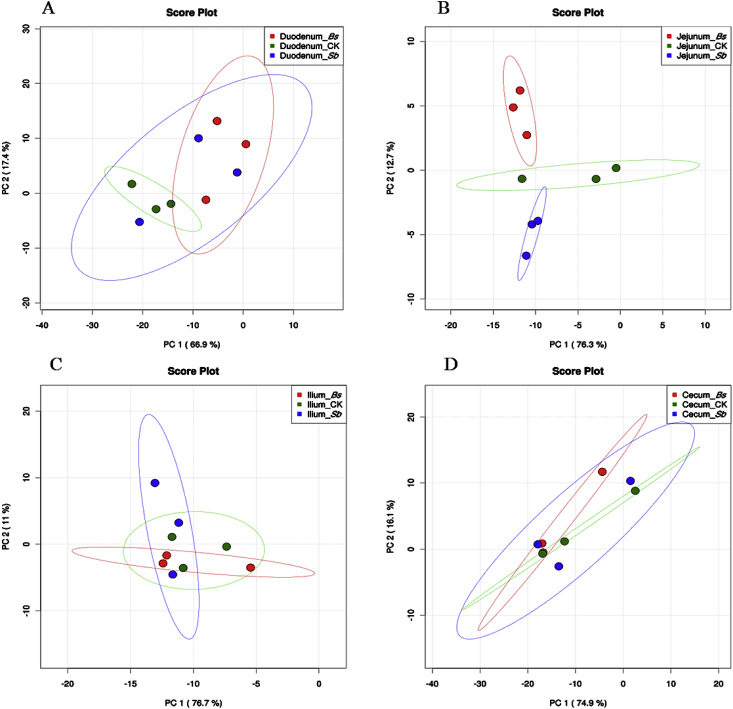
For microbial communities of digesta in the duodenum of broilers, no significant difference was observed among 3 groups at phylum level base on PCA (Fig. 5A); However, at the genus levels, the microbial communities in the Bs and control groups formed 2 different clusters in the duodenum (Fig. 6A). The abundance of Lactobacillus in the Bs and Sb groups was much higher than that of the control group (Fig. 3, P < 0.05), and the abundance of Clostridium in the Bs group was also higher than those of the control and Sb groups (Fig. 3, P < 0.05). However, the abundance of Bacteroides and Oscillospira in the Bs and Sb groups was lower than that of the control group (Fig. 3, P < 0.05).
Fig. 5.
Gut microbiome sequencing data of treatments and controls analyzed by principal component (PC) analyses at phyla level (A) duodenum, (B) jejunum, (C) ileum and (D) cecum CK: birds fed the basal diet supplemented with virginiamycin; Bs: birds fed the basal diet supplemented with Bacillus subtilis B10; Sb: birds fed the basal diet supplemented with Saccharomyces boulardii.
Fig. 6.
Gut microbiome sequencing data of treatments and controls analyzed by principal component (PC) analyses at genus level (A) duodenum, (B) jejunum, (C) ileum, and (D) cecum. CK: birds fed the basal diet supplemented with virginiamycin; Bs: birds fed the basal diet supplemented with Bacillus subtilis B10; Sb: birds fed the basal diet supplemented with Saccharomyces boulardii.
In the jejunum, the microbial community structure was similar to that of the duodenum at the phylum level. According to the PCA, there was no significant difference between the probiotic treatment and control groups at the phylum level (Fig. 5B). However, at the genus level, the microbial communities among the 3 groups formed 3 different clusters in the jejunum (Fig. 6B). At the genus level, the microbial diversity was increased, and the abundance of other bacteria was also increased at the expense of Lactobacillus in the Sb treatment group compared with the control and Bs treatment groups (Fig. 3B). The abundance of Streptococcus was higher in Bs group than those in the control and Sb groups (Fig. 3, P < 0.05).
In the Ileum and cecum, the bacterial community structure was not much different and relatively stable among the 3 groups (Fig. 2, Fig. 3). In the Ileum, no significant difference was observed among the 3 treatment groups based on PCA at phylum or genus level (Fig. 5, Fig. 6). In the cecum, no significant difference was observed based on PCA in phyla levels (Fig. 5D). Moreover, neither Bs administration nor Sb administration changed the cecum microbial community at the genus level compared with the control (Fig. 3), and the same results were obtained by the PCA (Fig. 6D).
3.4. Clustering of the bacterial community based on the category of metabolism
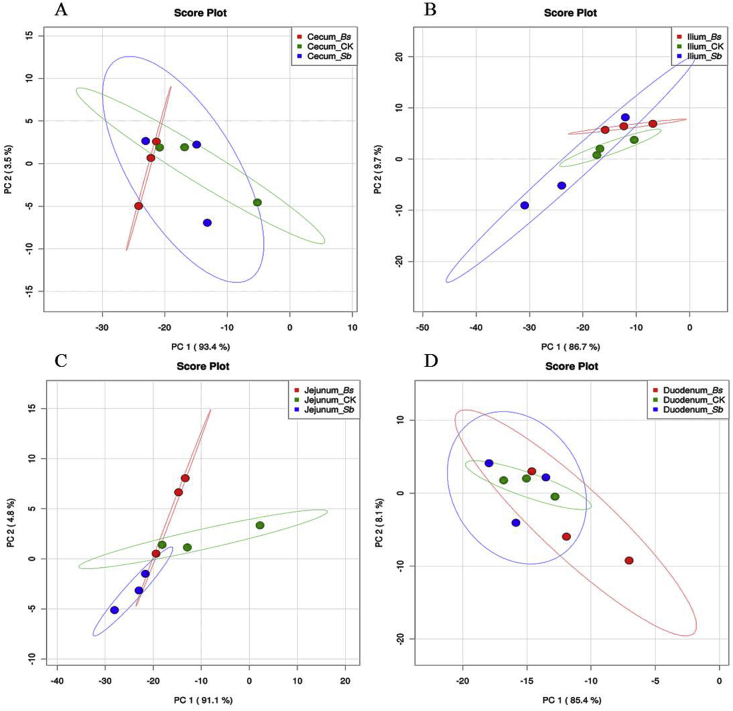
The OTU were assigned from taxonomic to phenotypic mapping using the METAGENassist webserver tool for multiple phenotype (about 21) categories classified based on habitat, metabolism, oxygen requirements, energy source, and other factors. Based on the category of metabolism, the PCA revealed that the microbial community of the 3 treatment groups formed into 3 different clusters in the jejunum and ileum (Figs. 7B and C). However, no difference was observed in either the cecum or duodenum (Figs. 7A and D). Notably, we also observed different microbial communities in different sections of the intestinal tract, based on the metabolism category analyses (Fig. 8). The microbial communities in the ileum and jejunum were separated from the cecum and duodenum in the control group (Fig. 8A). In the Bs group, the bacterial community in the cecum differed from those in the ileum and jejunum (Fig. 8B). Whereas in the Sb group, the bacterial community in the cecum differed from those in the jejunum and duodenum (Fig. 8C).
Fig. 7.
Gut microbiome sequencing data of treatments and controls analyzed by principal component (PC) analyses base on the category of metabolism (A) cecum, (B) ileum, (C) jejunum, and (D) duodenum. CK: birds fed the basal diet supplemented with virginiamycin; Bs: birds fed the basal diet supplemented with Bacillus subtilis B10; Sb: birds fed the basal diet supplemented with Saccharomyces boulardii.
Fig. 8.
Microbial community analyzed by principal component (PC) analyses based on metabolism. (A) CK: birds fed the basal diet supplemented with virginiamycin; (B) Bs: birds fed the basal diet supplemented with Bacillus subtilis B10; (C) Sb: birds fed the basal diet supplemented with Saccharomyces boulardii.
4. Discussion
The intestinal microbiota has an enormous metabolic potential and affects the host's state of health and nutrition (Rinttila and Apajalahti, 2013). Manipulation of the intestinal microbiota is a way to improve animal health and growth performance. Antibiotics are considered to modulate the microbial community within the GI tract, which subsequently promotes growth promotion and reduces disease occurrence when administered at subtherapeutic levels (Angelakis et al., 2013, Danzeisen et al., 2011). Virginiamycin is one of the antibiotic growth promoters that are widely used in agricultural animal production (Dumonceaux et al., 2006, Miles et al., 1984). However, antibiotics resistance and health problems make the trend to ban the use of them in animal agriculture. Our previous research indicated that Bs or Sb administration resulted in a higher body weight in broilers compared with virginiamycin treatment (Rajput et al., 2013a). This paper is aimed to interpret growth promotion effects (as observed in our previous study) of the 2 probiotic strains in the gut microbiota perspective.
The bacterial communities of different sections of the GI tract are markedly different, and it has been suggested that they should be considered separate ecosystems (van der Wielen et al., 2002). Moreover, the microbial densities and diversities vary in the different sections of GI tract, being maximal in the ceca of broilers, where fermentation is most active, reviewed by Yeoman and White (2014). Our results in this study also support this point of views. The microbial diversity in duodenum and cecum was much higher than that in the jejunum and ileum in all treatment groups. In addition, Bs or Sb administration did not affect gut microbial diversity compared with the control (except in the duodenums of Bs treatment group). What is more, according to the PCA, the microbial communities from different sections of the GI tract trended to be separated from each other, except in the Sb treatment group. The microbiota were parallel among the sections of the GI tract after the Sb administration, but the mechanism was not clear.
The ability to balance the host gut microbiota with probiotics has been documented. Chicken performance is linked to the gut microbiota. According to Torok's research, there are 8 common performance-linked OTU identified within both the ileum and cecum of chickens based on 16S rRNA gene sequences, which belong to the phyla Firmicutes, Bacteroidetes, Proteobacteria, and Verrucomicrobia (Torok et al., 2011). Interestingly, our results showed that the major phyla of Firmicutes, Bacteroidetes, Proteobacteria, and Verrucomicrobia were observed along the GI tract in all groups, especially in the duodenum and cecum. Furthermore, Bacteroidetes, Proteobacteria, and Verrucomicrobia were observed much higher abundance in the jejunum and ileum of the Sb group compared with the control and Bs groups. When the sequences were classified further into the genus levels, our present results were also similar to those of Torok et al. (2011). At the genus level, we found that Akkermansia, Bacteroides and Lactobacillus, which were performance-related genera (Torok et al., 2011) observed in the duodenum (except for Lactobacillus in the control and Bacteroides in the Bs group) and cecum at a much higher abundance compared with other intestinal sections. A low abundance of Akkermansia and Bacteroides was in the jejunum and cecum, whereas Lactobacillus was enriched in the jejunum and ileum, which was consistent with previous studies (Stanley et al., 2012, van der Hoeven-Hangoor et al., 2013). In addition, we also observed a very high abundance of Prevotella in the duodenum and cecum among the 3 treatment groups. Prevotella has been shown to be the dominant genus in both ruminants and swine (Kim et al., 2011, Stevenson and Weimer, 2007). Recently, Kang et al. (2013) demonstrated significantly lower abundances of the genera Prevotella, Coprococcus, and unclassified Veillonellaceae in samples from autistic children. This finding indicated that Prevotella may play an important role in animal behavior and health.
The gut microbiota plays an important role in host metabolism and nutrient absorption. The microbes help break down and digest the food ingested by the host. In ruminant livestock, the gut microbiota are required to fulfill approximately 70% of the animal's daily energy requirements (Flint and Bayer, 2008). The small intestine, which consists of the duodenum, jejunum, and ileum, is the compartment where most of the digestion and absorption of nutrients occurs (Renner, 1965). Previous studies showed that the activities of jejunal digestive enzymes (Na+ K+ ATPase, lipase and γ-glutamyl transpeptidase [γGT]) were significantly increased in the Sb treatment group, and the activity of γGT in the Bs treatment group also significantly increased compared with the control (Rajput et al., 2013b). Moreover, the villus height and width in the jejunum and ileum increased after Sb administration (Rajput et al., 2013a). Interestingly, our results showed that the microbial community in jejunum significantly differed among the Bs, Sb, and control groups at the genus level based on the PCA, forming 3 different clusters. The same result was also observed when it was analyzed base on the category of metabolism by PCA. These findings indicated that Sb and Bs administrations, especially that of Sb, could enhance food digestion and nutrient absorption by regulating or optimizing the gut microbial community composition, subsequently improve growth performance.
In conclusion, the results of the present study revealed that Sb and Bs can modulate a healthier microbial ecosystem, subsequently enhance the health status of broilers, and eventually improve the growth performance.
Acknowledgments
This study was supported by the 12th Five-Year-Plan in National Science and Technology for Rural Development in China (2013BAD10B03), the National 863 Project in China (2013AA102803D) and the Key Science and Technology Program of Zhejiang Province, China (No. 2006C12086).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.aninu.2018.03.004.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- Angelakis E., Merhej V., Raoult D. Related actions of probiotics and antibiotics on gut microbiota and weight modification. Lancet Infect Dis. 2013;13:889–899. doi: 10.1016/S1473-3099(13)70179-8. [DOI] [PubMed] [Google Scholar]
- Apajalahti J., Kettunen A., Graham H. Characteristics of the gastrointestinal microbial communities, with special reference to the chicken. World Poult Sci J. 2004;60:223–232. [Google Scholar]
- Arndt D., Xia J., Liu Y., Zhou Y., Guo A.C., Cruz J.A., Sinelnikov I., Budwill K., Nesbo C.L., Wishart D.S. METAGENassist: a comprehensive web server for comparative metagenomics. Nucleic Acids Res. 2012;40:W88–W95. doi: 10.1093/nar/gks497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aureli P., Capurso L., Castellazzi A.M., Clerici M., Giovannini M., Morelli L., Poli A., Pregliasco F., Salvini F., Zuccotti G.V. Probiotics and health: an evidence-based review. Pharmacol Res. 2011;63:366–376. doi: 10.1016/j.phrs.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Badri D.V., Chaparro J.M., Zhang R.F., Shen Q.R., Vivanco J.M. Application of natural blends of phytochemicals derived from the root exudates of Arabidopsis to the soil reveal that phenolic-related compounds predominantly modulate the soil microbiome. J Biol Chem. 2013;288:4502–4512. doi: 10.1074/jbc.M112.433300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani P.D., Bibiloni R., Knauf C., Waget A., Neyrinck A.M., Delzenne N.M., Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Pena A.G., Goodrich J.K., Gordon J.I. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek A.A., Binek M. Chicken intestinal microbiota function with a special emphasis on the role of probiotic bacteria. Pol J Vet Sci. 2014;17:385–394. doi: 10.2478/pjvs-2014-0057. [DOI] [PubMed] [Google Scholar]
- Claesson M.J., Cusack S., O'Sullivan O., Greene-Diniz R., de Weerd H., Flannery E., Marchesi J.R., Falush D., Dinan T., Fitzgerald G. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci USA. 2011;108:4586–4591. doi: 10.1073/pnas.1000097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzeisen J.L., Kim H.B., Isaacson R.E., Tu Z.J., Johnson T.J. Modulations of the chicken cecal microbiome and metagenome in response to anticoccidial and growth promoter treatment. PLoS One. 2011;6 doi: 10.1371/journal.pone.0027949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Cochetiere M.F., Durand T., Lalande V., Petit J.C., Potel G., Beaugerie L. Effect of antibiotic therapy on human fecal microbiota and the relation to the development of Clostridium difficile. Microb Ecol. 2008;56:395–402. doi: 10.1007/s00248-007-9356-5. [DOI] [PubMed] [Google Scholar]
- Degirolamo C., Rainaldi S., Bovenga F., Murzilli S., Moschetta A. Microbiota modification with probiotics induces hepatic bile acid synthesis via downregulation of the Fxr-Fgf15 axis in mice. Cell Rep. 2014;7:12–18. doi: 10.1016/j.celrep.2014.02.032. [DOI] [PubMed] [Google Scholar]
- Dibner J.J., Richards J.D. Antibiotic growth promoters in agriculture: history and mode of action. Poult Sci. 2005;84:634–643. doi: 10.1093/ps/84.4.634. [DOI] [PubMed] [Google Scholar]
- Dumas M.E., Barton R.H., Toye A., Cloarec O., Blancher C., Rothwell A., Fearnside J., Tatoud R., Blanc V., Lindon J.C. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci USA. 2006;103:12511–12516. doi: 10.1073/pnas.0601056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumonceaux T.J., Hill J.E., Hemmingsen S.M., Van Kessel A.G. Characterization of intestinal microbiota and response to dietary virginiamycin supplementation in the broiler chicken. Appl Environ Microbiol. 2006;72:2815–2823. doi: 10.1128/AEM.72.4.2815-2823.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint H.J., Bayer E.A. Plant cell wall breakdown by anaerobic microorganisms from the Mammalian digestive tract. Ann N Y Acad Sci. 2008;1125:280–288. doi: 10.1196/annals.1419.022. [DOI] [PubMed] [Google Scholar]
- Forder R.E., Howarth G.S., Tivey D.R., Hughes R.J. Bacterial modulation of small intestinal goblet cells and mucin composition during early posthatch development of poultry. Poult Sci. 2007;86:2396–2403. doi: 10.3382/ps.2007-00222. [DOI] [PubMed] [Google Scholar]
- Frank D.N., Robertson C.E., Hamm C.M., Kpadeh Z., Zhang T., Chen H., Zhu W., Sartor R.B., Boedeker E.C., Harpaz N. Disease phenotype and genotype are associated with shifts in intestinal-associated microbiota in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17:179–184. doi: 10.1002/ibd.21339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskins H.R., Collier C.T., Anderson D.B. Antibiotics as growth promotants: mode of action. Anim Biotechnol. 2002;13:29–42. doi: 10.1081/ABIO-120005768. [DOI] [PubMed] [Google Scholar]
- Guarner F. Enteric flora in health and disease. Digestion. 2006;73(Suppl. 1):5–12. doi: 10.1159/000089775. [DOI] [PubMed] [Google Scholar]
- Guarner F., Malagelada J.R. Gut flora in health and disease. Lancet. 2003;361:512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- Hamer H.M., De Preter V., Windey K., Verbeke K. Functional analysis of colonic bacterial metabolism: relevant to health? Am J Physiol Gastrointest Liver Physiol. 2012;302:G1–G9. doi: 10.1152/ajpgi.00048.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia W., Li H., Zhao L., Nicholson J.K. Gut microbiota: a potential new territory for drug targeting. Nat Rev Drug Discov. 2008;7:123–129. doi: 10.1038/nrd2505. [DOI] [PubMed] [Google Scholar]
- Kang D.W., Park J.G., Ilhan Z.E., Wallstrom G., LaBaer J., Adams J.B., Krajmalnik-Brown R. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One. 2013;8 doi: 10.1371/journal.pone.0068322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.B., Borewicz K., White B.A., Singer R.S., Sreevatsan S., Tu Z.J., Isaacson R.E. Longitudinal investigation of the age-related bacterial diversity in the feces of commercial pigs. Vet Microbiol. 2011;153:124–133. doi: 10.1016/j.vetmic.2011.05.021. [DOI] [PubMed] [Google Scholar]
- Kohl K.D. Diversity and function of the avian gut microbiota. J Comp Physiol B Biochem Syst Environ Physiol. 2012;182:591–602. doi: 10.1007/s00360-012-0645-z. [DOI] [PubMed] [Google Scholar]
- Ley R.E., Turnbaugh P.J., Klein S., Gordon J.I. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- Louis P., Scott K.P., Duncan S.H., Flint H.J. Understanding the effects of diet on bacterial metabolism in the large intestine. J Appl Microbiol. 2007;102:1197–1208. doi: 10.1111/j.1365-2672.2007.03322.x. [DOI] [PubMed] [Google Scholar]
- Miles R.D., Janky D.M., Harms R.H. Virginiamycin and broiler performance. Poult Sci. 1984;63:1218–1221. doi: 10.3382/ps.0631218. [DOI] [PubMed] [Google Scholar]
- Neufeld K.M., Kang N., Bienenstock J., Foster J.A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neuro Gastroenterol Motil. 2011;23:255–264. doi: 10.1111/j.1365-2982.2010.01620.x. e119. [DOI] [PubMed] [Google Scholar]
- Noverr M.C., Huffnagel G.B. Does the microbiota regulate immune responses outside the gut? Trends Microbiol. 2004;12:562–568. doi: 10.1016/j.tim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- O'Toole P.W., Claesson M.J. Gut microbiota: changes throughout the lifespan from infancy to elderly. Int Dairy J. 2010;20:281–291. [Google Scholar]
- Oakley B.B., Morales C.A., Line J., Berrang M.E., Meinersmann R.J., Tillman G.E., Wise M.G., Siragusa G.R., Hiett K.L., Seal B.S. The poultry-associated microbiome: network analysis and farm-to-fork characterizations. PLoS One. 2013;8 doi: 10.1371/journal.pone.0057190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajput I.R., Li L.Y., Xin X., Wu B.B., Juan Z.L., Cui Z.W., Yu D.Y., Li W.F. Effect of Saccharomyces boulardii and Bacillus subtilis B10 on intestinal ultrastructure modulation and mucosal immunity development mechanism in broiler chickens. Poult Sci. 2013;92:956–965. doi: 10.3382/ps.2012-02845. [DOI] [PubMed] [Google Scholar]
- Rajput I.R., Li Y.L., Xu X., Huang Y., Zhi W.C., Yu D.Y., Li W. Supplementary effects of Saccharomyces boulardii and Bacillus subtilis B10 on digestive enzyme activities, antioxidation capacity and blood homeostasis in broiler. Int J Agri Biol. 2013;15:231–237. [Google Scholar]
- Renner R. Site of fat absorption in the chick. Poult Sci. 1965;44:861–864. doi: 10.3382/ps.0440861. [DOI] [PubMed] [Google Scholar]
- Ridlon J.M., Kang D.J., Hylemon P.B. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- Rinttila T., Apajalahti J. Intestinal microbiota and metabolites-Implications for broiler chicken health and performance. J Appl Poult Res. 2013;22:647–658. [Google Scholar]
- Round J.L., Mazmanian S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal B.S., Lillehoj H.S., Donovan D.M., Gay C.G. Alternatives to antibiotics: a symposium on the challenges and solutions for animal production. Anim Health Res Rev. 2013;14:78–87. doi: 10.1017/S1466252313000030. [DOI] [PubMed] [Google Scholar]
- Stanley D., Denman S.E., Hughes R.J., Geier M.S., Crowley T.M., Chen H.L., Haring V.R., Moore R.J. Intestinal microbiota associated with differential feed conversion efficiency in chickens. Appl Microbiol Biotechnol. 2012;96:1361–1369. doi: 10.1007/s00253-011-3847-5. [DOI] [PubMed] [Google Scholar]
- Stevenson D.M., Weimer P.J. Dominance of Prevotella and low abundance of classical ruminal bacterial species in the bovine rumen revealed by relative quantification real-time PCR. Appl Microbiol Biotechnol. 2007;75:165–174. doi: 10.1007/s00253-006-0802-y. [DOI] [PubMed] [Google Scholar]
- Torok V.A., Hughes R.J., Mikkelsen L.L., Perez-Maldonado R., Balding K., MacAlpine R., Percy N.J., Ophel-Keller K. Identification and characterization of potential performance-related gut microbiotas in broiler chickens across various feeding trials. Appl Environ Microbiol. 2011;77:5868–5878. doi: 10.1128/AEM.00165-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoeven-Hangoor E., van der Vossen J.M.B.M., Schuren F.H.J., Verstegen M.W.A., de Oliveira J.E., Montijn R.C., Hendriks W.H. Ileal microbiota composition of broilers fed various commercial diet compositions. Poult Sci. 2013;92:2713–2723. doi: 10.3382/ps.2013-03017. [DOI] [PubMed] [Google Scholar]
- van der Wielen P.W., Keuzenkamp D.A., Lipman L.J., van Knapen F., Biesterveld S. Spatial and temporal variation of the intestinal bacterial community in commercially raised broiler chickens during growth. Microb Ecol. 2002;44:286–293. doi: 10.1007/s00248-002-2015-y. [DOI] [PubMed] [Google Scholar]
- Wei S., Morrison M., Yu Z. Bacterial census of poultry intestinal microbiome. Poult Sci. 2013;92:671–683. doi: 10.3382/ps.2012-02822. [DOI] [PubMed] [Google Scholar]
- Wise M.G., Siragusa G.R. Quantitative analysis of the intestinal bacterial community in one- to three-week-old commercially reared broiler chickens fed conventional or antibiotic-free vegetable-based diets. J Appl Microbiol. 2007;102:1138–1149. doi: 10.1111/j.1365-2672.2006.03153.x. [DOI] [PubMed] [Google Scholar]
- Yeoman C.J., White B.A. Gastrointestinal tract microbiota and probiotics in production animals. Annu Rev Anim Biosci. 2014;2:469–486. doi: 10.1146/annurev-animal-022513-114149. [DOI] [PubMed] [Google Scholar]
- Zhang X., Shu M., Wang Y., Fu L., Li W., Deng B., Liang Q., Shen W. Effect of photosynthetic bacteria on water quality and microbiota in grass carp culture. World J Microbiol Biotechnol. 2014;30:2523–2531. doi: 10.1007/s11274-014-1677-1. [DOI] [PubMed] [Google Scholar]
- Zoetendal E.G., Akkermans A.D., De Vos W.M. Temperature gradient gel electrophoresis analysis of 16S rRNA from human fecal samples reveals stable and host-specific communities of active bacteria. Appl Environ Microbiol. 1998;64:3854–3859. doi: 10.1128/aem.64.10.3854-3859.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.