Abstract
A novel variant of an iridium-based organometallic catalyst was synthesized and used to enhance the NMR signals of pyridine in a heterogeneous phase by immobilization on polymer microbead solid supports. Upon administration of parahydrogen (pH2) gas to a methanol mixture containing the HET-SABRE catalyst particles and the pyridine, up to fivefold enhancements were observed in the 1H NMR spectra after sample transfer to high field (9.4 T). Importantly, enhancements were not due to any residual catalyst molecules in solution, thus supporting the true heterogeneity of the SABRE process. Further significant improvements may be expected by systematic optimization of experimental parameters. Moreover, the heterogeneous catalyst is easy to separate and recycle, thus opening a door to future potential applications varying from spectroscopic studies of catalysis, to imaging metabolites in the body without concern of contamination from expensive and potentially toxic metal catalysts or accompanying organic molecules.
Keywords: NMR Spectroscopy, SABRE, heterogeneous catalysis, imaging agents, parahydrogen, hyperpolarization
Magnetic resonance imaging (MRI) of metabolic markers offers a powerful method for screening and diagnosing diseases, as well as gauging response to treatment. Yet at Boltzmann equilibrium, spin polarizations of conventional MR (on the order of ca. 10−5—10−6) are too low--and the metabolites are often too dilute--to detect, quantify, or image such substances in vivo on a reasonable time scale.[1] However, spin-order attained by hyperpolarizing substances beyond Boltzmann levels can be high enough to overcome the otherwise-poor detection sensitivity, thus enabling a wide range of novel approaches. Furthermore, because the high nuclearspin polarization is independent of the magnetic field, strong magnetic fields are unnecessary for some applications, thus permitting low/zero-field MRS/MRI,[2] and even remotely detected MRS/MRI.[3] Well-known hyperpolarization approaches include dynamic nuclear polarization (DNP)[4] and optical pumping.[5] However, another route to address the NMR/MRI sensitivity problem is to use parahydrogen (pH2) as the hyperpolarization source, as is done in a family of techniques referred to collectively as PHIP (parahydrogen-induced polarization).[6] There are two varieties of PHIP: In traditional PHIP, molecular precursors with unsaturated chemical bonds are hydrogenated by molecular addition of pH2, thereby transferring the nuclear spin-order to the molecular products. In a more recent variant, dubbed SABRE (signal amplification by reversible exchange),[3b,7] spin-order may be transferred from pH2 to target molecules during the lifetime of transient molecular complexes (ostensibly[7g]) without permanent chemical change. Importantly, catalysis plays a critical role in both PHIP approaches, as they each generally require an organometallic catalyst to facilitate the underlying chemical reactions. These reactions typically take place under conditions of homogeneous catalysis, a process wherein the catalyst molecules are dissolved within the same phase as the reagents. Thus, the wider biological application of PHIP for production of highly polarized liquids is constrained by the difficulty of separating the potentially toxic and expensive catalyst substances from the created hyperpolarized (HP) agents. Furthermore, the chemistry of both liquid-phase SABRE and aqueous PHIP first requires catalyst activation, thus often resulting in the release of chelating substances (e.g. octadiene, norbornadiene, or derivatives) into the liquid phase.
Following the progress with conventional PHIP[8] and Overhauser DNP,[9] we report herein the first observation of SABRE enhancement of NMR signals in solution using a heterogeneous catalyst. The novel HET-SABRE catalyst is prepared by immobilizing an iridium-based organometallic catalyst onto micropolymer beads. Upon administration of pH2 gas and sample transfer to high field (9.4 T), up to about fivefold enhancements were observed in the 1H NMR spectra of the substrate (pyridine). The emissive peak enhancements were qualitatively similar to those observed with the corresponding homogeneous SABRE catalyst. It is also shown by NMR spectroscopy, AAS, and MS that such enhancements were not due to any residual catalyst molecules in solution, thus supporting the true heterogeneity of the SABRE process.
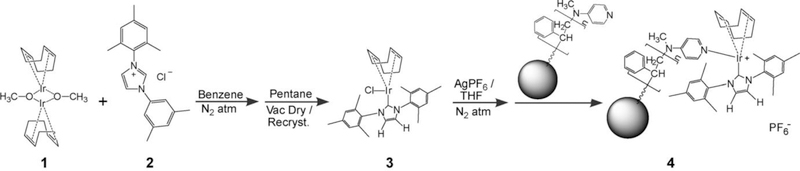
Relevant chemical structures are shown in Scheme 1, and the syntheses and characterizations are summarized with details provided in the Supporting Information. Briefly, the first steps provide synthesis of an N-heterocyclic carbene (NHC)/iridium catalyst[10] (itself used in various previous homogenous SABRE experiments[7c,d,g]): The iridium dimer 1 was reacted with the NHC derivative 2 in benzene and an inert atmosphere. After vacuum-drying and recrystallization in pentane, [IrCl(cod)(IMes)] (3) was obtained [IMes=1,3-bis(2,4,6-trimethylphenyl)imidazol-2-ylidene; cod=cyclooctadiene]. In subsequent steps, inspired by an approach in reference 11, 3 is first reacted with AgPF6 in THF and an inert atmosphere, wherein precipitation of AgCl drives the removal of the Cl moiety from 3 and the weak association of the PF6− ion. Filtration of the solution and addition of commercial polymer microbeads functionalized with 4-dimethylaminopyridine resulted in the creation of the final HET-SABRE catalyst 4. This approach was designed to exploit the fact that one of the pyridine-accepting positions on the catalyst’s iridium center is not labile.[7a,b,f]
Scheme 1.
Relevant structures and a synthetic summary for preparation of the HET-SABRE catalyst studied herein. a)Preparation of the [IrCl(cod)(IMes)] SABRE catalyst (3), which is a precursor for the preparation of the covalently linked SABRE catalyst 4.
Successful immobilization of the iridium complex onto the polymer microbeads was supported by AAS, IR, and MS experiments. According to AAS, the iridium complex comprises about 4% of the total HET-SABRE catalyst particles by weight (see the S upporting Information).
Results from SABRE demonstration experiments are provided in Figure 1. About 14 mg of 4 (corresponding to about 0.0015 mmol of the iridium complex) were added to a custom stopcock-sealed 5 mm Pyrex NMR tube with 200 μL [D4]MeOH and 0.03 mmol [H5]pyridine ([H5]Py). A typical thermally polarized 1H NMR signal, obtained after degassing the sample, is shown in Figure 1a. After addition of about 2400 Torr (ca. 320 kPa) of pH2 gas (pH2 fraction: ca. 64%) to the sample and a brief period of vigorous shaking in the fringe field of a wide-bore 9.4 T magnet (local field: ca. 100G), the NMR tube was immediately inserted into the magnet and a corresponding HET-SABRE-enhanced 1H NMR spectrum was obtained (Figure 1b). Clear and unambiguous HET-SABRE enhancement was observed, as manifested by: 1)the larger signal, and 2)the emissive peaks for the substrate pyridine resonances in a pattern qualitatively similar to that typically achieved with the homogeneous version of the catalyst 3 (see the Supporting Information). Note that during the shaking, the particles are distributed throughout the sample liquid, however, as soon as the shaking is finished, the particles quickly settle out onto the bottom of the NMR tube, thus effectively separating themselves from the liquid in the coil region. Indeed, the chemical shifts of the 1HNMR resonances for the substrate pyridine, in Figures 1a,b, are all for free (unbound) pyridine. Resonances for bound pyridine are also typically observed in the case of homogeneous SABRE. Quantification of enhancements (ε) is impeded by the reduced shim quality (from the sample shaking, heterogeneous sample contents, and reduced liquid level to facilitate mixing), which gives rise to imperfect spectral line shapes. Nevertheless, testing different integration limits allows estimated values of ε=(−)5.2±0.3, (−)4.1±0.3, and (−)2.7±0.2 respectively for ortho, para, and meta positions around the pyridine ring to be obtained using: ε=(Senhanced-Sthermal)/Sthermal, where S is a given integrated spectral intensity from Figures 1a,b (in good quantitative agreement with simple peak-height measurements). These results may be compared with corresponding SABRE enhancements obtained with the homogeneous SABRE catalyst 3, with ε values of up to about (−)70-fold being achieved under otherwise comparable conditions, but with a similar pattern of relative enhancements (see the Supporting Information).
Figure 1.

a) Proton NMR spectrum from a degassed mixture containing [D4]MeOH, the HET-SABRE catalyst particles, and the [H5]Py substrate thermally polarized at 9.4T. b) Corresponding HET-SABRE spectrum obtained from the sample immediately after administration of pH2 gas, agitation at about 100G, and rapid transfer to the 9.4T NMR magnet. c) Thermally polarized proton NMR spectrum from a supernatant solution. The corresponding control experiment using the supernatant solution is shown in (d) and no SABRE enhancement is observed. All spectra shown were acquired with a single scan (90° pulse). Peaks at about δ=3.3 and 4.9ppm are from residual protons from the deuterated solvent. The peak at δ=4.5ppm in (b) and (d) is from H2 gas. The peak at δ=1.3 is assigned to cyclooctane (C8H16).[7g] Spectra from an additional supernatant control experiment, as well as additional experimental details, are provided in the Supporting Information.
Particularly given the larger enhancements observed under homogeneously catalytic conditions, it is important to confirm that the results in Figure 1b are not simply a result of conventional (i.e. homogeneous) SABRE, involving catalyst molecules which have leached from the solid supports and become dissolved in solution. Following the experiment in Figure 1b, the supernatant liquid was separated from 4, and a similar SABRE experiment was performed on that liquid in the absence of 4. Figure 1c shows a thermally polarized 1HNMR spectrum from the supernatant solution, and can be compared to that obtained after the administration of 2400 Torr (ca. 320 kPa) of pH2 (ca. 59% pH2 enrichment), agitation at about 100 G, and insertion into the 9.4 T magnet (Figure 1d). No SABRE enhancement is observed in Figure 1d. Moreover, AAS, MS, and long-time 1H NMR acquisition did not indicate the presence of leached catalyst in the supernatant solution (within detection limits). Furthermore, signatures of substrate [H5]Py, solvent ([D4]MeOH), and cyclooctane were present in all four spectra (Figure 1; H2(g) is also observed in the SABRE experiments Figure 1b,d). Cyclooctadiene is hydrogenated as a byproduct and removed from the iridium center[7g] as part of catalyst activation.
Taken together, these results strongly support the conclusion that the enhancements in Figure 1b were not due to residual homogeneous catalyst floating freely in solution, but instead are the result of a true heterogeneous SABRE process. Finally, the HET-SABRE experiment in Figure 1b was repeated using [D5]pyridine as the substrate. No discernable SABRE enhancement of the substrate resonances could be detected and no evidence of H/D isotopic exchange was observed under the present conditions, results that are consistent with the traditional (nonhydrogen exchange) explanation of SABRE[7a] for the HET-SABRE results in Figure 1b (the recent observation of D/H exchange between substrate and pH2 during high-field studies of homogeneous SABRE with 3 occurred over longer experimental durations and under conditions of stopped-flow pH2 gas delivery[7g]).
In summary, we have reported a novel polymer-supported organometallic iridium catalyst and used it to demonstrate the feasibility of HET-SABRE by achieving enhancement of the 1H NMR signals of the substrate pyridine--the first such results of which we are aware. While the fivefold enhancements in the NMR signals of the substrate are modest compared to previous results obtained with homogeneous SABRE, it should be pointed out that these pilot experiments were performed under unfavorable conditions compared to those of homogeneous SABRE:[7a] 1) significantly higher substrate-to-catalyst ratio, 2) about 64% pH2 used, and 3) localized nature of active iridium centers on the surface of the solid support. Thus, given that other groups have achieved much larger enhancements than the corresponding homogeneous SABRE enhancements reported above, the present HET-SABRE enhancements should not represent a fundamental limit of the HET-SABRE approach. Indeed, it should be possible to achieve much larger HET-SABRE enhancements in the future by improving the delivery of both pH2 gas and the substrate to the catalyst materials (e.g. under much higher pH2 pressure and continuous flow), as well as by optimizing catalyst material design (e.g. to improve the solvent accessibility and surface density of activated catalyst sites) and other experimental conditions (including temperature, concentrations, and magnetic field). Furthermore, the substrate-to-catalyst ratio can be decreased by orders of magnitude, because the catalyst can be recycled and used for polarization of multiple batches of substrate. Finally, we note that the HET-SABRE catalyst should be easy to separate from the solution and recycle, and it should also now be possible to solve the issue of unwanted release of chelates into solution by preactivating the catalyst particles before use. Thus these results, combined with the ready potential of SABRE to achieve relatively low-cost, rapid, and high-throughput production of hyperpolarized substances, open a door to the straightforward preparation of chemically pure HP agents. Future efforts will investigate the potential translation of this approach to a range of biological, in vivo, and ultimately clinical spectroscopic and imaging studies.
Supplementary Material
Sending a signal:
A novel variant of an iridium-based organometallic catalyst was synthesized and immobilized on polymer microbeads. Upon administration of parahydrogen (pH2) gas to a solution containing the catalyst and pyridine, up to a fivefold enhancement is observed in the 1HNMR spectra owing to the title process (HET-SABRE). The catalyst is easy to recycle and holds potential for applications varying from spectroscopic studies of catalysis to imaging metabolites.
Acknowledgments
B.M.G. and F.S. thank Kyle Plunkett (SIUC) for advice and access to his glove box, and Xinju Zhu and Zhexiong Lee for assistance. We also thank Mary Kinsel (SIUC Mass Spectrometry Facility) for assistance with MS, and Pamela Ubaldo (SIUC) for assistance with AAS. B.M.G. is a member of the SIUC Materials Technology Center. This work was supported in part by the DoD CDMRP Era of Hope Award (W81XWH-12-1-0159/BC112431) and SIUC OSPA.
References
- [1].Kurhanewicz J, Vigneron DB, Brindle K, Chekmenev EY, Comment A, Cunningham CH, DeBerardinis RJ, Green GG, Leach MO, Rajan SS, Rizi RR, Ross BD, Warren WS, Malloy CR, Neoplasia 2011, 13, 81–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Coffey AM, Truong ML, Chekmenev EY, J. Magn. Reson 2013, 237, 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3](a).Goodson BM, Phys. World 2006, 19, 28–33; [Google Scholar]; (b) Gong QX, Gordji-Nejad A, Blumich B, Appelt S, Anal. Chem 2010, 82, 7078–7082; [DOI] [PubMed] [Google Scholar]; (c) Telkki V-V, Zhivonitko VV, Ahola S, Kovtunov KV, Jokisaari J, Koptyug IV, Angew. Chem 2010, 122, 8541–8544; Angew. Chem. Int. Ed. 2010, 49, 8363–8366. [DOI] [PubMed] [Google Scholar]
- [4](a).Abragam A, Goldman M, Rep. Prog. Phys 1978, 41, 395–467; [Google Scholar]; (b) Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K, Proc. Natl. Acad. Sci. USA 2003, 100, 10158–10163; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Carver T, Slichter C, Phys. Rev 1953, 92, 212–213. [Google Scholar]
- [5](a).Walker TG, Happer W, Rev. Mod. Phys 1997, 69, 629–642; [Google Scholar]; (b) Goodson BM, J. Magn. Reson 2002, 155, 157–216; [DOI] [PubMed] [Google Scholar]; (c) Nikolaou P, Coffey AM, Walkup LL, Gust BM, Whiting N, Newton H, Barcus S, Muradyan I, Dabaghyan M, Moroz GD, Rosen M, Patz S, Barlow MJ, Chekmenev EY, Goodson BM, Proc. Natl. Acad. Sci. USA 2013, 110, 14150–14155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6](a).Bowers CR, Weitekamp DP, Phys. Rev. Lett 1986, 57, 2645–2648; [DOI] [PubMed] [Google Scholar]; (b) Bowers CR, Weitekamp DP, J. Am. Chem. Soc 1987, 109, 5541–5542; [Google Scholar]; (c) Eisenschmid TC, Kirss RU, Deutsch PP, Hommeltoft SI, Eisenberg R, Bargon J, Lawler RG, Balch AL, J. Am. Chem. Soc 1987, 109, 8089–8091; [Google Scholar]; (d) Natterer J, Bargon J, Prog. Nucl. Magn. Reson. Spectrosc 1997, 31, 293–315. [Google Scholar]
- [7](a).Adams RW, Aguilar JA, Atkinson KD, Cowley MJ, Elliott PIP, Duckett SB, Green GGR, Khazal IG, Lopez-Serrano J, Williamson DC, Science 2009, 323, 1708–1711; [DOI] [PubMed] [Google Scholar]; (b) Atkinson KD, Cowley MJ, Elliott PI, Duckett SB, Green GG, López-Serrano J.n., Whitwood AC, J. Am. Chem. Soc 2009, 131, 13362–13368; [DOI] [PubMed] [Google Scholar]; (c) Cowley MJ, Adams RW, Atkinson KD, Cockett MC, Duckett SB, Green GG, Lohman JA, Kerssebaum R, Kilgour D, Mewis RE, J. Am. Chem. Soc 2011, 133, 6134–6137; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Zeng H, Xu J, Gillen J, McMahon MT, Artemov D, Tyburn J-M, Lohman JAB, Mewis RE, Atkinson KD, Green GGR, Duckett SB, vanZijl PCM, J. Magn. Reson 2013, 237, 73–78; [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Glöggler S, Müller R, Colell J, Emondts M, Dabrowski M, Blümich B, Appelt S, Phys. Chem. Chem. Phys 2011, 13, 13759–13764; [DOI] [PubMed] [Google Scholar]; (f) Dücker EB, Kuhn LT, Münnemann K, Griesinger C, J. Magn. Reson 2012, 214, 159–165; [DOI] [PubMed] [Google Scholar]; (g) Barskiy DA, Kovtunov KV, Koptyug IV, He P, Groome KA, Best QA, Shi F, Goodson BM, Shchepin RV, Coffey AM, Waddell KW, Chekmenev EY, J. Am. Chem. Soc 2014, 136, 3322–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8](a).Koptyug IV, Kovtunov KV, Burt SR, Anwar MS, Hilty C, Han S, Pines A, Sagdeev RZ, J. Am. Chem. Soc 2007, 129, 5580–5586; [DOI] [PubMed] [Google Scholar]; (b) Kovtunov KV, Beck IE, Bukhtiyarov VI, Koptyug IV, Angew. Chem 2008, 120, 1514–1517; Angew. Chem. Int. Ed. 2008, 47, 1492–1495; [DOI] [PubMed] [Google Scholar]; (c) Kovtunov KV, Koptyug IV in Magnetic Resonance Microscopy. Spatially Resolved NMR Techniques and Applications (Eds.: Codd S, Seymour JD), Wiley-VCH, Weinheim, 2008, pp.101–115; [Google Scholar]; (d) Kovtunov KV, Zhivonitko VV, Corma A, Koptyug IV, J. Phys. Chem. Lett 2010, 1, 1705–1708; [Google Scholar]; (e) Kovtunov KV, Beck IE, Zhivonitko VV, Barskiy DA, Bukhtiyarov VI, Koptyug IV, Phys. Chem. Chem. Phys 2012, 14, 11008–11014; [DOI] [PubMed] [Google Scholar]; (f) Lysova AA, Koptyug IV, Chem. Soc. Rev 2010, 39, 4585–4601; [DOI] [PubMed] [Google Scholar]; (g) Roth M, Kindervater P, Raich HP, Bargon J, Spiess HW, Munnemann K, Angew. Chem 2010, 122, 8536–8540; Angew. Chem. Int. Ed. 2010, 49, 8358–8362. [DOI] [PubMed] [Google Scholar]
- [9].Lingwood MD, Siaw TA, Sailasuta N, Abulseoud OA, Chan HR, Ross BD, Bhattacharya P, Han S, Radiology 2012, 265, 418–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10](a).Vazquez-Serrano LD, Owens BT, Buriak JM, Inorg. Chim. Acta 2006, 359, 2786–2797; [Google Scholar]; (b) Kownacki I, Kubicki M, Szubert K, Marciniec B, J. Organomet. Chem 2008, 693, 321–328. [Google Scholar]
- [11].Torres O, Martin M, Sola E, Organometallics 2009, 28, 863–870. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.