Abstract
This study investigated the effects of xylanase supplementations with cereal-based diets on nutrient digestibility and gut microbiota of growing pigs. A total of 96 individually penned pigs (initial BW = 22.7 ± 0.65 kg) were allotted to 12 treatments and subjected to a completely randomized block design experiment. Pigs in each treatment were fed an isocaloric wheat-based or corn-based diet with or without 1 of 5 types of xylanase supplements (XA, XB, XC, XD, XE). On d 42, all piglets were euthanized to obtain ileal and cecal digesta for microbial analysis, which involved high-throughput sequencing of the V1 — V3 regions of 16S rRNA gene. Corn- and wheat-based diets differed (P < 0.05) in digestion characteristics. Dietary treatments affected the alpha- and beta-diversities of microbiota in the cecum but not in the ileum. The wheat-based diet increased (P < 0.05) alpha-diversity and clustered separately (P < 0.05) compared with the corn-based diet. Wheat-based diet also promoted the relative abundance of genus (g.) Succinivibrio while corn-based diet promoted the proportion of family (f.) Veillonellaceae in the community. Among xylanases, only XC within the wheat-based diet altered (P < 0.05) the beta-diversity of the cecal microbiota compared with control. For each cereal-based diet and compared with the controls, xylanase treatments affected (P < 0.05) the proportions of 5 bacterial taxa in the ileum (f. Peptostreptococcaceae, order [o.] Streptophyta, f. Clostridiaceae, g. Clostridium and g. Streptococcus) and 8 in the cecum (g. Lactobacillus, g. Streptococcus, class [c.] Clostridia, f. Clostridiaceae, g. Megasphaera, g. Prevotella, g. Roseburia and f. Ruminococcaceae). Network analysis showed that across diets under control treatments, Bacteroidetes was the most influential phylum promoting cooperative relationships among members of the ileum and cecum microbiota. Xylanase treatment, however, reduced the influence of Bacteroidetes and promoted a large number of hub taxa majority of which belonged to the Firmicutes phylum. To maximize the efficiency of xylanase supplementation, our data suggest that xylanase C originated from Bacillus subtilis was more effective when applied to wheat-based diets, while xylanase A originated from Fusarium verticillioides was more beneficial when applied to corn-based diets.
Keywords: Piglets, Microbiota, Xylanase, Arabinoxylans, Digestibility
1. Introduction
By-products of cereal processing, such as wheat bran, wheat middlings, and corn distillers dried grains, are frequently used as alternative ingredients to reduce the cost of pig feedstuff (Yanez et al., 2011, Rosenfelder et al., 2013). These cereal by-products are rich sources of non-starch polysaccharides (NSP) such as arabinoxylans (AX) (Rosenfelder et al., 2013). Due to their structural characteristics (i.e., large molecular weight and semi-flexible random coil conformation), AX exhibit very high viscosity in aqueous solutions, leading to increased viscosity of digesta (Izydorcszyk and Biliaderis, 2007). In addition, non-ruminant animals do not produce enzymes for the hydrolysis of NSP (Barrera et al., 2004); therefore, the NSP in pigs' diets encapsulate other nutrients and can act as a physical barrier limiting nutrient digestibility in the small intestine (Nortey et al., 2007). The encapsulated nutrients and NSP are then mainly hydrolyzed in the large intestine via microbial fermentation (Nielsen et al., 2014).
Exogenous enzyme supplementation is a common method for reducing intestinal viscosity and improving digestive utilization of nutrients from NSP-rich feedstuffs for growing pigs (Nortey et al., 2008, Rosenfelder et al., 2013). Xylanases are key enzymes in carbohydrate metabolism. They can be isolated from bacteria and fungi (Beaugrand et al., 2004). For cereal AX degradation, xylanases attack the xylan backbone, randomly cleaving internal β-(1,4)-linkages and penetrating the cell wall network, hence, reducing molecular weight distribution (Zhang et al., 2016). While some pig studies have shown that xylanase supplementation increases the average daily gain (ADG) and gain:feed ratio (G:F) (Barrera et al., 2004, Diebold et al., 2004, Omogbenigun et al., 2004), others reported that the addition of xylanases to cereal-based diets did not affect growth performance (Mavromichalis et al., 2000, Widyaratne et al., 2009). These contrasting results may be due to differences in the amount of AX in the diets, variation in the cereal fraction, and/or different enzymatic hydrolysis activities. Nevertheless, the reasons for the differing results have not been conclusively determined and needs to be further investigated.
Pigs' intestines are colonized by vast numbers of diverse microbes that have an important impact on gut health (Wijtten et al., 2011, Xiao et al., 2016). NSP-rich diets strongly influence the gut environment by reducing gut transit time and pH, and increasing substrates that are slowly degradable by the microbiota in the large intestines (Murphy et al., 2012). Gut microbial fermentation of NSP primarily produce short chain fatty acids (SCFA) including acetate, propionate and butyrate (Topping and Clifton, 2001), which play key roles in intestinal epithelium proliferation, and thus gut barrier function (Scott et al., 2013). Apart from SCFA, gut microbiota produces a range of small metabolites and neurotransmitters, such as γ-aminobutyric acid (by members of Bifidobacteria and Lactobacilli), acetylcholine (by Lactobacilli), dopamine (by Bacillus), noradrenalin (by Escherichia), and serotonin (by Enterococcus and Streptococcus) that could regulate many intestinal physiological functions including intestinal fluid secretion and motility in the small and large intestines (Bourassa et al., 2016). In pigs, cereal-based diets with high NSP have been found to strongly impact microbiota composition, increasing relative abundances of Lactobacillus spp., Ruminococcus spp., Prevotella spp. and Roseburia spp. in the large intestine to name a few (Durmic et al., 1998, Murphy et al., 2012). It is speculated that high NSP diets supplemented with xylanases increase gut transit time and availability of nutrients for the host, therefore, differentially altering microbiota diversity and composition in the large intestine. Whether or not such alteration in microbiota composition is of benefit to pig performance requires further investigation. Thus, the current study aimed to evaluate the effects of various exogenous bacterial and fungal xylanases with cereal-based diets on nutrient digestibility, gut microbiota and their association with performance parameters in growing pigs.
2. Materials and methods
2.1. Ethics statements
The animal experiment for this study was approved and performed according to the ethical guidelines specified by the Animal Care Committee of the University of Manitoba (Reference Number: F09-008/1/2) and standard guidelines of the Canadian Council on Animal Care (CCAC, 2009).
2.2. Pigs, diets, experimental design, housing and sampling
The animal experiment was performed as previously described (Ndou et al., 2015). Briefly, 96 Genesus gilts ([Yorkshi-re − Landrace] × Duroc, initial BW = 22.7 ± 0.65 kg) from Glenlea Swine Research Unit, University of Manitoba were individually penned for 42 d, and allotted to 12 treatments (n = 8 per treatment) in a complete randomized block design with factorial arrangement (diets and xylanases). Pigs received 1 of the 2 basal diets: 1) corn with 40% corn distillers dried grains, or 2) wheat with 25% wheat co-products. The diets were supplemented with or without 75 mg/kg of 1 of 5 xylanases (xylanase A [XA], xylanase B [XB], xylanase C [XC], xylanase D [XD], and xylanase E [XE]). The original microorganism source and the molecular weight (MW) of xylanases were as follow: Fusarium verticillioides for XA (MW = 33 kDa), Aspergillus clavatus for XB (MW = 21 kDa), Bacillus subtilis for XC (MW = 23 kDa), Trichoderma reesei for XD (MW = 21 kDa) and a modified strain of T. reesei for XE (MW = 23 kDa). All xylanases were expressed in T. reesei except XC was expressed in B. subtilis. The pepsin stabilities of xylanases were reported in Ndou et al. (2015). The formulation and nutrient composition of the 2 basal diets, and xylanase products information, were also reported previously (Ndou et al., 2015). All diets were isocaloric having a net energy of approximately 10 MJ/kg and contained supplemental microbial phytase at the rate of 500 FTU/kg (AxtraPHY, Marlborough, Wilts, UK).
Each pen measured 1.5 m × 1.2 m was equipped with a stainless steel self-feeder and a low-pressure nipple drinker that allowed unlimited access to feed and water throughout the experiment. The weight of each pig on d 0 and 42, and feed intake during the experimental period, were measured to calculate average daily feed intake (ADFI), ADG and G:F. These performance characteristics have been reported previously (Ndou et al., 2015). Fecal samples were collected on the last 2 d of the experimental period, and stored immediately at −20 °C for later analysis of apparent total tract digestibility (ATTD).
On d 42, all pigs were sedated by intramuscular injection of 20 mg/kg BW of ketamine and 2 mg/kg BW of kylazine and euthanized by intracardiac injection of 110 mg/kg BW sodium pentobarbital (Bimeda-MTC Animal Health Inc., Cambridge, ON, Canada). The abdominal cavity was opened from sternum to pubis to expose the gastrointestinal tract without damaging the wall of the digestive tract. The small intestine was stripped free of its mesentery, and ileal and cecal digesta samples were obtained. The ileum digesta samples were collected at 5 cm from ileocecal junction. Samples were divided into 2 sub-samples and transferred to sterile sample bags. One sub-sample of the digesta was kept on ice and then transferred to −20 °C for later analysis of VFA and ATTD. The second sub-sample was immediately frozen in liquid nitrogen and transferred to −80 °C until used for DNA extraction and further microbial analyses.
2.3. Apparent total tract digestibility
Ileal and cecal digesta samples were thawed, homogenized in a blender (Waring Commercial, Tor-rington, CT, USA) and freeze dried. Fecal samples from the last 2 d of experiment were oven dried at 60 °C for 4 d, pooled per pig and subsampled. Both digesta and fecal samples were then finely ground in a Thomas Wiley mill model 4 (Labwrench, Midland, ON, Canada) and mixed prior to chemical analysis. The diets and fecal samples were analyzed for dry matter (DM), gross energy (GE), crude protein (CP) and fat contents. Gross energy was determined using an automated adiabatic oxygen bomb calorimeter (Parr Instrument Co., Moline, IL, USA) with benzoic acid as the reference material. The CP values were determined by multiplying the assayed N values by a factor of 6.25. Fat content was derived using ANKOM XT 20 Extractor (Ankom Technology, Fairport, NY). VFA concentrations were determined by gas chromatography as the method reported by Bhandari et al. (2007). The ATTD were calculated as described (Ndou et al., 2015).
2.4. DNA extraction
Approximately 150 mg of each ileum or cecum digesta sample was used for genomic DNA extraction using a ZR Fecal DNA extraction kit (D6100; Zymo Research., Orange, CA, USA), which included a bead-beating step for the mechanical lysis of the microbial cells. DNA was quantified using a NanoDrop 2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). DNA samples were normalized to 20 ng/μL, and quality was checked by PCR amplification of the 16S rRNA gene using universal primers 27F (5′-GAAGAGTTTGATCATGGCTCAG-3′) and 342R (5′-CTGCTGCCTCCCGTAG-3′) as described by Khafipour et al. (2009). Amplicons were verified by agarose gel electrophoresis.
2.5. Library construction and pyrosequencing
Ninety-six DNA samples (n = 8) were pyrosequenced as previously described (Plaizier et al., 2017), using the bacterial tag-encoded GS FLX-Titanium amplicon. Briefly, a mixture of Hot Start, HotStar high fidelity Taq polymerases, and Titanium reagents, were used to perform a one-step PCR amplification with 35 cycles, using the 28f (5′-GAGTTTGATCNTGGCTCAG-3′) and 519r (5′-GTNTTACNGCGGCKGCTG-3′) primers, which covered the hypervariable V1—V3 regions of the bacterial 16S rRNA genes (Plaizier et al., 2017). The pyrosequencing procedures were carried out at the Research and Testing Laboratory (Lubbock, TX; http://www.Researchandtesting.com).
2.6. Bioinformatic analysis
Sequencing data were binned using sample-specific barcode sequences, and filtered using QIIME 1.9.1 (Caporaso et al., 2010a). All sequences <200 bp, with ambiguous nucleotide bases, or a homopolymer length longer than 7 bp were removed from downstream analyses. Chimeric reads were filtered using UCHIME (Edgar et al., 2011) and sequences were assigned to operational taxonomic units (OTU) using the QIIME implementation of UCLUST (Edgar, 2010) at 97% pairwise identity threshold. Taxonomies were assigned to the representative sequence of each OTU using the RDP classifier (Wang et al., 2007) and aligned with the Greengenes Core reference database (DeSantis et al., 2006) using PyNAST algorithms (Caporaso et al., 2010a, Caporaso et al., 2010b). While the majority of OTU were classified at the genus (g.) level, some were classified only at the family (f.), order (o.), class (c.), or phylum (p.) levels.
Within-community diversity (alpha-diversity) was calculated by both Shannon and Simpson indices using the open source software QIIME 1.9.1. An even depth of 1,000 and 1,021 sequences per sample was used to calculate the diversity indices for the ileal and cecal digesta, respectively. To compare microbial composition between samples, beta-diversity was measured by calculating the Bray–Curtis distances (Lozupone and Knight, 2005) in QIIME. Nonmetric-multidimensional scaling (nMDS) plots were generated by R (3.3.1 version) with the VEGAN package (Oksanen et al., 2013) to visualize changes in microbiota diversity. Hierarchical clustering analysis was performed using R to show a visual interpretation heatmap of the similarity of bacterial taxa among diets and enzyme treatments (Derakhshani et al., 2016a, Derakhshani et al., 2016b). Normalized relative abundance of bacterial taxa (row normalize length transformation, PAST, version 2.17) was used to generate the clustering heatmap.
Correlation network analysis (CoNet) was used to investigate microbial co-occurrence/co-exclusion correlation between OTU and identify hub/influential bacterial taxa that show the highest number of connections with other taxa (Faust and Raes, 2016). The network analysis applied permutation computation with a combination of correlation and dissimilarity measures (Pearson's, Spearman's correlation coefficients, Bray–Curtis and Kullback–Leibler dissimilarities) based on relative abundances data to generate a primary microbial network. In the permutation stage, distributions of all pair-wise scores between the nodes (a node representing the relative abundance of a non-singleton OTU) were computed. Permutation was set as 500 and in combination with a renormalization step for Pearson's and Spearman's measures in order to address the issue of compositionality introduced by different sequencing depths for each sample. Furthermore, the measure-specific P-values were then computed as the probability of the null value (represented by the mean of the null distribution) under a Gauss curve generated from the mean and standard deviation of the bootstrap distribution. Measure-specific P-values were then merged using Simes' method (Simes, 1986), and after using Benjamini–Hochberg's false discovery rate (FDR) correction, only edges with merged P-values below 0.05 were kept. Edges with scores outside the 95% confidence interval defined by the bootstrap distribution and not supported by at least two measures were also discarded.
Spearman's non-parametric correlation analysis was used to explore the associations between bacterial taxa, production performance [extracted from Ndou et al., (2015)] and ATTD of pigs fed either corn or wheat-based diets supplemented with different xylanases. The correlation heatmaps were created using R.
2.7. Statistical analysis
Statistical analyses of the ATTD values, VFA concentrations, and alpha-diversity indices were tested using the MIXED procedure (Tukey studentized range adjustment) of SAS version 9.4 (SAS Inst. Inc., Cary, NC) with the xylanase treatment and basal diet as 2 fixed factors and the individual animal as a random factor. The non-normally distributed data were transformed and analyzed using the GLIMMIX procedure (Tukey studentized range adjustment) by the SAS. Permutational multivariate analysis of variance (PERMANOVA, Primer 7 [Anderson, 2005]) was used to do the statistical analyses of Bray–Curtis distances across xylanase treatments and diets (two fixed factors). Multivariate Association with Linear Models (MaAsLin, R V.0.0.3) was used to analyze the difference in bacteria taxa between the two cereal diets (Morgan et al., 2012). Correlations between bacterial taxa and physiological factors were calculated by non-parametric Spearman's rank correlation analysis using JMP version 10 (SAS Inst. Inc., Cary, NC). The differences between treatments were considered significant at P < 0.05.
3. Results
3.1. Nutrient apparent digestibility and VFA concentrations
Apparent total tract digestibility and VFA concentrations of the ileal and cecal digesta are reported in Tables 1 and 2. Most digestion characteristics showed significant differences between corn- and wheat-based diets, with the exception of cecum VFA. Pigs fed a wheat-based diet had higher (P < 0.05) DM, GE and CP digestibility, but lower (P < 0.05) fat digestibility and ileum VFA concentration than pigs fed a corn-based diet. Compared with various xylanase treatments, XC supplementation with a wheat-based diet yielded the highest (P < 0.05) CP digestibility of all xylanase treatments and controls.
Table 1.
Effects of different xylanase supplementations within basal diets on the apparent total tract digestibility of nutrients and VFA concentrations in the ileal and cecal digesta of piglets.1
| Item | Corn-based diet2 |
Wheat-based diet2 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | XA | XB | XC | XD | XE | SEM | Control | XA | XB | XC | XD | XE | SEM | |
| Digestibility, % | ||||||||||||||
| DM | 71.04 | 73.26 | 70.42 | 68.93 | 68.81 | 68.75 | 1.65 | 71.90 | 73.79 | 75.35 | 79.18 | 73.73 | 73.81 | 1.77 |
| GE | 71.01 | 72.89 | 70.54 | 68.78 | 68.49 | 68.29 | 1.64 | 72.91 | 73.44 | 76.21 | 80.39 | 74.83 | 74.50 | 1.69 |
| CP | 70.98 | 73.78 | 72.45 | 70.02 | 72.70 | 71.86 | 1.88 | 73.70a | 77.06a,b | 80.31a,b | 84.09b | 78.11a,b | 78.46a,b | 1.68 |
| Fat | 50.29 | 58.30 | 50.73 | 52.94 | 41.92 | 50.24 | 7.08 | 31.63 | 26.72 | 39.40 | 17.70 | 53.51 | 47.21 | 12.25 |
| VFA concentration, mmol/L | ||||||||||||||
| Ileum | 10.66 | 10.31 | 11.44 | 12.64 | 12.53 | 12.33 | 1.10 | 6.02 | 5.95 | 8.00 | 8.21 | 7.60 | 7.28 | 1.00 |
| Cecum | 42.75 | 46.80 | 39.15 | 42.53 | 43.84 | 44.25 | 4.76 | 42.59 | 40.52 | 42.67 | 35.40 | 33.80 | 34.79 | 4.07 |
SEM = standard error of the mean.
a, b Mean values within a row with different superscripts were significantly different (P < 0.05).
Reported values are least-squares means.
The basal diets were supplemented with or without 75 mg/kg of 1 of 5 types of xylanase supplements (xylanase A [XA], xylanase B [XB], xylanase C [XC], xylanase D [XD], and xylanase E [XE]) from various original microorganisms.
Table 2.
Effects of different diet types and xylanase treatments on the apparent total tract digestibility of nutrients and VFA concentrations in the ileal and cecal digesta of piglets.1
| Item | Diets |
Xylanases2 |
P-value |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Corn | Wheat | SEM | Control | XA | XB | XC | XD | XE | SEM | Diet | Xylanase | Diet × Xylanase | |
| Digestibility, % | |||||||||||||
| DM | 70.20a | 74.63b | 0.69 | 71.47 | 73.52 | 72.88 | 74.06 | 71.27 | 71.28 | 1.20 | <0.001 | 0.39 | 0.06 |
| GE | 70.00a | 75.38b | 0.68 | 71.96 | 73.16 | 73.37 | 74.59 | 71.66 | 71.40 | 1.17 | <0.001 | 0.36 | 0.02 |
| CP | 71.96a | 78.62b | 0.73 | 72.34 | 75.42 | 76.38 | 77.05 | 75.40 | 75.16 | 1.26 | <0.001 | 0.15 | 0.02 |
| Fat | 50.74b | 36.03a | 4.13 | 40.96 | 42.51 | 45.07 | 35.32 | 47.72 | 48.72 | 7.16 | 0.04 | 0.98 | 0.45 |
| VFA concentration, mmol/L | |||||||||||||
| Ileum | 11.65b | 7.18a | 0.46 | 8.34 | 8.13 | 9.72 | 10.43 | 10.07 | 9.80 | 0.80 | <0.001 | 0.23 | 0.98 |
| Cecum | 43.22 | 39.13 | 1.84 | 45.17 | 43.66 | 40.91 | 38.97 | 38.82 | 39.52 | 3.18 | 0.12 | 0.61 | 0.37 |
SEM = standard error of the mean.
a, b Mean values within a row with different superscripts were significantly different (P < 0.05).
Reported values are least-squares means.
The basal diets were supplemented with or without 75 mg/kg of 1 of 5 types of xylanase supplements (xylanase A [XA], xylanase B [XB], xylanase C [XC], xylanase D [XD], and xylanase E [XE]) from various original microorganisms.
3.2. Gut microbiota diversity
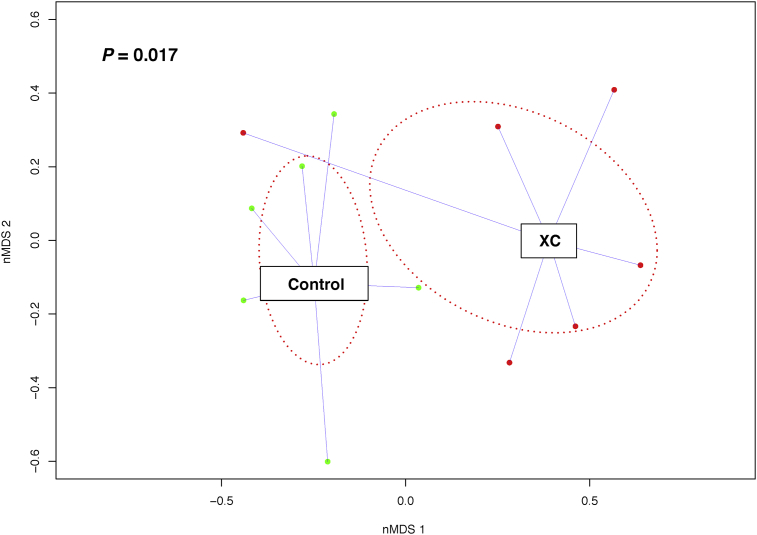
A total of 87 ileal and 87 cecal digesta samples were bioinformatically analyzed. Nine ileum digesta samples and 9 cecum digesta samples were eliminated due to low sequence reads (Appendix Table 1 for distribution of eliminated samples among treatments). Alpha-diversity indices of the ileum and cecum microbiota were not affected (P > 0.05) by xylanase treatments (Appendix Tables 2–6). The alpha-diversity of ileum microbiota was not affected by diets either (Appendix Table 6). However, the cecum microbiota had higher (P < 0.05) alpha-diversity in the wheat-based than in the corn-based diet (Table 3). For the beta-diversity, among the xylanase treatments, only XC had a different (P < 0.05) diversity compared with control in the cecum of pigs fed the wheat-based diet (Fig. 1). Among the basal diets, only the beta-diversity of ileum and cecum microbiota under the corn-based diet was found to differ (P < 0.05; PERMANOVA results) from that under the wheat-based diet (Figs. 2 and 3).
Table 3.
Alpha-diversity of cecal microbiota in piglets across diets and xylanase treatments.1
| Diversity indices | Diets |
Xylanases2 |
P-values |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Corn | Wheat | SEM | Control | XA | XB | XC | XD | XE | SEM | Diet | Xylanase | Diet × Xylanase | |
| Shannon | 4.39b | 4.91a | 0.17 | 4.61 | 4.56 | 4.69 | 4.57 | 4.92 | 4.65 | 0.29 | 0.03 | 0.94 | 0.67 |
| Simpson | 0.84b | 0.89a | 0.01 | 0.86 | 0.85 | 0.88 | 0.86 | 0.89 | 0.85 | 0.03 | 0.04 | 0.95 | 0.37 |
SEM = standard error of the mean.
a, b Mean values within a row with different superscripts were significantly different (P < 0.05).
Reported values are least-squares means.
The basal diets (corn- or wheat-based) were supplemented with or without 75 mg/kg of 1 of 5 types of xylanase supplements (xylanase A [XA], xylanase B [XB], xylanase C [XC], xylanase D [XD], and xylanase E [XE]) originated from various microorganisms.
Fig. 1.
Comparison of beta-diversity of cecum microbiota between control and xylanase C (XC) in pigs fed a wheat-based diet. The nonmetric-multidimensional scaling (nMDS) plots were generated using Bray–Curtis distances. P-value was obtained from PERMANOVA.
Fig. 2.
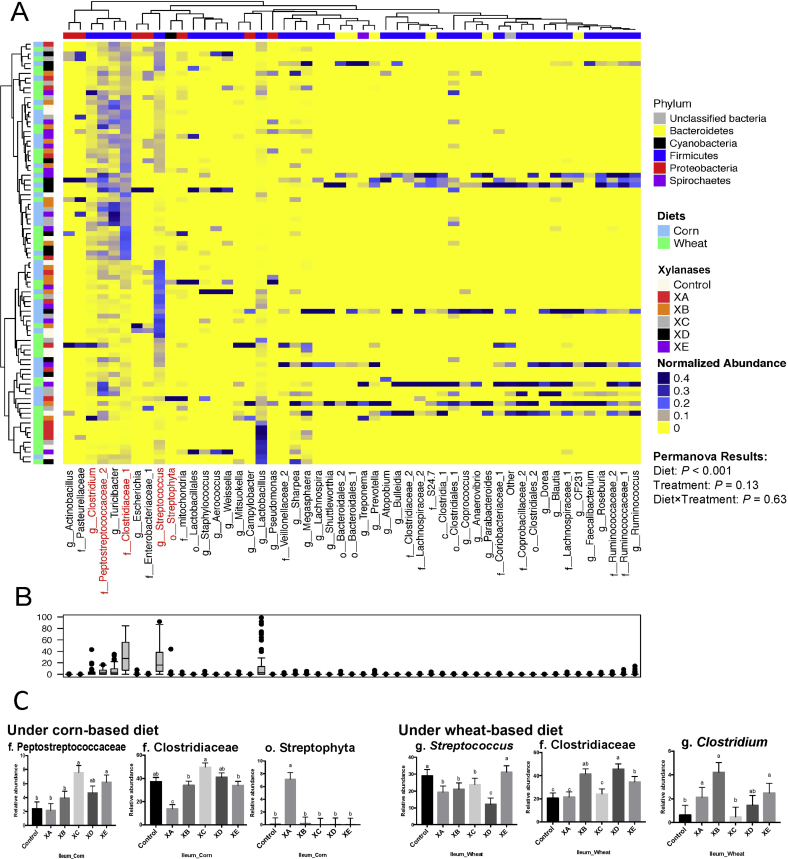
Compositions of bacterial community in the ileum of pigs fed corn- and wheat-based diets and supplemented with different xylanases. (A) Each row is representing a sample while each column is representing an operational taxonomic units (OTU) . The sample identifiers on the left branches are color coded based on diets and xylanase treatments. Top branches are color coded indicating the taxonomical assignment of the OTU at the phylum level. OTU with relative abundance above 0.01% of community are presented. While majority of OTU were classified at the genus level, some could only be classified to family (f.), order (o.), class (c.), or phylum (p.) levels. The normalized relative abundances of OTU obtained from 16S rRNA gene sequences in each ileum sample is reflected by the color of the scale (yellow to red). The dendrogram on the left shows how the samples are clustered based on the Bray–Curtis dissimilarity measure, averaged by diet and xylanase treatment. The dendrogram on the top shows clustering of bacterial taxa data based on the Spearman's rank correlation. (B) Box-Plot showing the relative abundances of bacterial taxa in ileum microbiota. (C) Column plots showing the effect of xylanase treatments on the abundance of bacterial taxa that significantly differed among treatments. Values are presented as least square means. Error bars are standard error of the mean. Significant relative abundance pertaining to changes in the different treatments are also indicated in the heatmap A. The basal diets (corn- or wheat-based) were supplemented with or without 75 mg/kg of 1 of 5 types of xylanase supplements (xylanase A [XA], xylanase B [XB], xylanase C [XC], xylanase D [XD], and xylanase E [XE]) originated from various microorganisms.
Fig. 3.
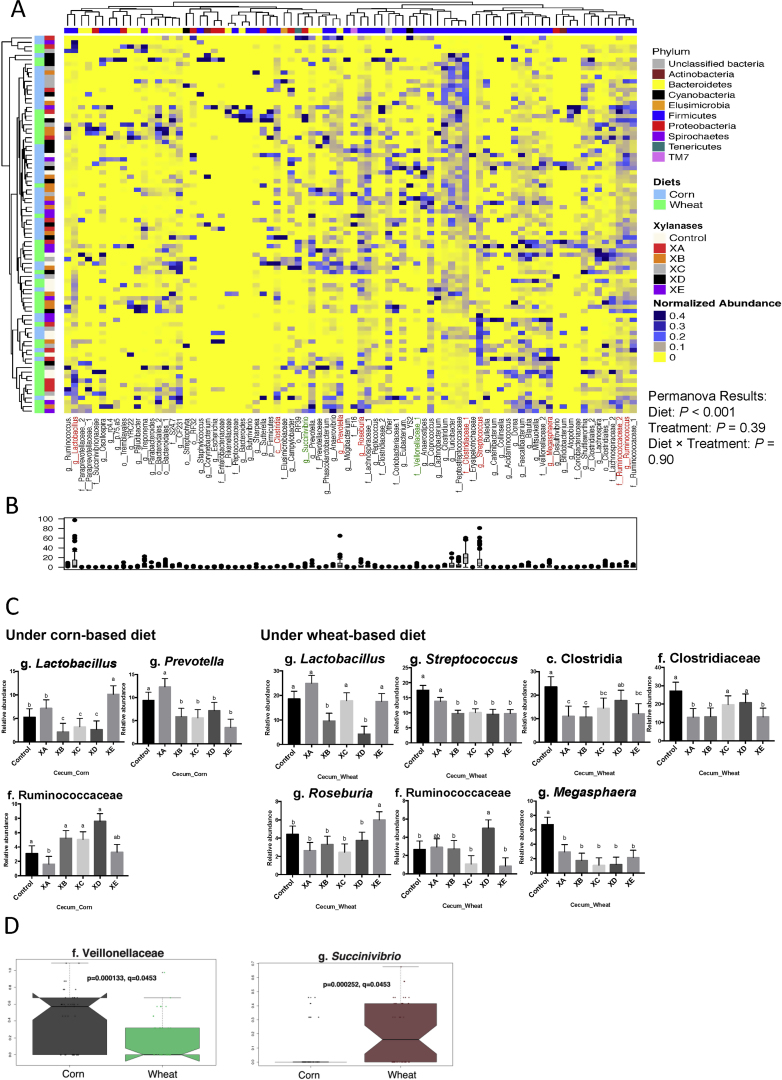
Compositions of bacterial community in the cecum of pigs fed corn- and wheat-based diets and supplemented with different xylanases. (A) Each row is representing a sample while each column is representing an operational taxonomic unit (OTU). The sample identifiers on the left branches are color coded based on diets and xylanase treatments. Top branches are color coded indicating the taxonomical assignment of the OTU at the phylum level. OTU with relative abundance above 0.01% of community are presented. While majority of OTU were classified at the genus level, some could only be classified to family (f.), order (o.), class (c.), or phylum (p.) levels. The normalized relative abundances of OTU obtained from 16S rRNA gene sequences in each cecum sample is reflected by the color of the scale (yellow to red). The dendrogram on the left shows how the samples are clustered based on the Bray–Curtis dissimilarity measure, averaged by diet and xylanase treatment. The dendrogram on the top shows clustering of bacterial taxa data based on the Spearman's rank correlation. (B) Box-Plot showing the relative abundances of bacterial taxa in cecum microbiota. (C) Column plots showing the effect of xylanase treatments on the abundance of bacterial taxa that significantly differed among treatments. Values are presented as least square means. Error bars are standard error of the mean. Significant relative abundance pertaining to changes in the different treatments are also indicated in the heatmap A. (D) The MaAsLin plots show the relative abundance of bacterial taxa that significantly differed between the 2 diet groups (P < 0.05). The basal diets (corn- or wheat-based) were supplemented with or without 75 mg/kg of 1 of 5 types of xylanase supplements (xylanase A [XA], xylanase B [XB], xylanase C [XC], xylanase D [XD], and xylanase E [XE]) originated from various microorganisms.
3.3. Effects of xylanases on the composition of gut microbiota
The cluster heatmaps (Figs. 2 and 3) revealed the distribution patterns of bacterial taxa in the ileum and cecum of pigs fed a corn- or wheat-based diet supplemented with different xylanases. Across treatments, 5 major phyla and 50 genera (mean relative abundance > 0.01% of community) were observed in the ileal microbiota (Fig. 2A) whilst the cecal microbiota appeared to be more diverse, characterized by 9 major phyla and 81 genera (Fig. 3A). Firmicutes was the dominant bacterial phylum in the ileum and cecum, with the g. Streptococcus and g. Lactobacillus being the most abundant genera (Figs. 2B and 3B). Based on clustering analysis, within the entire ileal or cecal communities, neither the diets nor xylanase treatments showed any significant dissimilarity (P > 0.05) at the bacterial phylum level. However, at the genus level xylanase treatments and/or the diets significantly affected the abundances of several bacterial taxa. In the ileum (Fig. 2C), the abundances of 5 bacterial taxa (f. Peptostreptococcaceae, o. Streptophyta, f. Clostridiaceae, g. Clostridium and g. Streptococcus) were altered (P < 0.05) depending on the xylanase treatment compared with control groups. In the cecum (Fig. 3C and D), the abundances of 8 bacterial taxa (g. Lactobacillus, g. Streptococcus, c. Clostridia, f. Clostridiaceae, g. Megasphaera, g. Prevotella, g. Roseburia and f. Ruminococcaceae) were affected (P < 0.05) by xylanase treatments compared with controls. Additionally, pigs fed the wheat-based diet had a lower (P < 0.05) proportion of f. Veillonellaceae and a higher proportion of g. Succinivibrio than pigs fed the corn-based diet (Fig. 3D).
3.4. Effects of xylanase treatments on the microbial networks in the cecum
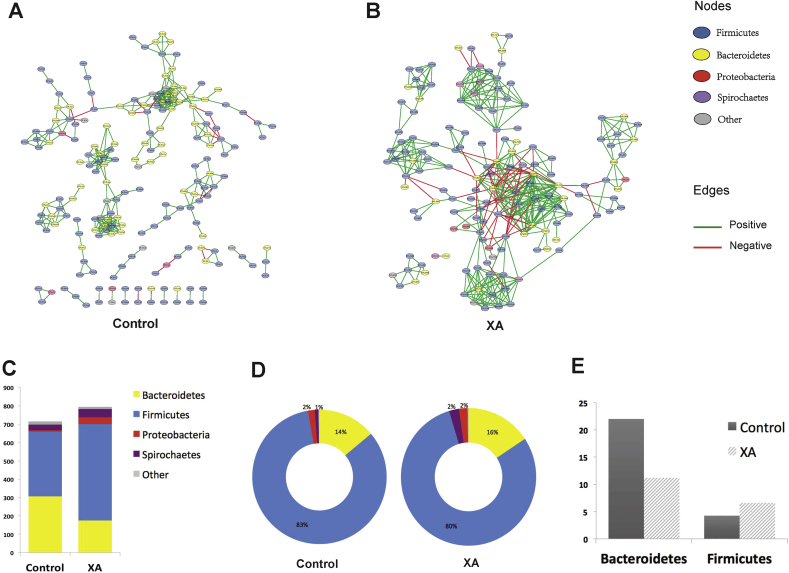
Xylanase C in the wheat-based diet and XA in the corn-based diet were selected to investigate the effect of xylanase on microbial networks in the cecum. These selections were based on the fact that only XC treatment in the wheat-based diet altered (P < 0.05) the beta-diversity of the cecal microbiota compared with control (Fig. 1), and that in the corn-based diet. Although xylanase treatments did not change (P > 0.05) the alpha- or beta-diversities, XA treatment showed the greatest improvement in digestibility compared with other xylanase treatments and control (Table 1). Additionally, the growth performance data previously reported by Ndou et al. (2015) showed that XC treatment in the wheat-based diet, and XA in the corn-based diet promoted (P < 0.05) ADG and NSP digestibility of the growing pigs compared with other xylanase treatments.
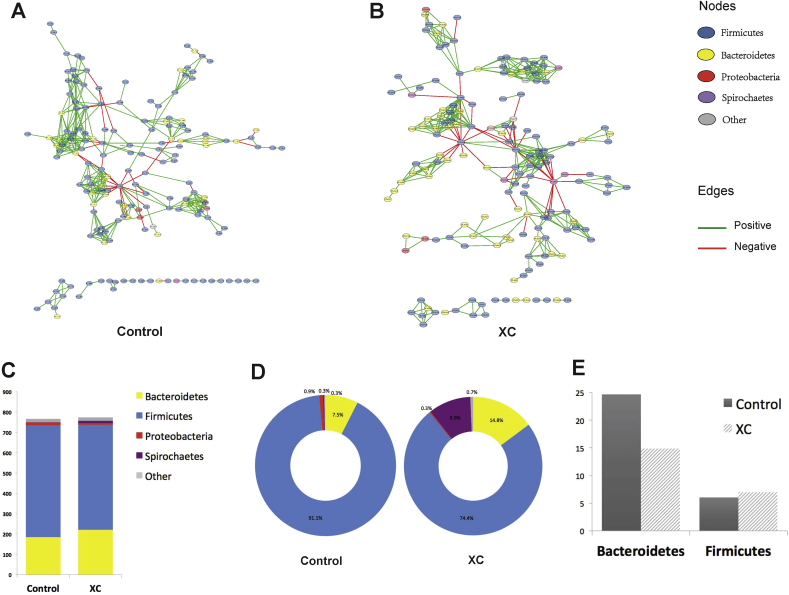
At the phylum level, microbial networks were formed among Firmicutes, Bacteroidetes, Proteobacteria and Spirochaetes (Figs. 4A, B, 5A, B). Majority of correlations (above 87%) were co-occurrences (positive edges shown by green lines) between the OTU (nodes). Depending on the treatments, Firmicutes contributed to 50% to 72% of positive connections in the microbial networks followed by Bacteroidetes which contributed to 22% to 43% (Figs. 4C and 5C).
Fig. 4.
Impact of xylanase A (XA) on the interaction profile of cecum microbiota in pigs fed a corn-based diet. (A), (B) Significant interactions between bacterial taxa within cecum microbiota under control (A) and XA (B) treatments. Each node represents an operational taxonomic unit (OTU), colored based on the originating phylum. Each edge represents a significant positive (green line) or negative (red line) interaction between nodes. (C) Positive degree of interactions (numbers of positive edges) of phyla in control and XA microbiota. (D) Relative abundances of bacterial phyla in control and XA microbiota. (E) Influential capability of Bacteroidetes and Firmicutes. The bars represent the normalized value of positive degree of interactions of Bacteroidetes and Firmicutes which calculated by dividing the positive degree of interaction of each phylum by its mean relative abundance.
Fig. 5.
Impact of xylanase C (XC) on the interaction profile of cecum microbiota in pigs fed a wheat-based diet. (A), (B) Significant interactions between bacterial taxa within cecum microbiota under control (A) and XC (B) treatments. Each node represents an operational taxonomic unit (OTU) colored based on the originating phylum level. Each edge represents a significant positive (green line) or negative (red line) interaction between nodes. (C) Positive degree of interactions (numbers of positive edges) of phyla in control and XC microbiota. (D) Relative abundances of bacterial phyla in control and XC microbiota. (E) Influential capability of Bacteroidetes and Firmicutes. The bars represent the normalized value of positive degree of interactions of Bacteroidetes and Firmicutes which calculated by dividing the positive degree of interaction of each phylum by its mean relative abundance.
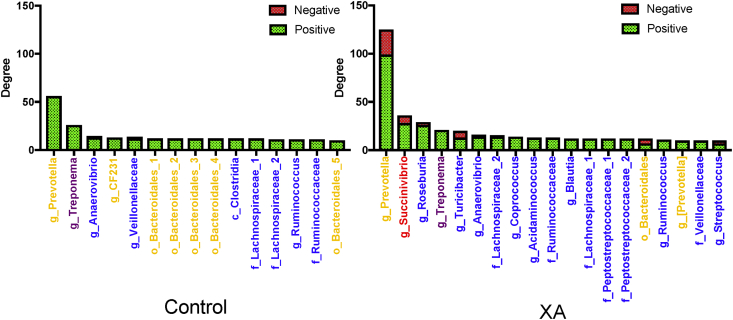
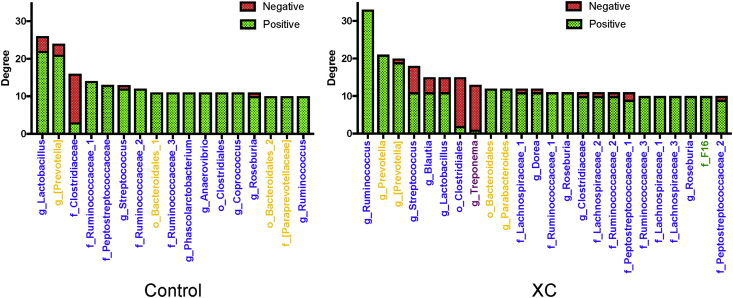
The relative abundances of bacterial phyla are presented in Figs. 4D and 5D. As relative abundances of taxa do not explain their influential capacity in the microbial network, data were normalized by dividing the degree of positive connectedness of each taxa by its relative abundance (Figs. 4E and 5E) (Trosvik and de Muinck, 2015). In both corn- and wheat-based diets under control conditions, Bacteroidetes had a higher influential capability (or a higher normalized degree of positive connectedness) than Firmicutes; however, Bacteroidetes influence declined under xylanase treatment (Figs. 4E and 5E). The hub bacterial taxa within the cecum microbiota are presented in Figs. 6 and 7. In pigs fed corn-based diets, 15 taxa with high degrees of interactions were identified in the cecum microbiota of control group compared with 19 taxa in the XA group (Fig. 6). Among these taxa, Prevotella had the highest normalized degree of interactions in both control and XA but its number of positive interactions was higher under xylanase treatment (Fig. 6). In pigs fed wheat-based diets, 17 taxa with high normalized degrees of interactions were identified in the cecum microbiota of control compared to 24 taxa in the XC (Fig. 7). It was noted that in pigs fed wheat-based diet, Lactobacillus emerged to be as the most influential taxa in the control and rank 6th in the XA treatment, while it was not placed among the top 15 hub bacterial taxa under the corn-based diet. Prevotella remained an important member of the cecum microbiota of pigs fed wheat-based diets affecting ecosystem network while holding the second highest normalized number of positive interactions in the community.
Fig. 6.
Hub bacterial taxa within the cecum microbiota of pigs fed a corn-based diet with or without xylanase A (XA). Bar plots show taxa with greater than 10 significant interactions (number of edges). Bacterial taxa were colored based on their originating phylum: yellow – Bacteroidetes; blue – Firmicutes; red – Proteobacteria; and purple - Spirochaetes.
Fig. 7.
Hub bacterial taxa within the cecum microbiota of pigs fed a wheat-based diet with or without xylanase C (XC). Bar plots show taxa with greater than 10 significant interactions (number of edges). Bacterial taxa were colored based on their originating phylum: yellow – Bacteroidetes; blue – Firmicutes; red – Proteobacteria; purple – Spirochaetes; and green – TM7.
3.5. Correlations between gut bacterial taxa and physiological factors
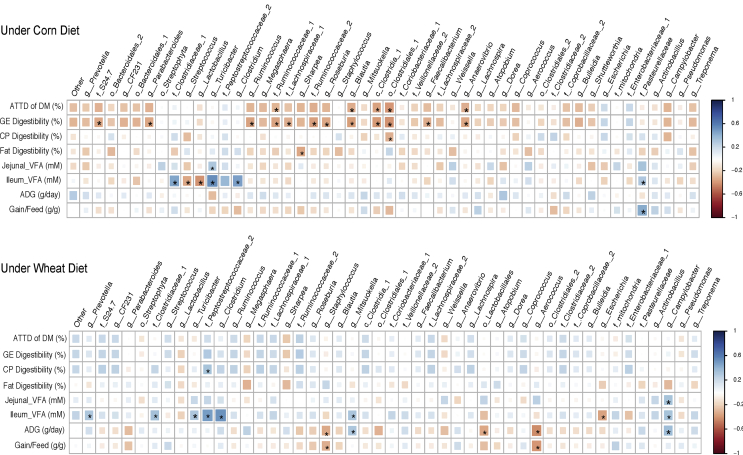
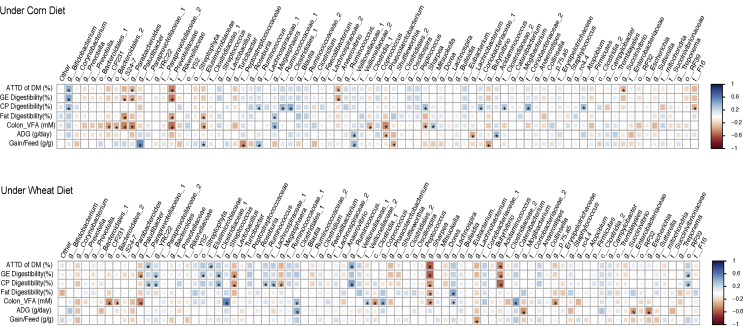
The correlation data between members of ileum and cecum microbiota and pigs' physiological parameters are presented in Appendix Figs. 1 and 2. In the ileum microbiota under both cereal diets, the relative abundance of f. Clostridiaceae and g. Clostridium were positively correlated (P < 0.05) with ileum total VFA concentration. In the cecum microbiota under wheat-based diets (Fig. 5), the relative abundance of g. Lactobacillus was negatively correlated (P < 0.05) with GE and CP digestibility; g. Streptococcus and c. Clostridia were positively correlated (P < 0.05) with colon VFA concentration; g. Megasphaera was negatively correlated (P < 0.05) with CP digestibility; and f. Clostridiaceae was positively correlated with CP and GE digestibility. In the cecum microbiota under the corn-based diet, f. Ruminococcaceae was positively correlated (P < 0.05) with CP digestibility.
4. Discussion
Diet, particularly macronutrients such as carbohydrates, has a critical role in shaping the composition and functionality of gut microbiota (Conlon and Bird, 2014). Xylanases hydrolyze the NSP of cereal-based diets and are widely used to improve nutrient digestibility and performance of pigs in the global swine industry (Kiarie et al., 2013, Munyaka et al., 2016). The resulting alterations to nutrient availability and gut environments may change the gut microbiota, which in turn can improve animal performance and health. A previous study in our group (Ndou et al., 2015) reported that XA supplementation in the corn-based diet and XC in the wheat-based diet significantly increased ADG and NSP digestibility in piglets. Our results further elucidated that in pigs fed wheat-based diets, XC also enhanced the digestibility of CP compared with other xylanase treatments and control. We further evaluated the effects of XA supplementation in pigs fed a corn-based diet, and XC in pigs fed a wheat-based diet, on microbial networks in the cecum and compared them with their corresponding control groups. In the cecum microbiota of control groups in both corn- and wheat-based diets, Bacteroidetes had the highest normalized degree of interactions with other community members. It is documented that Bacteroidetes have higher mean genes encoding for glycoside hydrolases, such as β-xylosidases, endo-1,4-β-xylanases and α-N-arabinofuranosidases, and polysaccharide lyases per genome compared with the members of the phylum Firmicutes or any other bacterial phyla in the gastrointestinal tract making them the primary degraders of complex polysaccharides in the piglet gut (El Kaoutari et al., 2013, Frese et al., 2015). Therefore, the high levels of polysaccharides in the corn- and wheat-based diets promoted Bacteroidetes influence and their number of interactions with other members of the cecum microbiota. When comparing xylanase treatments with controls, the influential capability of Bacteroidetes diminished under xylanase supplementations in both corn- and wheat-based diets, while that of Firmicutes increased. These results perhaps indicate that the xylanases supplementation aided in the breakdown of NSP, such as AX, to monosaccharides which in turn reduced the polysaccharides' availability to Bacteroidetes. Furthermore, at the lower taxonomical level, the microbial network outcome showed that both xylanase treatments had a greater number of hub bacterial taxa with positive interactions than their respective control groups in the cecum of piglets. The degree of positive connectedness, or co-occurrence with other members of community, for a bacterial taxon is known to represent its importance in ecosystem structure and ability to occupy an ecological niche (Trosvik and de Muinck, 2015, Braga et al., 2016). Therefore, presence of more hub members contributes to form a more diverse, balanced and stable microbial community, which aids in enhancing ecosystem functionality and resistance to invasion (Trosvik and de Muinck, 2015). Our results revealed that supplementation of xylanases increased hub members of cecal microbiota and may result in a more robust and stable microbial ecosystem, which is vital for promoting gut health. That being said, these results should be interpreted with care as they were based on correlation analyses, which might not represent true ecological interactions.
Comparison of alpha-diversity indices of the ileum and cecum microbiota showed that these indices did not change following xylanase supplementation. In pigs fed wheat-based diets, xylanase treatments (XB, XC, XD and XE) decreased the relative abundance of Streptococcus, which is one of the most abundant and hub genera in the cecum microbiota of piglets (Figs. 6 and 7). The digestibility data showed that pigs fed xylanase, particularly XC, had higher ileal digestibility of insoluble AX and insoluble NSP than control group (Ndou et al., 2015). Therefore, it can be speculated that supplemental xylanases improved the dietary polysaccharides digestibility in the ileum and in turn decreased the availability of such polysaccharides in the cecum, hence affecting the abundances of certain bacterial populations such as members of g. Streptococcus. and c. Clostridia. A significant reduction in abundance of Megasphaera in the cecum was observed in pigs with XC treatment and this was negatively correlated with CP digestibility. Megasphaera is a member of the Veillonellaceae family and is a common bacterial genera in the gut microbiota of pigs (Molbak et al., 2007, Bermingham et al., 2013). Hooda et al. (2012) reported a similar finding in cats, with the amount of CP in the digesta influencing the relative abundance of Megasphaera in the gut microbiota. This may be because xylanase contributed to the hydrolysis of internal β-(1,4)-linkages in dietary polysaccharides, particularly AX, in the cereal diet, which therefore eliminated the nutrient-encapsulating effect. This increased the availability of protein in the small intestine (Kiarie et al., 2013), which resulted in a low amount of residual CP in the large intestine and altered the relative abundance of Megasphaera in the cecum microbiota.
When comparing the cereal sources of basal diets, there were significant differences in most digestion characterizations between corn- and wheat-based diets. In term of the alpha-diversity of microbiota, our data showed that the Shannon and Simpson indices of cecum microbiota under the wheat-based diet were significantly higher (P < 0.05) than those under the corn-based diet. This indicates that the cecum microbiota under the wheat-based diet is more diverse and has higher richness. The cluster analysis also found that the beta-diversity of ileum and cecum microbiota across the 2 diets is significantly different. The reason may be that there is a larger proportion of insoluble AX in the corn-based diet than in the wheat-based diet (Ndou et al., 2015), which are covalently and non-covalently linked to other AX molecules and to other cell wall components, such as cellulose, lignin and proteins (Fengler and Marquardt, 1988). This linkage may have negatively impacted the solubilization and depolymerization of AX in the corn-based diet, which can obstruct nutrient absorption. Lending support to this hypothesis, our previous study also found that the digestibility of soluble AX was significantly higher in pigs fed the wheat-based diet compared with those fed the corn-based diet (Zhang et al., 2014, Ndou et al., 2015). As diet plays an essential role in maintaining the diversity of gut microbiota (Conlon and Bird, 2014), we speculate that cereal-based diets that contain higher NSP promote a more diverse nutritional niche, and hence, more diverse microbiota in the cecum.
5. Conclusion
Our data revealed that the cereal source was an important factor affecting the efficiency of xylanase supplementation and nutrient digestibility in the piglets gut. Consequently, this led to changes in the gut environments and residual nutrient availability in the cecum that influenced the diversity of piglet gut microbiota. To maximize the efficiency of xylanase supplementation, our data suggests that xylanase C –originated from B. subtilis– was more effective when applied to wheat-based diets, while xylanase A – originated from F. verticillioides – was more beneficial when applied to corn-based diets.
Conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
The present research is supported by grants from Growing Forward 2 – Agricultural Rural Development Initiative Program of the Province of Manitoba, Canada, and DuPont.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.aninu.2018.06.007.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Correlations between physiological factors and relative abundances of bacterial taxa in the ileum. The * symbol indicates a statistically significant correlation (P < 0.05). The scale colors (Spearman’s ρ from -1 to +1) indicate whether the correlation is positive (blue colored squares) or negative (red colored squares) between the taxa and physiological factors. ADG, average daily gain; ATTD, apparent total tract digestibility; GE, gross energy; CP, crude protein; VFA, volatile fatty acids.
figs1.
figs2.
Correlations between physiological factors and relative abundances of bacterial taxa in the cecum. The * symbol indicates a statistically significant correlation (P < 0.05). The scale colors (Spearman’s ρ from -1 to +1) indicate whether the correlation is positive (blue colored squares) or negative (red colored squares) between the taxa and physiological factors. ADG, average daily gain; ATTD, apparent total tract digestibility; GE, gross energy; CP, crude protein; VFA, volatile fatty acids.
References
- Anderson M. vol. 24. Department of Statistics, University of Auckland; New Zealand: 2005. (PERMANOVA: a FORTRAN computer program for permutational multivariate analysis of variance). [Google Scholar]
- Barrera M., Cervantes M., Sauer W.C., Araiza A.B., Torrentera N., Cervantes M. Ileal amino acid digestibility and performance of growing pigs fed wheat-based diets supplemented with xylanase. J Anim Sci. 2004;82:1997–2003. doi: 10.2527/2004.8271997x. [DOI] [PubMed] [Google Scholar]
- Beaugrand J., Cronier D., Thiebeau P., Schreiber L., Debeire P., Chabbert B. Structure, chemical composition, and xylanase degradation of external layers isolated from developing wheat grain. J Agric Food Chem. 2004;52:7108–7117. doi: 10.1021/jf049529w. [DOI] [PubMed] [Google Scholar]
- Bermingham E.N., Young W., Kittelmann S., Kerr K.R., Swanson K.S., Roy N.C. Dietary format alters fecal bacterial populations in the domestic cat (Felis catus) Microbiologyopen. 2013;2:173–181. doi: 10.1002/mbo3.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari S.K., Ominski K.H., Wittenberg K.M., Plaizier J.C. Effects of chop length of alfalfa and corn silage on milk production and rumen fermentation of dairy cows. J Dairy Sci. 2007;90:2355–2366. doi: 10.3168/jds.2006-609. [DOI] [PubMed] [Google Scholar]
- Bourassa M.W., Alim I., Bultman S.J., Ratan R.R. Butyrate, neuroepigenetics and the gut microbiome: can a high fiber diet improve brain health? Neurosci Lett. 2016;625:56–63. doi: 10.1016/j.neulet.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga R.M., Dourado M.N., Araujo W.L. Microbial interactions: ecology in a molecular perspective. Braz J Microbiol. 2016;47(Suppl 1):86–98. doi: 10.1016/j.bjm.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadian Council on Animal Care (CCAC). Guidelines on the care and use of farm animals in research, teaching and testing. in: CCAC (Ed.), Ottawa, Ontario, Canada; 2009.
- Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J.G., Bittinger K., Bushman F.D., DeSantis T.Z., Andersen G.L., Knight R. PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics. 2010;26:266–267. doi: 10.1093/bioinformatics/btp636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon M.A., Bird A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. 2014;7:17–44. doi: 10.3390/nu7010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derakhshani H., Tun H.M., Cardoso F.C., Plaizier J.C., Khafipour E., Loor J.J. Linking peripartal dynamics of ruminal microbiota to dietary changes and production parameters. Front Microbiol. 2016;7:2143. doi: 10.3389/fmicb.2016.02143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derakhshani H., Tun H.M., Khafipour E. An extended single-index multiplexed 16S rRNA sequencing for microbial community analysis on MiSeq illumina platforms. J Basic Microbiol. 2016;56:321–326. doi: 10.1002/jobm.201500420. [DOI] [PubMed] [Google Scholar]
- DeSantis T.Z., Hugenholtz P., Larsen N., Rojas M., Brodie E.L., Keller K. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diebold G., Mosenthin R., Piepho H.P., Sauer W.C. Effect of supplementation of xylanase and phospholipase to a wheat-based diet for weanling pigs on nutrient digestibility and concentrations of microbial metabolites in ileal digesta and feces. J Anim Sci. 2004;82:2647–2656. doi: 10.2527/2004.8292647x. [DOI] [PubMed] [Google Scholar]
- Durmic Z., Pethick D.W., Pluske J.R., Hampson D.J. Changes in bacterial populations in the colon of pigs fed different sources of dietary fibre, and the development of swine dysentery after experimental infection. J Appl Microbiol. 1998;85:574–582. doi: 10.1046/j.1365-2672.1998.853539.x. [DOI] [PubMed] [Google Scholar]
- Edgar R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Edgar R.C., Haas B.J., Clemente J.C., Quince C., Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kaoutari A., Armougom F., Gordon J.I., Raoult D., Henrissat B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat Rev Microbiol. 2013;11:497–504. doi: 10.1038/nrmicro3050. [DOI] [PubMed] [Google Scholar]
- Faust K., Raes J. CoNet app: inference of biological association networks using Cytoscape. F1000Res. 2016;5:1519. doi: 10.12688/f1000research.9050.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fengler A.I., Marquardt R.R. Water-soluble pentosans from rye. II. Effects on the rate of dialysis and the retention of nutrients by the chick. Cereal Chem. 1988;65:298–302. [Google Scholar]
- Frese S.A., Parker K., Calvert C.C., Mills D.A. Diet shapes the gut microbiome of pigs during nursing and weaning. Microbiome. 2015;3:28. doi: 10.1186/s40168-015-0091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooda S., Boler B.M.V., Dowd S.E., Swanson K.S. The gut microbiome of kittens is affected by dietary protein: carbohydrate ratio and correlated with blood metabolite and hormone concentrations. FASEB J. 2012;26 doi: 10.1017/S0007114512003479. 375.375-375.375. [DOI] [PubMed] [Google Scholar]
- Izydorcszyk M.S., Biliaderis C.G. Taylor & Francis Group; Boca Raton, Londra, New York: 2007. Arabynoxylans: technologically and nutritionally functional plant polysaccharides, in: functional food carbohydrates. [Google Scholar]
- Khafipour E., Li S.C., Plaizier J.C., Krause D.O. Rumen microbiome composition determined using two nutritional models of subacute ruminal acidosis. Appl Environ Microbiol. 2009;75:7115–7124. doi: 10.1128/AEM.00739-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiarie E., Romero L.F., Nyachoti C.M. The role of added feed enzymes in promoting gut health in swine and poultry. Nutr Res Rev. 2013;26:71–88. doi: 10.1017/S0954422413000048. [DOI] [PubMed] [Google Scholar]
- Lozupone C., Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 2005;71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavromichalis I., Hancock J.D., Senne B.W., Gugle T.L., Kennedy G.A., Hines R.H. Enzyme supplementation and particle size of wheat in diets for nursery and finishing pigs. J Anim Sci. 2000;78:3086–3095. doi: 10.2527/2000.78123086x. [DOI] [PubMed] [Google Scholar]
- Molbak L., Thomsen L.E., Jensen T.K., Bach Knudsen K.E., Boye M. Increased amount of Bifidobacterium thermacidophilum and Megasphaera elsdenii in the colonic microbiota of pigs fed a swine dysentery preventive diet containing chicory roots and sweet lupine. J Appl Microbiol. 2007;103:1853–1867. doi: 10.1111/j.1365-2672.2007.03430.x. [DOI] [PubMed] [Google Scholar]
- Morgan X.C., Tickle T.L., Sokol H., Gevers D., Devaney K.L., Ward D.V. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13:R79. doi: 10.1186/gb-2012-13-9-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munyaka P.M., Nandha N.K., Kiarie E., Nyachoti C.M., Khafipour E. Impact of combined beta-glucanase and xylanase enzymes on growth performance, nutrients utilization and gut microbiota in broiler chickens fed corn or wheat-based diets. Poult Sci. 2016;95:528–540. doi: 10.3382/ps/pev333. [DOI] [PubMed] [Google Scholar]
- Murphy P., Bello F.D., O'Doherty J.V., Arendt E.K., Sweeney T., Coffey A. Effects of cereal beta-glucans and enzyme inclusion on the porcine gastrointestinal tract microbiota. Anaerobe. 2012;18:557–565. doi: 10.1016/j.anaerobe.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Ndou S., Kiarie E., Agyekum A., Heo J., Romero L., Arent S. Comparative efficacy of xylanases on growth performance and digestibility in growing pigs fed wheat and wheat bran-or corn and corn DDGS-based diets supplemented with phytase. Anim Feed Sci Technol. 2015;209:230–239. [Google Scholar]
- Nielsen T.S., Laerke H.N., Theil P.K., Sorensen J.F., Saarinen M., Forssten S. Diets high in resistant starch and arabinoxylan modulate digestion processes and SCFA pool size in the large intestine and faecal microbial composition in pigs. Br J Nutr. 2014;112:1837–1849. doi: 10.1017/S000711451400302X. [DOI] [PubMed] [Google Scholar]
- Nortey T., Patience J., Sands J., Zijlstra R. Xylanase supplementation improves energy digestibility of wheat by-products in grower pigs. Livest Sci. 2007;109:96–99. [Google Scholar]
- Nortey T.N., Patience J.F., Sands J.S., Trottier N.L., Zijlstra R.T. Effects of xylanase supplementation on the apparent digestibility and digestible content of energy, amino acids, phosphorus, and calcium in wheat and wheat by-products from dry milling fed to grower pigs. J Anim Sci. 2008;86:3450–3464. doi: 10.2527/jas.2007-0472. [DOI] [PubMed] [Google Scholar]
- Oksanen J., Blanchet F.G., Kindt R., Legendre P., Minchin P.R., O’hara R.B. Package ‘vegan’. Commun Ecol Packag Version. 2013;2:9. [Google Scholar]
- Omogbenigun F.O., Nyachoti C.M., Slominski B.A. Dietary supplementation with multienzyme preparations improves nutrient utilization and growth performance in weaned pigs. J Anim Sci. 2004;82:1053–1061. doi: 10.2527/2004.8241053x. [DOI] [PubMed] [Google Scholar]
- Plaizier J.C., Li S.C., Tun H.M., Khafipour E. Nutritional models of experimentally-induced subacute ruminal acidosis (SARA) differ in their impact on rumen and hindgut microbiota in dairy cows. Front Microbiol. 2017;7:2128. doi: 10.3389/fmicb.2016.02128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfelder P., Eklund M., Mosenthin R. Nutritive value of wheat and wheat by-products in pig nutrition: a review. Anim Feed Sci Technol. 2013;185:107–125. [Google Scholar]
- Scott K.P., Gratz S.W., Sheridan P.O., Flint H.J., Duncan S.H. The influence of diet on the gut microbiota. Pharmacol Res. 2013;69:52–60. doi: 10.1016/j.phrs.2012.10.020. [DOI] [PubMed] [Google Scholar]
- Simes R.J. An improved Bonferroni procedure for multiple tests of significance. Biometrika. 1986;73:751–754. [Google Scholar]
- Topping D.L., Clifton P.M. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81:1031–1064. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- Trosvik P., de Muinck E.J. Ecology of bacteria in the human gastrointestinal tract--identification of keystone and foundation taxa. Microbiome. 2015;3:44. doi: 10.1186/s40168-015-0107-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Garrity G.M., Tiedje J.M., Cole J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widyaratne G., Patience J., Zijlstra R. Effect of xylanase supplementation of diets containing wheat distiller's dried grains with solubles on energy, amino acid and phosphorus digestibility and growth performance of grower-finisher pigs. Can J Anim Sci. 2009;89:91–95. [Google Scholar]
- Wijtten P.J., van der Meulen J., Verstegen M.W. Intestinal barrier function and absorption in pigs after weaning: a review. Br J Nutr. 2011;105:967–981. doi: 10.1017/S0007114510005660. [DOI] [PubMed] [Google Scholar]
- Xiao L., Estelle J., Kiilerich P., Ramayo-Caldas Y., Xia Z., Feng Q. A reference gene catalogue of the pig gut microbiome. Nat Microbiol. 2016;16161 doi: 10.1038/nmicrobiol.2016.161. [DOI] [PubMed] [Google Scholar]
- Yanez J.L., Beltranena E., Cervantes M., Zijlstra R.T. Effect of phytase and xylanase supplementation or particle size on nutrient digestibility of diets containing distillers dried grains with solubles cofermented from wheat and corn in ileal-cannulated grower pigs. J Anim Sci. 2011;89:113–123. doi: 10.2527/jas.2010-3127. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Smith C., Li W. Extraction and modification technology of arabinoxylans from cereal by-products: a critical review. Food Res Int. 2014;65:423–436. [Google Scholar]
- Zhang Z., Smith C., Li W., Ashworth J. Characterization of nitric oxide modulatory activities of alkaline-extracted and enzymatic-modified arabinoxylans from corn bran in cultured human monocytes. J Agric Food Chem. 2016;64:8128–8137. doi: 10.1021/acs.jafc.6b02896. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correlations between physiological factors and relative abundances of bacterial taxa in the ileum. The * symbol indicates a statistically significant correlation (P < 0.05). The scale colors (Spearman’s ρ from -1 to +1) indicate whether the correlation is positive (blue colored squares) or negative (red colored squares) between the taxa and physiological factors. ADG, average daily gain; ATTD, apparent total tract digestibility; GE, gross energy; CP, crude protein; VFA, volatile fatty acids.