Abstract
Abstract
Extracellular matrix (ECM) molecules play important roles in regulating processes such as cell proliferation, migration, differentiation and survival. Decorin is a proteoglycan that binds to (‘decorates’) collagen fibrils in the ECM. Decorin also interacts with many growth factors and their receptors, the most notable of these interactions being its inhibitory activity on TGF‐β, the growth factor responsible for fibrosis formation. We have generated a recombinant, multi‐functional, fusion‐protein consisting of decorin as a therapeutic domain and a vascular homing and cell‐penetrating peptide as a targeting vehicle. This recombinant decorin (CAR‐DCN) accumulates at the sites of the targeted disease at higher levels and, as a result, has substantially enhanced biological activity over native decorin. CAR‐DCN is an example of how molecular engineering can give a compound the ability to seek out sites of disease and enhance its therapeutic potential. CAR‐DCN will hopefully be used to treat severe human diseases.
Linked Articles
This article is part of a themed section on Translating the Matrix. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.1/issuetoc
Abbreviations
- AAA
abdominal aorta aneurysm
- BGN
biglycan
- BMP‐4
bone morphogenetic protein‐4
- DAMP
danger‐associated molecular patterns
- DCN
decorin
- ECM
extracellular matrix
- EGFR
EGF receptor
- GAG
glycosaminoglycan
- HSPG
heparan sulfate proteoglycan
- KO
knockout
- NRP‐1
neuropilin‐1
- PAH
pulmonary arterial hypertension
- RTK
receptor tyrosine kinase
- SLRP
small leucine‐rich proteoglycan
- TGF‐β
transforming growth factor‐β
Introduction
Our tissues are not made up solely from cells. A substantial part of every tissue is composed of extracellular matrix (ECM), a network of macromolecules occupying the space between cells. ECM accounts for as little as 5% of the tissue volume in some epithelial tissues, whereas it makes up the bulk, up to 95%, of mesenchymal tissues such as the bone, tendon and cartilage. More than 300 different ECM molecules have been identified, and various combinations of these proteins, together with carbohydrates such as hyaluronic acid and glycosaminoglycans (GAGs), form unique ECMs that provide structural and functional properties needed by any given tissue. The first ECM molecules identified were the major structural constituents of the human body. For a long period of time, the primary function of ECM proteins was thought to be related to the structural integrity of the tissue. However, our understanding on the ECM biology has dramatically expanded from that traditional view, and a far larger role of ECM molecules as regulators of key cellular processes is now appreciated (Ruoslahti and Yamaguchi, 1991). Demonstrations of direct interactions between ECM molecules and key growth factors signalling pathways have raised the prospect of using them as pharmacological molecules to control aberrant, growth factor‐induced, signalling in human diseases (Ruoslahti and Yamaguchi, 1991; Järvinen and Prince, 2015).
Collagens and proteoglycans are the two major constituents of the ECM. Decorin is the most studied member of the small leucine‐rich proteoglycan (SLRP) family. Initially identified as an ECM molecule that ‘decorates’ collagen fibrils (Krusius and Ruoslahti, 1986), it regulates collagen fibrillogenesis and is crucial for the mechanical integrity of such tissues as skin, tendon and ligaments (Danielson et al., 1997). Furthermore, it interacts with many growth factors or their receptors and has tumour suppressive, anti‐fibrotic and anti‐inflammatory effects that make it an appealing pharmaceutical agent to treat a number of human diseases (Järvinen and Prince, 2015).
Structure of decorin
Decorin is a proteoglycan with a protein core of 42 kDa and a single chondroitin/dermatan sulfate GAG chain attached to it close to the N terminus (Krusius and Ruoslahti, 1986) (Figures 1 and 2). The main structural domain of the decorin core protein consists of 12 tandem leucine‐rich repeats, and it is flanked on both sides by a cysteine‐rich region (Järvinen and Prince, 2015). Decorin exists as a dimer in physiological solutions (Scott et al., 2004), but the dimerization is reversible and the concave face of this protein is involved in dimerization or collagen binding, which means that decorin binds to collagen as a monomer (Islam et al., 2013) (Figure 1).
Figure 1.
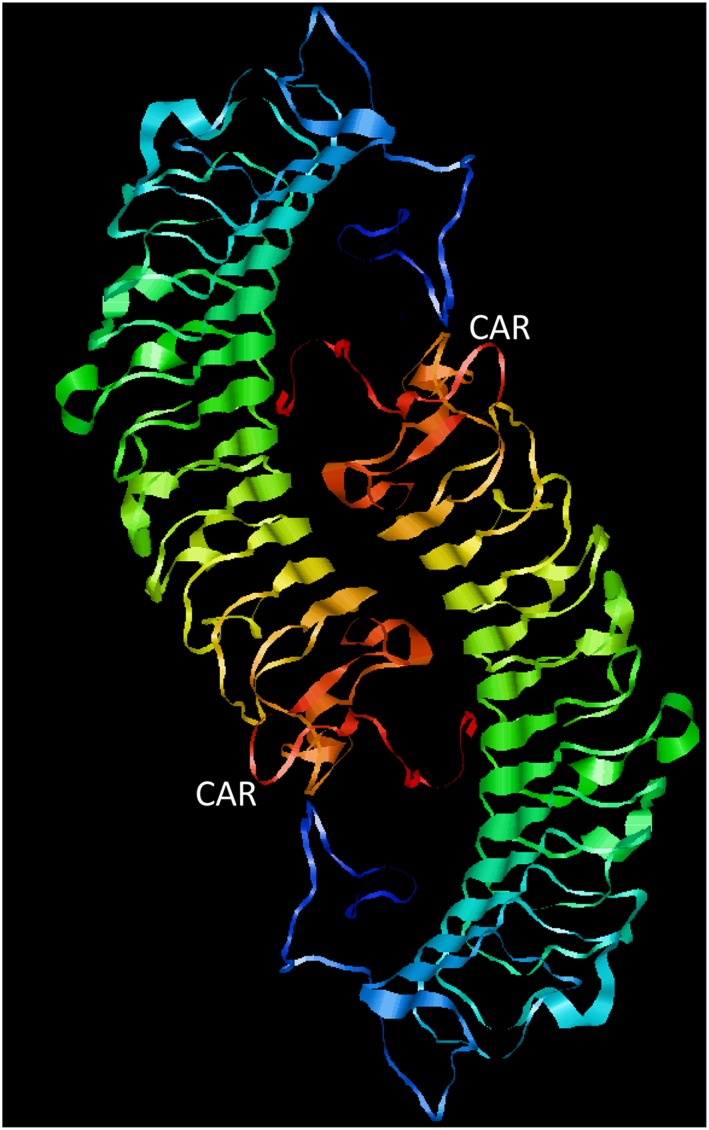
Structure of the engineered decorin variant. Human decorin is a proteoglycan with a monomeric protein core and a single chondroitin/dermatan sulfate GAG chain attached to it. Decorin exists as a dimer in physiological solutions. Structurally, it has a domain of 12 leucine‐rich repeats, flanked on both sides by cysteine‐rich regions. We have re‐engineered it by creating a recombinant fusion protein where a vascular homing and cell‐penetrating peptide ‘CAR’ has been cloned to the C‐terminus of the decorin (the fusion molecule is called CAR‐DCN). The outcome is a multi‐functional recombinant anti‐fibrotic molecule with enhanced biological activity and target tissue selectivity after systemic administration. CAR‐DCN structure as a dimer (from PDB 1XKU). Images were prepared with JMOL program.
Figure 2.

Decorin inhibits several growth factor signalling pathways involved in fibrosis formation. The diagram illustrates all four domains of decorin as well as the GAG side chain binding to the protein core. Decorin is a proteoglycan with one GAG attachment site near the core protein N‐terminus. The major structural domain is made out of 12 leucine‐rich repeats, which are flanked by cysteine‐rich regions on both sides. Decorin interacts with a wide set of different signalling molecules; among them are different isoforms of TGF‐β, PDGF, EGFR and ErbB1–4 RTKs, myostatin (MyoS), connective tissue growth factor/CCN2 and thrombospondin (Thbs) involved in scar and fibrosis formation. The binding sites in decorin for TGF‐β, CCN2, c‐Met and EGFR neutralization reside in different parts of the decorin molecule. Thus, in theory, a single DCN molecule could simultaneously sequester several mediators of fibrosis. Owing to these multiple interactions, the anti‐fibrotic effects of decorin are likely to be the sum of a number of molecular‐binding activities.
Decorin knockout (KO) mice are fertile and show no morphological malformations in the tissues (Danielson et al., 1997), whereas mutations in the human decorin gene cause congenital stromal corneal dystrophy (Chen et al., 2013), a rare ocular disease with corneal opacities and vision impairment. The decorin KO mice have fragile skin and mechanically weak tendons, reflecting the role of decorin in collagen fibrillogenesis (Danielson et al., 1997). A double‐KO mouse lacking both decorin and the closely related SLRP, biglycan, has a more severe phenotype than the decorin KO (Corsi et al., 2002). The phenotype is reminiscent of a specific subtype of Ehlers‐Danlos syndrome (Corsi et al., 2002), a connective tissue disorder characterized by skin hyperextensibility, joint hypermobility and tissue fragility. Thus, biglycan shares some of the functions of decorin and compensates for the loss of decorin in the decorin KO mice. In this context, it is worth noting that asporin, yet another SLRP, shares the collagen binding site with decorin (Kalamajski et al., 2009). Asporin, in turn, was able to compensate for both decorin and biglycan in a double‐KO mouse and alleviated the phenotype related to aberrant collagen fibrillogenesis in the absence of decorin and biglycan (Corsi et al., 2002).
Decorin and its interactions with signalling molecules
Decorin interacts with a wide variety of growth factors and growth factor receptors (Järvinen and Prince, 2015). TGF‐β was the first growth factor shown to interact with decorin (Yamaguchi and Ruoslahti, 1988; Yamaguchi et al., 1990) (Figure 2). Strikingly at the time, it was found that a proteoglycan, human decorin, was capable of inhibiting TGF‐β‐induced cancer cell spreading and proliferation (Yamaguchi and Ruoslahti, 1988; Yamaguchi et al., 1990). Later, it was shown that decorin binds to all isoforms of TGF‐β, ‐β1, ‐β2 and ‐β3 (Hildebrand et al., 1994). The binding to TGF‐β is by the decorin core protein, and the GAG side chain actually slightly interferes with the binding of the core protein to TGF‐β (Hildebrand et al., 1994). It appears that decorin traps TGF‐β in the ECM before TGF‐β can bind to its receptors on the cells (Ruoslahti and Yamaguchi, 1991). In addition to neutralizing the different isoforms of TGF‐β, decorin also binds and neutralizes another member of the TGF‐β superfamily member, myostatin (Miura et al., 2006), a growth factor suppressing skeletal muscle regeneration (Figure 2).
Another group of important signalling molecules that decorin interferes with are the EGF receptors (EGFR/ErbB1) and the rest of the ErbB receptor TKs (RTKs), as ErbB2‐4 has also been shown to be inhibited by decorin (Iozzo et al., 1999b) (Figure 2). Multiple ligands can bind to these receptors, which then form hetero‐ or homodimers that initiate signalling cascade for migration and proliferation. The ErbB‐receptors are frequently over‐expressed (and sometimes their genes amplified) in a large number of tumours, most notably in breast cancer, where the ErbB2 gene at 17q12 is amplified in 20% of the human breast cancers and drives cancer progression in these patients. After binding to decorin, the ErbB receptors dimerize, are internalized and then undergo rapid degradation (Iozzo et al., 1999b).
Decorin also interacts with the other RTKs outside the ErbB‐family. Its binding to c‐Met, hepatocyte growth factor receptor (Figure 2), induces a rapid, yet short‐lived, activation of the receptor but subsequently causes intracellular receptor degradation (Goldoni et al., 2009) (Figure 2).
In addition to interactions with TGF‐β and the RTKs, decorin also interacts with a large set of different signalling molecules. Decorin also binds and neutralizes PDGF, connective tissue growth factor/CCN2, low‐density lipoprotein receptor‐related protein 1, thrombospondin, periostin and Wnt‐1‐induced secreted protein 1 (Järvinen and Prince, 2015) (Figure 2). All of these signalling molecules have been implicated in either fibrosis formation or cancer progression.
Many proteases, such as different members of the MMP family, cleave decorin and make it inactive (Järvinen and Prince, 2015). Protease‐mediated cleavage of decorin has been implicated in the pathogenesis of different diseases (Järvinen and Prince, 2015; Turner et al., 2017). Whereas neonatal skin is completely devoid of catabolic fragments of decorin, inactive, cleaved decorin fragments accumulate in the skin during aging (Carrino et al., 2003). Recently, the decorin cleavage was directly associated with skin wrinkle formation, as UV light exposure on skin induced the expression of granzyme B, which cleaves decorin and leads to the appearance of wrinkles (Parkinson et al., 2015). Cleavage of decorin by granzyme B in models of aortic aneurysms, in turn, leads to aneurysm ruptures and subsequent deaths of the animals (Ang et al., 2011). Interestingly, granzyme B also activates TGF‐β (Turner et al., 2017).
The cleavage of decorin by proteases and cytokines is of special significance because the cleavage fragments of decorin can act as danger‐associated molecular patterns (DAMPs) through direct interactions with pattern recognition receptors including Toll‐like receptors 4 and 2 (Frevert et al., 2018). The interaction can trigger sterile inflammation or prolong a pathogen‐induced response. Thus, the granzyme B cleavage of anti‐fibrotic decorin into pro‐inflammatory DAMP‐active fragments could turn on a pro‐fibrotic and inflammatory programme (Järvinen and Prince, 2015; Turner et al., 2017). However, it is worth noting that the phenotype in the absence of decorin (the KO) is always pro‐inflammatory, and the overall effect of treatment with exogenous decorin is anti‐inflammatory (Järvinen and Prince, 2015).
The binding sites of decorin for key signalling molecules, such as TGF‐β, CCN2, c‐Met and EGFR, reside in different parts of the decorin molecule (Järvinen and Prince, 2015) (Figure 2). Thus, a single decorin molecule can simultaneously sequester multiple important growth factors/receptors and antagonize multiple signalling pathways crucial for tumour growth and fibrosis formation. Thus, owing to this multi‐functionality, decorin may exert its biological effects through multiple molecular approaches that all contribute to a varying degree to its effects on different pathological processes (Järvinen and Prince, 2015) (Figure 2).
Decorin – a promising anti‐tumour molecule
Despite the well‐documented compensatory effect of other SLRPs on the function of decorin, 30% of decorin KO mice developed spontaneous intestinal tumours (Bi et al., 2008) and chemically induced hepatic carcinogenesis is also accelerated in the KO mice (Horvath et al., 2014). Decorin and p53 tumour suppressor gene double‐KO mice succumb to lymphomas substantially earlier than p53 KO mice (Iozzo et al., 1999a). Thus, decorin appears to act as a tumour suppressor gene (Zhang et al., 2018).
A large number of studies have shown that decorin is a potent therapeutic molecule against cancer (Järvinen and Prince, 2015; Sainio and Järvelainen, 2018; Zhang et al., 2018). Decorin administration has been shown to inhibit tumour growth and progression in a large number of in vivo tumour models (Järvinen and Prince, 2015; Sainio and Järvelainen, 2018; Zhang et al., 2018). Virus‐mediated gene therapies encoding for decorin inhibit the growth and the progression of various types of cancers in vivo (Xu et al., 2015; Zhang et al., 2018). In addition to gene therapy studies, tumour treatment studies with a recombinant decorin protein have been carried out with considerable success (Seidler et al., 2006; Zhang et al., 2018). Systemic administration of recombinant decorin protein effectively inhibited tumour growth and reduced metastatic spreading of breast cancer cell lines in vivo (Seidler et al., 2006).
Anti‐fibrotic and anti‐inflammatory effects of decorin
TGF‐β is the growth factor responsible for scar and fibrosis formation in human diseases (Border and Ruoslahti, 1992). The demonstration that decorin is a natural antagonist to TGF‐β (Yamaguchi and Ruoslahti, 1988; Yamaguchi et al., 1990) led to the testing of recombinant decorin in a fibrotic kidney disease model, in which decorin inhibited the fibrosis characteristic of experimental glomerulonephritis (Border et al., 1992). More recent studies using either recombinant decorin protein or decorin gene therapy have confirmed the anti‐fibrotic activity of decorin in various scarring and fibrosis‐models in a number of organs (Järvinen and Ruoslahti, 2013). Furthermore, the fibrosis and scarring were more severe in decorin KO mice than in the WT mice (Järveläinen et al., 2006; Baghy et al., 2011). Altogether, these studies have established decorin as a natural anti‐fibrotic molecule.
Inflammation contributes to the fibrosis and scar formation. Decorin has a substantial anti‐inflammatory effect in inflammatory diseases associated with fibrosis. Genetic absence of decorin (KO mice) is pro‐inflammatory (and pro‐fibrotic) (Järveläinen et al., 2006; Baghy et al., 2011; Järvinen and Prince, 2015), whereas the treatment trials with recombinant decorin or decorin gene therapy consistently report anti‐inflammatory effects (Border et al., 1992; Järvinen and Prince, 2015).
Decorin in tissue regeneration
Decorin has also substantial regenerative potential and decorin KO mice show impaired tissue regeneration after injuries (Järveläinen et al., 2006; Baghy et al., 2011). Some of this defect is attributable to TGF‐β inhibition, but there are regenerative effects not related to the effect of decorin on TGF‐β. Decorin stimulates skeletal muscle regeneration after injury, and the underlying mechanism is myostatin inhibition (Zhu et al., 2007). Decorin has also been identified among the angiocrine (endothelial cell‐derived growth factor for organ‐specific tissue regeneration) factors for endothelial cell‐driven liver regeneration (Nolan et al., 2013). Decorin also promotes robust axon regeneration by multiple molecular mechanisms not related to the inhibition of TGF‐β (Minor et al., 2008).
Vascular heterogeneity – zip code system in vasculature enables tissue‐specific drug delivery
One of the goals of modern pharmaceutical treatment is to be as target specific as possible; drugs should be highly active against the disease while having as few side effects in the healthy parts of the body as possible (Järvinen et al., 2017). This goal is usually obtained by developing drugs that act on molecules selectively expressed in the diseased organ. However, any drug can be converted to a target‐specific one by a targeted delivery to the desired location. The vascular system provides a natural platform for doing that. Expanding understanding of the molecular composition of blood vessels has shown that organ‐specific molecular signatures exist in the blood vessels in each tissue, essentially forming a postal code system (‘vascular zip codes’) within the vasculature in our body (Ruoslahti, 2004; Ruoslahti, 2017). Moreover, various diseases induce the expression of disease‐specific, unique molecular signatures in their vasculature, providing an appealing target for disease‐specific delivery of systemically administered drugs (Ruoslahti, 2004; Ruoslahti, 2017). This is particularly evident in conditions such as cancer and tissue injuries, both of which are associated with tissue hypoxia, which induces new blood vessel growth by a process called angiogenesis (Ruoslahti, 2004). The angiogenic blood vessels are structurally and molecularly different from the dormant blood vessels elsewhere in the body and provide accessible and abundant targets for organ‐specific delivery of therapeutics (Ruoslahti, 2004; Ruoslahti, 2017). These organ‐ and disease‐specific vascular zip codes can be engaged by systemically administered affinity ligands that seek out, that is ‘home in’, to their target by binding to the corresponding vascular zip code receptor (Pasqualini and Ruoslahti, 1996; Ruoslahti, 2004; Ruoslahti, 2017). A particularly useful method for discovering affinity ligands for vascular zip codes has been in vivo phage display. A phage library is constructed by introducing a random oligonucleotide sequence into the gene of a bacteriophage surface protein. As a result, each phage particle will express one random peptide fused to the phage coat protein. The diversity of a library is close to the total number of possible sequence permutations in a peptide (typically about one billion distinct peptide sequences when using the most common seven amino acid long peptide library). Thus, the phage provides a physical link between the inserted peptide (or protein) and the gene encoding it. In a screen, the phage library is injected intravenously into the living, anaesthetised animals, followed by the harvesting of the target tissue and rescue of the bound phage particles for subsequent rounds of screening (Järvinen, 2012). The peptides are identified by sequencing the peptide‐encoding insert in the phage genome. High‐throughput sequencing (comparing profiles of phage pools rescued from the target and multiple control organs by next‐generation sequencing) now makes it possible to limit the in vivo screening to a single round of selection (Mann et al., 2017). Peptide sequences enriched in the selected phage pool are likely candidates for peptides that bind to the target (homing peptides). The major benefits of in vivo phage display are that (i) the target tissue (primarily blood vessels in it) is probed exactly as it exist in vivo, circumventing the problems that arise from changes in gene expression that result from the removal of cells from the natural habitat; and (ii) the screen contains a built‐in subtractive element, removal of non‐specific phage particles by all non‐target organs (Pasqualini and Ruoslahti, 1996; Ruoslahti, 2017). In vivo phage display has yielded numerous peptides (and, in some cases, proteins or protein fragments) that specifically home to their target organ or site of disease after systemic administration (Pasqualini and Ruoslahti, 1996; Ruoslahti, 2004; Ruoslahti, 2017).
CAR‐DCN, a systemically administered, anti‐fibrotic molecule
Injured tissue provides an excellent target for ligand‐mediated delivery of systemically administered therapeutic agents (Järvinen et al., 2017). The first actual sign of tissue regeneration after the injury and subsequent inflammatory period is massive angiogenesis in the injured area. The formation of angiogenic capillaries is induced by the hypoxia resulting from disrupted circulation in the damaged tissue (Barker et al., 2017; Järvinen et al., 2017). Hypoxia triggers the production of angiogenic growth factors, which induce angiogenesis in the injured tissue. The abundance of angiogenic blood vessels in the granulation tissue, the loose connective tissue that produced early at the site of injury, is well illustrated by the fact that the word ‘granulation’ is actually derived from the granular appearance of the angiogenic capillaries. This rich microvascular bed that essentially fills up the injured area presents an overwhelming number of target organ‐specific receptors for systemically administered ligands to bind and deliver therapeutics to the regenerating tissue in a target‐specific fashion. To obtain a ‘vehicle’ for targeting tissues undergoing repair/regeneration, we screened a random peptide library (1.0 × 109) by in vivo phage display for injury‐homing peptides (Järvinen and Ruoslahti, 2007). The method primarily produces peptides that target blood vessels in the target, and the screening yielded a number of peptides that homed to the angiogenic blood vessels in the newly formed granulation tissue (Järvinen and Ruoslahti, 2007). The most potent homing peptide among them had the sequence CARSKNKDC and was dubbed ‘CAR’. The CAR sequence contains a classical heparin‐binding motif that shows high homology with the heparin‐binding domain of bone morphogenetic protein‐4 (BMP‐4), a well‐established angiogenic growth factor. The CAR peptide accumulates in skin and tendon wounds and injuries at levels that are as much as 100–200 times higher than the levels of controls. Moreover, the homing is injury‐specific and no CAR peptide is detected in normal tissues. Importantly, the CAR peptide is a potent cell and tissue penetrating peptide that it effectively accumulates deep in tissue parenchyma (Järvinen and Ruoslahti, 2007). CAR binds, through its heparin binding motif, to the GAG component of cell surface heparan sulfate proteoglycans (HSPGs) and uses the HSPGs as receptors for the cell binding and penetration into cells and deep into the target tissue (Järvinen and Ruoslahti, 2007). The molecular basis of the specificity of the CAR peptide is not clear. As cell surface HSPGs are ubiquitous in cells, it may be that CAR recognizes a subtype of HS (a specific sulfation pattern could be the explanation) that is specific for regenerating tissues. It has subsequently been demonstrated that in addition to angiogenesis, CAR also recognizes inflammatory vasculature and shows remarkable homing specificity after systemic administration in conditions of pulmonary arterial hypertension (PAH), myocardial infarction and abdominal aortic aneurysm (AAA) (Urakami et al., 2011; Kean et al., 2012; Toba et al., 2014; Schneider et al., 2017; Shen et al., 2017).
To make use of the disease‐homing properties of CAR, we generated a multi‐functional therapeutic recombinant protein, in which the CAR peptide sequence is fused to the decorin core protein, which we have called CAR‐DCN (Järvinen and Ruoslahti, 2010) (Figures 1 and 3). The CAR peptide functions (i) as a homing device in the fusion protein that delivers the therapeutic molecule to a target, (ii) mediates cell and tissue penetration and (iii) attaches its payload to target cells and ECM through HSPGs (Järvinen and Ruoslahti, 2010) (Figure 3). The decorin core, in turn, functions as a therapeutic molecule that suppresses scar/fibrosis formation and reduces inflammation. We have demonstrated that CAR‐DCN is substantially more active than native decorin in suppressing TGF‐β bioactivity (Järvinen and Ruoslahti, 2010). Whereas decorin binds to all isoforms of TGF‐β (Hildebrand et al., 1994), CAR‐DCN is significantly more active than decorin in neutralization of TGF‐β1 and ‐β2, but shows no activity against TGF‐β3 (Järvinen and Ruoslahti, 2010). The differential inhibitory activity of CAR‐DCN against the TGF‐β isoforms could be clinically highly relevant, as TGF‐β1 is the growth factor responsible for scar formation, while TGF‐β2 enhances the profibrotic activity of TGF‐β1 (Järvinen and Ruoslahti, 2013). TGF‐β3, in turn, could have substantial anti‐scarring activity. A molecular basis for this selectivity could be the different HSPG‐binding properties of CAR‐peptide and the TGF‐β isoforms. CAR‐peptide, TGF‐β1 and ‐β2 all bind HSPG, but TGF‐β3, does not. The binding to the HSPGs increases the biological activity of TGF‐β1 and ‐β2. Thus, the CAR‐mediated binding to HSPGs in CAR‐DCN may enhance the neutralizing effect of the fusion protein by bringing it to the proximity of the heparin‐binding and scar‐inducing isoforms of TGF‐βs.
Figure 3.
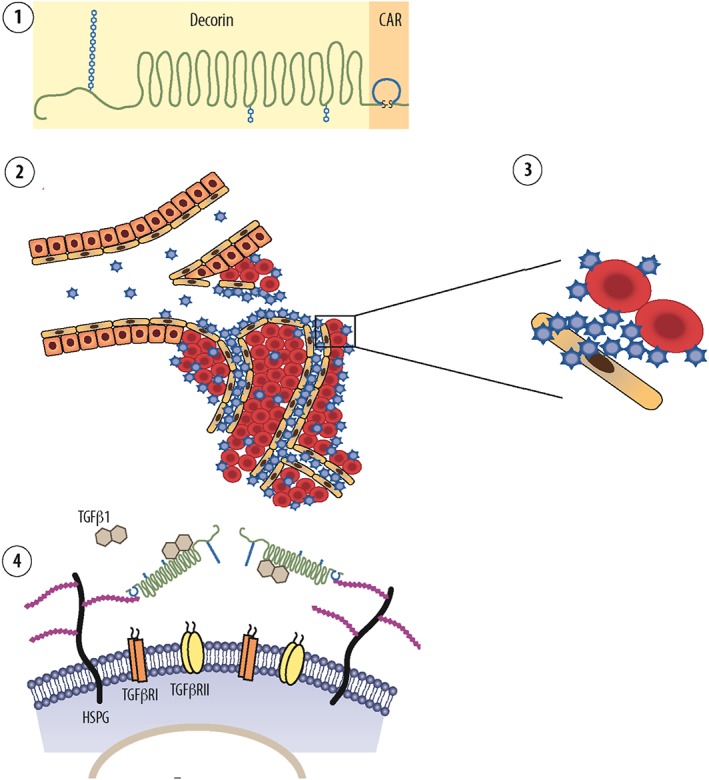
Mechanism of action of multi‐functional re‐engineered decorin in inhibition of scar formation. The diagram shows the recombinant fusion protein consisting of decorin and the vascular homing and cell‐penetrating peptide CAR. The CAR‐DCN ① is a multifunctional biotherapeutic agent that inhibits numerous growth factor signalling pathways involved in fibrosis. The systemically administered molecule is targeted by CAR peptide to the inflammatory or angiogenic vasculature in any organ of the body ②. The CAR homing peptide binds to its receptor (‘zip code’) in angiogenic or inflammatory vasculature ②, and as a cell‐penetrating peptide, it delivers the fusion molecule deep in the target organ parenchyme ③. The CAR peptide then binds to heparin sulfate proteoglycans on the cell surface of the stromal cells ③. This binding facilitates the neutralization of scar‐forming isoforms TGF‐β1 and ‐β2 by the therapeutic part of the molecule, DCN ④. This mechanism results in a therapeutic response, that is, reduction of scar formation in skin wound. Diagram by Helena Schmidt; reproduced with permission from Finnish Medical Journal Duodecim (originally published in Duodecim, 2011, 127: 50–51).
CAR‐DCN delivers increased amounts of decorin to skin wounds after systemic administration (Järvinen and Ruoslahti, 2010). CAR‐DCN reduced scar formation in skin wounds to a significantly greater extent than native decorin supplied at higher doses (Järvinen and Ruoslahti, 2010). More recently, it was shown that CAR‐DCN suppressed the progression of aneurysm formation substantially and prolonged survival significantly, in a mouse AAA model (Shen et al., 2017).
The CAR peptide was identified by in vivo phage screening as a wound‐homing peptide. Because CAR binds to heparin (Järvinen and Ruoslahti, 2007) and its sequence has homology with the heparin‐binding site of BMP‐4, it is likely that CAR recognizes a structural feature in heparan sulfate that is specific for the various pathologies CAR peptide that has been shown to target (Järvinen and Ruoslahti, 2010; Toba et al., 2014; Shen et al., 2017). This binding activity brings CAR peptide and its therapeutic fusion partner not only to the target tissue but also to the cell surfaces of parenchymal cells and ECM of that tissue, explaining the increased therapeutic activity (Järvinen and Ruoslahti, 2010; Shen et al., 2017). Another example of this approach is engineering of ‘super’ growth factors that are more potent than the native growth factors. This was accomplished by fusing a growth factor with a heparin‐binding domain from placental growth factor (Martino et al., 2014; Martino et al., 2015). Similar to CAR‐DCN, the heparin‐binding domain makes the chimeric, ‘super’ growth factors more active than the native ones by providing them with enhanced cell binding and presentation to the growth factor receptors.
Expressing a short homing peptide as part of a larger recombinant fusion protein makes it possible to circumvent two major pharmacological disadvantages of peptides, short half‐life in the circulation and low binding affinity towards the target molecule (Järvinen et al., 2017). Thus, multiple therapeutic multi‐functional recombinant fusion proteins consisting of vascular targeting element and a therapeutic molecule have been described in the biomedical literature, and the most advanced ones have already moved to clinical trials (Phase III) in humans. A concern when a vascular homing peptide is used as a targeting domain for a therapeutic protein is the potential immunogenicity of the resulting fusion protein. The vascular homing peptides are generally short (<10 amino acids) and, as such, not very likely to be immunogenic. For example, they are shorter that the complementary determining regions inserted into therapeutic antibodies, which usually do not cause immune reaction and are considered safe as drugs. Furthermore, vascular homing peptides sometimes share sequence homology with parts of human and mammalian proteins, as is the case with CAR peptide, which is also likely to reduce immunogenicity (Järvinen et al., 2017).
Bystander effect – a novel approach to drug delivery
Systemically administered drugs can be rendered target organ or disease‐specific by co‐administering the drug with certain homing peptides, without binding or chemical coupling to the peptide (Sugahara et al., 2010; Ruoslahti, 2017). The mechanism is activation of an endocytic transcytosis and trans‐tissue transport pathway by the peptide (Pang et al., 2014; Liu et al., 2017; Ruoslahti, 2017). The drug is swept into this pathway and transported into the diseased tissue targeted by the peptide (‘bystander effect’). This effect was first discovered with an integrin‐binding vascular homing peptide that has tumour‐penetrating properties (Teesalu et al., 2009; Sugahara et al., 2010). The CendR‐peptide binds to its integrin receptor, which is specific for tumour endothelium, and becomes proteolytically processed and releases a cryptic binding motif to another receptor, neuropilin‐1 (NRP‐1) (Teesalu et al., 2009; Sugahara et al., 2010). The NRP‐1 binding by CendR‐motif activates the transport pathway (CendR pathway), which transports the peptide and a co‐administered (or coupled) drug out of vessels and deep nto target tissue (Pang et al., 2014; Ruoslahti, 2017). Even antibodies and nanoparticles can be transported in a tumour‐specific fashion using the tumour‐penetrating peptides (Sugahara et al., 2010).
The CAR peptide has homing and cell penetration properties resembling those of CendR‐peptides described above. It also penetrates into its target cells and tissues, and it is capable of inducing a bystander effect on drugs administered simultaneously (Toba et al., 2014; Järvinen et al., 2017). The main difference is that the internalization of heparin‐binding CAR peptide is mediated by HSPGs and not by NRP‐1 (Pang et al., 2015).
So far, the CAR bystander effect has been demonstrated in PAH (Toba et al., 2014) (Figure 4). A fundamental problem with drugs for the treatment of PAH, which is a lethal disease, is that the disease is very resistant to vasodilation induced by blood pressure‐lowering drugs. As no anti‐hypertensive drug that would be selective for the pulmonary circulation exists, the anti‐hypertensive drugs need to be used in high doses in PAH. This causes an unwanted drop in systemic blood pressure (hypotensive shock) and limits the utility of these drugs in PAH. Co‐administering CAR peptide with blood pressure‐lowering drugs has been shown to convert these drugs to become tissue specific in their action (Toba et al., 2014). The CAR peptide targets the inflamed pulmonary blood vessels in PAH (but not normal vessels) and penetrates deep into the vessel wall in experimental models of PAH, and its bystander effect increases the accumulation of co‐administered drugs in the vessels affected by PAH (Urakami et al., 2011; Toba et al., 2014) (Figure 4). Doses as low as 10% of the dose used when drugs are administered alone were effective in lowering pulmonary blood pressure in PAH, while having little or no effect on the systemic circulation (Toba et al., 2014).
Figure 4.
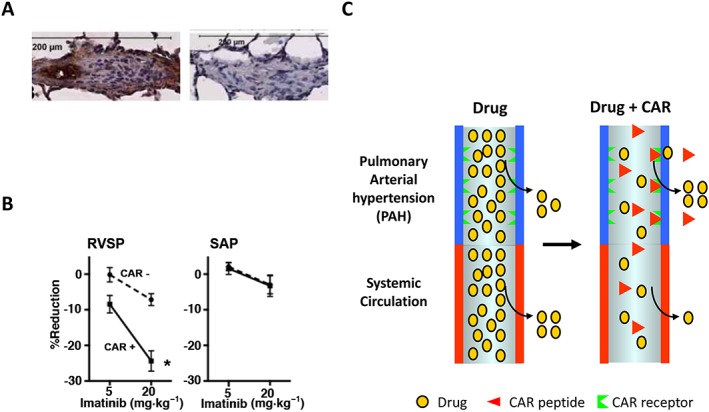
Vascular homing peptide‐induced tissue‐selective vasodilation in PAH. (A) Vascular homing and cell‐penetrating peptide CAR homes to pulmonary arteries in disease‐specific fashion in experimental models of PAH (Urakami et al., 2011; Toba et al., 2014). A small amount of control peptide (mutant CAR peptide) binding can be seen to similar blood vessels in PAH. (B) Effect of a mixture of CAR (0.3 mg·kg−1) and of the Rho‐kinase inhibitor Y27632 (1 mg·kg−1) on right ventricle (RVSP, right ventricle systolic pressure) and left ventricle (SAP, systemic arterial pressure) systolic pressure in PAH. The CAR/Y27632 combination treatment induced a marked pulmonary‐specific vasodilation, i.e., a fall in RVSP with only a minimum effect on SAP, in PAH. (C) Diagram of the ‘bystander effect’, that is, target organ‐specific drug delivery, in PAH by vascular homing peptide CAR (Toba et al., 2014).
Selective vasodilation in PAH has also been achieved with various types of nanoparticles coated with CAR for targeting and loaded with an anti‐hypertensive drug that is released slowly from the nanoparticles (Nahar et al., 2014; Gupta et al., 2014a; Gupta et al., 2014b; Gupta et al., 2017). The nanoparticle approach eliminates a major limitation of the CAR peptide co‐administration, short half‐life of the peptide and the drug. CAR has also been used to deliver mesenchymal stem cells, which were ‘painted’ with CAR to provide delivery (Kean et al., 2012; Schneider et al., 2017).
Future perspectives
Ligand‐mediated targeting of pharmaceutical agents shows promise in the treatment of different diseases. This review deals with is an example of a homing peptide, the CAR peptide, that can mediate target‐specific delivery of payloads as a part of multi‐functional recombinant protein, as well as without attachment of the payload to the peptide, through the so‐called bystander effect. The outcome of the targeted delivery is the improved specificity and efficacy of the targeted drug.
The therapeutic payload that we have used with the CAR peptide, the ECM proteoglycan decorin, is a curious example of a pharmacologically active compound that has not reached the clinic despite a considerable amount of scientific evidence showing its efficacy in experimental models of some serious human diseases with an end stage of tissue fibrosis. Hopefully, the targeted and the biologically more active version of the decorin created by making use of the disease‐targeting properties of CAR peptide will change this in the near future.
In a more general sense, the pharmacological potential of native ECM proteins, such as decorin, may have been overlooked and may present a treasure trove of opportunities for future drug discovery and development.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018) and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a,b).
Conflict of interest
T.J. has nothing to declare. E.R. is a shareholder of Vascular Biosciences Pharmaceuticals Inc., which holds a licence to the CAR peptide.
Acknowledgements
The authors thank Dr Stuart Prince, for drawing the decorin picture and Dr Ulrike May, for her thoughtful comments. The work was funded by the Academy of Finland, Päivikki and Sakari Sohlberg Foundation, Pirkanmaa Hospital District Research Foundation, Diabetes Wellness Foundation and Tampere Tuberculosis Foundation.
Järvinen, T. A. H. , and Ruoslahti, E. (2019) Generation of a multi‐functional, target organ‐specific, anti‐fibrotic molecule by molecular engineering of the extracellular matrix protein, decorin. British Journal of Pharmacology, 176: 16–25. 10.1111/bph.14374.
References
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: Catalytic receptors. Br J Pharmacol 174: S225–S271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Other proteins. Br J Pharmacol 174 (Suppl 1): S1–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang LS, Boivin WA, Williams SJ, Zhao H, Abraham T, Carmine‐Simmen K et al (2011). Serpina3n attenuates granzyme B‐mediated decorin cleavage and rupture in a murine model of aortic aneurysm. Cell Death Dis 2: e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghy K, Dezso K, Laszlo V, Fullar A, Peterfia B, Paku S et al (2011). Ablation of the decorin gene enhances experimental hepatic fibrosis and impairs hepatic healing in mice. Lab Invest 91: 439–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker H, Aaltonen M, Pan P, Vähätupa M, Kaipiainen P, May U et al (2017). Role of carbonic anhydrases in skin wound healing. Exp Mol Med 49: e334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi X, Tong C, Dockendorff A, Bancroft L, Gallagher L, Guzman G et al (2008). Genetic deficiency of decorin causes intestinal tumor formation through disruption of intestinal cell maturation. Carcinogenesis 29: 1435–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Border WA, Noble NA, Yamamoto T, Harper JR, Yamaguchi Y, Pierschbacher MD et al (1992). Natural inhibitor of transforming growth factor‐β protects against scarring in experimental kidney disease. Nature 360: 361–364. [DOI] [PubMed] [Google Scholar]
- Border WA, Ruoslahti E (1992). Transforming growth factor‐β in disease: the dark side of tissue repair. J Clin Invest 90: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrino DA, Onnerfjord P, Sandy JD, Cs‐Szabo G, Scott PG, Sorrell JM et al (2003). Age‐related changes in the proteoglycans of human skin. Specific cleavage of decorin to yield a major catabolic fragment in adult skin. J Biol Chem 278: 17566–17572. [DOI] [PubMed] [Google Scholar]
- Chen S, Sun M, Iozzo RV, Kao WW, Birk DE (2013). Intracellularly‐retained decorin lacking the C‐terminal ear repeat causes ER stress: a cell‐based etiological mechanism for congenital stromal corneal dystrophy. Am J Pathol 183: 247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi A, Xu T, Chen XD, Boyde A, Liang J, Mankani M et al (2002). Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers‐Danlos‐like changes in bone and other connective tissues. J Bone Miner Res 17: 1180–1189. [DOI] [PubMed] [Google Scholar]
- Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV (1997). Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol 136: 729–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frevert CW, Felgenhauer J, Wygrecka M, Nastase MV, Schaefer L (2018). Danger‐associated molecular patterns derived from the extracellular matrix provide temporal control of innate immunity. J Histochem Cytochem 66: 213–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldoni S, Humphries A, Nystrom A, Sattar S, Owens RT, McQuillan DJ et al (2009). Decorin is a novel antagonistic ligand of the Met receptor. J Cell Biol 185: 743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Ibrahim HM, Ahsan F (2014a). Peptide‐micelle hybrids containing fasudil for targeted delivery to the pulmonary arteries and arterioles to treat pulmonary arterial hypertension. J Pharm Sci 103: 3743–3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Patel B, Nahar K, Ahsan F (2014b). Cell permeable peptide conjugated nanoerythrosomes of fasudil prolong pulmonary arterial vasodilation in PAH rats. Eur J Pharm Biopharm 88: 1046–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Rashid J, Nozik‐Grayck E, McMurtry IF, Stenmark KR, Ahsan F (2017). Cocktail of superoxide dismutase and fasudil encapsulated in targeted liposomes slows PAH progression at a reduced dosing frequency. Mol Pharm 14: 830–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand A, Romaris M, Rasmussen LM, Heinegard D, Twardzik DR, Border WA et al (1994). Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor β. Biochem J 302 (Pt 2): 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath Z, Kovalszky I, Fullar A, Kiss K, Schaff Z, Iozzo RV et al (2014). Decorin deficiency promotes hepatic carcinogenesis. Matrix Biol 35: 194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo RV, Chakrani F, Perrotti D, McQuillan DJ, Skorski T, Calabretta B et al (1999a). Cooperative action of germ‐line mutations in decorin and p53 accelerates lymphoma tumorigenesis. Proc Natl Acad Sci U S A 96: 3092–3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo RV, Moscatello DK, McQuillan DJ, Eichstetter I (1999b). Decorin is a biological ligand for the epidermal growth factor receptor. J Biol Chem 274: 4489–4492. [DOI] [PubMed] [Google Scholar]
- Islam M, Gor J, Perkins SJ, Ishikawa Y, Bachinger HP, Hohenester E (2013). The concave face of decorin mediates reversible dimerization and collagen binding. J Biol Chem 288: 35526–35533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järveläinen H, Puolakkainen P, Pakkanen S, Brown EL, Hook M, Iozzo RV et al (2006). A role for decorin in cutaneous wound healing and angiogenesis. Wound Repair Regen 14: 443–452. [DOI] [PubMed] [Google Scholar]
- Järvinen TAH, Rashid J, May U, Valmari T, Ahsan F (2017). Systemic targeted delivery of multi‐functional recombinant proteins and nanoparticles in regenerative medicine. ACS Biomat Sci Eng 3: 1273–1282. [DOI] [PubMed] [Google Scholar]
- Järvinen TA (2012). Design of target‐seeking antifibrotic compounds. Methods Enzymol 509: 243–261. [DOI] [PubMed] [Google Scholar]
- Järvinen TA, Prince S (2015). Decorin: a growth factor antagonist for tumor growth inhibition. Biomed Res Int 2015: 654765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järvinen TA, Ruoslahti E (2007). Molecular changes in the vasculature of injured tissues. Am J Pathol 171: 702–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järvinen TA, Ruoslahti E (2010). Target‐seeking antifibrotic compound enhances wound healing and suppresses scar formation in mice. Proc Natl Acad Sci U S A 107: 21671–21676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järvinen TA, Ruoslahti E (2013). Targeted antiscarring therapy for tissue injuries. Adv Wound Care (New Rochelle) 2: 50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalamajski S, Aspberg A, Lindblom K, Heinegard D, Oldberg A (2009). Asporin competes with decorin for collagen binding, binds calcium and promotes osteoblast collagen mineralization. Biochem J 423: 53–59. [DOI] [PubMed] [Google Scholar]
- Kean TJ, Duesler L, Young RG, Dadabayev A, Olenyik A, Penn M et al (2012). Development of a peptide‐targeted, myocardial ischemia‐homing, mesenchymal stem cell. J Drug Target 20: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusius T, Ruoslahti E (1986). Primary structure of an extracellular matrix proteoglycan core protein deduced from cloned cDNA. Proc Natl Acad Sci U S A 83: 7683–7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Lin P, Perrett I, Lin J, Liao YP, Chang CH et al (2017). Tumor‐penetrating peptide enhances transcytosis of silicasome‐based chemotherapy for pancreatic cancer. J Clin Invest 127: 2007–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann AP, Scodeller P, Hussain S, Braun GB, Molder T, Toome K et al (2017). Identification of a peptide recognizing cerebrovascular changes in mouse models of Alzheimer's disease. Nat Commun 81403–017–01096‐0: 1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino MM, Briquez PS, Guc E, Tortelli F, Kilarski WW, Metzger S et al (2014). Growth factors engineered for super‐affinity to the extracellular matrix enhance tissue healing. Science 343: 885–888. [DOI] [PubMed] [Google Scholar]
- Martino MM, Briquez PS, Maruyama K, Hubbell JA (2015). Extracellular matrix‐inspired growth factor delivery systems for bone regeneration. Adv Drug Deliv Rev 94: 41–52. [DOI] [PubMed] [Google Scholar]
- Minor K, Tang X, Kahrilas G, Archibald SJ, Davies JE, Davies SJ (2008). Decorin promotes robust axon growth on inhibitory CSPGs and myelin via a direct effect on neurons. Neurobiol Dis 32: 88–95. [DOI] [PubMed] [Google Scholar]
- Miura T, Kishioka Y, Wakamatsu J, Hattori A, Hennebry A, Berry CJ et al (2006). Decorin binds myostatin and modulates its activity to muscle cells. Biochem Biophys Res Commun 340: 675–680. [DOI] [PubMed] [Google Scholar]
- Nahar K, Absar S, Gupta N, Kotamraju VR, McMurtry IF, Oka M et al (2014). Peptide‐coated liposomal fasudil enhances site specific vasodilation in pulmonary arterial hypertension. Mol Pharm 11: 4374–4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan DJ, Ginsberg M, Israely E, Palikuqi B, Poulos MG, James D et al (2013). Molecular signatures of tissue‐specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell 26: 204–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang HB, Braun GB, Friman T, Aza‐Blanc P, Ruidiaz ME, Sugahara KN et al (2014). An endocytosis pathway initiated through neuropilin‐1 and regulated by nutrient availability. Nat Commun 5: 4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang HB, Braun GB, Ruoslahti E (2015). Neuropilin‐1 and heparan sulfate proteoglycans cooperate in cellular uptake of nanoparticles functionalized by cationic cell‐penetrating peptides. Sci Adv 1: e1500821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson LG, Toro A, Zhao H, Brown K, Tebbutt SJ, Granville DJ (2015). Granzyme B mediates both direct and indirect cleavage of extracellular matrix in skin after chronic low‐dose ultraviolet light irradiation. Aging Cell 14: 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualini R, Ruoslahti E (1996). Organ targeting in vivo using phage display peptide libraries. Nature 380: 364–366. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E (2004). Vascular zip codes in angiogenesis and metastasis. Biochem Soc Trans 32: 397–402. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E (2017). Tumor penetrating peptides for improved drug delivery. Adv Drug Deliv Rev 110‐111: 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E, Yamaguchi Y (1991). Proteoglycans as modulators of growth factor activities. Cell 64: 867–869. [DOI] [PubMed] [Google Scholar]
- Sainio AO, Järvelainen HT (2018). Decorin‐mediated oncosuppression – a potential future adjuvant therapy for human epithelial cancers. Br J Pharmacol. 176: 5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Angele P, Järvinen TAH, Docheva D (2017). Rescue plan for Achilles: therapeutics steering the fate and functions of stem cells in tendon wound healing. Adv Drug Deliv Rev S0169‐409X:30319–8. [DOI] [PubMed] [Google Scholar]
- Scott PG, McEwan PA, Dodd CM, Bergmann EM, Bishop PN, Bella J (2004). Crystal structure of the dimeric protein core of decorin, the archetypal small leucine‐rich repeat proteoglycan. Proc Natl Acad Sci U S A 101: 15633–15638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler DG, Goldoni S, Agnew C, Cardi C, Thakur ML, Owens RT et al (2006). Decorin protein core inhibits in vivo cancer growth and metabolism by hindering epidermal growth factor receptor function and triggering apoptosis via caspase‐3 activation. J Biol Chem 281: 26408–26418. [DOI] [PubMed] [Google Scholar]
- Shen Y, Russo V, Zeglinski MR, Sellers SL, Wu Z, Oram C et al (2017). Recombinant decorin fusion protein attenuates murine abdominal aortic aneurysm formation and rupture. Sci Rep 7: 15857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Greenwald DR et al (2010). Coadministration of a tumor‐penetrating peptide enhances the efficacy of cancer drugs. Science 328: 1031–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teesalu T, Sugahara KN, Kotamraju VR, Ruoslahti E (2009). C‐end rule peptides mediate neuropilin‐1‐dependent cell, vascular, and tissue penetration. Proc Natl Acad Sci U S A 106: 16157–16162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toba M, Alzoubi A, O'Neill K, Abe K, Urakami T, Komatsu M et al (2014). A novel vascular homing peptide strategy to selectively enhance pulmonary drug efficacy in pulmonary arterial hypertension. Am J Pathol 184: 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CT, Lim D, Granville DJ (2017). Granzyme B in skin inflammation and disease. Matrix Biol S0945‐053X (17)30335–9. [DOI] [PubMed] [Google Scholar]
- Urakami T, Järvinen TA, Toba M, Sawada J, Ambalavanan N, Mann D et al (2011). Peptide‐directed highly selective targeting of pulmonary arterial hypertension. Am J Pathol 178: 2489–2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Neill T, Yang Y, Hu Z, Cleveland E, Wu Y et al (2015). The systemic delivery of an oncolytic adenovirus expressing decorin inhibits bone metastasis in a mouse model of human prostate cancer. Gene Ther 22: 247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Mann DM, Ruoslahti E (1990). Negative regulation of transforming growth factor‐β by the proteoglycan decorin. Nature 346: 281–284. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Ruoslahti E (1988). Expression of human proteoglycan in Chinese hamster ovary cells inhibits cell proliferation. Nature 336: 244–246. [DOI] [PubMed] [Google Scholar]
- Zhang W, Ge Y, Cheng Q, Zhang Q, Fang L, Zheng J (2018). Decorin is a pivotal effector in the extracellular matrix and tumour microenvironment. Oncotarget 9: 5480–5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Li Y, Shen W, Qiao C, Ambrosio F, Lavasani M et al (2007). Relationships between transforming growth factor‐β1, myostatin, and decorin: implications for skeletal muscle fibrosis. J Biol Chem 282: 25852–25863. [DOI] [PubMed] [Google Scholar]


