Abstract
Background
Machine perfusion techniques offer a solution to the serious organ shortage. However, to assess the effects of machine perfusion, many detailed studies are required. In this study, an ex vivo reperfusion model using diluted autologous blood was confirmed to evaluate the utility of machine preservation for livers donated after cardiac death (DCD). In particular, beneficial effects of the oxygenated hypothermic machine perfusion (HMP) for DCD porcine livers are evaluated.
Material/Methods
Porcine livers were procured under warm ischemia time (WIT) of 60 min. The livers were preserved by hypothermic machine perfusion (HMP) or static cold storage (CS) for 4 h. After the preservation, the livers were perfused for 2 h using the ex vivo reperfusion model with diluted blood oxygenated by a membrane oxygenator at 35–38°C.
Results
At 2 h of ex vivo reperfusion with 60 min of warm ischemic time (WIT), the portal vein pressure for CS was higher than HMP (18.8±15.9 vs. 7.5±3.9 [mmHg] in 60 min). Furthermore, LDH in CS was higher than HMP (528.5±149.8 vs. 194.1±32.2 [IU/L/100 g liver] in 60 min. P<0.05). Lactate after CS (60) was significantly higher than HMP (60) (8.67±0.39 vs. 5.68±0.60 [mmol/L] at 60 min. p<0.01).
Conclusions
The ex vivo reperfusion model can be used to evaluate the utility of machine perfusion. Advantages of HMP for DCD livers are evaluated with this model.
MeSH Keywords: Liver Transplantation, Organ Preservation, Reperfusion Injury
Background
The current criterion standard method of organ preservation, simple cold storage (CS), has been widely accepted. Liver machine perfusion preservation (MP) techniques for ex vivo organ preservation have been suggested as a potential alternative to CS and can preserve and resuscitate organs donated after cardiac death (DCD) or obtained from extended criteria donors (ECD) grafts, which may expand the donor pool. However, the use of DCD liver grafts is associated with increased risk of primary non-function or ischemia-reperfusion injury.
Hypothermic machine perfusion (HMP) provides continuous circulation of preservation solution and metabolic substances. A washout effect removes and dilutes waste products from direct endothelial/parenchymal contact due to stabilization of the microcirculation. There is strong evidence that renal HMP improves the early graft function [1] and improves the tissue ATP concentrations upon reperfusion [2]. HMP also allows for pharmacological manipulation and the pretransplant assessment of grafts [2] and has enhanced the use of ECD kidneys [1]. Furthermore, recent reports suggest a long-term graft survival benefit for HMP kidneys [2,3].
A clinical pilot study was performed with normal livers compared with CS alone and with CS followed by HMP [4], showing that the rates of early allograft dysfunction, postoperative transaminases, bilirubin, and hospital stay were significantly lower in the CS+HMP group compared with the CS-only group. The same authors later performed HMP in ECD livers and reported the safety and efficacy of HMP in such a scenario [5]. In addition, the utility of normothermic machine perfusion (NMP) recently showed the possibility of MP, showing that the rate of graft injury, as measured by hepatocellular enzyme release and organ discard was lower and mean preservation time was longer in the NMP group compared with the CS group [6].
However, the optimum temperatures and conditions for organ preservation remain controversial. Studies on MP have been performed in the following 3 temperature categories: hypothermic (6–10°C), subnormothermic (20–23°C), and normothermic (37°C) [6–8]. However, more detailed large-animal studies with a sufficient number of subjects are required to confirm the utility of these techniques and evaluate the preservation results. Furthermore, from the perspective of animal ethics, the development and implementation of alternatives to animal use should be considered when designing experiments.
In the present study, an isolated ex vivo reperfusion model (IRM) simulating reperfusion after transplantation using diluted autologous blood was evaluated for its utility in MP for DCD livers ex vivo instead of in transplantation. This technique can reduce the need for animal experiments of transplantation. In particular, the beneficial effects of oxygenated HMP for DCD porcine livers were evaluated using an ex vivo reperfusion model.
Material and Methods
Animals
Experiments were performed on porcine liver grafts (domestic, female, cross-bred Large-Yorkshire, Landrace, and Duroc pigs, 2 to 3 months old, 20 kg; Tokyo Experimental Animals Supply Co., Ltd., Tokyo, Japan). These harvesting procedures were conducted under inhalation anesthesia with isoflurane (Forane VR; Abbot Japan, Tokyo, Japan), and hemorrhagic shock and the removal of ventilation induced cardiac arrest. The Institutional Animal Ethics Committee of the Clinical Research Center, National Center for Child Health and Development (Permit no. 2010-010 A) approved all experimental procedures.
Perfusion preservation machine
The grafts were perfused using an original MP system (Figure 1) [9]. This system consists of the hepatic artery (HA) and portal vein (PV) perfusion circuits. Each circuit consists of a roller-type pump (MasterFlex7520-40; Cole-Parmer, IL, USA), an electrical flow meter (VN05; Aichi Tokei, Japan for PV; FD-SS02; Keyence, Osaka, Japan for HA), a ceramic capacitance pressure sensor (KL76; Nagano Keiki, Nagano, Japan), and a custom-made air trap. An oxygenator (HP0-06 H-C; Senko Medical Instrument, Tokyo, Japan) was installed in the circuit for the PV and HA. Two optical oxygen sensors (NeoFox; Ocean Optics, FL, USA) were installed to measure the oxygen concentration of the perfusate in the PV circuit. The perfusate temperature was controlled by a heat exchanger and ice-cold water. The flow rate of the PV was 0.22 mL/min/g and HA was 0.06 mL/min/g. The oxygen consumption (mg/min/100 g liver) of the organ was calculated as normalized values with the mass of the organ (mg/min/100 g liver) from the difference in the oxygen concentration at the inlet port of the PV circuit and the outlet port positioned near the hepatic vein (HV).
Figure 1.
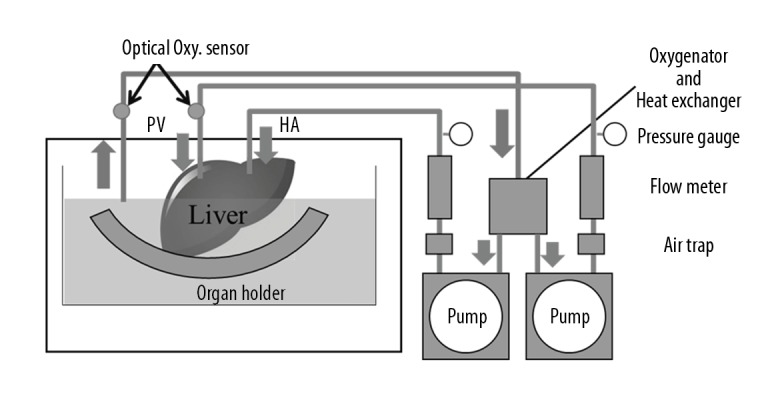
Machine perfusion system. This system consists of 2 separated perfusion circuits for the hepatic artery (HA) and portal vein (PV). Each circuit is equipped with a roller pump, a flow meter, a pressure sensor, and an air trap. An oxygenator was installed in the circuit for the PV and HA to measure the oxygen concentration of the perfusate. The temperature in the organ chamber was controlled by a heat exchanger and ice-cold water.
Oxygenation
Oxygenated perfusate supplied oxygen to the liver grafts during MP under HMP conditions using an oxygenator. The oxygen concentrations were controlled with the blending volume conditions of oxygen and air using the air blender of the oxygenator. The condition of the oxygen concentration was maintained to hold the minimum dissolved oxygen concentration for the calculation of the oxygen consumption at the outlet port. The oxygen consumption (mg/min/100 g liver) of the organ was calculated as normalized values with the mass of the organ (mg/min/100 g liver) from the difference in the oxygen concentration at the inlet port of the PV circuit and the outlet port positioned near the HV.
Experimental design
Grafts were procured under a warm ischemia time (WIT) of 0 or 60 min and were preserved under CS or HMP for 4 h. The perfusate used a modified gluconate University of Wisconsin solution. We used 4 perfusion conditions in the present study (Figure 2):
Figure 2.

Experimental groups. Group 1: The livers were procured under warm ischemia time of 0 min and preserved by CS (4–8°C, n=4). Group 2: The livers were procured under warm ischemia time of 60 min and preserved by CS (4–8°C, n=4). Group 3: The livers were procured under warm ischemia time of 0 min and preserved by HMP (6–10°C, n=3). Group 4: The livers were procured under warm ischemia time of 60 min and preserved by HMP (6–10°C, n=4).
CS (0) group: liver grafts were preserved immediately after cardiac arrest (WIT 0) with simple CS (n=4).
CS (60) group: liver grafts were preserved after 60 min of cardiac arrest (WIT 60) with CS for 4 h (n=4).
HMP (0) group: grafts were perfused with HMP for 4 h after WIT 0 (n=3).
HMP (60): liver grafts were perfused with HMP for 4 h after WIT 60 (n=4).
The liver grafts were treated for 2 h using an isolated liver perfusion model, with the outcomes compared among 4 groups.
Isolated reperfusion model (IRM)
Following the preservation, all organs were rinsed with 500 mL of cold Euro-Collins solution for 20 min, and subsequently exposed at room temperature for 10 min without perfusion to imitate the slow rewarming of the graft during surgical implantation in vivo.
The livers were then reperfused with oxygenated diluted autologous blood at 38°C. The autologous blood was diluted with saline (40%), dextran (20%), calciol (5%), sodium bicarbonate, heparin sodium, and potassium. The hematocrit was maintained at around 10–12%. The oxygenator was regulated to achieve physiological blood gas values (pO2, approximately 150–200 mmHg; pCO2 approximately 30–50 mmHg). HA perfusion was set at 80 mmHg and automatically maintained by a roller pump connected to a pressure sensor placed in the inflow line immediately before the arterial cannula. Perfusion of the PV was maintained in a flow-constant manner (0.8 mL/min/g).
The viability assessment during MP
Aspartate aminotransferase (AST) and lactate dehydrogenase (LDH) were taken from the perfusate every 30 min and analyzed to determine the viability of the preserved liver grafts using standard biochemical methods (clinical grade). Blood gas and lactate concentrations in perfusate were measured using the iStat program (Abbot, NJ, USA).
Statistical analyses
Statistical analyses were performed using Microsoft Excel 2013 (Microsoft, WA, USA). The results are presented as mean ± standard deviation (SD). After proving the assumption of normality, differences between groups were tested by t test. A p-value of <0.05 was considered to indicate statistical significance.
Results
Vascular pressure of the PV and HA during IRM
The PV pressure (PVP) and HA pressure (HAP) under each perfusion condition during IRM are shown in Figures 3 and 4, respectively. The HAP after CS (0) was higher than that after HMP (0) (151.0±17.9 vs. 50.4±41.9 mmHg at 30 min and 143.8±27.4 vs. 47.0±37.2 mmHg at 60 min after reperfusion). Furthermore, the HAP after CS (60) was significantly higher than that after HMP (60) (227.0±25.6 vs. 115.8±15.3 mmHg at 60 min and 171.5±27.2 vs. 109.8±9.8 mmHg at 120 min after reperfusion).
Figure 3.

Portal vein pressure after reperfusion. The PV pressure (PVP) after each perfusion condition during IRM are shown. The PVP after CS (0) was higher than after HMP (0).
Figure 4.

Hepatic artery pressure after reperfusion. HA pressure (HAP) after each perfusion condition during IRM are shown. The HAP after CS (0) was higher than after HMP (0). The HAP after CS (60) was significantly higher than after HMP (60). CS (0) vs. HMP (0): * p<0.05, ** p<0.01, CS (60) vs. HMP (60): # p<0.05, ## p<0.01
In addition, the PVP after CS (60) was higher than that after HMP (60) (18.8±15.9 vs. 7.5±3.9 mmHg at 60 min and 16.6±14.6 vs. 8.1±5.3 mmHg at 120 min after reperfusion).
AST, LDH, and lactate during IRM
The AST and LDH levels during IRM after CS (0), HMP (0), CS (60) and HMP (60) are shown in Figures 5 and 6. The AST level after CS (60) was significantly higher than that after HMP (60) at every point after reperfusion (# p<0.05, vs. IRM after CS (60) and HMP (60); Figure 6). Even at WIT 0 min, a significant difference was noted in the AST level between CS (0) and HMP (0) at every point after IRM (136.4±25.5 vs. 69.1±6.7 IU/L/100 g liver at 60 min and 218.9±27.4 vs. 120.2±41.4 IU/L/100 g liver at 120 min after reperfusion). Furthermore, the LDH level with IRM after CS (60) was significantly higher than that after HMP (60) (528.5±149.8 vs. 194.1±32.2 IU/L/100 g liver at 60 min and 576.8±150.6 vs. 226.4±50.5 IU/L/100 g liver at 120 min after reperfusion; p<0.05). The lactate level after CS (60) was significantly higher than that after HMP (60) at every point after reperfusion (8.67±0.39 vs. 5.68±0.60 mmol/L at 60 min and 11.48±0.84 vs. 7.29±0.67 mmol/L at 120 min, respectively; p<0.01; Figure 7).
Figure 5.

AST after reperfusion. AST after each perfusion condition during IRM are shown. AST after CS (60) was significantly higher than HMP (60) at every point after reperfusion. Even at 0 min of WIT, there was a statistically significant difference between these CS (0) and HMP (0) group: CS (60) vs. HMP (60): # p<0.05, ## p<0.01.
Figure 6.
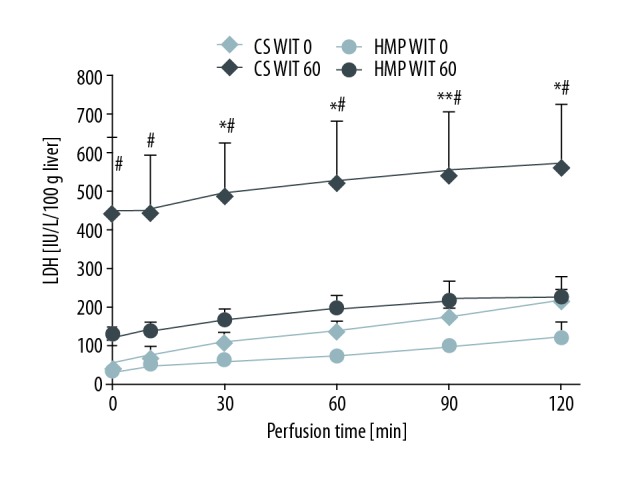
LDH after reperfusion. LDH after each perfusion condition during IRM are shown. LDH with IRM after CS (60) was remarkably higher compared with HMP (60). CS (0) vs. HMP (0): * p<0.05, ** p<0.01, CS (60) vs. HMP (60): # p<0.05, ## p<0.01.
Figure 7.

Lactate after reperfusion. Lactate after CS (60) was significantly higher than HMP (60) at every point after reperfusion: ** p<0.01 CS 60 vs. HMP 60: # p<0.05, ## p<0.01.
Discussion
The utility of MP techniques for preserving the organ viability and recovering the organ function for organ transplantation has been reported previously [2,3], and the potential for HMP to increase the donor pool using marginal livers has also been reported [10–12].
Several different IRMs have been established to predict transplantation results [9,11,13]. In the present study, we established an IRM that reproduced ischemia-reperfusion injury to evaluate the utility of MP with DCD livers. With 2 h of reperfusion, the PVP, HAP, and LDH in the HMP (0) group remained relatively low, and better preservation conditions compared to CS (0) were noted. In contrast, in the CS (60) group, vascular pressure and LDH fluctuated drastically early after reperfusion, and obvious ischemia-reperfusion injury was noted. These differences in the perfusion flow dynamics and outflow of liver enzymes at the early stage of reperfusion might aid in rapid evaluation of organ function.
The continuous flow in MP allows for an influx of nutrients and oxygen while flushing cytokines, proteins, and toxins from the liver, thereby preventing the damage cascade buildup that occurs within a static preserved liver. The washout effects of MP and the continuous delivery of metabolic substances and antioxidants to the parenchyma and endothelium has been demonstrated to improve organ function [2,5].
Temperature modulation (e.g., preservation at normothermic and subnormothermic temperatures) offers a new means of improving organ preservation techniques with oxygenation to maintain graft viability and function. The advantages of normothermic MP (NMP) include physiological preservation [7]. A phase 1 clinical trial evaluating the efficacy of NMP [8] showed that NMP was safe as well as feasible, from retrieval to transplantation, including transportation. In addition, a clinical pilot study has recently reported the utility of normothermic machine perfusion (NMP) [6]. Several groups have examined the effects of setting the perfusion temperature to a subnormothermic temperature, which an intermediate temperature (20–33°C) between hypothermic and normothermic temperature conditions. Bruinsma et al. evaluated the efficacy of subnormothermic MP for ex vivo preservation and recovery of human livers for transplantation by assessing the perfusion outcomes with a metabolomics analysis [8]. In addition, our group showed the beneficial effects of subnormothermic MP in a pig model based on the flow dynamics during perfusion [14]. However, the oxygen carrier containing perfusion solution used during subnormothermic conditions remains an issue to be addressed. HMP may therefore offer several advantages over normothermic conditions, including a reduced oxygen requirement and nutrition consumption and the avoidance of complicated systems for maintaining the temperature and high metabolic requirements [15,16].
Shear stress occurs due to the reaction of oxygen with increased concentrations of intracellular redox-active iron, which triggers free-radical-mediated cell injury [17]. The present findings suggest that oxidative stress and endothelial shear stress can be decreased by regulating the perfusion temperature and oxygenation. Regarding oxygenation during HMP, the mechanism of tissue protection involves the reactivation of mitochondrial respiration, which occurs prior to reperfusion, when HMP oxidizes mitochondrial electron complexes. Mitochondrial electron overload on reperfusion can be prevented by the decreased production of reactive oxygen species [18]. Other studies have suggested that injury also occurs in the sinusoidal endoplasmic reticulum during both cold and warm ischemia [19,20]. However, extended periods of preservation without oxygenation may also be associated with certain disadvantages and limitations of endoplasmic stress and alterations of the sinusoidal endothelial cells [9,16]. Regarding aggressive oxygenation, our study showed that active oxygenation was not required for HMP.
In summary, HMP appears to protect against injury in 2 ways. First, oxygenation under hypothermic conditions protects against mitochondrial and nuclear injury by downregulating mitochondrial activity before reperfusion. Second, cold perfusion itself, under low pressure, prevents endothelial damage, independent of oxygen.
Conclusions
Our ex vivo IRM may be useful for evaluating the utility of MP, particularly in large-animal models of DCD liver transplantation.
Acknowledgements
We thank all of the lab members and our colleagues for their helpful suggestions and assistance in the experiments.
Footnotes
Source of support: This work was supported by JSPS KAKENHI (#15H03922 to H.O. and N. Matsuno) from the Japan Society for the Promotion of Science (JSPS), Foundation, and a Grant-in-Aid for Innovative Research in Life Science from Asahikawa Medical University to N. Matsuno
Conflict of interest
None.
References
- 1.Moers C, Smits JM, Maathuis MH, et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. 2009;360:7–19. doi: 10.1056/NEJMoa0802289. [DOI] [PubMed] [Google Scholar]
- 2.Kwiatkowski A, Wszola M, Kosieradzki M, et al. Machine perfusion preservation improves renal allograft survival. Am J Transplant. 2007;7:1942–47. doi: 10.1111/j.1600-6143.2007.01877.x. [DOI] [PubMed] [Google Scholar]
- 3.Guarrera JV, Estevez J, Boykin J, et al. Hypothermic machine perfusion of liver grafts for transplantation: technical development in human discard and miniature swine models. Transplant Proc. 2005;37:323–25. doi: 10.1016/j.transproceed.2004.12.094. [DOI] [PubMed] [Google Scholar]
- 4.Guarrera JV, Henry SD, Samstein B, et al. Hypothermic machine preservation in human liver transplantation: The first clinical series. Am J Transplant. 2010;10:372–81. doi: 10.1111/j.1600-6143.2009.02932.x. [DOI] [PubMed] [Google Scholar]
- 5.Guarrera JV, Henry SD, Samstein B, et al. Hypothermic machine preservation facilitates successful transplantation of “orphan” extended criteria donor livers. Am J Transplant. 2015;15:161–69. doi: 10.1111/ajt.12958. [DOI] [PubMed] [Google Scholar]
- 6.Nasralla D, Coussios CC, Merqental H, et al. A randomized trial of normothermic preservation in liver transplantation. Nature. 2018;7703:50–56. doi: 10.1038/s41586-018-0047-9. [DOI] [PubMed] [Google Scholar]
- 7.St Peter SD, Imber CJ, Lopez I, et al. Extended preservation of non-heart-beating donor livers with normothermic machine perfusion. Br J Surg. 2002;89(5):609–16. doi: 10.1046/j.1365-2168.2002.02052.x. [DOI] [PubMed] [Google Scholar]
- 8.Bruinsma BG, Yeh H, Ozer S, et al. Subnormothermic machine perfusion for ex vivo preservation and recovery of the human liver for transplantation. Am J Transplant. 2014;14(6):1400–9. doi: 10.1111/ajt.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morito N, Obara H, Matsuno N, et al. Regulated oxygenation of rewarming machine perfusion for porcine donation after cardiac death liver transplantation. Transpl Proc. 2016;48:1244–46. doi: 10.1016/j.transproceed.2015.12.098. [DOI] [PubMed] [Google Scholar]
- 10.Obara H, Matsuno N, Shigeta T, et al. Rewarming machine perfusion system for liver transplantation. J Med Devices. 2013;7:1–7. doi: 10.1016/j.transproceed.2013.01.087. [DOI] [PubMed] [Google Scholar]
- 11.Compagnon P, Levesque E, Hentati H, et al. An oxygenated and transportable machine perfusion system fully rescues liver grafts exposed to lethal ischemic damage in a pig model of DCD liver transplantation. Transplantation. 2017;101(7):e205–13. doi: 10.1097/TP.0000000000001764. [DOI] [PubMed] [Google Scholar]
- 12.Raikumar R, Jassem W, Mergental HL. Liver transplantation after ex vivo normothermic machine preservation: A phase 1 (first-in-man) clinical trial. 2016;16:1779–87. doi: 10.1111/ajt.13708. [DOI] [PubMed] [Google Scholar]
- 13.Minor T, Efferz P, Fox M, et al. Controlled oxygenated rewarming of cold stored liver grafts by thermally graduated machine perfusion prior to reperfusion. Am J Transplant. 2013;13:1450–60. doi: 10.1111/ajt.12235. [DOI] [PubMed] [Google Scholar]
- 14.Furukori M, Matsuno N, Watanabe K, et al. Subnormothermic machine perfusion preservation with rewarming for donation after cardiac death liver grafts in pigs. Transpl Proc. 2016;48(4):1239–43. doi: 10.1016/j.transproceed.2015.12.076. [DOI] [PubMed] [Google Scholar]
- 15.Henry SD, Guarrera JV. Protective effects of hypothermic ex vivo perfusion on ischemia/reperfusion injury and transplant outcomes. Transplant Rev (Orlando) 2012;26(2):163–67. doi: 10.1016/j.trre.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Matsuno N, Uchida K, Furukawa H. Impact of machine perfusion preservation of liver grafts from donation after cardiac death. Transpl Proc. 2014;46:1099–110. doi: 10.1016/j.transproceed.2013.11.135. [DOI] [PubMed] [Google Scholar]
- 17.Rauen U, Petrat F, Li T, De Groot H. Hypothermia injury/cold-induced apoptosis – evidence of an increase in chelatable iron causing oxidative injury in spite of low O2-/H2O2 formation. FASEB J. 2000;14:1953–64. doi: 10.1096/fj.00-0071com. [DOI] [PubMed] [Google Scholar]
- 18.Du C, Fang M, Li Y, et al. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;1:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 19.Koetting M, Lüer B, Efferz P, et al. Optimal time for hypothermic reconditioning of liver grafts by venous systemic oxygen persufflation in a large animal model. Transplantation. 2011;91:42–47. doi: 10.1097/TP.0b013e3181fed021. [DOI] [PubMed] [Google Scholar]
- 20.Minor T, Manekeller S, Sioutis M, Dombrowski F. Endoplasmic and vascular surface activation during organ preservation: Refining upon the benefits of machine perfusion. Am J Transplant. 2006;6:1355–66. doi: 10.1111/j.1600-6143.2006.01338.x. [DOI] [PubMed] [Google Scholar]


