Abstract
Background
STAT3 has emerged as a novel potential target for sorafenib, a multikinase inhibitor, in the context of cancer therapy. ARHGAP24 is a Rac-specific Rho GTPase-activating protein (Rho GAP), which can convert Rho GTPases to an inactive state. It has been proved to be an oncosuppressor protein in renal cancer. In the present study, we investigated its anti-cancer effect in breast cancer (BC).
Material/Methods
Quantitative real-time PCR (qRT-PCR) and Western blot analysis were performed to detect the expression of ARHGAP24 in clinical tissue samples. Then, BC MDA-MB-231 cells were virally transduced with ARHGAP24 silencing or overexpression lentiviral vectors in the absence or presence of sorafenib. Cell viability and metastatic ability were evaluated by using the Cell Counting Kit-8 (CCK-8) and Transwell assays. Proteins belonging to the STAT3 pathway were detected by Western blot.
Results
ARHGAP24 decreased in BC tissues compared with the adjacent normal tissues. Forced expression of ARHGAP24 and sorafenib treatment significantly suppressed the viability, migration, and invasion of MDA-MB-231 cells. Conversely, elimination of the endogenous ARHGAP24 with shRNA promoted cell viability, migration, and invasion. The phosphorylation of STAT3 and the expression of MMP-2 and MMP-9 were attenuated by ARHGAP24 ectopic expression and sorafenib treatment. Furthermore, forced expression of ARHGAP24 significantly enhanced sorafenib-induced decrease of cell viability, migration, and invasion of MDA-MB-231 cells, while elimination of the endogenous ARHGAP24 with shRNA inhibited it.
Conclusions
ARHGAP24 can suppress the development of MDA-MB-231 cells via the STAT3 signaling pathway, and sorafenib inhibits cell viability, migration, invasion, and STAT3 activation in MDA-MB-231 cells through ARHGAP24.
MeSH Keywords: Cell Migration Assays, Cell Survival, STAT3 Transcription Factor
Background
As the most common malignant tumor in women, breast cancer (BC), leads to a large percentage of cancer deaths worldwide [1,2]. BC mainly has 4 subtypes according to the gene expression profile: basal-like, human epidermal growth-factor receptor-2-positive/estrogen receptor-negative (HER2+/ER−), luminal A, and luminal B [3]. Distant metastasis is a serious threat to the survival of BC patients, especially for those with triple-negative BC, who tend to have early relapse and metastasis due to the lack of effective targeted therapy [4]. Better understanding of the molecular mechanisms of BC progression will contribute to the investigation of more effective therapies for metastatic BC.
Rho GTPases belong to the Ras-related small GTPases superfamily, with more than 20 members having been identified, of which RhoA, Rac1, and Cdc42 have been extensively studied. Activated Rho GTPases regulate the remodeling of the actin cytoskeleton, and then affect cell morphology, polarity, and migration though the downstream effectors [5–7]. Mounting evidence indicates that Rho GTPases play a crucial role in the formation and progression of numerous cancers [8,9]. High expression of Rho GTPases has been observed in BC tissues [10,11]. For example, Rac1 reduces the occurrence of apoptosis in BC cells in patients receiving radiotherapy [12]. Signal transducer and activator of transcription 3 (STAT3) is an extensively studied transcriptional factor. STAT3-mediated deregulation of nuclear gene expression is implicated in a variety of cellular functions and contributes to the malignant phenotypes of cells [13,14]. Notably, recent studies indicate that Rho GTPases are important regulators of STAT3 activation [15]. STAT3 has been demonstrated to be a target of sorafenib, a multikinase inhibitor that hampers cancer cell proliferation, migration, and invasion [16,17]. However, the regulation of sorafenib-induced STAT3 signaling in BC is not fully understood.
ARHGAP24 is a Rac-specific Rho GAP and is closely involved in cellular morphology change [18,19]. Downregulation of ARHGAP24 has been observed in renal cancer tissues. Moreover, forced expression of ARHGAP24 suppresses the formation and progression of renal cancer [20]. However, the specific effect of ARHGAP24 in human tumors and the possible regulatory role in sorafenib-induced STAT3 activity are still largely unknown. We performed the present study to investigate the potential function of ARHGAP24 in the formation and progression of BC and to discover the molecular mechanisms involved. Our results demonstrate that ARHGAP24 suppresses the development of MDA-MB-231 cells via the STAT3 signaling pathway and sorafenib inhibits cell viability, migration, invasion, and STAT3 activation in MDA-MB-231 cells through ARHGAP24.
Material and Methods
Tissues and cells
Thirty pairs of tumor tissues and adjacent normal tissues were obtained from BC patients at Yantai Affiliated Hospital of Binzhou Medical University with the permission of patients and Ethics Committee approval. MDA-MB-231 cells (American Type Culture Collection, ATCC, USA) were used for the in vitro experiments. Dulbecco’s modified Eagle’s medium (DMEM) (Hyclone, USA) containing 1% penicillin-streptomycin (PS) (Solarbio, Beijing, China) and 10% fetal bovine serum (FBS) (Gibco, USA) was used for cell culture. MDA-MB-231 cells were incubated in a 37°C incubator with 5% CO2.
Quantitative real-time PCR (qRT-PCR)
The expression level of ARHGAP24 was detected by qRT-PCR. The total RNA in tissues or cells was extracted by TRIzol reagent (Invitrogen, USA). After confirming the purity by a micro-spectrophotometer, RNA was reverse-transcribed using a cDNA Synthesis Kit (Thermo-Fisher, Saint Louis, MO, USA). QRT-PCR was conducted with SYBR Green Real-Time PCR Master Mixes (Thermo-Fisher). Results were evaluated using ABI Prism 7300 SDS software (Applied Biosystems, USA). ARHGAP24 mRNA levels were normalized to GAPDH and calculated by the 2−ΔΔCt method. Primers used in the study were as follows: ARHGAP24 (NM_001025616.2): forward: 5′-AACTCCTGTCGCTCTTCTACC-3′; reverse: 5′-GCTGTTGCCCACAAATGTCTC-3′. GAPDH (NM_001256799.1): forward: 5′-CACCCACTCCTCCACCTTTG-3′; reverse: 5′-CCACCACCCTGTTGCTGTAG-3′.
Cell transfection
To investigate the regulatory function of ARHGAP24 in MDA-MB-231 cells, we constructed the lentiviral vectors to reduce or increase ARHGAP24 expression. We designed the RNAi (RNA interference) sequence targeting position 727–749 (GATCGGATGACAGCAAATC) of human ARHGAP24 gene and synthesized the short hairpin RNA (shRNA). Then, ARHGAP24-shRNA was integrated into pLKO.1-puro (Addgen, Cambridge, MA, USA) and transferred into DH5α cells (TransGen, Beijing, China). The colonies were identified by PCR. Afterwards, pLKO.1-shARHGAP24, psPAX2, and pMD2G plasmids were extracted from the bacteria solution by E.Z.N.A.® Endo-free Plasmid Mini Kit I (Omega, USA) and cotransfected into 293T cells for 4–6 h. After 48-h transfection, the supernatant was collected for viral transduction in MDA-MB-231 cells.
Similarly, ARHGAP24 ectopic expression lentiviral vector was constructed by integrating the coding sequence (CDS) of ARHGAP24 into pLVX-Puro. The primers used to synthesize the CDS were as follows: forward: 5′-GCGAATTCATGGAGGAGAACAATGACT (EcoR I); reverse: 5′-CGGGATCCCTGAATCCATATTGTGTTT (BamH I) (underscoring indicates the restriction enzyme cutting site). Virus particles were produced by transfecting 293T cells with ARHGAP24-pLVX-Puro together with viral packaging vectors (psPAX2, pMD2G) as described above, and then MDA-MB-231 cells were infected with virus-containing supernatants.
Cell counting kit-8 (CCK-8) assay
MDA-MB-231 cells were seeded in 96-well plates (3×103 cell/well). Then, the wells were divided into 4 groups: in the WT group, MDA-MB-231 cells were cultured in normal medium; in the NC group, MDA-MB-231 cells were cultured with negative control lentiviral vectors viral transduction; in the shARHGAP24 group, MDA-MB-231 cells were cultured with ARHGAP24-shRNA lentiviral vectors viral transduction; and in the pLVX-ARHGAP24 group, MDA-MB-231 cells were cultured with ARHGAP24-pLVX-Puro lentiviral vectors viral transduction. After culturing for different lengths of time, CCK-8 solution was added to each well. Optical density (OD) value at 450 nm was detected to evaluate cell viability.
Transwell assays
To observe cell migration, MDA-MB-231 cells were divided into 4 groups and virally transduced with different recombinant lentiviral vectors as described above. Next, cells were digested with trypsin and were resuspended in DMEM supplemented with 1% FBS. Then, the upper layer of the Transwell chamber was filled with cell suspension (0.3 ml/chamber) and the lower layer was filled with 0.7 ml of DMEM supplemented with 10% FBS. Each group was tested in triplicate. After culturing for 48 h, the Transwell chambers were soaked in 4% paraformaldehyde for 10 min. Finally, the migrated cells were stained by crystal violet solution (Solarbio, Beijing, China) and photographed with light microscopy under ×200 magnification. Similar to in the migration assay, 80 μl of matrigel (BD, Franklin Lakes, USA) was first added to the upper layer. Then, the treated cells were seeded for invasion assay.
Western blot analysis
Protein samples were extracted from tissues or MDA-MB-231 cells by RIPA lysis buffer (Solarbio) and quantified using a BCA Protein Assay Kit (Thermo-Fisher). Then, the protein samples were separated with sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and were transferred to nitrocellulose membranes (Millipore, MA, USA). Protein bands were incubated with primary antibodies and paired secondary antibodies. The Tanon 5200 imaging system (Tanon, Shanghai, China) was used to analyze the protein levels. The antibodies used were as follows: ARHGAP24 (Abcam, Ab203874, diluted 1: 200), p-STAT3 (Abcam, Ab76315, diluted 1: 5000), STAT3 (CST, #9139, diluted 1: 2000), MMP-2 (Abcam, Ab97779, diluted 1: 1000), MMP-9 (Abcam, Ab38898, diluted 1: 2000), and GAPDH (CST, #5174, diluted 1: 2000).
Statistical analysis
All experiments were performed 3 times. Results are presented as mean ± standard deviation (SD) and were analyzed by GraphPad Prism 6 software (GraphPad, USA). The paired-samples t test was used to compare the differences in ARHGAP24 expression between tumor tissues and paired adjacent tissues. Other results were analyzed by the independent-samples t test. P<0.05 was considered to be significant.
Results
The expression of ARHGAP24 in BC tissues
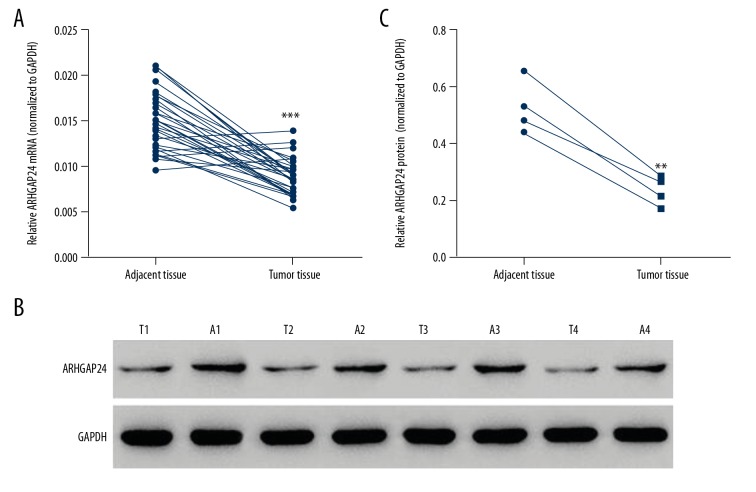
To investigate the expression of ARHGAP24 in BC tissues, 30 tumor tissues and paired adjacent tissues were collected. The clinicopathologic characteristics of the 30 BC patients enrolled in the study are shown in Table 1. We detected the protein and mRNA levels of ARHGAP24. According to the qRT-PCR results, ARHGAP24 expression level significantly decreased in tumor tissues with a lower mRNA level (Figure 1A). Western blot analysis demonstrated that ARHGAP24 protein was also reduced in tumor tissues (Figure 1B, 1C), which was consistent with qRT-PCR results. These results suggest that ARHGAP24 was downregulated in BC tissues. Whether ARHGAP24 downregulation is correlated with the development of BC needs further exploration.
Table 1.
Clinicopathological characteristics and follow-up data of 30 patients with BC.
| Characteristics | Number of patients/ number analyzed (%) |
|---|---|
| Age (years) | |
| <60 | 18/30 (60.0%) |
| ≥60 | 12/30 (40.0%) |
| TNM stage | |
| I | 8/30 (26.7%) |
| II | 12/30 (40.0%) |
| III | 10/30 (33.3%) |
| Lymph node metastasis | |
| Yes | 11/30 (36.7%) |
| No | 19/30 (63.3%) |
| ER status | |
| Positive | 12/30 (40.0%) |
| Negative | 18/30 (60.0%) |
| PR status | |
| Positive | 16/30 (53.3%) |
| Negative | 14/30 (46.7%) |
| HER2 status | |
| Positive | 13/30 (43.3%) |
| Negative | 17/30 (56.7%) |
ER – estrogen receptor; PR – progesterone receptor; HER2 – human epidermal growth factor receptor-2.
Figure 1.
ARHGAP24 was downregulated in BC tissues. Thirty BC tissue samples and the paired adjacent tissues were used to detect the expression of ARHGAP24. (A) qRT-PCR results of ARHGAP24 mRNA levels normalized to GAPDH. (B) Western blot results of ARHGAP24 protein levels. GAPDH was used as the loading control. T1–4, tumor tissues; A1–4, BC adjacent tissues. (C) Statistical analysis of the relative protein levels of ARHGAP24. ** P<0.01, *** P<0.001 compared with adjacent tissues.
The regulatory effect of ARHGAP24 on MDA-MB-231 cells
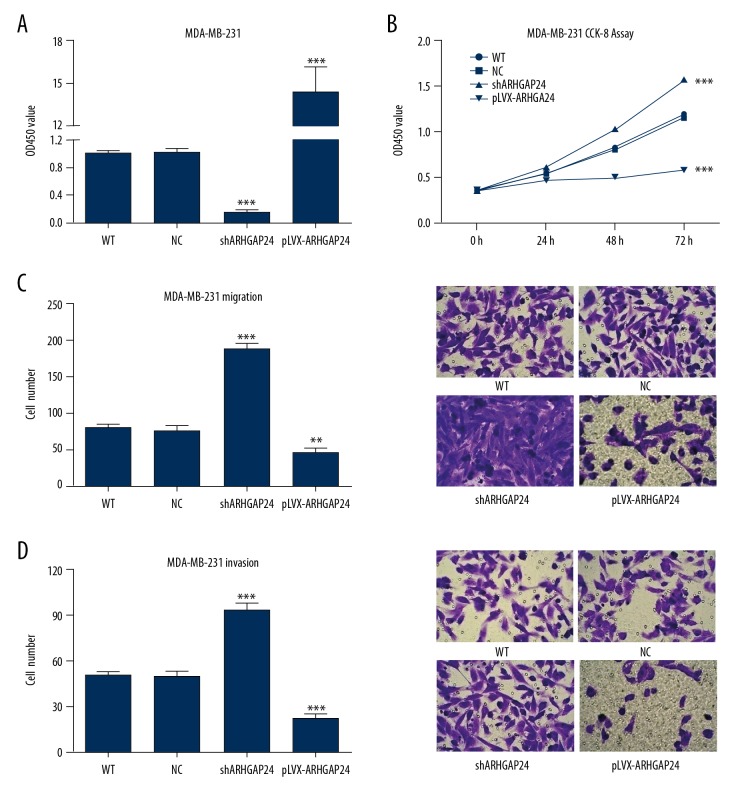
Due to the low-expression of ARHGAP24 in BC tissues, ARHGAP24 may be a negative regulator for BC formation. Next, we examined the regulatory function of ARHGAP24 in the BC cell line. MDA-MB-231 cells were virally transduced with ARHGAP24-shRNA lentiviral vectors or ARHGAP24-pLVX-Puro lentiviral vectors. QRT-PCR was performed to confirm the silencing or overexpression of ARHGAP24. As shown in Figure 2A, ARHGAP24 transcription was significantly suppressed due to the interference of ARHGAP24-shRNA. Conversely, ARHGAP24-pLVX elevated ARHGAP24 expression. The effect of ARHGAP24 on cell viability was evaluated by CCK-8 assay. Figure 2B shows that depletion of the endogenous ARHGAP24 by shRNA significantly promoted the viability of MDA-MB-231 cells. On the contrary, the cell viability was suppressed by the ectopic expression of ARHGAP24. Furthermore, Transwell assays revealed more migrated and invaded cells with the silencing of ARHGAP24, whereas forced expression of ARHGAP24 brought about the opposite results (Figure 2C, 2D). Taken together, our results show that ARHGAP24 suppressed the viability, migration, and invasion of MDA-MB-231 cells.
Figure 2.
ARHGAP24 suppressed proliferation, migration, and invasion in MDA-MB-231 cells. MDA-MB-231 cells were virally transduced with different recombinant lentiviral vectors to induce ARHGAP24 silencing or ectopic expression. (A) The relative fold change of ARHGAP24 mRNA levels confirmed by qRT-PCR. (B) MDA-MB-231 cell proliferation detected by CCK-8 assay after viral transduction for 24, 48, and 72 h. MDA-MB-231 cell migration and invasion were detected by Transwell assays after viral transduction for 48 h. (C) Left panel shows the statistical analysis of cell numbers. Right panel shows representative pictures of the migrated cells under ×200 magnification. (D) Left panel shows the statistical analysis of cell numbers. Right panel shows representative pictures of the invaded cells under ×200 magnification. ** P<0.01, *** P<0.001 compared with WT and NC.
The functional mechanism of ARHGAP24 suppressing viability, migration, and invasion in MDA-MB-231 cells
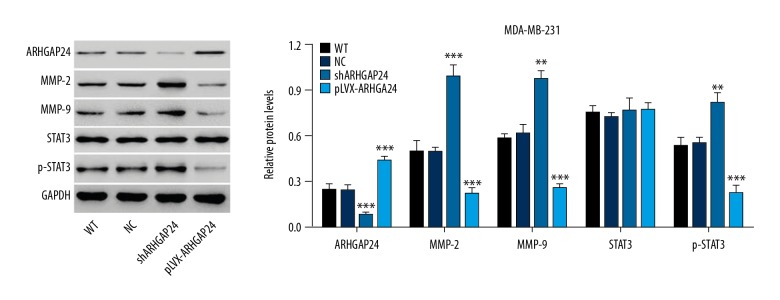
To investigate the underlying molecular mechanisms of the anti-cancer effect, Western blot analysis was performed to detect the related proteins. As shown in Figure 3, the silencing and overexpressing of ARHGAP24 were again confirmed by Western blot. Stimulating ARHGAP24 expression with ARHGAP24-pLVX-Puro lentiviral vectors reduced the protein level of p-STAT3 but had no effect on the total STAT3, indicating the suppression of STAT3 activation. Furthermore, the expression of MMP-2 and MMP-9 was also reduced. These results suggest that the decreased viability, migration, and invasion ability of MDA-MB-231 cells were due to the activation of STAT3. We obtained the opposite results when blocking ARHGAP24 expression with shRNA. Collectively, ARHGAP24 seems to exert, at least in part, its anti-cancer effect in MDA-MB-231 cells via the STAT3/MMP-2 and MMP-9 pathways.
Figure 3.
ARHGAP24 exerted anti-cancer effect via STAT3/MMP-2/MMP-9 signals in MDA-MB-231 cells. Western blot analysis was performed to detect the expression of several essential proteins. Left panel shows representative results of Western blot analysis. Right panel shows quantification of relative protein levels. The values were normalized to GAPDH. ** P<0.01, *** P<0.001 compared with WT and NC.
Effect of ARHGAP24 on sorafenib-induced decrease of cell viability, migration and invasion in MDA-MB-231 cells
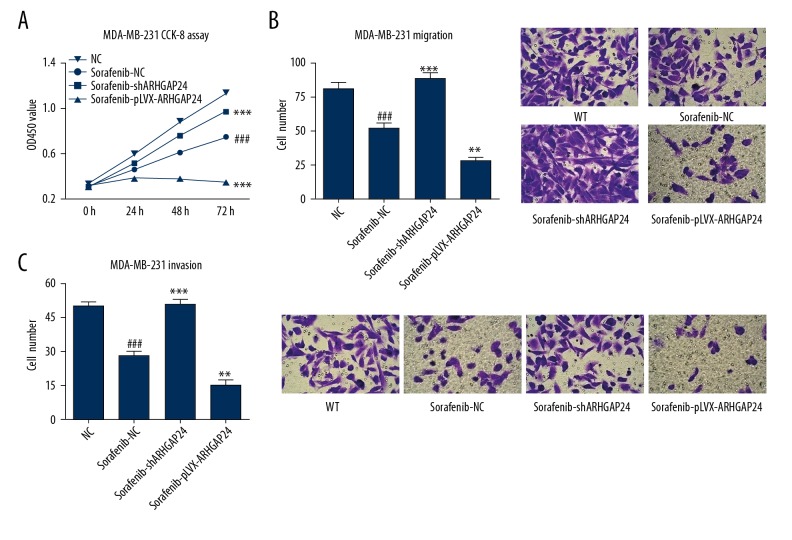
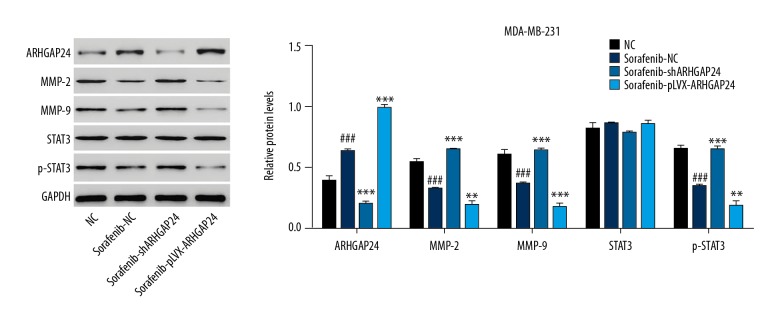
To evaluate the effect of sorafenib on BC cell viability, migration, and invasion and the potential mechanism involved, MDA-MB-231 cells were treated with sorafenib (1.5 μM) with or without pLKO.1-shARHGAP24 and ARHGAP24-pLVX-Puro viral transduction. CCK-8 and Transwell analysis showed that sorafenib treatment significantly inhibited the viability, migration, and invasion of MDA-MB-231 cells (Figure 4). Furthermore, stimulating ARHGAP24 expression with ARHGAP24-pLVX-Puro lentiviral vectors enhanced sorafenib-induced inhibition of cell viability, migration, and invasion. Western blot analysis demonstrated that sorafenib treatment significantly inhibited the STAT3 activation and the MMP-2 and MMP-9 expression, and increased the ARHGAP24 expression, which were enhanced by stimulating ARHGAP24 expression with ARHGAP24-pLVX-Puro lentiviral vectors (Figure 5). Conversely, we found the opposite results when blocking ARHGAP24 expression with shRNA. Taken together, our results suggest that ARHGAP24 is involved in the sorafenib-induced changes in MDA-MB-231 cell viability, migration, and invasion.
Figure 4.
Effect of ARHGAP24 on sorafenib-induced decrease of viability, migration and invasion in MDA-MB-231 cells. MDA-MB-231 cells were treated with sorafenib (1.5 μM) and virally transduced with or without different recombinant lentiviral vectors to induce ARHGAP24 silencing or ectopic expression. (A) MDA-MB-231 cell viability detected by CCK-8 assay after treatment for 24, 48, and 72 h. MDA-MB-231 cell migration and invasion were detected by Transwell assays after viral transduction for 48 h. (B) Left panel shows the statistical analysis of cell numbers, while the right panel shows representative pictures of the migrated cells under ×200 magnification. (C) Left panel shows the statistical analysis of cell numbers. Right panel shows representative pictures of the invaded cells under ×200 magnification. ### P<0.001 compared with NC. ** P<0.01, *** P<0.001 compared with sorafenib+NC.
Figure 5.
Effect of ARHGAP24 on sorafenib-mediated protein expression in MDA-MB-231 cells. MDA-MB-231 cells were treated with sorafenib (1.5 μM) and virally transduced with or without different recombinant lentiviral vectors to induce ARHGAP24 silencing or ectopic expression. Western blot analysis was performed to detect the expression of several essential proteins. The left panel shows representative results of Western blot analysis, while the right panel shows quantification of the relative protein levels. The values were normalized to GAPDH. ### P<0.001 compared with NC. **P<0.01, *** P<0.001 compared with sorafenib+NC.
Discussion
Few studies have focused on the effect of ARHGAP24 in human tumors. Nishi et al. demonstrated that ARHGAP24 was a novel prognostic factor for follicular lymphoma [21]. Xu et al. showed that ARHGAP24 induced cell cycle arrest and apoptosis and impaired invasion in renal cancer cell lines. In addition, it was reported that tumor formation was inhibited in vivo [20]. Moreover, RASAL2 negatively regulated ARHGAP24 activity by reducing the serine phosphorylation and then stimulated RAC1 activation to promote triple-negative BC progression. These reports indicate that ARHGAP24 appears to be not only a Rho GAP exerting the antagonism effect against Rac GTPases, but also is a downstream effector of Rho GTPases. Similar to these findings, in the present study, ARHGAP24 expression was downregulated in BC tissues. ARHGAP24 silencing by shRNA contributed to changes in MDA-MB-231 cell viability, migration, and invasion. Notably, ectopic expression of ARHGAP24 was confirmed as an effective method to suppress MDA-MB-231 cell viability, migration, and invasion.
Grandis et al. demonstrated the essential role of STAT3 in the regulation of the growth of cancer cells [22], and great efforts have been made to explore the effect of STAT3 in human tumors. An abundance of evidence confirms the aberrant activation of STAT3 in diverse human tumor tissues or cell lines and hyperactive Stat3 contributing to cell proliferation, survival, migration, invasion, and angiogenesis [14]. Moreover, targeting STAT3 is effective for cancer therapy [23,24]. Expectedly, the STAT3 signaling pathway is implicated in the development of BC, and excessive activation of STAT3 in BC tissues has been found [25,26]. In general, STAT3 is mainly activated in 3 ways: by cytokine receptors such as the interleukin-6 (IL-6) receptor, by growth-factor receptors such as epidermal growth factor receptor (EGFR), and by non-receptor tyrosine kinases such as SRC and ABL [14]. Interestingly, Rho GTPases are proved to be important for STAT3 activation. Previous studies showed that mutationally activated RhoA, Rac1, and Cdc42 can stimulate STAT3 activation [27–29]. In the present study, ARHGAP24 as a negative regulator of STAT3 activation significantly inhibited the expression of proteins belonging to the STAT3 pathway, such as MMP-2 and MMP-9.
As ARHGAP24 is downregulated in BC tissues, whether ARHGAP24 could be developed as a predictive or prognostic marker for BC deserves further study. In addition, the mechanism by which Rho GTPases activates STAT3 is controversial. Simon et al. showed that Rac1 bound directly to STAT3 and regulated STAT3 activity [30]. In another study, Rac1 activated Stat3 indirectly through IL-6 autocrine [28]. A recent study suggested that gp130 is required for the activation of Stat3 by Rac1 [31]. Moreover, MgcRacGAP as a kind of Rho GAP also negatively regulates STAT3 activation [32]. However, the present study only demonstrated the inhibitory effect of ARHGAP24 on STAT3 activation, and the underlying regulatory mechanism is still unclear. Therefore, more efforts should be made to investigate the detailed mechanisms involved.
BC mainly has 4 subtypes according to the gene expression profile – luminal A, luminal B, HER2+/ER−, and basal-like [3] – which affects the selection of therapy [33]. Systemic adjuvant treatment, such as chemotherapy, endocrine therapy, targeted therapy, or their combinations, plays a prominent role in the treatment of BC [34]. Unfortunately, the emergence of drug resistance limits the efficacy of these therapies [35,36]. Previous studies have shown that targeted therapies can trigger the feedback activation of STAT3, which eventually induces drug resistance [37,38]. Therefore, the combination of STAT3 inhibitors with the clinically approved targeted agents is a promising strategy to overcome drug resistance. Our results showed that sorafenib, a multikinase inhibitor, significantly inhibited MDA-MB-231 cell viability, migration, and invasion, along with the STAT3 activation and expression of MMP-2 and MMP-9, which is in line with previous studies showing that sorafenib inhibits pancreatic and liver cancer cell proliferation, migration, and invasion through inhibiting STAT3 activation [39,40]. In the present study, forced expression of ARHGAP24 enhanced sorafenib-induced inhibition of cell viability, migration, and invasion, along with the STAT3 activation and MMP-2 and MMP-9 expression. However, inhibiting ARHGAP24 suppressed the effects of sorafenib on MDA-MB-231 cell viability, migration, invasion, and related protein expression.
Conclusions
In conclusion, the present study preliminarily provides insights into the anti-cancer effect of ARHGAP24 in MDA-MB-231 cells. ARHGAP24 overexpression suppressed the viability, migration, and invasion of MDA-MB-231 cells via the STAT3 signaling pathway, and sorafenib inhibited cell viability, migration, invasion, and STAT3 activation in BC through ARHGAP24. Therefore, ARHGAP24 may be a promising candidate for new therapeutic targets for BC.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. Cancer J Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Sun H, Yin CQ, Liu Q, et al. Clinical significance of routine blood test-associated inflammatory index in breast cancer patients. Med Sci Monit. 2017;23:5090–95. doi: 10.12659/MSM.906709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jitariu AA, Cimpean AM, Ribatti D, Raica M. Triple negative breast cancer: The kiss of death. Oncotarget. 2017;8:46652–62. doi: 10.18632/oncotarget.16938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olson MF. Contraction reaction: Mechanical regulation of Rho GTPase. Trends Cell Biol. 2004;14:111–14. doi: 10.1016/j.tcb.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Tcherkezian J, Lamarche-Vane N. Current knowledge of the large RhoGAP family of proteins. Biol Cell. 2007;99:67–86. doi: 10.1042/BC20060086. [DOI] [PubMed] [Google Scholar]
- 8.Porter AP, Papaioannou A, Malliri A. Deregulation of Rho GTPases in cancer. Small GTPases. 2016;7:123–38. doi: 10.1080/21541248.2016.1173767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zandvakili I, Lin Y, Morris JC, Zheng Y. Rho GTPases: Anti- or pro-neoplastic targets? Oncogene. 2017;36:3213–22. doi: 10.1038/onc.2016.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fritz G, Just I, Kaina B. Rho GTPases are over-expressed in human tumors. Int J Cancer. 1999;81:682–87. doi: 10.1002/(sici)1097-0215(19990531)81:5<682::aid-ijc2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 11.Fritz G, Brachetti C, Bahlmann F, et al. Rho GTPases in human breast tumours: Expression and mutation analyses and correlation with clinical parameters. Br J Cancer. 2002;87:635–44. doi: 10.1038/sj.bjc.6600510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hein AL, Post CM, Sheinin YM, et al. RAC1 GTPase promotes the survival of breast cancer cells in response to hyper-fractionated radiation treatment. Oncogene. 2016;35:6319–29. doi: 10.1038/onc.2016.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–88. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 14.Yu H, Jove R. The STATs of cancer – new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 15.Raptis L, Arulanandam R, Geletu M, Turkson J. The R(h)oads to Stat3: Stat3 activation by the Rho GTPases. Exp Cell Res. 2011;317:1787–95. doi: 10.1016/j.yexcr.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou J, Chen L, Zhang Y, et al. Synergistic effect of EMS1-shRNA and sorafenib on proliferation, migration, invasion and endocytosis of SMMC-7721. J Mol Histol. 2014;45:205–16. doi: 10.1007/s10735-013-9543-2. [DOI] [PubMed] [Google Scholar]
- 17.Wei JC, Meng FD, Qu K, et al. Sorafenib inhibits proliferation and invasion of human hepatocellular carcinoma cells via up-regulation of p53 and suppressing FoxM1. Acta Pharmacol Sin. 2015;36:241–51. doi: 10.1038/aps.2014.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohta Y, Hartwig JH, Stossel TP. FilGAP, a Rho- and ROCK-regulated GAP for Rac binds filamin A to control actin remodelling. Nat Cell Biol. 2006;8:803–14. doi: 10.1038/ncb1437. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura F. FilGAP and its close relatives: A mediator of Rho-Rac antagonism that regulates cell morphology and migration. Biochem J. 2013;453:17–25. doi: 10.1042/BJ20130290. [DOI] [PubMed] [Google Scholar]
- 20.Xu G, Lu X, Huang T, Fan J. ARHGAP24 inhibits cell cycle progression, induces apoptosis and suppresses invasion in renal cell carcinoma. Oncotarget. 2016;7:51829–39. doi: 10.18632/oncotarget.10386. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Nishi T, Takahashi H, Hashimura M, et al. FilGAP, a Rac-specific Rho GTPase-activating protein, is a novel prognostic factor for follicular lymphoma. Cancer Med. 2015;4:808–18. doi: 10.1002/cam4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grandis JR, Drenning SD, Chakraborty A, et al. Requirement of Stat3 but not Stat1 activation for epidermal growth factor receptor- mediated cell growth in vitro. J Clin Invest. 1998;102:1385–92. doi: 10.1172/JCI3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bosch-Barrera J, Queralt B, Menendez JA. Targeting STAT3 with silibinin to improve cancer therapeutics. Cancer Treat Rev. 2017;58:61–69. doi: 10.1016/j.ctrv.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Seo HS, Ku JM, Choi HS, et al. Apigenin overcomes drug resistance by blocking the signal transducer and activator of transcription 3 signaling in breast cancer cells. Oncol Rep. 2017;38:715–24. doi: 10.3892/or.2017.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X, Xiao Q, Bai X, et al. Activation of STAT3 is involved in malignancy mediated by CXCL12-CXCR4 signaling in human breast cancer. Oncol Rep. 2014;32:2760–68. doi: 10.3892/or.2014.3536. [DOI] [PubMed] [Google Scholar]
- 26.Banerjee K, Resat H. Constitutive activation of STAT3 in breast cancer cells: A review. Oncol Rep. 2014;32:2760–68. doi: 10.1002/ijc.29923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aznar S, Valeron PF, del Rincon SV, et al. Simultaneous tyrosine and serine phosphorylation of STAT3 transcription factor is involved in Rho A GTPase oncogenic transformation. Mol Biol Cell. 2001;12:3282–94. doi: 10.1091/mbc.12.10.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faruqi TR, Gomez D, Bustelo XR, et al. Rac1 mediates STAT3 activation by autocrine IL-6. Proc Natl Acad Sci USA. 2001;98:9014–19. doi: 10.1073/pnas.161281298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Debidda M, Wang L, Zang H, et al. A role of STAT3 in Rho GTPase-regulated cell migration and proliferation. J Biol Chem. 2005;280:17275–85. doi: 10.1074/jbc.M413187200. [DOI] [PubMed] [Google Scholar]
- 30.Simon AR, Vikis HG, Stewart S, et al. Regulation of STAT3 by direct binding to the Rac1 GTPase. Science. 2000;290:144–47. doi: 10.1126/science.290.5489.144. [DOI] [PubMed] [Google Scholar]
- 31.Arulanandam R, Geletu M, Feracci H, Raptis L. Activated Rac1 requires gp130 for Stat3 activation, cell proliferation and migration. Exp Cell Res. 2010;316:875–86. doi: 10.1016/j.yexcr.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 32.van Adrichem AJ, Wennerberg K. MgcRacGAP inhibition stimulates JAK-dependent STAT3 activity. FEBS Lett. 2015;589:3859–65. doi: 10.1016/j.febslet.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 33.Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies – improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol. 2015;26:1533–46. doi: 10.1093/annonc/mdv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao W, Li X, Wang J, et al. Decreasing eukaryotic initiation factor 3C (EIF3C) suppresses proliferation and stimulates apoptosis in breast cancer cell lines through mammalian target of rapamycin (mTOR) Pathway. Med Sci Monit. 2017;23:4182–91. doi: 10.12659/MSM.906389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coley HM. Mechanisms and strategies to overcome chemotherapy resistance in metastatic breast cancer. Cancer Treat Rev. 2008;34:378–90. doi: 10.1016/j.ctrv.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Gu G, Dustin D, Fuqua SA. Targeted therapy for breast cancer and molecular mechanisms of resistance to treatment. Curr Opin Pharmacol. 2016;31:97–103. doi: 10.1016/j.coph.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 37.Lee HJ, Zhuang G, Cao Y, et al. Drug resistance via feedback activation of Stat3 in oncogene-addicted cancer cells. Cancer Cell. 2014;26:207–21. doi: 10.1016/j.ccr.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 38.Li G, Zhao L, Li W, et al. Feedback activation of STAT3 mediates trastuzumab resistance via upregulation of MUC1 and MUC4 expression. Oncotarget. 2014;5:8317–29. doi: 10.18632/oncotarget.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang S, Sinicrope FA. Sorafenib inhibits STAT3 activation to enhance TRAIL-mediated apoptosis in human pancreatic cancer cells. Mol Cancer Ther. 2010;9:742–50. doi: 10.1158/1535-7163.MCT-09-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gu FM, Li QL, Gao Q, et al. Sorafenib inhibits growth and metastasis of hepatocellular carcinoma by blocking STAT3. World J Gastroenterol. 2011;17:3922–32. doi: 10.3748/wjg.v17.i34.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]