Abstract
Background
Gemcitabine/cisplatin (GP) resistance displays a negative role in treating advanced and metastatic non-small cell lung cancer (NSCLC). Several studies found that the association existed between platelets and cancer antigen 125 (CA125) with anticancer drugs. But the exact correlation between GP resistance and platelet activation index remains poorly understood.
Material/Methods
Pre-chemotherapy platelet activation index and CA125 were retrospectively evaluated in 169 advanced and metastatic NSCLC patients. All variables were screened by chi-square test and then evaluated by log-rank test. Survival curves were generated by Kaplan-Meier analysis. Univariate and multivariate survival analysis were performed by using Cox proportional hazards model.
Results
The overall rate of GP resistance for NSCLC patients was 72.19%. Mean platelet volume (MPV) and plateletcrit (PCT) are negative predictors of GP resistance adenocarcinoma [Odds ratio (OR): 5.81, 95% confidence interval (CI): 1.082–31.195, P=0.004] and squamous cell carcinoma (PCT: R: 3.517, 95% CI: 1.087–11.387, P=0.036), respectively. But both were an independent factor associated with overall survival (OS). Moreover, only CA125 was a dependent factor associated with OS for squamous cell carcinoma [OS: hazard ratio (HR): 1.741, 95% CI: 1.002–3.024, P=0.049; GP resistance: OR: 4.862, 95% CI: 1.437–16.448, P=0.011].
Conclusions
Platelet activation index will be a potential marker for predicting GP resistance. Besides, CA125 ≥16.9 could be used as a potential marker for predicting GP resistance and OS, which was more sensitive than CA125 ≥35 for squamous cell carcinoma.
MeSH Keywords: Blood Platelets; Carcinoma, Non-Small-Cell Lung; Drug Resistance; Mean Platelet Volume
Background
Non-small cell lung cancer (NSCLC) continues to be the cancer with the highest incidence and cancer-related mortality among malignant tumors, accounting for approximately 80% of all diagnosed lung cancer cases. The main reason for poor prognosis is that the great majority of NSCLC patients are diagnosed at an advanced stage [1]. With the development of technology, many methods have been used to treat NSCLC [2–6]. Chemotherapy has become the standard approach in the treatment of advanced NSCLC [7]. Gemcitabine/cisplatin (GP) serves as first-line doublet chemotherapy for treating advanced NSCLC, with an objective response rate of 20%, median progression-free-survival of 6.1 months, and median overall survival (OS) time of 13.1 months [8]. Unfortunately, not all the sufferers receive clinical benefits from GP chemotherapy [8]. The majority of patients treated with GP who are GP resistance will eventually become deceased, which suggests that we need to find a suitable marker to predict GP resistance before its use.
Platelets count is commonly used to evaluate whether chemotherapy can be used for patients. Recently, several studies found complex interactions between platelets and tumor cells resulting in tumor progression and metastases [9,10]. Clinical studies reported that platelet activation index including platelet count (PLC), mean platelet volume (MPV), platelet distribution width (PDW), and plateletcrit (PCT) were associated with poor prognosis in solid tumors [11–15]. Theoretically, platelets display an anti-proliferative effect role through releasing various growth factors during chemotherapy [16]. In fact, laboratory research demonstrated that platelets increase the survival of tumor cells challenged with anticancer drugs [16]. Furthermore, a few researchers found that some anti-platelet drugs enhanced the effect of anticancer drugs, including gemcitabine, paclitaxel, and 5-fluorouracil, by a complicated mechanism that downregulates the phosphorylation of DNA repair proteins and epithelialization in cancer cells, while enhancing drug-induced cell cycle arrest [16–18]. It might imply an important link between platelet activation index and GP resistance for NSCLC patients.
Additionally, cancer antigen 125 (CA125) ≥35 is commonly considered a marker of disease progression and sometimes also a sign of ineffective chemotherapy by oncologists. In this study, we focused on the evaluation of GP resistance and prognosis via platelet activation index such as PLT, MPV, PCT, and PDW in NSCLC patients. The potential value of CA125 in predicting both GP resistance and OS time were also discussed.
Material and Methods
Enrolled population
This work was approved by Zhejiang Cancer Hospital Ethic Committee. Based on the patient enrollment criteria, 169 NSCLC patients were enrolled by a medical team at our hospital (to reduce the subjective differences in the treatment groups) from January 2008 to December 2010. The information about patient gender, age, smoking status, histology, tumor-node-metastasis (TNM) stage, PLT, MPV, PCT, PDW, and CA125 were collected based on the original patient records.
Cancer staging was assessed by the TNM classification criteria issued by the International Union Against Cancer (UIAC) in 2007. Peripheral blood was collected with EDTA tubes at 1 week before chemotherapy. The routine full blood test was performed by our hospital Clinical Laboratory Department.
Patient enrollment criteria were as follows: 1) without any treatment before GP chemotherapy; ≥2 cycles of GP chemotherapy (gemcitabine (GEM) 1000 mg/m2 day 1 and 8, + cisplatin (DDP) 35~45 mg/m2 day 1 and 2). 2) 18 years < age <75 years, life expectancy ≥3 months (90 days); pathological diagnosis of squamous cell carcinoma or adenocarcinoma. 3) Systemic functional status score (WHO ECOG) 0–2 or KPS score ≥70. 4) Bone marrow hematopoietic function is basically normal: peripheral blood leukocyte count ≥3.5×109/L, neutrophil absolute value number ≥1.5×109/L, hemoglobin ≥9.0 g/L, platelet count ≥100×109/L. 5) Liver and kidney function test: serum aminotransferase ≤2 times the upper limit of normal, total bilirubin ≤1.5 times the upper limit of normal, serum creatinine ≤1.5 times the upper limit of normal or serum creatinine clearance ≥50 mL/min. 6) No previous history of malignancy, organ function was normal, without serious complications, or died from lung cancer and related complications.
Exclusion criteria were as follows: 1) allergy and allergy to many drugs, 2) suffers from mental disorders, 3) severe infection or organic disease, 4) for women, pregnancy and lactation.
To determine OS, follow-up was conducted by telephone and during hospitalization from April 6, 2011.The survival time was calculated until the last follow-up (April 24, 2012).
Response evaluation
The primary drug resistance with GP chemotherapy were assessed after 2 cycles of chemotherapy, based on the rules established the Response by Evaluation Criteria in Solid Tumors (RECIST) [19]. The standard with GP chemotherapy resistance follows the principles described by Altan et al. [20].
PLR measurement
The PLC, MPV, PDW, PCT, and CA125 of peripheral blood were measured with an automatic hematology analyzer 1 week before GP chemotherapy.
Statistical analysis
Inter-group differences in categorical variables were assessed for significance using the chi-square test; differences in continuous variables were assessed using the Mann-Whitney U test or t-test. The optimal cutoff value for age, PLC, MPV, PDW, PCT, and CA125were determined by receiver operating characteristic (ROC) curve analysis. All variables were screened by chi-square test and then evaluated by log-rank test to confirm the risk factors eventually. Survival curves were generated by Kaplan-Meier analysis. Univariate and multivariate survival analysis were performed using Cox proportional hazards model. All data were analyzed by SPSS 16.0 software. (Version 16.0, purchase by SPSS software package). P<0.05 was considered as statistical significance.
Results
Patient characteristics
The characteristics of the patients according to histology are summarized in Table 1. Only 31 patients (24.627%) were alive after the last follow-up (April 24, 2012) among the 169 patients who were enrolled in our study. In total, 72.19% of NSCLC patients (122 out of 169) developed GP resistance. According to pathological histology, we divided the 169 cases into 2 groups: 90 cases (53.254%) had adenocarcinoma (ADC group) and 79 cases (46.746%) had squamous cell carcinoma (SqCC group). Among all the variables, gender, age (years), smoking status, CA125 (U/mL), and GP resistance were significantly different between the 2 groups, especially the factor of GP resistance.
Table 1.
Different variables according to histology with NSCLC.
| Variable | ADC (n=90) | SqCC (n=79) | P value | |
|---|---|---|---|---|
| Gender | ||||
| Female | 35 (38.889) | 12 (15.190) | 0.001 | |
| Male | 55 (61.111) | 67 (84.810) | ||
| Age (yr) | ||||
| Median (range) | 53.311±10.211 (27–73) | 57.038±6.991 (44–72) | 0.007 | |
| TNM stage | ||||
| IIIA | 8 (8.889) | 12 (15.190) | 0.378 | |
| IIIB | 28 (31.111) | 26 (32.911) | ||
| IV | 54 (60.000) | 41 (51.899) | ||
| Smoking | ||||
| Never smoker | 41 (45.556) | 21 (26.582) | 0.011 | |
| Current or former smoker | 49 (54.444) | 58 (73.418) | ||
| GP resistance | ||||
| PD+SD | 71 (78.889) | 51 (64.557) | 0.038 | |
| PR+CR | 19 (21.111) | 28 (35.443) | ||
| State | ||||
| Alive | 15 | 16 | 0.548 | |
| Death | 75 | 63 | ||
| Median survival time [MST] | 505 days | 434 days | ||
| PLT (109/L) | Median (range) | 249.689±77.664 (123–484) | 260.430±87.46 (120–483) | 0.402 |
| MPV (fl) | Median (range) | 10.639±1.594 (7.1–18.5) | 10.162±1.708 (6.5–14.7) | 0.064 |
| PCT (%) | Median (range) | 0.263±0.079 (0.15–0.62) | 0.257±0.070 (0.13–0.46) | 0.586 |
| PDW (%) | Median (range) | 13.662±2.826 (8.1–22.7) | 13.585±3.033 (8.5–23.7) | 0.88 |
| CA125 (U/mL) | Median (range) | 93.451 (6.4–509.2) | 59.713 (6.5–677.3) | 0.001 |
NSCLC – non-small cell lung cancer; ADC – adenocarcinoma; SqCC – squamous cell carcinoma; GP – gemcitabine+cisplatin; PD – progressive disease; SD – stable disease; PR – partial response’s; CR – complete response; PLC – platelet count; MPV – mean platelet volume; PDW – platelet distribution width; PCT – plateletcrit, OS – overall survival time.
However, between the two groups, gender, age, smoking status, and CA12 were not the influence factors for GP resistance according to non-conditional logistic regression analysis (Table 2). Therefore, the study result that showed the incidence of GP resistance in the ADC group was higher than that in the SqCC group was reliable (71 out of 90 cases versus 51 out of 79 cases, P=0.038 at <0.05, Table 1).
Table 2.
The correlation between the parameters and GP resistance.
| Variable | B | OR | 95% CI | P value |
|---|---|---|---|---|
| Gender | 0.476 | 0.4 | 0.588–3.792 | 0.4 |
| Age (yr) | 0.02 | 0.169 | 0.988–1.071 | 0.169 |
| Smoking | 0.425 | 0.387 | 0.628–3.324 | 0.387 |
| CA125(U/mL) | 0.002 | 0.197 | 0.994–1.001 | 0.197 |
GP – gemcitabine+cisplatin; OR – odds ratio; CI – confidence interval.
Subgroup analysis for OS according to histology
First, we used ROC curve analysis to determine the optimal cutoff value for age, PLC, MPV, PDW, PCT, and CA125 for prediction of survival status, which was 44.5, 166.5, 11.05, 0.255, 13.15, and 19.8, respectively (Table 3, Figure 1). Second, we investigated the OS value of pathological histology type relative to gender, age, TNM stage, GP resistance, smoking status, PLT, MPV, PCT, PDW, and CA125 (Table 4). There was no significant correlation between the OS value and histology irrespective of parameters (gender, age, TNM stage, smoking status, PLT, MPV, PCT, PDW, and CA125). We found that the SqCC group had significantly shorter OS than the ADC group once the GP resistance occurred (ADC: median survival time (MST)=554 days vs. SqCC: MST 356 days, P=0.047, Table 4, Figure 2).
Table 3.
The Age, PLT, MPV, PCT, PDW, CA125 markers for prediction of survival status.
| Variable | AUC (95% CI) | SN, % | SP, % | Cut-off value |
|---|---|---|---|---|
| Age (yr) | 0.503 (0.395–0.611) | 0.877 | 0.968 | 44.5 |
| PLT (109/L) | 0.517 (0.404–0.63) | 0.899 | 0.806 | 166.5 |
| MPV (fl) | 0.504 (0.396–0.613) | 0.312 | 0.194 | 11.05 |
| PCT (%) | 0.544 (0.436–0.653) | 0.464 | 0.323 | 0.255 |
| PDW (%) | 0.54 (0.43–0.649) | 0.543 | 0.355 | 13.15 |
| CA125 (U/mL) | 0.652 (0.548–0.757) | 0.746 | 0.419 | 19.8 |
AUC – area under the curve; CI – confidence interval; SN – sensitivity; SP – specificity; PLC – platelet count; MPV – mean platelet volume; PDW – platelet distribution width; PCT – plateletcrit.
Figure 1.
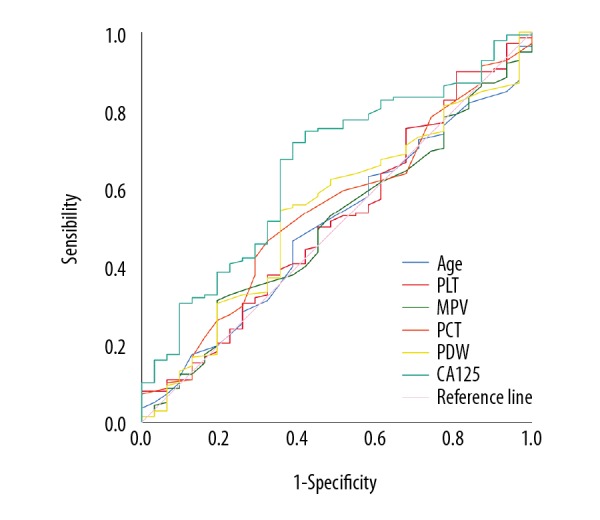
Overall survival in NSCLC group. ROC curve analysis was performed to analyze the optimal cutoff values of age, PLC, MPV, PCT, PDW, and CA125 for survival status prediction. NSCLC – non-small cell lung cancer; ROC – receiver operating characteristic; PLC – platelet count; MPV – mean platelet volume; PCT – plateletcrit; PDW – platelet distribution width; CA125 – cancer antigen 125.
Table 4.
Subgroup analysis for OS according to histology.
| Histology | OS, days | ||||
|---|---|---|---|---|---|
| N (%) | Median (SD) | 95%Cl | P value | ||
| Gender | |||||
| Female | ADC | 35 (74.468) | 626 (84.373) | 460.629–791.371 | 0.419 |
| SqCC | 12 (25.532) | 357 (175.604) | 12.817–701.183 | ||
| Male | ADC | 55 (45.082) | 460 (76.106) | 310.832–609.168 | 0.842 |
| SqCC | 67 (54.918) | 437 (65.476) | 308.668–565.332 | ||
| Age (yr) | |||||
| <44.5 | ADC | 11 (61.111) | 405 (125.51) | 159.001–650.999 | 0.487 |
| SqCC | 7 (38.889) | 456 (115.738) | 229.153–682.847 | ||
| ≥44.5 | ADC | 79 (52.318) | 562 (56.302) | 451.649–672.351 | 0.214 |
| SqCC | 72 (47.682) | 429 (63.109) | 305.306–552.694 | ||
| TNM stage | |||||
| IIIA | ADC | 8 (40) | 867 (166.651) | 540.364–1193.636 | 0.932 |
| SqCC | 12 (60) | 502 (233.48) | 44.378–959.622 | ||
| IIIB | ADC | 28 (51.852) | 697 (97.404) | 506.089–887.911 | 0.058 |
| SqCC | 26 (48.148) | 434 (95.607) | 246.611–621.389 | ||
| IV | ADC | 54 (56.842) | 436 (35.63) | 366.165–505.835 | 0.735 |
| SqCC | 41 (43.158) | 356 (11.068) | 334.307–377.693 | ||
| GP resistance | |||||
| PD+SD | ADC | 71 (58.197) | 554 (62.722) | 431.065–676.935 | 0.047 |
| SqCC | 51 (41.803) | 356 (17.068) | 322.546–389.454 | ||
| PR+CR | ADC | 19 (40.426) | 544 (156.353) | 237.548–850.452 | 0.618 |
| SqCC | 28 (59.574) | 480 (101.273) | 281.504–678.496 | ||
| Smoking | |||||
| Never smoker | ADC | 41 (66.129) | 606 (48.649) | 510.647–701.353 | 0.304 |
| SqCC | 21 (33.871) | 343 (166.587) | 16.489–669.511 | ||
| Current or former smoker | ADC | 49 (45.794) | 460 (36.052) | 389.339–530.661 | 0.667 |
| SqCC | 58 (54.206) | 437 (71.081) | 297.682–576.318 | ||
| PLT (109/L) | |||||
| <166.5 | ADC | 11 (55) | 455 (107.894) | 243.527–666.473 | 0.491 |
| SqCC | 9 (45) | 476 (58.138) | 362.05–589.95 | ||
| ≥166.5 | ADC | 79 (53.02) | 554 (65.425) | 425.768–682.232 | 0.214 |
| SqCC | 70 (46.98) | 429 (59.315) | 312.743–545.257 | ||
| MPV (fl) | |||||
| <11.05 | ADC | 61 (50.833) | 481 (68.456) | 346.827–615.173 | 0.64 |
| SqCC | 59 (49.167) | 474 (71.315) | 334.223–613.777 | ||
| ≥11.05 | ADC | 29 (59.184) | 606 (100.463) | 409.092–802.908 | 0.095 |
| SqCC | 20 (40.816) | 289 (105.654) | 81.917–496.083 | ||
| PCT (%) | |||||
| <0.255 | ADC | 46 (48.421) | 544 (77.432) | 392.234–695.766 | 0.826 |
| SqCC | 49 (51.579) | 474 (29.408) | 416.361–531.639 | ||
| ≥0.255 | ADC | 44 (59.459) | 505 (90.89) | 326.855–683.145 | 0.209 |
| SqCC | 30 (40.541) | 356 (19.855) | 317.084–394.916 | ||
| PDW (%) | |||||
| >13.15 | ADC | 44 (53.012) | 481 (75.528) | 332.964–629.036 | 0.204 |
| SqCC | 39 (46.988) | 429 (67.809) | 296.095–561.905 | ||
| ≤13.15 | ADC | 46 (53.488) | 570 (85.344) | 402.725–737.275 | 0.966 |
| SqCC | 40 (46.512) | 437 (88.544) | 263.454–610.546 | ||
| CA125 (U/mL) | |||||
| <19.8 | ADC | 19 (35.549) | 744 (156.327) | 437.599–1050.401 | 0.527 |
| SqCC | 34 (64.151) | 695 (129.722) | 440.744–949.256 | ||
| ≥19.8 | ADC | 71 (61.207) | 455 (37.323) | 381.848–528.152 | 0.07 |
| SqCC | 45 (38.793) | 357 (9.389) | 338.597–375.403 | ||
OS – overall survival time; SD – standard deviation; CI – confidence interval; ADC – adenocarcinoma; SqCC – squamous cell carcinoma; GP – gemcitabine+cisplatin; PD – progressive disease; SD – stable disease; PR – partial responses; CR – complete response; PLC – platelet count; MPV – mean platelet volume; PDW – platelet distribution width; PCT – plateletcrit.
Figure 2.
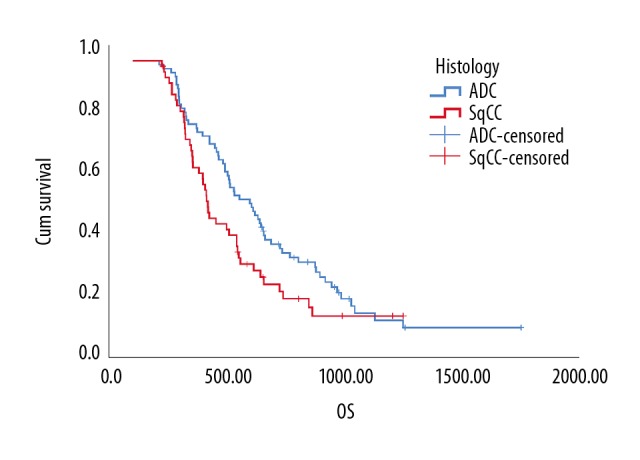
Kaplan-Meier analysis of the overall survival of histology difference in non-small cell lung cancer patients with gemcitabine/cisplatin resistance (P=0.047, n=122).
The impact of various factors on GP resistance to OS in the ADC group
First, the optimal cutoff value with GP resistance for age, PLT, MPV, PCT, PDW, and CA125 were determined by ROC curve analysis. And were 66.5, 235.5, 10.85, 0.355, 12.2, and 29.55, respectively (Table 5, Figure 3). Then, we assessed risk factors with GP resistance for the ADC group by univariate analysis and multivariate analysis. We found that the presence of GP resistance was an independent factor associated with MPV (≥10.85) factors [odds ratio (OR): 5.81, 95% confidence interval (CI): 1.082–31.195, P=0.004] eventually (Tables 6, 7). In addition, we found that there was no significant link between GP resistance and CA125, whether we used 35 or 29.55 as the optimal cutoff value for CA125 (Tables 6, 7).
Table 5.
The age, PLT, MPV, PCT, PDW, CA125 markers for prediction of GP resistance for ADC.
| Variable | AUC (95% CI) | SN, % | SP, % | Cut-off value |
|---|---|---|---|---|
| Age (yr) | 0.504 (0.353–0.656) | 0.085 | 0.211 | 65.5 |
| PLT (109/L) | 0.463 (0.321–0.604) | 0.437 | 0.632 | 235.5 |
| MPV (fl) | 0.616 (0.488–0.745) | 0.451 | 0.105 | 10.85 |
| PCT (%) | 0.499 (0.35–0.648) | 0.099 | 0.211 | 0.355 |
| PDW (%) | 0.579 (0.423–0.734) | 0.676 | 0.421 | 12.2 |
| CA125 (U/mL) | 0.459 (0.324–0.594) | 0.549 | 0.737 | 29.55 |
AUC – area under the curve; CI – confidence interval; SN – sensitivity; SP – specificity; PLC – platelet count; MPV – mean platelet volume; PDW – platelet distribution width; PCT – plateletcrit.
Figure 3.
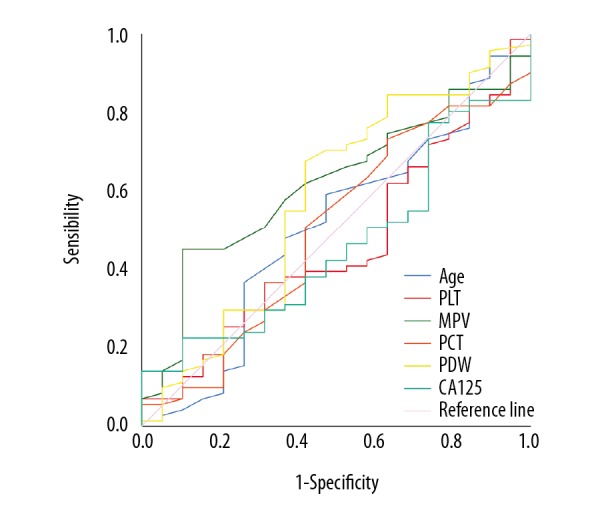
Gemcitabine/cisplatin resistance for adenocarcinoma. ROC curve analysis was used to measure the optimal cutoff values for gemcitabine/cisplatin resistance for age, PLT, MPV, PCT, PDW, and CA125. ROC – receiver operating characteristic; PLC – platelet count; MPV – mean platelet volume; PCT – plateletcrit; PDW – platelet distribution width; CA125 – cancer antigen 125.
Table 6.
Univariate analysis of risk factors for GP resistance in ADC.
| Variable | PD+SD (n, %) | PR+CR (n, %) | P value |
|---|---|---|---|
| Gender | |||
| Female | 28 (80) | 7 (20) | 0.837 |
| Male | 43 (78.182) | 12 (21.818) | |
| Age (yr) | |||
| <65.5 | 65 (81.25) | 15 (18.75) | 0.121 |
| ≥65.5 | 6 (60) | 4 (40) | |
| TNM stage | |||
| IIIA | 6 (75) | 2 (25) | 0.438 |
| IIIB | 20 (71.429) | 8 (28.571) | |
| IV | 45 (83.333) | 9 (16.667) | |
| Smoking | |||
| Never smoker | 32 (78.049) | 9 (21.951) | 0858 |
| Current or former smoker | 39 (79.592) | 10 (20.408) | |
| PLT (109/L) | |||
| ≥235.5 | 31 (72.093) | 12 (27.907) | 0.131 |
| <235.5 | 40 (85.106) | 7 (14.894) | |
| MPV (fl) | |||
| <10.85 | 39 (69.643) | 17 (30.357) | 0.006 |
| ≥10.85 | 32 (94.118) | 2 (5.882) | |
| PCT (%) | |||
| ≥0.355 | 7 (63.636) | 4 (36.364) | 0.186 |
| <0.355 | 64 (81.013) | 15 (18.987) | |
| PDW (%) | |||
| <12.2 | 23 (67.647) | 11 (32.353) | 0.042 |
| ≥12.2 | 48 (85.714) | 8 (14.286) | |
| CA125 (U/mL) | |||
| ≥29.55 | 39 (73.585) | 14 (26.415) | 0.14 |
| <29.55 | 32 (86.486) | 5 (13.514) | |
| CA125 (U/mL)-standard | |||
| <35 | 34 (85) | 6 (15) | 0.204 |
| ≥35 | 37 (74) | 13 (26) | |
PD – progressive disease; SD – stable disease; PR – partial responses; CR – complete response; PLC – platelet count; MPV – mean platelet volume; PDW – platelet distribution width; PCT – plateletcrit.
Table 7.
Multivariate analysis of risk factors for GP resistance in ADC.
| Factors | OR | 95%CI | P value |
|---|---|---|---|
| MPV(fl) (≥10.85) | 5.81 | 1.082–31.195 | 0.04 |
| PDW (%) (≥12.2) | 1.377 | 0.431–4.403 | 0.589 |
GP – gemcitabine+cisplatin; ADC – adenocarcinoma; OR – odds ratio; CI – confidence interval; MPV – mean platelet volume;
PDW – platelet distribution width.
Finally, we found there was no significant correlation between OS and MPV, with using 10.85 as the optimal cutoff value for MPV [hazard ratio (HR): 1.025, 95%Cl: 0.321–3.271, P=0.967, Table 8].
Table 8.
Cox proportional regression model for OS with ADC.
| Variables | OS, days | |||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| HR (95%Cl) | P value | HR (95%Cl) | P value | |
| GP resistance (PD+SD vs. PR+CR) | 1.081 (0.604–1.935) | 0.793 | ||
| Gender (Male vs. Female) | 1.447 (0.897–2.334) | 0.13 | ||
| Age (yr) (<65.5 vs. ≥65.5) | 0.522 (0.235–1.159) | 0.11 | ||
| TNM stage (IIIA, IIIB, IV) | 2.015 (1.351–3.007) | 0.001 | 2.198 (1.458–3.313) | <0.001 |
| Smoking (Never smoker vs. current or former smoker) | 1.106 (0.701–1.744) | 0.665 | ||
| PLT (109/L) (<235.5 vs. ≥235.5) | 0.949 (0.602–1.496) | 0.821 | ||
| MPV (fl) (<10.85 vs. ≥10.85) | 1.025 (0.321–3.271) | 0.967 | ||
| PCT (%) (<0.355 vs. ≥0.355) | 0.835 (0.399–1.747) | 0.64 | ||
| PDW (%) (<12.2 vs. ≥12.2) | 1.094 (0.685–1.747) | 0.707 | ||
| CA125 (U/mL) (<29.55 vs. ≥29.55) | 0.53 (0.33–0.853) | 0.009 | 0.459 (0.282–0.749) | 0.002 |
| CA125 (U/mL)-standard (<35 vs. ≥35) | 1.757 (1.103–2.797) | 0.018 | 2.117 (1.311–3.418) | 0.002 |
OS – overall survival time; HR – hazard ratio; CI – confidence interval; GP – gemcitabine+cisplatin; PD – progressive disease; SD – stable disease; PR – partial responses; CR – complete response; PLC – platelet count; MPV – mean platelet volume; PDW – platelet distribution width; PCT – plateletcrit.
The impact of various factors on GP resistance to OS in the SqCC group
First, we determined the optimal cutoff value with GP resistance for age, PLT, MPV, PCT, PDW, and CA125 were 58.5, 229.5, 9.3, 0.235, 14.95, and 16.9 respectively, by ROC curve analysis (Table 9, Figure 4). Then, using 35 as the CA125 (U/mL) standard cutoff value, we confirmed PCT (%) (≥0.235) and CA125 (U/mL) (≥16.9) were dependent predictors of GP resistance in the lung SqCC group by chi-square test and log-rank test. At the same time, by comparison, we found GP resistance evaluated at a cutoff value of 16.9 for CA125 was more sensitive than using 35 as a cutoff value for CA125.
Table 9.
The Age, PLT, MPV, PCT, PDW, CA125 markers for prediction of GP resistance for SqCC.
| Variable | AUC (95% CI) | SN, % | SP, % | Cut-off value |
|---|---|---|---|---|
| Age (yr) | 0.377 (0.253–0.502) | 0.314 | 0.571 | 58.5 |
| PLT (109/L) | 0.578 (0.442–0.715) | 0.647 | 0.393 | 229.5 |
| MPV (fl) | 0.593 (0.454–0.733) | 0.804 | 0.571 | 9.3 |
| PCT (%) | 0.675 (0.549–0.802) | 0.667 | 0.286 | 0.235 |
| PDW (%) | 0.329 (0.206–0.452) | 0.235 | 0.571 | 14.95 |
| CA125 (U/mL) | 0.669 (0.539–0.798) | 0.765 | 0.429 | 16.9 |
AUC – area under the curve; CI – confidence interval; SN – sensitivity; SP – specificity; PLC – platelet count; MPV – mean platelet volume; PDW – platelet distribution width; PCT – plateletcrit.
Figure 4.
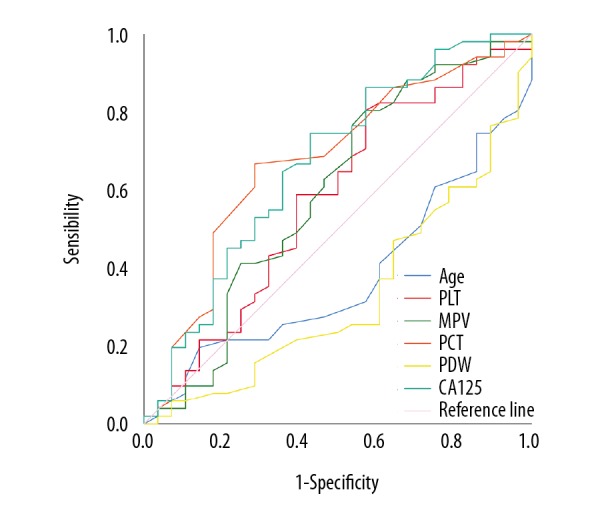
Gemcitabine/cisplatin resistance for squamous cell carcinoma. ROC curve analysis was used to access the optimal cutoff values for gemcitabine/cisplatin resistance for age, PLT, MPV, PCT, PDW, and CA125. ROC – receiver operating characteristic; PLC – platelet count; MPV – mean platelet volume; PCT – plateletcrit, PDW – platelet distribution width; CA125 – cancer antigen 125.
In addition, although PDW (%) (<14.95) and CA125 (≥16.9) were not dependent factors of GP resistance, we found they were both significantly associated with GP resistance (Table 10). Then, we assessed risk factors with GP resistance for the SqCC group by univariate analysis and multivariate analysis. We found the presence of GP resistance was an independent factor associated with PCT MPV (≥0.235) factors (OR: 3.517, 95% CI: 1.087–11.387, P=0.036), and CA125 (≥16.9) factors (OR: 4.862, 95% CI: 1.437–16.448, P=0.011) (Table 11).
Table 10.
Univariate analysis of risk factors for GP resistance in SqCC.
| Variable | PD+SD (n,%) | PR+CR (n,%) | P value |
|---|---|---|---|
| Gender | |||
| Female | 10 (75) | 2 (25) | 0.14 |
| Male | 41 (61.194) | 26 (38.806) | |
| Age (yr) | |||
| ≥58.5 | 16 (50) | 16 (50) | 0.026 |
| <58.5 | 35 (72.34) | 12 (27.66) | |
| TNM stage | |||
| IIIA | 7 (58.333) | 5 (41.667) | 0.757 |
| IIIB | 16 (61.538) | 10 (38.462) | |
| IV | 28 (65.854) | 13 (34.146) | |
| Smoking | |||
| Never smoker | 17 (76.19) | 4 (23.81) | 0.067 |
| Current or former smoker | 34 (58.621) | 24 (41.379) | |
| PLT (109/L) | |||
| <229.5 | 18 (51.429) | 17 (48.571) | 0.03 |
| ≥229.5 | 33 (72.727) | 11 (27.273) | |
| MPV (fl) | |||
| <9.3 | 10 (45.455) | 12 (54.545) | 0.027 |
| ≥9.3 | 41 (70.175) | 16 (29.825) | |
| PCT (%) | |||
| <0.235 | 17 (45.946) | 20 (54.054) | 0.001 |
| ≥0.235 | 33 (78.571) | 9 (21.429) | |
| PDW (%) | |||
| ≥14.95 | 13 (43.333) | 17 (56.667) | 0.002 |
| <14.95 | 38 (75.51) | 11 (24.49) | |
| CA125 (U/mL) | |||
| <16.9 | 14 (44.828) | 16 (55.172) | 0.005 |
| ≥16.9 | 38 (74) | 12 (26) | |
| CA125 (U/mL)-standard | |||
| <35 | 30 (57.692) | 22 (42.308) | 0.077 |
| ≥35 | 21 (77.778) | 6 (22.222) | |
PD – progressive disease; SD – stable disease; PR – partial responses; CR – complete response; PLC – platelet count; MPV – mean platelet volume; PDW – platelet distribution width; PCT – plateletcrit.
Table 11.
Multivariate analysis of risk factors for GP resistance in SqCC.
| Factors | OR | 95% CI | P value |
|---|---|---|---|
| Age (yr) (<58.5) | 2.92 | 0.932–9.148 | 0.066 |
| PLT (109/L) (≥229.5) | 0.932 | 0.161–5.389 | 0.937 |
| MPV (fl) (≥9.3) | 4.258 | 0.696–26.069 | 0.117 |
| PCT (%) (≥0.235) | 3.517 | 1.087–11.387 | 0.036 |
| PDW (%) (<14.95) | 1.267 | 0.282–5.689 | 0.757 |
| CA125 (U/mL) (≥16.9) | 4.862 | 1.437–16.448 | 0.011 |
OR – odds ratio; CI – confidence interval; PLC – platelet count; MPV – mean platelet volume; PDW – platelet distribution width; PCT – plateletcrit.
Finally, we found no difference in the OS of PCT (≥0.235) group and PCT (<0.235) group by Cox proportional regression model (P=0.439, Table 12). Therefore, the data presented in Table 12 for multivariate analysis were the results that incorporated PDW, TNM stage, and CA125 (not PDW, TNM stage, CA125, and GP resistance) into the Cox proportional regression model together. On the other hand, predicting GP resistance and OS evaluated at a cutoff value of 16.9 for CA125 was more sensitive than using 35 as a cutoff value for CA125 (cutoff =16.9: HR: 1.741, CI: 1.014–2.988, P=0.044; cutoff=35: HR: 1.365, CI: 0.816–2.284, P=0.236, Table 12). Additionally, the data analyzed from the Cox proportional regression model, which included GP resistance and TNM stage, showed that GP resistance was a prognostic factor independent of stage (HR: 1.858, Cl: 1.084–3.186, P=0.024, Table 12). Overall, a cutoff value of 16.9 for CA125 was significantly associated with GP resistance and OS for the SqCC group (Figure 5).
Table 12.
Cox proportional regression model for OS with SqCC.
| Variables | OS, days | |||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| HR (95%Cl) | P value | HR (95%Cl) | P value | |
| GP resistance (PD+SD vs. PR+CR) | 1.78 (1.042–3.041) | 0.035 | ||
| Gender (Male vs. Female) | 1.196 (0.569–2.515) | 0.636 | ||
| Age (yr) (<58.5 vs. ≥58.5) | 1.284 (0.775–2.126) | 0.332 | ||
| TNM stage (IIIA, IIIB, IV) | 1.546 (1.087–2.197) | 0.015 | 1.502 (1.064–2.119) | 0.021 |
| Smoking (Never smoker vs. current or former smoker) | 0.93 (0.519–1.666) | 0.807 | ||
| PLT (109/L) (<229.5 vs. ≥229.5) | 1.127 (0.681–1.862) | 0.642 | ||
| MPV (fl) (<9.3 vs. ≥9.3) | 1.629 (0.927–2.863) | 0.09 | ||
| PCT (%) (<0.235 vs. ≥0.235) | 1.217 (0.74–2.004) | 0.439 | ||
| PDW (%) (<14.95 vs. ≥14.95) | 1.74 (1.028–2.943) | 0.039 | 1.617 (0.952–2.748) | 0.076 |
| CA125 (U/mL) (<16.9 vs. ≥16.9) | 1.741 (1.014–2.988) | 0.044 | 1.741 (1.002–3.024) | 0.049 |
| CA125 (U/mL)-standard (<35 vs. ≥35) | 1.365 (0.816–2.284) | 0.236 | ||
OS – overall survival time; HR – hazard ratio; CI – confidence interval; GP – gemcitabine+cisplatin; PD – progressive disease; SD – stable disease; PR – partial responses; CR – complete response; PLC – platelet count; MPV – mean platelet volume; PDW – platelet distribution width; PCT – plateletcrit.
Figure 5.
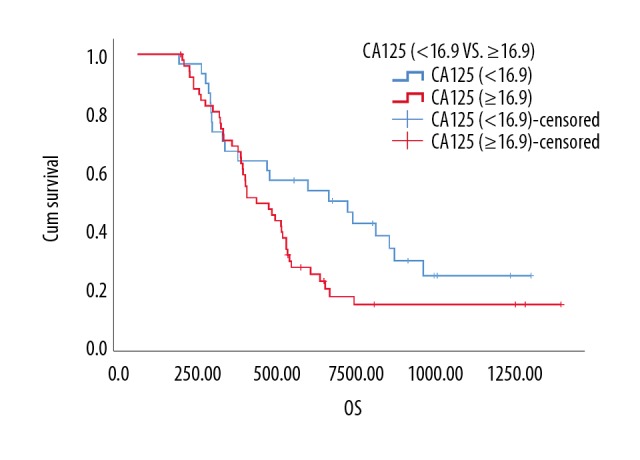
Kaplan-Meier analysis of the overall survival of CA125 (U/mL) in squamous cell carcinoma patients (P=0.049, n=79). CA125, cancer antigen 125.
Discussion
According to the NCCN and European Society for Medical Oncology guidelines, GP chemotherapy is one of the recommended first-line treatment options for patients with advanced and metastatic NSCLC [21,22]. The objective response rate with GP chemotherapy for NSCLC patients is approximately 30% [23], which is close to our result of 27.81%.
A few studies have demonstrated the ascendancy of GP for squamous cell carcinoma in ORR (objective response rate) and OS [24,25]. In our study, we also found ORR with GP chemotherapy for ADC patients was significantly lower than that in SqCC patients. Strangely, we found the SqCC patients had significant shorter OS than ADC patients with the condition of GP resistance. According to the existing literature reports, few follow-up treatment options are recommended for those with squamous cell and adenocarcinoma histology once the GP resistance happens [22]. It is seldom reported that squamous cell NSCLC patients got benefit from third generation EGFR-TKI targeted drugs or immunotherapy agents compared to adenocarcinoma histology. Therefore, it is particularly important to know the risk factors with GP resistance for lung squamous cell carcinoma, so that patients are not exposed to potentially harmful GP chemotherapy without benefits.
Recently, the relationship between GP resistance and platelet activation is getting more and more attention. The complex mechanism between platelets and GP resistance lies in epithelialization: increasingly activated platelets through direct contact or release of transforming growth factor beta 1 (TGFβ1) can activate epithelial-mesenchymal transition (EMT) in tumor cells [26–29]. Then a variety of adhesion proteins and transcriptional factors (Snail, Slug, and EMX2) are upregulated by epithelialization resulting in GP resistance [30–32].
PCT is the marker of platelet activation, which is obtained by multiplying PLT and MPV. Changes in the PCT have been reported in a small number of inflammatory diseases and cancer patients [33]. Higher PCT seems to be associated with tumorigenesis for epithelial ovarian cancer [34]. In this study, we found that PCT ≥0.235 had no significant link with outcomes but was a main risk factor for GP resistance for lung squamous cell carcinoma. PCT is a maker that seems to be mainly associated with platelet plaque formation [35], including tumor cell-induced platelet aggregation (TCIPA) through tumor cells and platelet-related interactions, resulting in the release of platelet cytokines to tumor cells, and finally affecting GP resistance [30,36]. Simultaneously, we found that MPV ≥10.85 was the only main risk factor with GP resistance for lung adenocarcinoma cell carcinoma. MPV level is regarded as a signal of abnormal platelet production and activation, especially for high platelet levels. Thus, larger platelets release more cytokines upon stimulation than smaller ones, and some of cytokines can hearten epithelial-mesenchymal transition (EMT) in tumor cells, leading to GP resistance [28,29,37,38]. This is the reason why MPV ≥10.85 was the only main risk factor with GP resistance for lung adenocarcinoma cell carcinoma in this research.
Generally, increasing the value of CA125 is often considered a sign of ineffective treatment after GP chemotherapy compared with before therapy. The mechanism involved may be related to enhance mesohaline-related EMT [38]. In our research, we found that CA125 ≥16.9 was more sensitive than CA125 ≥35 to predictive GP resistance for lung squamous cell carcinoma. Furthermore, we found that MPV ≥10.85 was more sensitive than CA125 ≥35 to predictive GP resistance for lung adenocarcinoma cell carcinoma, and PCT ≥0.235 was more sensitive than CA125 ≥35 to predictive GP resistance for lung squamous cell carcinoma. But we also found that CA125 ≥16.9 was more sensitive than PCT ≥0.235 to predictive GP resistance for lung squamous cell carcinoma. This suggests that GP resistance emerges at the cutoff value of 16.9 for CA125 but not 35.
Conclusions
PCT and MPV are important parameters showing platelet functions, and PCT and MPV decrease has been shown in earlier studies in colorectal cancer treated with bevacizumab [39]. In our study, MPV ≥10.85 was significantly related to GP resistance for lung adenocarcinoma cell carcinoma, and PCT ≥0.235 was significantly related to GP resistance for lung squamous cell carcinoma. Therefore, platelets and its activation index will be potential makers for predicting GP resistance. Furthermore, platelets and its activation index are likely to be used more extensively due to their low cost in comparison with serum tumor markers.
In clinical practice, the intense surveillance for platelet-related indicators of NSCLC is conducive to early understanding of the status of GP resistant for NSCLC, which is of great significance for disease assessment, treatment guidance, and complications prevention of NSCLC. With the pervasive application of platelet-related indicators in NSCLC, we expect to be able to effectively control the risk factors to reduce the morbidity and mortality of NSCLC.
To the best of our knowledge, this is the first study to use the ROC curve replaced Bonferroni test analysis. In this study, we confirmed that the ideal cutoff value of PCT was 0.235 for predicting GP resistance in patients with advanced stage IIIA–IV lung squamous cell carcinoma, and had a significantly higher area under the curve (AUC) value than CA125 ≥16.9; MPV was 10.85 for predicting GP resistance in patients with advanced stage IIIA–IV lung adenocarcinoma cell carcinoma. Besides, CA125 ≥16.9 was a potential marker for predicting GP resistance and OS, which was more sensitive than CA125 ≥35 as well.
There were some limitations to this study. This was a single-center, small sample, retrospective study; multicentric prospective studies are needed to reduce selection bias. In future research, we will make the value of platelet activation index and CA125 with GP resistance more precise and operable by increasing the clinical cases in future research.
Footnotes
Source of support: This research was financially supported by the National Key Research and Development Program of China (2017YFC0908602) and Fundamental Research Funds for the Central University, the National Natural Science Foundation of China (81403026 and 81430081), the Zhejiang Provincial Natural Sciences Foundation of China (Q18H290004 and LY17H310001), Zhejiang Province Medicine Health General Research Program (2015KYB071, 2017KY258, and 2018KY314), Zhejiang Pharmaceutical Association (2017ZYY14), Scientific Research Fund Project of Integrated Chinese and Western Medicine Institute in Zhejiang Province (2014LYZD017), the Zhejiang Provincial Program for the Cultivation of 151 Talents (Ping Huang), and the Zhejiang Provincial Program for the Cultivation of High-Level Innovative Heath Talents (Ping Huang), the 1022 Talent Training Program of Zhenjiang Cancer Hospital (Zeng Wang)
Conflict of interests
None.
References
- 1.McGuire S. World Cancer Report 2014. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO Press; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]; Adv Nutr. 2016;7(2):418–19. doi: 10.3945/an.116.012211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong J, Mao Y, Li J, He J. Stair-climbing test predicts postoperative cardiopulmonary complications and hospital stay in patients with non-small cell lung cancer. Med Sci Monit. 2017;23:1436–41. doi: 10.12659/MSM.900631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu D, Liang L, Nie L, et al. Efficacy, safety and predictive indicators of apatinib after multilines treatment in advanced nonsquamous non-small cell lung cancer: Apatinib treatment in nonsquamous NSCLC. Asia Pac J Clin Oncol. 2018 doi: 10.1111/ajco.12870. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Zhang K, Qin H, Pan F, et al. Nedaplatin or oxaliplatin combined with paclitaxel and docetaxel as first-line treatment for patients with advanced non-small cell lung cancer. Med Sci Monit. 2014;20:2830–36. doi: 10.12659/MSM.891318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin H, Wang C, Jiang Y, et al. Patients with single brain metastasis from non-small cell lung cancer equally benefit from stereotactic radiosurgery and surgery: A systematic review. Med Sci Monit. 2015;21:144–52. doi: 10.12659/MSM.892405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao Z, Li J, Jiang Y, et al. CLDN1 Increases drug resistance of non-small cell lung cancer by activating autophagy via up-regulation of ULK1 phosphorylation. Med Sci Monit. 2017;23:2906–16. doi: 10.12659/MSM.904177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kazaz SN, Oztop I. Immune checkpoint inhibitors in advanced-stage non-small cell lung cancer. Turk Thorac J. 2017;18(4):101–7. doi: 10.5152/TurkThoracJ.2017.17006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jang J, Kim HK, Cho BC, et al. Randomized phase II study comparing weekly docetaxel-cisplatin vs. gemcitabine-cisplatin in elderly or poor performance status patients with advanced non-small cell lung cancer. Cancer Chemother Pharmacol. 2017;79(5):873–80. doi: 10.1007/s00280-017-3289-6. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Gao J, Bai M, et al. The pretreatment platelet and plasma fibrinogen level correlate with tumor progression and metastasis in patients with pancreatic cancer. Platelets. 2014;25(5):382–87. doi: 10.3109/09537104.2013.827782. [DOI] [PubMed] [Google Scholar]
- 10.Buergy D, Wenz F, Groden C, Brockmann MA. Tumor-platelet interaction in solid tumors. Int J Cancer. 2012;130(12):2747–60. doi: 10.1002/ijc.27441. [DOI] [PubMed] [Google Scholar]
- 11.Liu W, Ha M, Yin N. Combination of platelet count and lymphocyte to monocyte ratio is a prognostic factor in patients undergoing surgery for non-small cell lung cancer. Oncotarget. 2017;8(42):73198–207. doi: 10.18632/oncotarget.18336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao L, Zhang H, Zhang, et al. Prognostic value of combination of preoperative platelet count and mean platelet volume in patients with resectable non-small cell lung cancer. Oncotarget. 2017;8(9):15632–41. doi: 10.18632/oncotarget.14921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui MM, Li N, Liu X, et al. Platelet distribution width correlates with prognosis of non-small cell lung cancer. Sci Rep. 2017;7(1):3456. doi: 10.1038/s41598-017-03772-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oncel M, Kiyici A, Oncel M, et al. Evaluation of platelet indices in lung cancer patients. Asian Pac J Cancer Prev. 2015;16(17):7599–602. doi: 10.7314/apjcp.2015.16.17.7599. [DOI] [PubMed] [Google Scholar]
- 15.He Y, Rozeboom L, Rivard CJ, et al. PD-1, PD-L1 protein expression in non-small cell lung cancer and their relationship with tumor-infiltrating lymphocytes. Med Sci Monit. 2017;23:1208–16. doi: 10.12659/MSM.899909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radziwon-Balicka A, Medina C, O’Driscoll L, et al. Platelets increase survival of adenocarcinoma cells challenged with anticancer drugs: Mechanisms and implications for chemoresistance. Br J Pharmacol. 2012;167(4):787–804. doi: 10.1111/j.1476-5381.2012.01991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng Q, Shi H, Wang H, et al. The ligation of aspirin to cisplatin demonstrates significant synergistic effects on tumor cells. Chem Commun (Camb) 2014;50(56):7427–30. doi: 10.1039/c4cc00419a. [DOI] [PubMed] [Google Scholar]
- 18.Zheng X, Carstens JL, Kim J, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527(7579):525–30. doi: 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 20.Altan B, Kaira K, Watanabe A, et al. Relationship between LAT1 expression and resistance to chemotherapy in pancreatic ductal adenocarcinoma. Cancer Chemother Pharmacol. 2018;81(1):141–53. doi: 10.1007/s00280-017-3477-4. [DOI] [PubMed] [Google Scholar]
- 21.Xu Y, Liu H, Chen J, Zhou Q. Comparisons between the National Comprehensive Cancer Network (NCCN) non-small-cell lung cancer (NSCLC) Clinical Practice Guidelines (Chinese version), the NCCN original edition, and the European Society for Medical Oncology NSCLC Guidelines in 2009. Thorac Cancer. 2010;1(2):83–86. doi: 10.1111/j.1759-7714.2010.00016.x. [DOI] [PubMed] [Google Scholar]
- 22.Novello S, Barlesi F, Califano R, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(Suppl 5):v1–27. doi: 10.1093/annonc/mdw326. [DOI] [PubMed] [Google Scholar]
- 23.Ai D, Guan Y, Liu XJ, et al. Clinical comparative investigation of efficacy and toxicity of cisplatin plus gemcitabine or plus Abraxane as first-line chemotherapy for stage III/IV non-small-cell lung cancer. Onco Targets Ther. 2016;9:5693–98. doi: 10.2147/OTT.S109683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schiller JH, Harrington D, Belani CP, et al. Cooperative Oncology, Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346(2):92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 25.Wu YL, Lu S, Cheng Y, et al. Efficacy and safety of pemetrexed/cisplatin versus gemcitabine/cisplatin as first-line treatment in Chinese patients with advanced nonsquamous non-small cell lung cancer. Lung Cancer. 2014;85(3):401–7. doi: 10.1016/j.lungcan.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20(5):576–90. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guillem-Llobat P, Dovizio M, Bruno MA, et al. Aspirin prevents colorectal cancer metastasis in mice by splitting the crosstalk between platelets and tumor cells. Oncotarget. 2016;7(22):32462–77. doi: 10.18632/oncotarget.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma D, Brummel-Ziedins KE, Bouchard BA, Holmes CE. Platelets in tumor progression: A host factor that offers multiple potential targets in the treatment of cancer. J Cell Physiol. 2014;229(8):1005–15. doi: 10.1002/jcp.24539. [DOI] [PubMed] [Google Scholar]
- 29.Naugler WE, Karin M. The wolf in sheep’s clothing: The role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med. 2008;14(3):109–19. doi: 10.1016/j.molmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Haslehurst AM, Koti M, Dharsee M, et al. EMT transcription factors snail and slug directly contribute to cisplatin resistance in ovarian cancer. BMC Cancer. 2012;12:91. doi: 10.1186/1471-2407-12-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsukasa K, Ding Q, Yoshimitsu M, et al. Slug contributes to gemcitabine resistance through epithelial-mesenchymal transition in CD133(+) pancreatic cancer cells. Human Cell. 2015;28(4):167–74. doi: 10.1007/s13577-015-0117-3. [DOI] [PubMed] [Google Scholar]
- 32.Yue D, Li H, Che J, et al. EMX2 is a predictive marker for adjuvant chemotherapy in lung squamous cell carcinomas. PLoS One. 2015;10(7):e0132134. doi: 10.1371/journal.pone.0132134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerday E, Baer VL, Lambert DK, et al. Testing platelet mass versus platelet count to guide platelet transfusions in the neonatal intensive care unit. Transfusion. 2009;49(10):2034–39. doi: 10.1111/j.1537-2995.2009.02253.x. [DOI] [PubMed] [Google Scholar]
- 34.Ma X, Wang Y, Sheng H, et al. Prognostic significance of thrombocytosis, platelet parameters and aggregation rates in epithelial ovarian cancer. J Obstet Gynaecol Res. 2014;40(1):178–83. doi: 10.1111/jog.12151. [DOI] [PubMed] [Google Scholar]
- 35.Mahdavi-Zafarghandi R, Shakiba B, Keramati MR, Tavakkoli M. Platelet volume indices in patients with varicocele. Clin Exp Reprod Med. 2014;41(2):92–95. doi: 10.5653/cerm.2014.41.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goubran HA, Stakiw J, Radosevic JM, Burnouf T. Platelet-cancer interactions. Semin Thromb Hemost. 2014;40(3):296–305. doi: 10.1055/s-0034-1370767. [DOI] [PubMed] [Google Scholar]
- 37.Kai H, Kitadai Y, Kodama M, et al. Involvement of proinflammatory cytokines IL-1beta and IL-6 in progression of human gastric carcinoma. Anticancer Res. 2005;25(2a):709–13. [PubMed] [Google Scholar]
- 38.Osada J, Rusak M, Kamocki Z, et al. Platelet activation in patients with advanced gastric cancer. Neoplasma. 2010;57(2):145–50. doi: 10.4149/neo_2010_02_145. [DOI] [PubMed] [Google Scholar]
- 39.Mutlu H, Berk V, Karaca H, et al. Treatment regimen with bevacizumab decreases mean platelet volume in patients with metastatic colon cancer. Clin Appl Thromb Hemost. 2012;18(5):546–48. doi: 10.1177/1076029611430958. [DOI] [PubMed] [Google Scholar]


